Abstract
Both objective and perceived social isolations were associated with future cognitive decline and increase risk of Alzheimer’s disease (AD). However, the impacts of perceived social isolation depending on different clinical stages of AD have not been elucidated. The aim of this study was to investigate the influence of perceived social isolation or loneliness on brain structure and future cognitive trajectories in patients who are living with or are at risk for AD. A total of 176 elderly patients (mean age of 78 years) who had complaint of memory problems (39 subjective cognitive decline [SCD], 53 mild cognitive impairment [MCI], 84 AD) underwent structural MRI and neuropsychological testing. Loneliness was measured by one binary item question “Do you often feel lonely?.” Voxel-based morphometry was conducted to evaluate regional gray matter volume (rGMV) difference associated with loneliness in each group. To evaluate individual differences in cognitive trajectories based on loneliness, subgroup analysis was performed in 51 patients with AD (n = 23) and pre-dementia status (SCD-MCI, n = 28) using the longitudinal scores of Alzheimer’s Disease Assessment Scale-cognitive component-Japanese version (ADAS-Jcog). Whole brain VBM analysis comparing lonely to non-lonely patients revealed loneliness was associated with decreased rGMV in bilateral thalamus in SCD patients and in the left middle occipital gyrus and the cerebellar vermal lobules I − V in MCI patients. Annual change of ADAS-Jcog in patients who reported loneliness was significantly greater comparing to these non-lonely in SCD-MCI group, but not in AD group. Our results indicate that perceived social isolation, or loneliness, might be a comorbid symptom of patients with SCD or MCI, which makes them more vulnerable to the neuropathology of future AD progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00584-6.
Keywords: Perceived social isolation, Subjective cognitive decline, Alzheimer’s disease, Cognitive trajectory, Voxel-based morphometry
Introduction
As the world’s aging population increases, the number of people with Alzheimer’s disease (AD) and other dementias is rising [1]. Dementia has been found to contribute to social isolation in later life [2]. Social isolation includes objective social isolation and perceived social isolation. The former usually is defined as an inadequate quality and quantity of social connectedness, such as living alone, lack of social activities, and a small social network [3, 4]. In contrast, perceived social isolation, or loneliness, refers to a discrepancy between one’s subjective expectancy regarding social situations and achieved levels of social connectedness [5, 6].
Previous findings on loneliness and AD were inconsistent [7–10]. For instance, evidence from two cohort studies found subjectively feeling lonely, but not the objective social isolation (charactered as living alone, small social network size, and lower social activity), was associated with increased risk of AD, even after adjusting for depression and the AD risk factors APOE ε4 [3, 11]. Moreover, in a recent 20-year cohort study, perceived social isolation was measured with a single question (“Do you feel loneliness?”) and was associated with an increased risk of all-cause dementia and AD [12]. Nevertheless, two studies reported that risk of dementia was not increased in these participants who subjectively reported loneliness [13, 14]. This inconsistence can be explained by the trajectory of loneliness. Different pattern of loneliness trajectory (persistent, incident, and transient) may play different role in the context of developing AD. In fact, in a recent study, Akhter-Khan et al. [15] using one item loneliness derived from the Center for Epidemiologic Studies Depression Scale found that persistent loneliness was independently associated with an increased risk of AD, while recovery from loneliness (transient loneliness) is protective for the development of AD.
Neuronal loss in AD predominantly arises in the medial temporal lobe, including the hippocampus and amygdala [16], as well as in the thalamus and putamen [17]. Magnetic resonance imaging (MRI) is a non-invasive and reliable tool that can be used to monitor disease progression in AD [18]. Voxel-based morphometry (VBM) is an automated and objective method of analyzing brain images, with highly reproducible results [18]. Several VBM studies have revealed that loneliness is correlated with reduced gray matter volume in brain regions related to cognitive processing and emotional regulation, such as the anterior hippocampus, amygdala, and left cerebellum [19]; the left posterior superior temporal sulcus [20, 21]; the dorsolateral prefrontal cortex [22]; and the cerebellar vermis lobule [23].
Recent studies have uncovered novel information regarding the neurophysiological characteristics of loneliness with respect to the onset of AD. In two cross-sectional studies using positron emission tomography imaging, loneliness was associated with a higher brain amyloid-β burden, especially in APOEε4 carriers [24], and greater tau pathology [25], suggesting that loneliness may be a sensitive clinical marker of AD pathological brain changes in older people. Furthermore, perceived loneliness predicted AD-related gene expression, even after controlling for baseline cognitive decline [26]. The regulatory loop theory of loneliness [27] suggests that feeling socially isolated may trigger increased sensitivity to social threats, which can lead to maladaptive cognition, such as holding more negative social expectations and more strongly remembering negative social events. This kind of dysregulation may generate differences in brain regions that are functionally involved in processing, expressing, and regulating emotionally and socially relevant information [3].
Although loneliness has been found to affect the risk of AD in cognitively healthy older adults, the manifestation of loneliness in brain structures at different stages of AD remains elusive. Exploring loneliness and brain structure at different stages of AD will contribute to our understanding on the impact of loneliness in the pathophysiology of AD and may help for prevention and novel treatment of AD. The aim of the present study was to investigate loneliness, gray matter volume, and future cognitive change in individuals living with or at risk of AD, including those with subjective cognitive decline (SCD), mild cognitive impairment (MCI), and AD.
Methods
Participants
We consecutively recruited 235 participants from the memory clinic at Tohoku University Hospital during the period from December 2018 to March 2021. All participants underwent a multidisciplinary diagnostic evaluation including a structured clinical interview, a neurological examination, neuropsychological testing, blood tests, and neuroimaging including structural MRI and N-isopropyl-p-123I-iodoamphetamine single photon emission computed tomography. Clinical diagnoses were determined by experienced clinicians. Diagnosis of AD was based on the criteria of the National Institute on Aging-Alzheimer’s Association (NIA-AA) working group [28]. We included both “possible” and “probable” AD patients. Diagnosis of MCI was based on the NIA-AA criteria: objective cognitive impairment related to memory as well as other cognitive domains, but with unaffected overall mental function and activities of daily life [29]. A diagnosis of SCD was given to individuals with subjective cognitive complaints but for whom the results of clinical assessments, including neuropsychological assessments and neuroimaging, did not indicate any neurological or psychiatric disorders [30]. Previous studies have shown that loneliness is highly correlated with depression scores [31, 32], and that, conceptually, depression is very similar to loneliness. Therefore, we used strict exclusion criteria to control for irrelevant variables, including depression. The exclusion criteria were cerebral stroke, brain injury, cerebral hemorrhage, cerebral arteriosclerosis, Parkinson’s disease, depression, schizophrenia, other non-AD dementias, and a lack of a clear diagnosis. We excluded 59 patients, leaving a total of 176 patients (39 SCD, 53 MCI, 84 AD) who were enrolled in the present study (for the data exclusion process see Fig. 1). Due to a limited number of study participants who had the 15-item Japanese version of the Geriatric Depression Scale (GDS) (n = 142 in total participants; n = 41 in longitudinal cognitive data), we conducted additional multiple regression analysis to examine whether depression score is a significant confounder in cognitive tests (Supplementary Table S1). As a result, we found that GDS score was not a significant predictor of any cognitive tests.
Fig. 1.
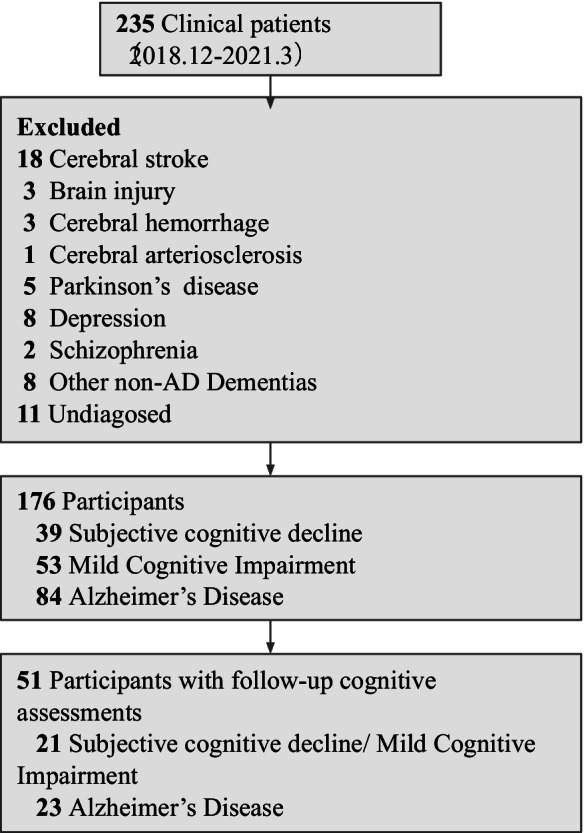
Subject flow diagram
This study adhered to the tenets of the Declaration of Helsinki, and the protocols were approved by the Clinical Research Ethics Committee of the Tohoku University Graduate School of Medicine (approval number: 2018–1-618). The requirement of informed consent was waived, and an opt-out method was used given the retrospective study design. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) on February 6, 2019 (UMIN000035762).
Assessment of loneliness
Loneliness was measured using one binary question, “Do you often feel lonely?,” administered as part of our neuropsychological assessments. Previous studies have also used binary questions to assess loneliness [15, 33], with similar results to those obtained with the three-item UCLA loneliness scale [34]. A single question or simple loneliness scale has been found to be more adaptable to research in older people. Furthermore, the one item measuring loneliness from the Center for Epidemiologic Studies Depression Scale predicted AD risk better than the sum of the remaining nine items [3]. We also included one item to determine living situation: living alone or not.
MRI acquisition and processing
All three-dimensional T1-weighted magnetization-prepared rapid acquisition gradient echo structural images were acquired using a 3-Tesla Philips Achieva scanner with a 32-channel head coil. The scanning parameters were as follows: repetition time, 8.70 ms; echo time, 3.1 ms; 8° flip angle; field of view, 256 × 256 × 180 mm; and voxel size, 0.7 × 0.7 × 0.7 mm. The structural MRI data were analyzed using Statistical Parametric Mapping software (SPM12; Welcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks, Inc., Natick, MA). Preprocessing entailed the following four main steps. First, T1-weighted structural images were reoriented using an automated reorienting script (https://www.nemotos.net/?p=1892) in MATLAB. Second, the reoriented T1-weighted images were segmented into six tissues including gray matter, white matter, cerebrospinal fluid, soft tissue, skull, and non-brain regions using the new segmentation algorithm implemented in SPM12. After segmentation, we used the DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) registration process to spatially normalize the tissue probability maps obtained by the aforementioned method to Montreal Neurological Institute (MNI) space. This yielded images with 1.5 × 1.5 × 1.5 mm3 voxels. Subsequently, all images were smoothed via convolution with an isotropic Gaussian kernel of 8 mm full width at half maximum. The total brain volume (TBV) was calculated by combining the total volume of the gray and white matter.
Assessment of longitudinal cognitive changes
To further investigate the relationships between social isolation and follow-up cognitive change in clinical AD patients, we included patients with longitudinal cognitive assessments. Specifically, we ask patients to undergo a follow-up visit every 12 months after the initial visit. The follow-up neuropsychological assessment only includes Alzheimer’s Disease Assessment Scale Cognitive Subscale Japanese version (ADAS-Jcog). Out of the 176 patients, 51 (28 SCD-MCI patients, 23 AD patients) underwent follow-up assessment of cognitive function (average follow-up time: 373 days). ADAS-Jcog is a validated and structured scale often used to measure cognitive change in clinical AD patients [35, 36]. The ADAS-Jcog score ranges from 0 to 70, with higher scores indicating worse cognitive function.
Statistical analysis
Data are presented as the mean ± SD for continuous variables and frequency and proportions for categorical variables, unless otherwise indicated. Analyses were performed using SPSS Statistics version 24.0 (IBM, Armonk, NY, USA) with the significance level determined by a two-tailed value of P < 0.05. In the descriptive analysis, the participants were divided into the SCD group, MCI group, and AD group; an analysis of variance was used for continuous variables; and the Chi-square test was used for categorical variables. For the follow-up cognitive assessments, annual cognitive changes were examined.
For the whole-brain VBM analysis, we conducted an independent t-test to detect differences in rGMV between lonely participants and non-lonely participants in the SCD group, MCI group, and AD group. Age, sex, Mini-Mental State Examination (MMSE) scores, and TBV were entered as covariates. To adjust for multiple comparisons, the significance threshold was set to the cluster level of the family wise error rate (FWE) < 0.05. Next, we created a region of interest (ROI) mask determined by the clusters with significant differences in the whole brain VBM analysis using WFU Pickatlas [37]. The gray matter volumes in these brain regions were then extracted using the ROI mask to further examine the rGMV difference in the three groups.
Results
The demographic data are summarized according to the diagnostic group in Table 1. We excluded 59 of the original 235 patients, leaving a total of 176 patients, who we divided into three groups: 39 patients with SCD (mean age: 73 years; lonely patients: 38%), 53 patients with amnestic MCI (mean age: 78 years; lonely patients: 32%), and 84 patients with AD (mean age: 78 years; lonely patients: 45%).
Table 1.
Demographic characteristics of the study participants for each dementia status1
| SCD | MCI | AD | All groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-lonely (n = 24) | Lonely (n = 15) | P | Non-lonely (n = 36) | Lonely (n = 17) | P | Non-lonely (n = 46) | Lonely (n = 38) | P | Non-lonely (n = 106) | Lonely (n = 70) | P | |
| Age (years) | 70.8 ± 12.6 | 78.6 ± 14.2 | 0.080 | 77.6 ± 6.6 | 79.5 ± 8.0 | 0.359 | 78.1 ± 7.4 | 81.0 ± 6.1 | 0.062 | 76.3 ± 9.0 | 80.1 ± 8.8 | 0.007 |
| Sex (male, %) | 14 (58%) | 7 (47%) | 0.480 | 15 (41%) | 2 (12%) | 0.029 | 15 (33%) | 13 (34%) | 0.877 | 44 (44%) | 22 (31%) | 0.192 |
| Education (years) | 13.6 ± 2.8 | 12.7 ± 2.4 | 0.341 | 13.0 ± 2.4 | 11.8 ± 2.3 | 0.090 | 13.1 ± 2.7 | 12.1 ± 2.4 | 0.085 | 13.2 ± 2.6 | 12.2 ± 2.3 | 0.010 |
| Immediate family members (n) | 3.3 ± 1.5 | 3.8 ± 1.3 | 0.328 | 3.5 ± 1.5 | 3.8 ± 1.5 | 0.479 | 4.0 ± 1.2 | 4.1 ± 1.4 | 0.540 | 3.7 ± 11.4 | 4.0 ± 11.4 | 0.135 |
| BMI | 24.2 ± 4.0 | 22.9 ± 3.9 | 0.304 | 21.7 ± 3.0 | 22.4 ± 3.2 | 0.410 | 21.7 ± 3.5 | 21.9 ± 3.0 | 0.823 | 22.3 ± 3.6 | 22.2 ± 3.3 | 0.900 |
| MMSE | 28.0 ± 2.5 | 25.9 ± 3.7 | 0.050 | 25.1 ± 2.7 | 24.2 ± 4.3 | 0.467 | 20.9 ± 5.1 | 19.4 ± 4.0 | 0.128 | 23.9 ± 4.9 | 22.0 ± 4.9 | 0.012 |
| Living alone (%) | 5 (21%) | 2 (13%) | 0.550 | 4 (11%) | 5 (30%) | 0.098 | 3 (7%) | 12 (32%) | 0.003 | 12 (11%) | 19 (27%) | 0.006 |
| Drinking habits2 | 17 (71%) | 6 (40%) | 0.057 | 11 (31%) | 4 (24%) | 0.596 | 20 (44%) | 17 (45%) | 0.908 | 48 (45%) | 27 (39%) | 0.408 |
| Smoking habits3 | 7 (29%) | 6 (40%) | 0.485 | 11 (31%) | 3 (18%) | 0.320 | 11 (24%) | 12 (32%) | 0.433 | 29 (2%) | 21 (30%) | 0.675 |
| TBV | 1000.8 ± 102.4 | 925.8 ± 138.9 | 0.060 | 915.0 ± 76.1 | 861.4 ± 94.2 | 0.031 | 861.2 ± 77.8 | 859.2 ± 72.9 | 0.904 | 911.5 ± 98.4 | 874.0 ± 97.8 | 0.140 |
| GMV | 554.2 ± 56.8 | 490.8 ± 89.3 | 0.010 | 503.3 ± 45.5 | 480.2 ± 49.4 | 0.099 | 474.3 ± 49.3 | 471.9 ± 46.2 | 0.815 | 502.4 ± 58.0 | 478.0 ± 58.2 | 0.007 |
1Values are expressed as mean ± SD. Calculated using a two tailed t-test for continuous variables and the χ2 test for categorical variables. 2Drinking habits were assessed by asking the participants whether they have a habit to drinking alcohol. 3Smoking habits were measured by asking the participants whether they have a habit of smoking cigarettes. AD, a patient group with Alzheimer’s disease; BMI, body mass index; GMV, gray matter volume; MCI, a participant group with mild cognitive impairment; MMSE, Mini-Mental State Examination; SCD, a participant group diagnosed as subjective cognitive decline; TBV, total brain volume
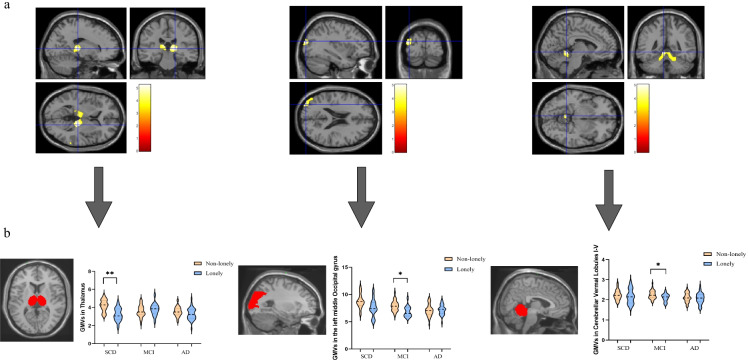
Table 1 shows tendencies that lonely patients were more likely to be older, female, have a lower MMSE score, and be less educated than non-lonely patients in each group. Additionally, lonely patients in each group also showed tendencies with lower TBV and lower GMV and were more likely to live alone, compared with non-lonely patients (Fig. 2).
Fig. 2.
Brain regions showing smaller gray matter volume in lonely subjects when compared with non-lonely subjects after controlled for age, sex, MMSE, and total brain volume. a Clusters of smaller gray matter volume in region of the thalamus in subjective cognitive decline group; clusters of smaller rGMV in the region of cerebellar vernal lobules and the left middle occipital gyrus in mild cognitive impairment group. b ROI masks were created for the thalamus, the middle occipital gyrus, and the cerebellar vermal lobules I − V, respectively. Then the gray matter volume of these three regions was extracted for further two-tail t-test controlled for age, sex, MMSE, and total brain volume in each group. *P < 0.05, **P < 0.001
Despite age in SCD group, sex in MCI group, and education years in all patients between lonely and non-lonely patients reached a significant level, caution is needed when interpreting these results due to a limited sample size and a failure to control other important confounders.
Out of the 176 patients, 51 for whom we had longitudinal ADAS-Jcog scores were classified into the SCD-MCI and AD group. This was done for two reasons. First, the ADAS-Jcog is not only a cognitive assessment used in clinical AD trials, but is also employed in pre-dementia patients, such as those with MCI and SCD, to detect cognitive changes at earlier stages of disease progression [38]. Second, in the present study, we had a limited number of longitudinal patients, and individuals with SCD and MCI are particularly at risk for developing AD. Therefore, we divided these patients into two groups instead of three to improve the statistical power of the analysis (Table 2).
Table 2.
Brain regions with smaller rGMV in lonely subjects diagnosed as pre-AD dementia status (SCD [n = 39] and MCI [n = 53]) compared with non-lonely subjects
| Brain regions | MNI coordinates | T value | P | Cluster size (voxels) | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| SCD | Right thalamus | 18 | − 26 | 4 | 5.27 | 0.020 (FWE-corr) | 1002 |
| Left thalamus | − 8 | − 24 | 12 | 4.77 | 0.050 (FWE-corr) | 771 | |
| MCI | Cerebellar vermal lobules | − 6 | − 45 | − 6 | 5.04 | 0.004 (FEW-corr) | 1474 |
| Left middle occipital gyrus | − 36 | − 88 | 18 | 4.86 | 0.031 (uncorrected) | 477 | |
AD, Alzheimer’s disease; FWE-corr, family wise error corrected; MCI, mild cognitive impairment; rGMV, regional gray matter volume; MNI coordinates, Montreal Neurological Institute coordinates; SCD, subjective cognitive decline
In the SCD-MCI group, the lonely patients were older, had fewer years of education, had lower ADAS-Jcog baseline scores, and had a greater annual degree of change in ADAS-Jcog score, although this was marginally significant (P = 0.08, Cohen’s d = − 0.801). In the AD group, the lonely patients had higher ADAS-Jcog baseline scores and a smaller degree of annual ADAS-Jcog change when compared with the non-lonely patients (Table 2). Our analysis of co-variants, after adjusting for age and sex, showed that loneliness was a significant predictor (P = 0.05) of annual ADAS-Jcog change in the SCD-MCI group and not the AD group (P = 0.40) (Fig. 3).
Fig. 3.
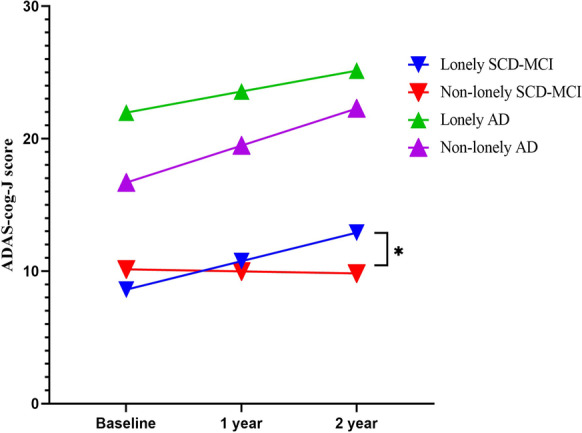
Prediction of annual ADAS-Jcog change in each group. The changes in follow-up ADAS-Jcog score in the SCD-MCI group indicate that the patients with loneliness had a significantly greater degree of serial cognitive decline compared with those without loneliness. *P < 0.05
The whole brain VBM analysis (Table 3) revealed decreased rGMV in bilateral thalamus (Fig. 2, left-side panel) in the SCD group of lonely participants.
Table 3.
Demographic characteristics associated with loneliness in participants diagnosed as AD and pre-AD dementia status (SCD-MCI)1
| SCD-MCI | P | AD | P | |||
|---|---|---|---|---|---|---|
| Non-lonely (n = 20) | Lonely (n = 8) | Non-lonely (n = 17) | Lonely (n = 6) | |||
| Age | 75.3 ± 9.8 | 80.1 ± 7.6 | 0.180 | 77.8 ± 9.4 | 79.3 ± 10.7 | 0.495 |
| Sex (male, %) | 10 (50%) | 4 (50%) | 1.000 | 5 (29%) | 2 (25%) | 0.858 |
| Education (years) | 13.2 ± 1.96 | 11.3 ± 2.6 | 0.082 | 13.3 ± 2.9 | 11.7 ± 3.1 | 0.256 |
| ADAS-Jcog scores | ||||||
| Baseline | 10.1 ± 3.7 | 8.6 ± 3.4 | 0.322 | 16.7 ± 7.6 | 22.0 ± 9.6 | 0.184 |
| Annual change | − 0.2 ± 3.2 | 2.2 ± 2.5 | 0.080 | 2.8 ± 5.8 | 1.2 ± 3.0 | 0.390 |
1Values are expressed as mean ± SD. Calculated using two-tailed t-tests for continuous variables and the χ2 test for categorical variables. AD, patients with Alzheimer’s disease; ADAS-Jcog, Alzheimer’s Disease Assessment Scale-cognitive component-Japanese version; SCD-MCI, group containing patients with subjective cognitive decline and those with mild cognitive impairment
In the MCI group, lonely patients exhibited decreased rGMV in the cerebellar vermal lobules I − V (Fig. 2, middle panel) and the region containing the left middle occipital gyrus (MOG) (Fig. 2, right-side panel). We found no significant difference in rGMV between the lonely and non-lonely patients in the AD group (Table 1).
Subsequently, we created ROI masks separately for the thalamus, the cerebellar vermal lobules, and the middle occipital gyrus, and extracted the rGMV in the corresponding three regions to conduct post-hoc two-tailed t-tests in the SCD, MCI, and AD groups separately. In the SCD group, the rGMV of the thalamus was decreased in lonely patients compared with non-lonely patients, after controlling for age, sex, MMSE scores, and TBV. Furthermore, patients with self-reported feelings of loneliness in the MCI group showed decreased rGMV in the cerebellar vermal lobules and the middle occipital gyrus (Fig. 2b).
Discussion
Our VBM analysis in which we compared lonely to non-lonely patients revealed that loneliness was associated with decreased rGMV: (1) in the bilateral thalamus in SCD patients and (2) in the left middle occipital gyrus and cerebellar vermal lobules I − V in MCI patients independent of age, sex, MMSE, and TBV. Additionally, the follow-up cognitive change data indicated that patients in the SCD-MCI group who reported subjective feelings of social isolation had a significantly greater degree of annual ADAS-Jcog change compared with those who were not lonely. To the best of our knowledge, this study is the first to examine loneliness, brain structure, and cognitive change in clinical AD patients.
Lonely patients with SCD showed decreased rGMV in the bilateral thalamus when compared with non-lonely patients with SCD. Thalamus is involved in multiple cognitive functions [39], including declarative memory [40]. Moreover, the anterior thalamic nucleus is a key component of the Papez circuit, which, if damaged, often gives rise to problems with episodic memory and is highly correlated with memory impairment and the evolution of AD [41]. Decreased GMVs and WMVs in thalamus have been consistently reported in amnestic MCI [42, 43], and the severity of thalamus atrophy is positively correlated with MMSE scores [44]. However, previous VBM studies investigating the relationship between loneliness and decreased rGMV have mainly reported changes in the medial and dorsolateral prefrontal cortex, anterior insula, amygdala, hippocampus, posterior superior temporal lobe, ventral striatum, and cerebellum [45]. This discrepancy could be explained by the difference in the health status of the participants. Most previous studies mainly focused on cognitively healthy adults, whereas our study targeted clinical patients with subjectively reported cognitive decline. Furthermore, using detailed medical history, we implemented strict exclusion criteria to avoid the influences of other related diseases or procedures, such as cerebral stroke and brain surgery.
Another main finding was that the patients with MCI who experienced loneliness showed decreased rGMV in the left MOG and cerebellar vermal lobules. The MOG is involved in visual information processing and the perception of facial emotion [46]. This result partially supports the findings of Li and colleagues [47], who reported decreased functional connectivity strength in the left-MOG in patients with MCI who developed AD compared with those who did not. The cerebellar vermal lobules are not only related to motor control, but also influence multiple domains of cognitive function, such as cognitive flexibility and working memory [48]. Notably, the cerebellum is also involved in visuomotor coordination [49]. Given our finding of decreased rGMV in the left-MOG, loneliness may influence AD via changes in visual-related brain function. Previous neuroimaging studies have suggested that experiences of loneliness can alter activity in brain regions related to visual, attentional, and emotional processes [27, 50]. Consistent with this idea, empirical behavioral studies have suggested that the maintenance of loneliness is associated with interpersonal cognitive biases regarding social threat-related information, which usually manifests as visual biases [32, 51, 52].
The changes in follow-up ADAS-Jcog scores in the SCD-MCI group indicate that the patients with loneliness had a significantly greater degree of serial cognitive decline compared with those without loneliness. For lonely patients in the early stages of AD, the neuropathology of loneliness may influence neural systems related to cognition and memory that could increase susceptibility to the deleterious effects of early AD neuropathology [3]. Inadequate social interaction may affect the neurogenesis and synaptic density of the brain, making individuals less able to compensate for other neural systems compromised by AD-related neuropathology [27].
In this study, we could not detect significant associations between loneliness and gray matter volume, or significant cognitive changes in the AD group. Emotional alterations such as severe loss of empathy are common symptoms in the progression of AD. An impaired ability to recognize and share subjective emotional experiences is important in generating loneliness feeling [4]. Therefore, loneliness may have a lesser impact on brain morphology and cognitive trajectories in AD patients. Another alternative explanation is that the one-item loneliness scale may not be sufficiently accurate in terms of measuring the real experience of loneliness in patients with AD. Some clinical assessments of neuropsychiatric AD symptoms require a certain level of cognitive ability. For instance, a previous study suggested that the Depressive Signs Scale is not suitable or accurate for assessing depressive symptoms among patients with AD [53]. A combination of measurements is needed to assess the real-life experience of loneliness and its relationship with AD.
Our study had some limitations. First, despite the clinical assessments measuring loneliness, the single self-reported loneliness question might not have accurately measured the real-life experience of loneliness. This loneliness question, which directly asks about subjective feelings of loneliness, has been widely used in previous research. Other multidimensional scales of loneliness focus on personal experiences related to social networks and social relationships. The concept of loneliness may be difficult to understand for those who have severe AD and may vary according to cultural background and identity. Second, our study was based on a limited sample size because there were only 176 clinical patients included, with longitudinal cognitive scores from 51 patients. We assessed loneliness at a single time-point, and thus its transient effect, which has shown to be associated with lower risk for AD [15], was uncertain. Thus, our methods should be replicated in a larger longitudinal study. Third, despite both individuals with SCD and MCI are particularly at risk for developing AD, we recognized that patients with SCD and patients with MCI were classified into one group due to a limited number of longitudinal data. Our results underline the detrimental effect of loneliness in the neuropathology of AD progression among those who are at high risk of AD. However, we failed to clarify in which pre-dementia status (SCD or MCI) loneliness may lead to greater detrimental impact on greater future cognitive decline, even converting to future AD. Future research could elucidate the impact of loneliness on different stages of AD and its conversion into AD through a large-scale follow-up study. Fourth, despite SCD was defined as self-perceived decline without objectively impaired cognitive function and the diagnosis of SCD was being made by experienced clinicians based on multidisciplinary diagnostic evaluation, we recognize that some SCD patients in our study showed an unexpected lower MMSE score. MMSE is not a standardized cognitive test included in the diagnosis of SCD, but a mandatory criterion for SCD is that individuals maintain normal cognitive function [30]. Therefore, an unexpected lower MMSE score may, to some extent, suggest a possibility of cognitive impairment among these SCD patients. Given the fact that subjectively reported decline in cognition is a core criterion both in the diagnosis of SCD and MCI and there is a lack of common concept and standardized measurements in clinical diagnosis of SCD, we cannot preclude the possibility that some of the SCD patients may overlap with patients with MCI. More concrete guidelines or standardized cognitive tests are needed to distinguish SCD from MCI in the context of different cultural backgrounds. Lastly, despite trying to control the possible confounding effect of depressive scores by excluding 8 patients with depression and our additional analysis (see supplementary Table S1) has clarified the minor effect of GDS on cognitive tests in our study sample, our results should be treated with caution due to lack of controlling some important confounders, such as depressive scores.
Conclusion and perspective
In the context of the increasing AD population and the long-lasting effects of the COVID-19 pandemic, an increasing number of individuals with AD-related conditions are likely facing social disconnectedness and loneliness. While previous studies have mainly focused on cognitively normal older adults, the effect of loneliness on patients who are at high risk of AD is not well-understood. Our study demonstrates that loneliness may be a comorbid symptom in patients with SCD or MCI, making them potentially more vulnerable to the neuropathology of future AD progression. Specific assessments are needed to identify loneliness among clinical patients who are at high risk of AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Sayaka Makabe and Kiyoe Katsura (Institute of Development, Aging and Cancer, Tohoku University) for their clerical support and data management. We thank Sydney Koke, MFA, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- AD
Alzheimer’s disease
- ADAS-Jcog
Alzheimer’s Disease Assessment Scale-cognitive component-Japanese version
- DARTEL
Diffeomorphic anatomical registration through exponentiated lie algebra
- FEW
Family wise error rate
- MCI
Mild cognitive impairment
- MMSE
Mini-mental state examination
- MNI
Montreal Neurological Institute
- MOG
Middle occipital gyrus
- MRI
Magnetic resonance imaging
- NIA-AA
National Institute on Aging-Alzheimer’s Association
- rGMV
Regional gray matter volume
- SCD
Subjective cognitive decline
- TBV
Total brain volume
- UMIN-CTR
University Hospital Medical Information Network Clinical Trials Registry
- VBM
Voxel-based morphometry
Author contribution
ZY, YTat, and TM co-designed the study. YTat and TNag were responsible for scanning the subjects and supporting the image technology. YTat, NT, SY, TN, MM, and YTaka collected the data. ZY and LY analyzed the data. ZY wrote the first draft of the manuscript. TM and YTaki supervised this study. All authors have contributed to and approved the final manuscript.
Funding
This work was supported by the Advanced Graduate Program for Future Medicine and Health Care, Tohoku University. Yasuko Tatewaki was supported by the TUMUG Support Project (Project to Promote Gender Equality and Female Researchers) of Tohoku University. This work was partly supported by the JST COI (Center of Innovation Science) Program Grant Number JPMJCE1303. Per contractual agreement, the funder had no role in the design of the study; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Data availability
Anonymized data of patients are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was in accordance with the Helsinki Declaration and national ethical standards. The institutional review board of our center approved the study protocol (approval number: 2018–1-618). This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) on February 6, 2019 (UMIN000035762).
Consent to participate
The requirement of informed consent was waived, and an opt-out method was used given the retrospective study design.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garre-Olmo J. Epidemiology of Alzheimer’s disease and other dementias. Rev Neurol. 2018;66(11):377–86. 10.33588/rn.6611.2017519. [PubMed]
- 2.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 4.Zavaleta D, Samuel K, Mills C. Social isolation: a conceptual and measurement proposal. OPHI WORKING PAPER 2014 January 2014:Available at: http://www.ophi.org.uk/social-isolation-a-conceptual-and-measurement-proposal/.
- 5.Jackson J, Cochran SD. Loneliness and psychological distress. J Psychol. 1991;125(3):257–262. doi: 10.1080/00223980.1991.10543289. [DOI] [PubMed] [Google Scholar]
- 6.Shibata M, Ohara T, Hosoi M, Hata J, Yoshida D, Hirabayashi N, Morisaki Y, Nakazawa T, Mihara A, Nagata T, et al. Emotional loneliness is associated with a risk of dementia in a general Japanese older population: the Hisayama study. J Gerontol B Psychol Sci Soc Sci. 2021;76(9):1756–1766. doi: 10.1093/geronb/gbaa196. [DOI] [PubMed] [Google Scholar]
- 7.Penninkilampi R, Casey AN, Singh MF, Brodaty H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2018;66(4):1619–1633. doi: 10.3233/jad-180439. [DOI] [PubMed] [Google Scholar]
- 8.Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J Alzheimers Dis. 2019;70(s1):S119–S144. doi: 10.3233/jad-180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lara E, Martín-María N, De la Torre-Luque A, Koyanagi A, Vancampfort D, Izquierdo A, Miret M. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res Rev. 2019;52:7–16. doi: 10.1016/j.arr.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Victor CR. Is loneliness a cause or consequence of dementia? A public health analysis of the literature. Front Psychol. 2020;11:612771. doi: 10.3389/fpsyg.2020.612771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holwerda TJ, Deeg DJ, Beekman AT, van Tilburg TG, Stek ML, Jonker C, Schoevers RA. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL) J Neurol Neurosurg Psychiatry. 2014;85(2):135–142. doi: 10.1136/jnnp-2012-302755. [DOI] [PubMed] [Google Scholar]
- 12.Sundström A, Adolfsson AN, Nordin M, Adolfsson R. Loneliness increases the risk of all-cause dementia and Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2020;75(5):919–926. doi: 10.1093/geronb/gbz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickel H, Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med. 1994;24(1):179–192. doi: 10.1017/s0033291700026945. [DOI] [PubMed] [Google Scholar]
- 14.He YL, Zhang XK, Zhang MY. Psychosocial risk factors for Alzheimer’s disease: the Hong Kong College of Psychiatrists Ltd., 2000.
- 15.Akhter-Khan SC, Tao Q, Ang TFA, Itchapurapu IS, Alosco ML, Mez J, Piers RJ, Steffens DC, Au R, Qiu WQ. Associations of loneliness with risk of Alzheimer’s disease dementia in the Framingham Heart Study. Alzheimers Dement. 2021;17(10):1619–1627. doi: 10.1002/alz.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage. 2010;49(1):1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, de Bruin PW, Middelkoop HA, van Buchem MA, van der Grond J. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain. 2008;131(Pt 12):3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer’s disease. Expert Rev Neurother. 2008;8(11):1691–1702. doi: 10.1586/14737175.8.11.1691. [DOI] [PubMed] [Google Scholar]
- 19.Düzel S, Drewelies J, Gerstorf D, Demuth I, Steinhagen-Thiessen E, Lindenberger U, Kühn S. Structural brain correlates of loneliness among older adults. Sci Rep. 2019;9(1):13569. doi: 10.1038/s41598-019-49888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. Brain structure links loneliness to social perception. Curr Biol. 2012;22(20):1975–1979. doi: 10.1016/j.cub.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian X, Hou X, Wang K, Wei D, Qiu J. Neuroanatomical correlates of individual differences in social anxiety in a non-clinical population. Soc Neurosci. 2016;11(4):424–437. doi: 10.1080/17470919.2015.1091037. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Wang Y, Liu W, Wei D, Yang J, Du X, Tian X, Qiu J. Neuroanatomical correlates of attitudes toward suicide in a large healthy sample: a voxel-based morphometric analysis. Neuropsychologia. 2016;80:185–193. doi: 10.1016/j.neuropsychologia.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Wong NML, Shao R, Wu J, Tao J, Chen L, Lee TMC. Cerebellar neural markers of susceptibility to social isolation and positive affective processing. Brain Struct Funct. 2019;224(9):3339–3351. doi: 10.1007/s00429-019-01965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, Johnson KA, Sperling RA. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiat. 2016;73(12):1230–1237. doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d'OleireUquillas F, Jacobs HIL, Biddle KD, Properzi M, Hanseeuw B, Schultz AP, Rentz DM, Johnson KA, Sperling RA, Donovan NJ. Regional tau pathology and loneliness in cognitively normal older adults. Transl Psychiatry. 2018;8(1):282. doi: 10.1038/s41398-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canli T, Yu L, Yu X, Zhao H, Fleischman D, Wilson RS, De Jager PL, Bennett DA. Loneliness 5 years ante-mortem is associated with disease-related differential gene expression in postmortem dorsolateral prefrontal cortex. Transl Psychiatry. 2018;8(1):2. doi: 10.1038/s41398-017-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Misra N. Loneliness, depression and sociability in old age. Ind Psychiatry J. 2009;18(1):51–55. doi: 10.4103/0972-6748.57861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging. 2010;25(2):453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, Ong A, Kernbach JM, Wiecki TV, Ge T, et al. The default network of the human brain is associated with perceived social isolation. Nat Commun. 2020;11(1):6393. doi: 10.1038/s41467-020-20039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiovitz-Ezra S, Ayalon L. Use of direct versus indirect approaches to measure loneliness in later life. Res Aging. 2011;34(5):572–591. doi: 10.1177/0164027511423258. [DOI] [Google Scholar]
- 35.Awata S, Bech P, Koizumi Y, Seki T, Kuriyama S, Hozawa A, Ohmori K, Nakaya N, Matsuoka H, Tsuji I. Validity and utility of the Japanese version of the WHO-Five Well-Being Index in the context of detecting suicidal ideation in elderly community residents. Int Psychogeriatr. 2007;19:77–88. doi: 10.1017/S1041610206004212. [DOI] [PubMed] [Google Scholar]
- 36.Raghavan N, Samtani MN, Farnum M, Yang E, Novak G, Grundman M, Narayan V, DiBernardo A. The ADAS-Cog revisited: novel composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials. Alzheimers Dement. 2013;9(1 Suppl):S21–31. doi: 10.1016/j.jalz.2012.05.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. A Narrative Review. J Alzheimers Dis. 2018;63(2):423–444. doi: 10.3233/jad-170991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 40.Van der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39(4–5):1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- 41.Aggleton JP, Pralus A, Nelson AJ, Hornberger M. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain. 2016;139(Pt 7):1877–1890. doi: 10.1093/brain/aww083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chételat G, Landeau B, Eustache F, Mézenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Nowrangi MA, Rosenberg PB. The fornix in mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2015;7:1. doi: 10.3389/fnagi.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrarini L, Palm WM, Olofsen H, van der Landen R, Jan Blauw G, Westendorp RG, Bollen EL, Middelkoop HA, Reiber JH, van Buchem MA, et al. MMSE scores correlate with local ventricular enlargement in the spectrum from cognitively normal to Alzheimer disease. Neuroimage. 2008;39(4):1832–1838. doi: 10.1016/j.neuroimage.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Lam JA, Murray ER, Yu KE, Ramsey M, Nguyen TT, Mishra J, Martis B, Thomas ML, Lee EE. Neurobiology of loneliness: a systematic review. Neuropsychopharmacology. 2021;46(11):1873–1887. doi: 10.1038/s41386-021-01058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng C, Zhou J, Ma H, Tan Y, Wu X, Guan C, Qiao H, Li J, Zhong Y, Wang C, et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry. 2018;18(1):370. doi: 10.1186/s12888-018-1955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wang X, Li Y, Sun Y, Sheng C, Li H, Li X, Yu Y, Chen G, Hu X, et al. Abnormal resting-state functional connectivity strength in mild cognitive impairment and its conversion to Alzheimer’s disease. Neural Plast. 2016;2016:4680972. doi: 10.1155/2016/4680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, Prince JL, Onyike CU. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain. 2017;140(3):707–720. doi: 10.1093/brain/aww327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown SH, Kessler KR, Hefter H, Cooke JD, Freund HJ. Role of the cerebellum in visuomotor coordination. I. Delayed eye and arm initiation in patients with mild cerebellar ataxia. Exp Brain Res. 1993;94(3):478–488. doi: 10.1007/bf00230206. [DOI] [PubMed] [Google Scholar]
- 50.Feng C, Wang L, Li T, Xu P. Connectome-based individualized prediction of loneliness. Soc Cogn Affect Neurosci. 2019;14(4):353–365. doi: 10.1093/scan/nsz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qualter P, Rotenberg K, Barrett L, Henzi P, Barlow A, Stylianou M, Harris RA. Investigating hypervigilance for social threat of lonely children. J Abnorm Child Psychol. 2013;41(2):325–338. doi: 10.1007/s10802-012-9676-x. [DOI] [PubMed] [Google Scholar]
- 53.Katona CL, Aldridge CR. The dexamethasone suppression test and depressive signs in dementia. J Affect Disord. 1985;8(1):83–89. doi: 10.1016/0165-0327(85)90076-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data of patients are available from the corresponding author on reasonable request.