Abstract
Phosphorus (P) is an essential nutrient for diverse biological processes, which aggregate to the animal's requirement for P, and nutritionists strive to meet this requirement accurately. The P demand for a growing pig comprises requirements for maintenance and tissue deposition. The P in feed ingredients, however, must be digested and absorbed before its ultimate partition between the 2 aforementioned requirement components. Phosphorus from various sources could behave differently during digestion and absorption, which results in their disparate bioavailability for pigs. The system of standardized total tract digestibility reflects true total tract digestibility of P and feed ingredient effects on specific endogenous P loss with relative ease of implementation, and this system guarantees satisfactory additivity in digestible P among the ingredients in a diet—the foundation for diet formulation. The basal endogenous P loss, which is much easier to measure than the specific endogenous P loss, is considered as part of the pig's maintenance requirement. With this arrangement, a digestibility framework is established both for measuring the P-providing capacity of various feed ingredients and for describing the pig's P requirement. This framework entails basic understanding of the function, digestion, absorption, excretion, and homeostasis of P as support pillars. Understanding the workings of this framework enables potential integration of factors such as environment conditions and disease status in future P requirement models. The current review discusses dietary sources, digestion, absorption, bioavailability and requirement of P for growing pigs to understand the status quo, revealing the points of consensus as well as those of debate, and to encourage further investigation to provide more clarity.
Keywords: Bioavailability, Digestibility, Phosphorus, Pig, Requirement
1. Introduction
Elemental phosphorus (P) is highly reactive and complexes with oxygen to form the tetrahedral phosphate when exposed to air (Ehret, 1948). Thus, phosphate becomes the nutritional currency of P for plants and animals. This currency can be mined from rock phosphate in nature, an irreplaceable and nonrenewable resource, and its misuse for farm animals can result in poor productivity of farm operations attributable to P deficiency or oversupply. In the case of oversupply, P runoff through waterways could occur with serious ecological repercussions. The avoidance of these disastrous scenarios entails understanding of general principles of P nutrition in farm animals and the exceptions to these principles to achieve high accuracy in P nutrition (Liu et al., 2016).
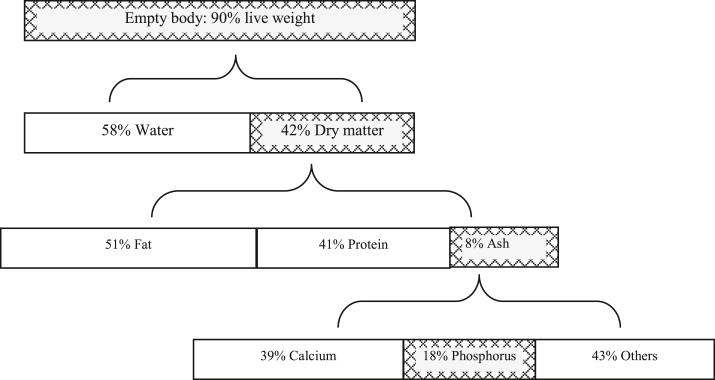
In general, P pools within the body could be envisaged as comprising a small pool circulating in body fluids for short-term vital functions, and a large pool within the skeleton, which by continuous deposition and resorption provides finite buffering capacity for maintaining constant levels in the small pool (Fernández, 1995). This large pool harbors approximately 77% of the whole-body P in a pig, which contrasts with 99% for calcium (Just Nielsen, 1973). The mass percentage of P in bone varies according to the status of bone. Phosphorus accounts for 18% of the ashed bone, 10% of the dry fat-free bone, and 4.5% of the wet bone (Crenshaw, 2001). A chemical distribution of P in the whole body of a 90-kg pig is illustrated in Fig. 1. In this illustration, P is 0.54% of the pig live weight. In reality, the percentage of P in the pig live weight decreased from 0.64% to 0.43% when the pig grew from 8.5 to 146 kg (Mahan, 1998).
Fig. 1.
An illustration of the chemical distribution of phosphorus in the body of a 90-kg pig (Just Nielsen, 1973; Crenshaw, 2001).
Phosphorus is required for diverse biological processes (Berndt and Kumar, 2009). The skeletal system— built with constituents in the chemical formula of Ca10(PO4)6(OH)2—maintains the shape of the body, provides protection to internal organs, and functions as levers for body movement. The cell membrane is made of phospholipids, molecules that are usually portrayed as spheres, each with 2 tails hanging down with phosphate as the sphere. The phospholipid-based bilayer structure provides cell structural integrity and controls the flow of molecules in and out of the cell. The importance of DNA is supreme because of its sole commitment to transferring genetic information from generation to generation, and the backbone of DNA is made of sugars and phosphate groups joined by ester bonds. Adenosine-5′-triphosphate is dubbed the molecular currency unit for intracellular energy transfer, which entails the enzyme-catalyzed transfer of phosphoryl group (Knowles, 1980). Many key regulatory proteins in cells exist as either phosphorylated or dephosphorylated in reflection of the action of protein kinases or phosphatases, which are essential for the transduction of biological messages (Cohen, 1988).
The microbes colonizing the pig gut present another dimension of the pig's requirement for P despite its smaller amount in comparison to the need of host. The microbes in the small intestine of pigs mainly engage in the digestion and absorption of nutrients whereas the microbes in the large intestine, as the main force of gut microbiota, participate in the degradation of fiber (Liu et al., 2017; Wang et al., 2020). Dietary fiber provides food for bacteria, promoting fermentation and multiplication of bacteria in the gastrointestinal tract, which might increase the bacterial utilization of P in the large intestine (Metzler and Mosenthin, 2008). A reduction in P available for bacteria in the large intestine due to enhanced P absorption in the small intestine with supplemental phytase could be related to the lower cellulase activity in feces of pigs (Metzler, 2007). Additionally, the P supply could interact with the health status and immune system of pigs. It has been shown that a low P diet has an effect on immune features in jejunum (Just et al., 2018), and receptor-activator of NF-κB ligand (RANK) might contribute to linking P homeostasis and immunity in swine (Oster et al., 2016).
2. Dietary sources of phosphorus
wDietary P is primarily provided by plant and animal feedstuffs and inorganic P supplements (Waldroup, 1999). Each source comprises different chemical forms of P which could be organic or inorganic, depending on the presence or absence of carbon in the compound.
2.1. Phosphorus of plant source
Phosphorus in plant seed is stored primarily in the form of phytic acid, also known as phytate or phytin (Eeckhout and De Pape, 1994). The terms phytic acid, phytate and phytin refer to free acid, salt, and calcium/magnesium/potassium salt, respectively, and have been used interchangeably in the literature (Cowieson et al., 2016). Phytic acid loosely binds bi- and trivalent cations under the acidic conditions of the stomach or precipitates as phytate at the neutral pH of small intestine, inhibiting the intestinal absorption of trace elements, and has thus been regarded as an antinutrient for decades (Schlemmer et al., 2001). Phytate-bound P is only partially degradable in swine because of the minimal secretion of phytase along the gastro-intestinal tract and the unfavorable chemical environment of a complete diet. As a consequence, dietary phytate concentration becomes a bottleneck and governs the magnitude of the animal's response to exogenous supplementation of phytase (Selle and Ravindran, 2008).
The accumulation site of phytin in seeds and the content of phytate P vary appreciably among feedstuffs. Accumulation of phytin in seeds occurs during the maturation phase of seed development, the period of rapid cell expansion (Lott et al., 1995). The accumulation sites of phytin in monocotyledonous and dicotyledonous seeds are the aleurone layer and globoids (one of the inclusions of the protein body), respectively (Reddy et al., 1982). Corn is an exception, with 88% of the phytate P in a kernel contained in the germ fraction (O'Dell et al., 1972). Soybean has phytin contained in protein bodies distributed throughout the seed, but phytin is concentrated only in crystalloid and globoid substructures in peanuts, cotton seed and sunflower seeds (Baker and Stein, 2013). Moreover, phytate in individual feedstuffs might have different chemical properties, such as stability and solubility, which could affect their susceptibility to phytase. The concentration of phytate in cereal grains is generally lower than that of oilseeds; however, phytate P concentration as a percentage of total P content is usually higher in cereal grains than in oilseeds. As measured and reported by Eeckhout and de Paepe (1994), the phytate P concentration in oats, wheat, triticale, maize, barley, rye and sorghum ranged from 0.19% to 0.25%; whereas phytate P as a percentage of total P content varied from 59% to 70%. On the contrary, the meals of peanut, rapeseed, sunflower and soybean contained 0.32% to 0.44% phytate P, which accounted for 36% to 53% of the total P. Attempts to predict the phytate P content from total P for different categories of feedstuffs have met with limited success for cereal byproducts and oilseeds (Table 1).
Table 1.
Linear relationship between total phosphorus (P) and phytate P (%).
| Type of feedstuffs | Regression equation (Y: phytate P; X: total P) | R2 |
|---|---|---|
| Cereals1 | Y = 0.42 × X + 0.08 | 0.20 |
| Cereals2 | Y = 0.49 × X + 0.05 | 0.52 |
| Cereals byproducts1 | Y = 0.85 × X - 0.04 | 0.95 |
| Wheat + wheat by-products2 | Y = 0.83 × X - 0.06 | 0.95 |
| Maize + maize by-products2 | Y = 0.50 × X + 0.04 | 0.93 |
| Legume seeds1 | Y = 0.43 × X + 0.02 | 0.62 |
| Legume seeds1 | Y = 0.54 × X - 0.04 | 0.79 |
| Oilseeds1 | Y = 0.95 × X - 0.20 | 0.95 |
| Oilseed meals2 | Y = 0.24 × X + 0.18 | 0.42 |
Novel varieties of grain and oilseed species have been developed to decrease the phytate concentration and to increase bioavailability of P in feedstuffs. For example, the digestibility of P in low-phytate corn was approximately 26 percentage points higher than in normal corn (Bohlke et al., 2005), and the bioavailability of P in low-phytate soybean meal (SBM) was 12 to 26 percentage points higher than in conventional SBM (Sands et al., 2003). It is noteworthy that substantial amounts of intrinsic phytase and acid phosphatase activities were found in rye, wheat, rye bran, and wheat bran (Viveros et al., 2000). The efficacy of cereal phytase, however, was determined to be only 40% of that of microbial phytase (Zimmermann et al., 2002). Fortunately, the effects of cereal and microbial phytases on apparent P absorption in pigs were found to be additive (Zimmermann et al., 2003).
2.2. Phosphorus of animal and mineral sources
Feedstuffs of animal origin are a great source of energy, amino acids and minerals for pigs (Olukosi and Adeola, 2009; Sulabo and Stein, 2013; Kerr et al., 2019). These feedstuffs are rich in P, and have relatively higher P digestibility ranging from 68% to 91% (Jongbloed and Kemme, 1990). Structural analysis of meat and bone meal ashes revealed calcium hydroxyapatite (Ca10(PO4)6(OH)2) as the major inorganic constituent, which is characteristic of bones (Deydier et al., 2005). Hydroxyapatite is known to dissolve readily in acids and the gastric acid of pigs should be pivotal to its dissolution and releasing both Ca and phosphate. The bioavailability of P in meat and bone meal, relative to that in monocalcium phosphate, was approximately 91% for growing pigs, and meat and bone meal can serve as the sole source of supplemental P for finishing pigs (Traylor et al., 2005). Sulabo and Stein (2013) reported that P in meat and bone meal was about 70% as digestible as the P in monosodium phosphate. Limitations to its use include the regulatory restriction due to concerns for bovine spongiform encephalopathy, as evidenced by the ban of feeding all processed animal proteins to all farm animals in EU since 2001 (the ban on processed animal protein as feed to non-ruminants has recently been lifted), and the high variability in gross composition and protein quality that challenges the precision formulation of diets. Almost all the variation in protein and ash composition of meat and bone meal was due to the variation in the ratios of bones and soft tissue (Garcia and Phillips, 2009). In addition, whey power, whey permeates and low-ash whey permeate have excellent digestible P with standardized total tract digestibility (STTD) of P measured to be 91.2%, 93.1% and 91.8%, respectively (Kim et al., 2012).
Inorganic sources of P are highly digestible and consistent in quality and therefore are widely used as a supplemental form of P. However, variation in P digestibility exists both within and between feed phosphates (Eeckhout and De Pape, 1997; Poulsen, 2007). A review by Eeckhout and de Paepe (1997) showed that the apparent P digestibility varied between 52% and 71% for dehydrated dicalcium phosphate, between 54% and 87% for anhydrous dicalcium phosphate, and between 64% and 91% for monocalcium phosphate. Using a regression technique, the digestibility of P was determined to be 92% for monocalcium phosphate, 63% for anhydrous dicalcium phosphate and 73% for hydrated dicalcium phosphate (Eeckhout and de Paepe, 1997). With a regression method, the absorption of P was determined to be 71% to 75% for monocalcium phosphate in comparison to the highest availability of 79% for monosodium phosphate (Poulsen, 2007). Using a P-free basal diet, true total tract digestibility (TTTD) of P was measured to be 88% for dicalcium phosphate, 98% for monosodium phosphate and 89% to 95% for monocalcium phosphate with a declared purity of 50% to 100% (Petersen and Stein, 2006). The coexistence of multiple phosphate forms in individual commercial products was usually not suggested in the labels (Soares, Jr., 1995). The relative concentrations of dicalcium phosphate and monocalcium phosphate in a specific source of feed phosphate might not be important because both forms of P are equally digestible, but the bioavailability of P in anhydrous dicalcium phosphate appeared to be 88% of that in the hydrated form, because the dissolution in stomach may be slower for anhydrous dicalcium phosphate (Grimbergen et al., 1985).
3. Phosphorus digestion and absorption
Phosphate is absorbed in inorganic form from both inorganic and organic sources after hydrolysis (Jongbloed, 1987). The different solubility of P sources reflects the difference in digestion and absorption of P by animals. The P solubility of the inorganic sources in weak acid solution is higher than those of animal origin. Plant P sources have low P solubility, and the more soluble sources tend to have higher P digestibility (Lineva et al., 2018). The solubility of the inorganic P sources, however, cannot be assumed as a surrogate for their digestion and absorption in animals because the dissociated phosphate could be complexed or chelated in the digesta by antagonists such as phytate. Moreover, it has been demonstrated that there was no correlation between the bioavailability of P in the defluorinated phosphate in pigs and their solubility in neutral ammonium citrate, which contrasted with a slight positive relationship for chicks (Coffey et al., 1994). Incidentally, the particle size of defluorinated phosphate appeared not to affect the bioavailability of P (Cromwell et al., 1987). Phytate — the primary form of organic P in plant feedstuffs — needs to become soluble in the gastrointestinal tract of pigs for hydrolysis to occur.
3.1. Hydrolysis of phytate
Hydrolysis of phytic acid proceeds in a stepwise manner, producing free phosphates and various intermediate products that may be absorbed to a small extent (Jongbloed et al., 1992; Kemme et al., 2006). Phytate degradation through the whole gastrointestinal tract was nearly 100% for diets with or without intrinsic phytase due to hindgut microbial activities (Jongbloed et al., 1992; Schlemmer et al., 2001; Angel et al., 2005). The main site of phytate hydrolysis by supplemented phytase is the stomach because the pH conditions of the stomach more closely match the pH optima of most commercial phytases than the small intestinal pH. As shown in Table 2, the greatest difference in the degradation of phytic acid or IP6 attributable to phytase presence or supplementation was observed in either the stomach or duodenum, and this difference seemed to maintain throughout the small intestine before further action by microbes in the large intestine. This pattern holds true for phytases of both plant and microbial origins. For a diet rich in intrinsic phytase, 58% of total inositol phosphates in the stomach were phytate hydrolysis products, whereas for the diet with inactivated phytase, the phytate hydrolysis products only accounted for 17% (Schlemmer et al., 2001). In a diet with inactivated intrinsic phytase, almost no phytate degradation occurred in the stomach, whereas in a diet with 150 FTU/kg Aspergillus niger phytase, 22% of the dietary phytate was hydrolyzed, and the phytate degradation increased further up to 52% with the supplemental phytase at 900 FTU/kg (Kemme et al., 2006). With the supplementation of 1,500 FTU/kg A. niger phytase to a corn-soybean meal diet, an increase of approximately 48 percentage units in gastroduodenal hydrolysis of phytic acid was found, which compares to an uplift of 50 percentage units at the end of ileum (Jongbloed et al., 1992).
Table 2.
Degradation (%) of phytic acid or myo-inositol hexakisphosphate (IP6) throughout the gastro-intestinal tract of pigs.
| References | Analyte | Phytase, U/kg | Stomach | Duodenum | Ileum | Total tract |
|---|---|---|---|---|---|---|
| Jongbloed et al. (1992, corn-soybean meal diet) | Phytic acid | 0 | 21.5 | 9.6 | ||
| 1,500 | 69.2 | 59.7 | ||||
| Jongbloed et al. (1992, Dutch diet) | Phytic acid | 0 | 1.2 | −1.4 | ||
| 1,500 | 93.2 | 74.0 | ||||
| Kemme et al. (2006) | IP6 | 0 | 7 | 27 | ||
| 150 | 22 | 43 | ||||
| 900 | 52 | 65 | ||||
| Schlemmer et al. (2001) | Phytic acid | 0.2 | 16.8 | 97.4 | ||
| 43.1 | 58.1 | 97.7 | ||||
| Rosenfelder-Kuon et al. (2020) | IP6 | 0 | 18.4 | 96.6 | ||
| 750 | 76.3 | 97.5 | ||||
| 1,500 | 83.2 | 98.6 | ||||
| 3,000 | 85.0 | 97.4 | ||||
| Rosenfelder-Kuon et al. (2020) | IP6 | 0 | 30.1-31.2 | 98.5-98.9 | ||
| 1,500 | 92.1-92.3 | 98.9 |
3.2. Absorption of P
It is generally accepted that the small intestine is the major site for P absorption in pigs as well as other simple-stomach species (Moore and Tyler, 1955; Partridge, 1978; Breves and Schröder, 1991). The large intestine, however, might also contribute to P homeostasis in pigs because the expression of some sodium/phosphate transporters were detected in the colon (Wubuli et al., 2019). A significant absorption of P occurred in the large intestine of pigs (Liu et al., 2000, 2017). The large intestine may play an important role in whole-body P homeostasis by recycling endogenous P secreted in the upper gastrointestinal tract (Fan et al., 2001), but the endogenous P secretion is of minor importance to gastrointestinal P turnover in nonruminants relative to ruminants (Breves and Schröder, 1991). Depending on the source of carbohydrate, net absorption or secretion of P could occur; cellulose and pectin caused a net secretion, but starch induced a net absorption (Baumgärtel et al., 2008). Liu et al. (2017) reported higher P absorption in the large intestine in practical diets than in semi-purified diets. The heavier pigs were found to absorb more P in the large intestine, but varying dietary P levels supplied by dicalcium phosphate was not an influencing factor (Liu et al., 2018a).
The general models for absorption of P have been described (Breves and Schröder, 1991; Sabbagh et al., 2011; Goff, 2018). Briefly, P can either go through the tight junctions by electrochemical gradient or solvent drag, a process known as paracellular absorption, or pass through the epitheliums via a transcellular process. Eto et al. (2006) used a voltage-clamp technique, which allowed the differentiation of P permeation through transcellular and paracellular routes in rat intestine, and found that the active component transcellular route accounted for 78% of the total transport as opposed to 22% for paracellular route. The passive transport route might predominate at higher luminal P concentrations. The transcellular P transport comprises at least 3 steps: (1) P entry across the lumen brush-border membrane into the enterocyte, (2) intracellular P transport to the basolateral side of the cell, and (3) P extrusion across the basolateral membrane (Cross et al., 1990). The apical P uptake represents the major rate-limiting step of the overall transepithelial P transport (Schröder et al., 1998). So far, 9 genes from 3 subfamilies (type I, II and III sodium/phosphate co-transporters) were annotated as sodium-dependent P co-transporters in pigs, and SLC34A3 of the NaPi-II appeared to be the most prominent intestinal P transporter in pigs (Wubuli et al., 2019). Sodium-dependent phosphate uptake, however, was only stimulated post-transcriptionally as the concentration of P decreases in the diet for pigs without alterations in mRNA expression of the NaPi-IIb transporter (Saddoris et al., 2010).
3.3. Phosphorus excretion
The reasons for the excretion of P are the low P digestibility and the excessive dietary content of digestible P (Poulsen et al., 1999; Knowlton et al., 2004). Fecal P consists of undigested portions of phytate-bound and non-phytate P from plant sources, undigested portions of P from animal byproducts and mineral supplements, and surplus amount of bioavailable P in excess of animal needs (Waldroup, 1999). There are 3 factors influencing the excretion of P via feces: 1) inevitable P losses, which depend on the body weight of pigs, 2) the availability of dietary P, which relates to the dietary origin of the P, and 3) the regulatory P excretion, which is due to the adaptation in absorption and/or endogenous secretion to the level of P supply (Rodehutscord et al., 1999b). Fecal P excretion amounted to about 52%, 46%, and 55% of P intake in sows, weaners, and growing pigs, respectively, and in general, the growing period contributed up to 75% of the total P excretion (Poulsen et al., 1999). The urinary excretion of P depends on the P status of the pig (Rodehutscord et al., 1999b). As a major means of manure disposal, land application has endangered the quality of ground water due to the relative excess of P in manure than that required by crops and the subsequent buildup of P in soil, which potentiates the movement of P during soil erosion and in surface water runoff (Kornegay and Verstegen, 2001). This concern calls for minimizing P excretion from pig farming with more efficient utilization of P in feed and meeting pig's requirement for P without unnecessary waste.
3.4. Phosphorus homeostasis
Systemic P homeostasis is achieved by orchestrating intestinal absorption, bone formation and resorption, and kidney excretion and reabsorption through a hormonal network (Razzaque; 2009; Sabbagh et al., 2011; Martin et al., 2012). The main hormones implicated in the regulation of P homeostasis are parathyroid hormone (PTH), vitamin D3 (cholecalciferol), and fibroblast growth factor-23 (FGF23) with its cofactor klotho (Lederer, 2014). Parathyroid hormone is more associated with P regulation in kidney and bone, whereas P absorption in the small intestine is stimulated by vitamin D (Breves and Schröder, 1991). In contrast to PTH, FGF23 inhibits renal tubular P reabsorption and suppresses circulating calcitriol (the active form of vitamin D3) concentrations (Martin et al., 2012). There are numerous reviews on the hormonal homeostasis of P in intestine, bone and kidney in the literature with an emphasis on human nutrition and health. In pigs, the onset of vitamin D-dependent mechanism for active P absorption does not occur until weaning (Schröder et al., 1998). With the Ussing chamber technique, Lee et al. (1986) proved there are also vitamin D-independent local mechanisms governing the intestinal active P transport. Hormonal regulation may play a role only in the long-term regulation of P homeostasis, and the short-term postprandial responses that occur independently of hormones may play a larger role than previously appreciated (Berndt and Kumar, 2009). Vitamin D3 can be synthesized in skin through the action of ultraviolet irradiation. Vitamin D3 is hydroxylated in the liver to form 25-hydroxycholecalciferol, the major circulating metabolite, which is further hydroxylated in the renal tubule cells to the most active metabolite of vitamin D3, 1, 25-dihydroxycholecalciferol or to a less-active metabolite, 24,25-dihydroxycholecalciferol (Sommerville et al., 1978). A confined housing environment with insufficient sunlight could precipitate vitamin D deficiency resulting in a disturbance in the absorption and metabolism of Ca and P (NRC, 1998). Supplementing vitamin D3 to pig diets did not affect the growth performance (Li et al., 1998; O'Doherty et al., 2010; Lindemann et al., 2012), but the vitamin D3 supplementation to a low-Ca, low-P diet produced similar growth performance as the diet with adequate Ca and P when fed to 20 kg pigs (Adeola et al., 1998). Serum 25-hydroxycholecalciferol concentration, which is the specific vitamin D metabolite to indicate animal's vitamin D status, was increased by oral gavage or intramuscular injection of vitamin D (Lindemann et al., 2012). Dietary administration of vitamin D3 to pregnant and lactating sows is necessary to ensure normal skeletal mineralization in young pigs (Witschi et al., 2011). Dietary supplementation of 25-hydroxy cholecalciferol tended to promote normal endochondral ossification and inhibit osteochondrosis progression in pigs (Sugiyama et al., 2013).
4. Phosphorus bioavailability
Bioavailability is an abstract concept to describe the extent to which a nutrient in a feedstuff is absorbed and metabolized by the animal (Stein et al., 2007) and has been used without due prudence. Bioavailability of P could be interpreted as the amount of P in a feedstuff that is released during digestion, absorbed from the gastrointestinal tract, and used for maintenance and growth of grower pigs. Bioavailability cannot be measured in absolute terms, but can be quantified in a comparative sense relative to a standard.
4.1. Relative bioavailability of P
Relative bioavailability of P is derived from slope-ratio assays providing information on the P-providing capacity of a feed ingredient in comparison to a standard P source. The slope derived from the linear response variable with the test ingredient (bt) is compared with the slope of the response variable in the basal diets supplemented with the standard ingredient (bs), and the percentage value (bt/bs × 100) is referred to as the availability of that particular source of P (Littell et al., 1997). This kind of assay is usually considered as the ultimate standard against which other methods are judged (Lewis and Bayley, 1995), but suffers from the drawbacks of being costly, time consuming, and inefficient. Moreover, it requires a strict test for fundamental validity to determine whether regression lines for the reference and test ingredients intersect at the point of the basal diet, and a test for statistical validity to verify whether responses to the reference and test ingredients are linear and without curvature (Adeola, 2009). For availability assays where linearity of response is imperative, the dietary concentration of available P should not exceed 2 g/kg of feed (Ketaren et al., 1993). There are occasions where linear regression may not be sufficient to explain the majority of the total variance; nonlinear (asymptotic or sigmoidal) regression could be tried for a better fitting to the data (Ravindran et al., 1995). Bioavailability is usually estimated using P in monosodium, monocalcium or dicalcium phosphate as the standard. The response variables could be parameters describing growth performance and bone characteristics. Bone parameters are supposed to be very accurate because about 80% of the retained P is deposited in bone. In pigs, there was a strong dependency of P digestibility and bone measurements on supplemental P, but the blood parameters (alkaline phosphatase and plasma inorganic P) and chemical property (solubility) were less correlated (Dellaert et al., 1990). Bone ash concentration appeared to be more responsive than growth performance and P retention to dietary P supply (Ketaren et al., 1993). It is noteworthy that bones are not equally optimal for determination of P supply and various bone segments also mineralize differently (Sørensen et al., 2019). Either non-defatted or defatted bone processing methods can be used to assess bone mineralization; however, the defatted bone processing is preferred for grower -finisher pigs to minimize variation in mature pigs (Wensley et al., 2020). Dual-energy X-ray absorptiometry (DEXA) has been used successfully to evaluate bone mineral content and density in live pigs and generated comparable results with chemical and physical analysis (Bernau et al., 2020; Schlegel and Gutzwiller, 2020).
4.2. Phosphorus digestibility
Bioavailability of P reflects the net effects of digestion, absorption and post-absorptive utilization of P by tissues and organs. Digestion and absorption are prerequisites for bioavailability whereas utilization of the absorbed is proof of bioavailability (Sibbald, 1987). The marginal efficiency of using digestible P for post-absorption net requirement is approximately 95% (NRC, 2012). Therefore, P digestibility can be considered as an estimate of P bioavailability to a large extent. In theory, the digestibility of P in an ingredient can be calculated by multiplying its relative bioavailability of P with the digestibility of P in the mineral phosphate (the standard to which the test ingredient is compared), but such conversion has been challenged by relevant studies comparing digestibility and bioavailability of P. Low-phytate corn was determined to contain at least 5 times as much available P in comparison to 3 times as much digestible P as normal corn (Spencer et al., 2000). Baker et al. (2013) compared STTD and relative bioavailability of P in pigs: the STTD of P in dicalcium phosphate and DDGS were determined to be 93.1% and 63.1%; indicating a relative bioavailability of 67.8% of P in DDGS to that in dicalcium phosphate, but the relative bioavailability of P in DDGS was measured to be 86% to 88% of that in dicalcium phosphate. In broilers, P digestibility and absorption also disagrees with relative bioavailability; P digestibility and absorption values obtained for some ingredients using diets that are semisynthetic or semi-purified, particularly when they contain low Ca levels, may not represent diets used in commercial practices (Munoz et al., 2020). Despite the discrepancy, the system of STTD of P has predominated over the system of P bioavailability in pigs as signaled by its adoption in Nutrient Requirement Council (NRC, 2012).
To measure P digestibility, 2 operational decisions are required regarding the use of marker or the classical quantitative comparison between input and output, and the choice between ileal and fecal sampling. Firstly it has been shown that the large intestine does not seem to play an important role in the digestion of P and there is no difference in true P digestibility between ileal and total tract levels (Fan et al., 2001; Shen et al., 2002; Ajakaiye et al., 2003; Dilger and Adeola, 2006), which contrasts the modifying effects of hindgut microbes on amino acids and the consequent preference of measuring amino acids digestibility at the ileal level over the total tract level (Sauer and Ozimek, 1986). Therefore, the determination of P digestibility from mouth to anus is justified by accuracy as well as ease of operation. The P secretion or absorption in the large intestine cannot yet be easily integrated in the current digestibility system, and their influence on the precision of diet formulation under practical conditions may be negligible. Secondly, indigestible markers could be included in feed and measured in digesta/feces as conceptualized in the index method for measuring P digestibility. The index method is laudable due to its labor-saving advantage via measuring the concentrations of the indigestible indicator in both feed and feces in replacement of the exact collection and records of feed intake and fecal output with great care to detail (Adeola, 2001; Agudelo et al., 2010). The index method also enables grab fecal sampling from pigs housed in groups in reflection of commercial housing conditions considering that the pigs housed individually and in groups have different feed intake patterns which have different effect on digestibility (de Haer and de Vries, 1993). Additionally, the markers have little to no impact on microbial metabolism and growth performance of pigs (Kerr et al., 2015).
The index method, however, could underestimate digestibility of dry matter and energy, which relates to the low recovery of marker and the property of feed (Adeola, 2001; Jang et al., 2014; Li et al., 2016). In terms of fecal P digestibility, the apparent total tract digestibility (ATTD) of P (38.5%) using the indicator method was significantly higher than that (36.7%) of the total collection method in Kemme et al. (1997b), and the opposite was reported by Agudelo et al. (2010) and Jang et al. (2014). In terms of sensitivity to treatment difference in fecal P digestibility, discrepancy also appeared to exist between the 2 methods (Kemme et al., 1997b; Agudelo et al., 2010). It is becoming more accepted that the type of marker is an influencing factor for measuring nutrient digestibility (Wang and Adeola, 2018), but the most appropriate choice among the markers is still debatable considering the conflicting results in the literature (McCarthy et al., 1974; Jagger et al., 1992; Brestenský et al., 2017; Prawirodigdo et al., 2021). Moreover, the comparison among markers was based more on the digestibility of dry matter, organic matter, gross energy or amino acids without due attention to minerals. It is well-known that pigs should be adapted to the diets over several days for the marker to stabilize in feces before sample collection, and the fecal samples should be grabbed daily for at least 2 to 5 consecutive days and then composited to make a representative sample (Moughan et al., 1991; Agudelo et al., 2010; Jang et al., 2014; Liu et al., 2018b; Choi and Kim, 2019). Different digestibility systems (apparent, standardized, or true) were explored, and their differences have been discussed extensively in previous review papers (Sibbald, 1987; Stein et al., 2007; Adeola et al., 2016; She et al., 2017; Zhang and Adeola, 2017). An important point of debate is the additivity of nutrient digestibility among feed ingredients, and the essence of additivity boils down to whether the nutrient digestibility can be affected or not by the varying inclusion levels of each nutrient-providing feed ingredient.
The modifier “apparent” before P digestibility reflects the calculation that both the undigested P from feed as well as the endogenous loss from the pig have been deducted from the dietary P supply, or in other words, the true P-providing ability of feed has been diminished by the endogenous loss from the pig. Poor additivity and large variability of apparent P digestibility have been reported in the literature. Rodehutscord et al. (1996) and Fan and Sauer (2002) found that the ATTD values of P measured for feed ingredients are not always additive when used in diet formulation for pigs. In previous studies where graded levels of P were investigated using index method, the ATTD values for P in corn ranged from −41.4% to 39.1% with 25 kg pigs (Shen et al., 2002), and from 18.8% to 42.5% in soybean meal with 6.8 kg pigs (Fan et al., 2001) and from 3.7% to 48.1% with 40 kg pigs (Ajakaiye et al., 2003). On the contrary, the ATTD of P in monocalcium phosphate did not change with increasing inclusion rate of monocalcium phosphate using either the direct method (Petersen and Stein, 2006) or the difference method (Stein et al., 2008). The ATTD of P in SBM and canola meal were also not affected by the inclusion level of test ingredient (Akinmusire and Adeola, 2009). Paradoxically, Dilger and Adeola (2006) found significant linear and quadratic effects of inclusion level of low-phytate SBM on the ATTD of P, but not of conventional SBM. The endogenous loss of P from pigs accounts for different proportions of the P supply due to varying inclusion rates of the feed ingredients and thereby challenges the additivity of apparent P digestibility. This exasperation for nutritionists could be moderated with the standardized P digestibility system.
The modifier “standardized” reflects the calculation that the undigested P from feed as well as the endogenous P loss from the pig specific to the feed have been deducted from the feed P supply; whereas, the inevitable basal endogenous P loss is considered as a factor of the pig's requirement. NRC (2012) derived STTD of P for feed ingredients using basal endogenous P losses of 190 mg/kg dry matter intake to correct the ATTD of P in the literature. Indirectly, Almeida and Stein (2010) showed that STTD values for P are additive because no significant difference was observed among 4 diets formulated using values for STTD of P. NRC (2012) also asserted that using values for the STTD of P in practical diet formulation, additivity among feed ingredients is achieved, and diets are more accurately formulated compared with using values for ATTD of P. She et al. (2018) demonstrated that the STTD of P in corn, SBM and canola meal are more additive than their ATTD counterparts in pigs.
The modifier “true” before P digestibility means the true P-providing ability of feed as reflected by the calculation that only the feed-derived undigested P has been deducted from the P supply in feed. Different methods have been used to measure the true P digestibility. Fan et al. (2001) determined the gastrointestinal endogenous P output and true P digestibility values in plant ingredients based on the regression technique and index method. Based on the total collection method, Dilger and Adeola (2006) reevaluated the regression method to determine endogenous P losses and true digestibility of P . Fang et al. (2007a) determined the TTTD of P using a substitution method, which was not different from the regression method. Liu et al. (2014) proved that the measured TTTD of P in soybean and canola meals with the regression method was not affected by the inclusion of cornstarch or corn in the diet. Petersen and Stein (2006) estimated endogenous P losses and true digestibility of P in inorganic P sources based on a P-free diet. Fang et al. (2007b) demonstrated the additivity of TTTD values for P in some cereals and oilseeds using the substitution method. Finally, Zhai and Adeola (2013a) proved the additivity of TTTD values for P in corn and soybean meal using the regression method. The primary challenge for true P digestibility is the difficulty in measuring the specific endogenous loss of P associated with each feed ingredient and taking it into account as part of the pig's requirement. In contrast, the standardized P digestibility system, which corrects apparent digestible P for basal endogenous P loss, appeared to be the most practical choice.
The influencing factors for P digestibility could be associated with animals, feed ingredients and dietary composition and housing environments. Zhai and Adeola (2013b,c) determined TTTD of P in monocalcium phosphate using the regression method based on a corn-soybean meal diet and found that the TTTD of P increased from 67.5% for pigs at 16 kg to 84.3% for pigs at 25 kg. This concurs with the conclusion from Rodehutscord et al. (1999a), that the apparent P digestibility increased when body weight (BW) increased from 15 to 35 kg, especially when the P mainly originated from monocalcium phosphate. Pettey et al. (2006) determined the TTTD of P in monocalcium phosphate using the regression method based on a highly digestible, semi-purified diet fortified with minerals (except P) and vitamins and found the TTTD of P declined from 97% for pigs at 27 kg to 94% and 89% for pigs at 59 and 98 kg, respectively. When the dietary P was marginal and solely from organic sources, the apparent digestibility of P increased when BW of pigs increased from 30 to 60 kg and then remained relatively stable until 100 kg BW (Kemme et al., 1997a). On the contrary, the TTTD of P in soybean meal was determined to be 48.5% (Fan et al., 2001) for weanling pigs and to be 51.3% for 40 to 58 kg pigs indicating no significant difference associated with BW of pig (Ajakaiye et al., 2003). Comprehensive research tracking the progression of pigs from weaning to market will shed more light on the factor of BW on digestibility of P from both mineral and plant sources. The digestibility of P in SBM tested in the 1st decade of the 21st century was much higher than previous varieties partially due to the changes in produced soybean per se as surmised by Goebel and Stein (2011), and it is necessary to regularly update P digestibility for contemporary cereal grains. It is noteworthy that there can be significant variation among different genotypes of the same grain species (Schemmer et al., 2020). Interestingly, the apparent P digestibility was higher for pigs housed in pens compared with pigs housed in metabolic crates, which might be due to coprophagy (Kemme et al., 1997b) and begs for attention to the potential gap between digestibility assays and practical production conditions.
Additionally, a wide Ca to P ratio lowers P absorption, resulting in reduced growth and bone mineralization, especially if the diet is marginal in P (NRC, 2012). In finishing pigs, the detrimental effect of a wide Ca:P ratio, compared with a narrow ratio, was found to be more pronounced in conditions of marginal supply of P, which is not due to a decreased digestibility of P but rather to an increased urinary excretion of P (Wilde and Jourquin, 1992). Despite the presence of a moderate amount of wheat phytase, increasing dietary Ca levels did not result in any significant change in apparent P digestibility for growing-finishing pigs (Eeckhout et al., 1995). On the contrary, Stein et al. (2011) showed that the ATTD of P decreased linearly with increasing dietary Ca probably due to the binding between Ca and P in the intestinal tract. When the diet is supplemented with phytase, increasing Ca to P ratio decreased apparent digestibility of P probably due to the formation of insoluble Ca-phytate in small intestine which is less accessible to phytase action as well as the reduction in endogenous activities of phytase and alkaline phosphatase (Liu et al., 1998).
5. Requirement for P
The requirement for P could be estimated by factorial and empirical approaches. The factorial approach needs to quantify the components of net P requirement, which consists of maintenance P requirement to offset the inevitable P losses and the requirement for sustaining the growth of skeleton and tissues. Scientifically, the factorial method is advantageous because it can be applied to various systems of production (Jongbloed and Everts, 1992). An empirical approach uses the results of feeding trials with diets of various nutrient contents (Jongbloed, 1987). The empirical estimates could be used to validate the estimates derived from the factorial approach.
Until late 1980s when systematic research was initiated in the Netherlands to determine digestible P requirement for pigs and digestible P content in feedstuffs, most of the studies had been based on total P (Poulsen et al., 1999). The difference in the availability of P in the ingredients of diets results in a large variation in recommendations for total P (Ketaren et al., 1993). Apparent digestible P requirement has considered the fecal endogenous P excretion as a loss, so apparent digestible P requirement for maintenance equates to urinary P loss. However, to compensate for the lower digestibility of P when animals are fed above their requirement, Jongbloed and Everts (1992) suggested 6 mg fecal P loss/kg live BW added to the urinary loss of 1 mg/kg live BW as the endogenous loss. To estimate the P requirement for growth, Jongbloed and Everts (1992) used results of slaughter experiments between 1960 and 1985 and their own results and described the relationship between P content (g) and live BW (kg) using the equation: ln P = 1.494 + 1.108 × ln BW – 0.018 × (ln BW)2. This relationship was simplified as ln P = 1.418 + 1.009 × ln BW by Pettey et al. (2005). Bikker and Blok (2017) described body P mass (g) in relation to empty BW (kg) as ln P = 1.67 + 1.0004 × ln EBW. NRC (2012) used whole-body protein mass to estimate whole-body P mass, and thus the maximum rate of whole-body P retention. NRC (2012) estimated the standardized digestible P requirement using the equation: P requirement (g/d) = 0.85 × [(maximum whole-body P retention)/0.77 + 0.19 × feed dry matter intake + 0.007 × body weight]. This equation is based on the assumptions that the basal endogenous P losses are 190 mg/kg feed dry matter intake via feces and 7 mg/kg BW per day via urine, the marginal efficiency of using STTD-based P intake for whole-body P retention is 0.77 (instead of 0.95 to account for the variation among individual pigs in groups), and P requirement for maximum growth performance is equivalent to 0.85 of P requirement for maximum whole-body P retention. NRC (2012) preferred the body protein mass over live or empty body weight for prediction of body P content, but Bikker and Blok (2017) did not find significant difference between empty body weight and whole-body protein as the explanatory variable. Therefore, any change in body weight gain or lean growth rate might alter P requirement. For example, pigs treated with porcine somatropin require a higher daily amount of P than untreated pigs (NRC, 2012), the pigs selected for high lean deposition require more available P (Saraiva et al., 2012), the increased body weight gain in pigs fed a high dose of phytase might require additional P (Wu et al., 2019), and gilts have a higher P requirement than barrows (Thomas and Kornegay, 1981; Ekpe et al., 2002). However, pigs with a propensity to produce more lean tissue do not have greater body P content than those producing less muscle tissue (Wiseman et al., 2009). A recent mechanistic model that accounts for the distribution of P between bone and soft tissues showed lower standardized digestible P requirement than NRC (2012) for pigs between 29 and 93 kg of BW and higher for pigs with lighter and heavier BW (Lautrou et al., 2020).
Empirical P requirement estimates also reflect the disease challenges and environmental conditions specific to each feeding trial, which have not been captured by the existing modeling approach yet, and thus empirical studies can be used to validate and adjust the model-generated requirements. Zhai and Adeola (2013a,b,c, 2015) determined the TTTD of P in corn, soybean meal, and monocalcium phosphate using regression method, and estimated the TTTD-based P requirement to be 3.2, 3.1, 2.7, and 2.3 g/kg feed for 10 to 20, 20 to 40, 40 to 60, and 60 to 80 kg pigs of mixed sex. These requirement estimates were converted to their STTD equivalents (3.9, 3.2, 2.8 and 2.4 g/kg feed), which were higher than NRC (2012) recommendations for pigs under 33 kg and lower for pigs greater than 33 kg (Zhai, 2013). Recently, it has been validated that NRC (2012) recommendations for STTD P are reasonably accurate for 6 kg to 13 kg pigs when body weight gain was used as the response (Wu et al., 2019). In a cooperative study involving 11 participating stations, Adeola et al. (2015) estimated the STTD P requirements of 20 to 40 kg pigs and the results were higher than the recommendations by NRC (2012). Vier et al. (2019a, b) provided empirical evidence that the NRC (2012) accurately estimates the STTD P requirement for 11 to 23 kg pigs on a grams per day basis, but underestimates on a percentage of the diet for both 11 to 23 kg and 24 to 130 kg pigs. The divergent estimates of P requirement especially when expressed on a percentage basis of diet might be associated with the difference in feed intake related to environmental factors either suppressing or stimulating the pig's appetite. Saraiva et al. (2012) found that pigs under heat stress have reduced feed intake and possibly reduced protein accretion, resulting in reduced P requirement when expressed as a percentage of diet. The difference in feed intake associated with dietary energy density and the discrepancy between research and commercial conditions could lead to different P requirement estimates (Hastad et al., 2004). Adeola et al. (2015) pointed out the central factor for the discrepancy in estimates of P requirement for growth performance being the total dietary Ca concentration for the Ca to P ratio at which there was a breakpoint (requirement estimate) for digestible P.
6. Conclusions
Admittedly, a plethora of research has accumulated over the years with regards to P as a nutrient for growing pigs. This collective work has enabled a robust understanding about the key components of P nutrition of growing pigs: sources, digestion, absorption, bioavailability, excretion, and homeostasis, which culminated in defining and meeting the P requirement of pigs. The STTD P was recommended over the nebulous bioavailable P to accurately describe P requirement (Adeola and Cowieson, 2011) and has gained predominance in pigs. NRC (2012) updated the mathematical model to estimate requirement for STTD P of growing-finishing pigs between 20 and 140 kg live body weight and integrated in the model the requirements of pigs below 20 kg BW based on empirical studies. These model-generated requirements take into account the physiological state and genetic potential for growth, but don't reflect the environmental conditions as well as disease challenges (NRC, 2012). This calls for actual P requirements under practical production conditions, and the principles and algorithms for narrowing the gap between model-generated and actual requirements need to be ascertained. Collaborative initiatives at certain time intervals to update both the P digestibility in up-to-date feed ingredients as well as P requirements in pigs under continuous genetic selection pressure should be organized. Moreover, the study protocols among the participating stations should be aligned to minimize variance and to facilitate meta-analysis. Last but not the least, P nutrition could not be separated from Ca nutrition. The recent work on measuring STTD Ca as well as optimizing the Ca to P ratio on the standardized digestible basis have shown the direction for further effort.
Author contributions
Conceptualization: H. Zhai; Data curation: J. Liu; Formal analysis: J. Liu; Investigation: H. Zhai, J. Liu; Methodology: H. Zhai, O. Adeola; Resources: J. Liu; Writing - Original Draft: H. Zhai, O. Adeola; Writing - Review & Editing: J. Liu, O. Adeola; Supervision: J. Liu; Project administration: J. Liu; Funding acquisition: J. Liu.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by Sichuan Science and Technology Program (2021JDJQ0010).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Hengxiao Zhai, Email: heng-xiao.zhai@dsm.com.
Jingbo Liu, Email: liuswust@163.com.
References
- Adeola O. In: Swine nutrition. Lewis A.J., Southern L.L., editors. CRC Press; Washington, DC: 2001. Digestion and balance techniques in pigs; pp. 903–916. [Google Scholar]
- Adeola O. Bioavailability of threonine and tryptophan in peanut meal for starter pigs using slope-ratio assay. Anima. 2009;3:677–684. doi: 10.1017/S1751731109004066. [DOI] [PubMed] [Google Scholar]
- Adeola O., Azain M.J., Carter S.D., Crenshaw T.D., Estienne M.J., Kerr B.J., et al. A cooperative study on the standardized total-tract digestible phosphorus requirement of twenty-kilogram pigs. J Anim Sci. 2015;93:5743–5753. doi: 10.2527/jas.2015-9509. [DOI] [PubMed] [Google Scholar]
- Adeola O., Cowieson A.J. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Adeola O., Orban J.I., Ragland D., Cline T.R., Sutton A.L. Phytase and cholecalciferol supplementation of low-calcium and low-phosphorus diets for pigs. Can J Anim Sci. 1998;78:307–313. [Google Scholar]
- Adeola O., Xue P., Cowieson A., Ajuwon K. Basal endogenous losses of amino acids in protein nutrition research for swine and poultry. Anim Feed Sci Technol. 2016;221:274–283. [Google Scholar]
- Agudelo J.H., Lindemann M.D., Cromwell G.L. A comparison of two methods to assess nutrient digestibility in pigs. Livest Sci. 2010;133:74–77. [Google Scholar]
- Ajakaiye A., Fan M.F., Archbold T., Hacker R.R., Forsberg C.W., Phillips J.P. Determination of true digestive utilization of phosphorus and the endogenous phosphorus outputs associated with soybean meal for growing pigs. J Anim Sci. 2003;81:2766–2775. doi: 10.2527/2003.81112766x. [DOI] [PubMed] [Google Scholar]
- Akinmusire A.S., Adeola O. True digestibility of phosphorus in canola and soybean meals for growing pigs: influence of microbial phytase. J Anim Sci. 2009;87:977–983. doi: 10.2527/jas.2007-0778. [DOI] [PubMed] [Google Scholar]
- Almeida F.N., Stein H.H. Performance and phosphorus balance of pigs fed diets formulated on the basis of values for standardized total tract digestibility of phosphorus. J Anim Sci. 2010;88:2968–2977. doi: 10.2527/jas.2009-2285. [DOI] [PubMed] [Google Scholar]
- Angel C.R., Powers W.J., Applegate T.J., Tamim N.M., Christman M.C. Influence of phytase on water-soluble phosphorus in poultry and swine manure. J Environ Qual. 2005;34:563–571. doi: 10.2134/jeq2005.0563. [DOI] [PubMed] [Google Scholar]
- Baker S.R., Kim B.G., Stein H.H. Comparison of values for standardized total tract digestibility and relative bioavailability of phosphorus in dicalcium phosphate and distillers dried grains with solubles fed to growing pigs. J Anim Sci. 2013;91:203–210. doi: 10.2527/jas.2010-3776. [DOI] [PubMed] [Google Scholar]
- Baker D.H., Stein H.H. In: Sustainable swine nutrition. Chiba L.I., editor. Wiley-Blackwell Press; Ames, IA: 2013. Bioavailability of minerals and vitamins in feestuffs; pp. 341–364. [Google Scholar]
- Baumgärtel T., Metzler B.U., Mosenthin R., Greiner R., Rodehutscord M. Precaecal and postileal metabolism of P, Ca and N in pigs as affected by different carbohydrate sources fed at low level of P intake. Arch Anim Nutr. 2008;62:169–181. doi: 10.1080/17450390802028047. [DOI] [PubMed] [Google Scholar]
- Bernau M., Schrott J., Schwanitz S., Kreuzer L.S., Scholz A.M. “Sex” and body region effects on bone mineralization in male pigs. Arch Anim Breed. 2020;63:103–111. doi: 10.5194/aab-63-103-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt T., Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiol. 2009;24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker P., Blok M. Wageningen Livestock Research; 2017. Phosphorus and calcium requirements of growing pigs and sows. CVB documentation report 59. [Google Scholar]
- Bohlke R.A., Thaler R.C., Stein H.H. Calcium, phosphorus, and amino acid digestibility in low-phytate corn, normal corn, and soybean meal by growing pigs. J Anim Sci. 2005;83:2396–2403. doi: 10.2527/2005.83102396x. [DOI] [PubMed] [Google Scholar]
- Brestenský M., Nitrayová S., Heger J., Patráš P. Chromic oxide and acid-insoluble ash as markers in digestibility studies with growing pigs and sows. J Anim Physiol Anim Nutr. 2017;101:46–52. doi: 10.1111/jpn.12503. [DOI] [PubMed] [Google Scholar]
- Breves G., Schröder B. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr Res Rev. 1991;4:125–140. doi: 10.1079/NRR19910011. [DOI] [PubMed] [Google Scholar]
- Choi H., Kim B.G. A low-fiber diet requires a longer adaptation period before collecting feces of pigs compared with a high-fiber diet in digestibility experiments using the inert marker method. Anim Feed Sci Technol. 2019;256:114254. [Google Scholar]
- Coffey R.D., Mooney K.W., Cromwell G.L., Aaron D.K. Biological availability of phosphorus in defluorinated phosphates with different phosphorus solubilities in neutral ammonium citrate for chicks and pigs. J Anim Sci. 1994;72:2653–2660. doi: 10.2527/1994.72102653x. [DOI] [PubMed] [Google Scholar]
- Cohen P.C. Protein phosphorylation and hormone action. Proc Roy Soc Lond B. 1988;234:115–144. doi: 10.1098/rspb.1988.0040. [DOI] [PubMed] [Google Scholar]
- Crenshaw T.D. In: Swine nutrition. Lewis A.J., Southern L.L., editors. CRC Press; Boca Raton: 2001. Calcium, phosphorus, vitamin D, and vitamin K in swine nutrition; pp. 187–212. [Google Scholar]
- Cromwell G.L., Stahly T.S., Burnell T.W. Source and particle size of phosphorus supplements on bioavailability of phosphorus for pigs. J Anim Sci. 1987;65(Supp.1):134. [Abstr.] [Google Scholar]
- Cross H.S., Debiec H., Peterlik M. Metabolism and regulation of intestinal phosphate absorption. Miner Electrol Metabol. 1990;16:115–124. [PubMed] [Google Scholar]
- Cowieson A.J., Ruckebusch J.P., Knap I., Guggenbuhl P., Fru-Nji F. Phytate-free nutrition: a new paradigm in monogastric animal production. Anim Feed Sci Technol. 2016;222:180–189. [Google Scholar]
- de Haer L.C.M., de Vries A.G. Feed intake patterns of and feed digestibility in growing pigs housed individually or in groups. Livest Prod Sci. 1993;33:277–292. [Google Scholar]
- Deydier E., Guilet R., Sarda S., Sharrock P. Physical and chemical characterisation of crude meat and bone meal combustion residue: “waste or raw material?”. J Hazard Mater. 2005;121:141–148. doi: 10.1016/j.jhazmat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Dellaert B.M., Van Der Peet G.F.V., Jongbloed A.W., Beers S. A comparison of different techniques to assess the biological availability of feed phosphates in pig feeding. Neth J Agric Sci. 1990;38:555–566. [Google Scholar]
- Dilger R.N., Adeola O. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing pigs fed conventional and low-phytate soybean meal. J Anim Sci. 2006;84:627–634. doi: 10.2527/2006.843627x. [DOI] [PubMed] [Google Scholar]
- Eeckhout W., de Paepe M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Technol. 1994;77:53–60. [Google Scholar]
- Eeckhout W., de Paepe M. The digestibility of three calcium phosphates for pigs as measured by difference and by slope-ratio assay. J Anim Physiol Anim Nutr. 1997;77:53–60. [Google Scholar]
- Eeckhout W., de Paepe M., Warnants N., Bekaert H. An estimation of the minimal P requirements for growing-finishing pigs, as influenced by the Ca level of the diet. Anim Feed Sci Technol. 1995;52:29–40. [Google Scholar]
- Ehret W.F. Spontaneous combustion. J Chem Educ. 1948;25:250. [Google Scholar]
- Ekpe E.D., Zijlstra R.T., Patience J.F. Digestible phosphorus requirement of grower pigs. Can J Anim Sci. 2002;82:541–549. [Google Scholar]
- Eto N., Tomita M., Hayashi M. NaPi-mediated transcellular permeation in the dominant route in intestinal inorganic phosphate absorption in rats. Drug Metab Pharmacokiner. 2006;21:217–221. doi: 10.2133/dmpk.21.217. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Archbold T., Sauer W.C., Lackeyram D., Rideout T., Gao Y., et al. Novel methodology allows simultaneous measurement of true phosphorus digestibility and the gastrointestinal endogenous phosphorus outputs in studies with pigs. J Nutr. 2001;131:2388–2396. doi: 10.1093/jn/131.9.2388. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Sauer W.C. Additivity of apparent ileal and fecal phosphorus digestibility values measured in single feed ingredients for growing-finishing pigs. Can J Anim Sci. 2002;82:183–191. [Google Scholar]
- Fang R.J., Yin Y.L., Wang K.N., He J.H., Chen Q.H., Li T.J., et al. Comparison of the regression analysis technique and the substitution method for the determination of true phosphorus digestibility and faecal endogenous phosphorus losses associated with feed ingredients for growing pigs. Livest Sci. 2007;109:251–254. [Google Scholar]
- Fang R.J., Li T.J., Yin F.G., Yin Y.L., Kong X.F., Wang K.N., et al. The additivity of true or apparent phosphorus digestibility values in some feed ingredients for growing pigs. AJAS (Asian-Australas J Anim Sci) 2007;20:1092–1099. [Google Scholar]
- Fernández J.A. Calcium and phosphorus metabolism in growing pigs. II. Simultaneous radio-calcium and radio-phosphorus kinetics. Livest Prod Sci. 1995;41:243–254. [Google Scholar]
- Garcia R.A., Phillips J.G. Physical distribution and characteristics of meat and bone meal protein. J Sci Food Agric. 2009;89:329–336. [Google Scholar]
- Goebel K.P., Stein H.H. Phosphorus digestibility and energy concentration of enzyme-treated and conventional soybean meal fed to weanling pigs. J Anim Sci. 2011;89:764–772. doi: 10.2527/jas.2010-3253. [DOI] [PubMed] [Google Scholar]
- Goff J.P. Invited review: mineral absorption mechanisms, mineral interactions that affect acid-base and antioxidant status, and diet considerations to improve mineral status. J Dairy Sci. 2018;101:2763–2813. doi: 10.3168/jds.2017-13112. [DOI] [PubMed] [Google Scholar]
- Grimbergen A.H.M., Cornelissen J.P., Stappers H.P. The relative availability of phosphorus in inorganic feed phosphates for young turkeys and pigs. Anim Feed Sci Technol. 1985;13:117–130. [Google Scholar]
- Hastad C.W., Dritz S.S., Tokach M.D., Goodband R.D., Nelssen J.L., DeRouchey J.M., et al. Phosphorus requirements of growing-finishing pigs reared in a commercial environment. J Anim Sci. 2004;82:2945–2952. doi: 10.2527/2004.82102945x. [DOI] [PubMed] [Google Scholar]
- Jagger S., Wiseman J., Cole D.J.A., Craigon J. Evaluation of inert markers for the determination of ileal and faecal apparent digestibility values in the pig. Br J Nutr. 1992;68:729–739. doi: 10.1079/bjn19920129. [DOI] [PubMed] [Google Scholar]
- Jang Y.D., Lindemann M.D., Agudelo-Trujillo J.H., Escobar C.S., Kerr B.J., Inocencio N., et al. Comparison of direct and indirect estimates of apparent total tract digestibility in swine with effort to reduce variation by pooling of multiple day fecal samples. J Anim Sci. 2014;92:4566–4576. doi: 10.2527/jas.2013-6570. [DOI] [PubMed] [Google Scholar]
- Jongbloed A.W. Wageningen Agricultural University; 1987. Phosphorus in the feeding of pigs: effect of diet on the absorption and retention of phosphorus by growing pigs. [Doctor Degree Dissertation] [Google Scholar]
- Jongbloed A.W., Everts H. Apparent digestible phosphorus in the feeding of pigs in relation to availability, requirement and environment. 2. The requirement of digestible phosphorus for piglets, growing-finishing pigs and breeding sows. Neth J Agric Sci. 1992;40:123–136. [Google Scholar]
- Jongbloed A.W., Kemme P.A. Apparent digestible phosphorus in the feeding of pigs in relation to availability, requirement and environment. 1. Digestible phosphorus in feedstuffs from plant and animal origin. Neth J Agric Sci. 1990;38:567–575. [Google Scholar]
- Jongbloed A.W., Mroz Z., Kemme P.A. The effect of supplementary Aspergillus Niger phytase in diets for pigs on concentration and apparent digestibility of dry matter, total phosphorus, and phytic acid in different sections of the alimentary tract. J Anim Sci. 1992;70:1159–1168. doi: 10.2527/1992.7041159x. [DOI] [PubMed] [Google Scholar]
- Just F., Oster M., Büsing K., Borgelt L., Murani E., Ponsuksili S., et al. Lowered dietary phosphorus affects intestinal and renal gene expression to maintain mineral homeostasis with immunomodulatory implications in weaned piglets. BMC Genom. 2018;19:1–11. doi: 10.1186/s12864-018-4584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just Nielsen A. Anatomical and chemical composition of Danish Landrace pigs slaughtered at 90 kilograms live weight in relation to litter, sex and feed composition. J Anim Sci. 1973;36:476–483. [Google Scholar]
- Kemme P.A., Jongbloed A.W., Mroz Z., Beynen A.C. The effect of Aspergillus Niger phytase in rendering phytate phosphorus available for absorption in pigs is influenced by pig physiological status. J Anim Sci. 1997;75:2129–2138. doi: 10.2527/1997.7582129x. [DOI] [PubMed] [Google Scholar]
- Kemme P.A., Radcliffe J.S., Jongbloed A.W., Mroz Z. Factors affecting phosphorus and calcium digestibility in diets for growing-finishing pigs. J Anim Sci. 1997;75:2139–2146. doi: 10.2527/1997.7582139x. [DOI] [PubMed] [Google Scholar]
- Kemme P.A., Schlemmer U., Mroz Z., Jongbloed A.W. Monitoring the stepwise phytate degradation in the upper gastrointestinal tract of pigs. J Sci Food Agric. 2006;86:612–622. [Google Scholar]
- Kerr B.R., Urriola P.E., Jha R., Thomson J.E., Curry S.M., Shurson G.C. Amino acid composition and digestible amino acid content in animal protein by-product meals fed to growing pigs. J Anim Sci. 2019;97:4540–4547. doi: 10.1093/jas/skz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B.J., Weber T.E., Ziemer C.J. Dietary marker effects on fecal microbial ecology, fecel VFA, nutrient digestibility coefficients, and growth performance in finishing pigs. J Anim Sci. 2015;93:2183–2190. doi: 10.2527/jas.2014-8633. [DOI] [PubMed] [Google Scholar]
- Ketaren P.P., Batterham E.S., White E. Phosphorus studies in pigs 1. Available phosphorus requirements of grower/finisher pigs. Br J Nutr. 1993;70:249–268. doi: 10.1079/bjn19930121. [DOI] [PubMed] [Google Scholar]
- Kim B.G., Lee J.W., Stein H.H. Energy concentration and phosphorus digestibility in whey powder, whey permeate, and low-ash whey permeate fed to weanling pigs. J Anim Sci. 2012;90:289–295. doi: 10.2527/jas.2011-4145. [DOI] [PubMed] [Google Scholar]
- Knowles J.R. Enzyme-catalyzed phophoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Knowlton K.F., Radcliffe J.S., Novak C.L., Emmerson D.A. Animal management to reduce phosphorus losses to the environment. J Anim Sci. 2004;82:173–195. doi: 10.2527/2004.8213_supplE173x. [DOI] [PubMed] [Google Scholar]
- Kornegay E.T., Verstegen M.W.A. In: Swine nutrition. Lewis A.J., Southern L.L., editors. CRC Press; Boca Raton: FL: 2001. Swine nutrition and environmental pollution and odor control; p. 609. [Google Scholar]
- Lautrou M., Pomar C., Dourmad J.-Y., Narcy A., Schmidely P., Létourneau-Montminy M.P. Phosphorus and calcium requirements for bone mineralization of growing pigs predicted by mechanistic modeling. Anima. 2020;14:313–322. doi: 10.1017/S1751731120001627. [DOI] [PubMed] [Google Scholar]
- Lederer E. Regulation of serum phosphate. J Physiol. 2014;18:3985–3995. doi: 10.1113/jphysiol.2014.273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.B.N., Walling W., Barautbar N. Intestinal phosphate absorption: influence of vitamin D and non-vitamin D factors. Am J Physiol. 1986;250:369–373. doi: 10.1152/ajpgi.1986.250.3.G369. [DOI] [PubMed] [Google Scholar]
- Lewis A.J., Bayley H.S. In: Bioavailability of nutrients for animals: amino acids, minerals, and vitamins. Ammerman C.B., Baker D.H., Lewis A.J., editors. Academic Press; New York: 1995. Amino acid bioavailability; p. 35. [Google Scholar]
- Li D., Che X., Wang Y., Hong C., Thacker P.A. Effect of microbial phytase, vitamin D3, and citric acid on growth performance and phosphorus, nitrogen and calcium digestibility in growing swine. Anim Feed Sci Technol. 1998;73:173–186. [Google Scholar]
- Li Y.S., Tran H., Bundy J.W., Burkey T.E., Kerr B.J., Nielsen M.K., et al. Evaluation of collection method and diet effects on apparent digestibility and energy values of swine diets. J Anim Sci. 2016;94:2415–2424. doi: 10.2527/jas.2016-0275. [DOI] [PubMed] [Google Scholar]
- Lindemann M.D., Monnegue H.J., Jang Y.D. Proc. Midwest swine nutr. Conf. 2012. Vitamin D status of pigs from birth to weaning as affected by gavage or injection; pp. 59–65. Indianapolis. [Google Scholar]
- Lineva A., Kirchner R., Kienzle E., Kamphues J., Dobenecker B. A pilot study on in vitro solubility of phosphorus from mineral sources, feed ingredients and compound feed for pigs, poultry, dogs and cats. J Anim Physiol Anim Nutr. 2018;103:317–323. doi: 10.1111/jpn.12986. [DOI] [PubMed] [Google Scholar]
- Littell R.C., Henry P.R., Lewis A.J., Ammerman C.B. Estimation of the relative bioavailability of nutrients using SAS procedures. J Anim Sci. 1997;75:2672–2683. doi: 10.2527/1997.75102672x. [DOI] [PubMed] [Google Scholar]
- Liu J., Bollinger D.W., Ledoux D.R., Venum T.L. Lowering the dietary calcium to total phosphorus ratio increases phosphorus utilization in low-phosphorus corn-soybean meal diets supplemented with microbial phytase for growing-finishing pigs. J Anim Sci. 1998;76:808–813. doi: 10.2527/1998.763808x. [DOI] [PubMed] [Google Scholar]
- Liu J., Bollinger D.W., Ledoux D.R., Venum T.L. Effects of dietary calcium:phosphorus ratios on apparent absorption of calcium and phosphorus in the small intestine, cecum, and colon of pigs. J Anim Sci. 2000;78:106–109. doi: 10.2527/2000.781106x. [DOI] [PubMed] [Google Scholar]
- Liu J.B., Yang Y.K., He J., Zeng F.K. Comparison of two diet types in the estimation of true digestibility of phosphorus in soybean and canola meals for growing pigs by the regression method. Livest Sci. 2014;167:269–275. [Google Scholar]
- Liu J.B., Cao S.C., Chen L., Zhang H.F. Effect of dietary phosphorus level on the determination of standardized and true total tract digestibility of phosphorus for growing pigs. Anim Feed Sci Technol. 2016;215:117–123. [Google Scholar]
- Liu J.B., Shen X.Y., Zhai H.X., Chen L., Zhang H.F. Dietary sources of phosphorus affect postileal phosphorus digestion in growing pigs. J Anim Sci. 2017;95:4490–4498. doi: 10.2527/jas2017.1426. [DOI] [PubMed] [Google Scholar]
- Liu J.B., Xue P.C., Cao S.C., Liu J., Chen L., Zhang H.F. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim Feed Sci Technol. 2018;242:86–94. [Google Scholar]
- Liu J.B., Yan H.L., Cao S.C., Liu J., Zhang H.F. Effect of feed intake level on the determination of apparent and standardized total tract digestibility of phosphorus for growing pigs. Anim Feed Sci Technol. 2018;246:137–143. [Google Scholar]
- Lott J.N.A., Greenwood J.S., Batten G.D. In: Seed development and germination. Kigel J., Galili G., editors. Marcel Dekker; New York: NY: 1995. Mechanisms and regulation of mineral nutrient storage during seed development; pp. 215–235. [Google Scholar]
- Mahan D.C., Shields R.G., Jr. Macro- and micromineral composition of pigs from birth to 145 kilograms of body weight. J Anim Sci. 1998;76:506–512. doi: 10.2527/1998.762506x. [DOI] [PubMed] [Google Scholar]
- Martin A., David V., Quarles L.D. Regulation and function of the FGF23/Klotho endocrine pathways. Physiol Rev. 2012;92:132–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J.F., Aherne F.X., Okai D.B. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can J Anim Sci. 1974;54:107–109. [Google Scholar]
- Metzler B.U. University of Hohenheim; 2007. Effects of fermentable carbohydrates and dietary P supply on bacterial P incorporation, activity and composition. [Doctor Degree Dissertation] [Google Scholar]
- Metzler B.U., Mosenthin R. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. AJAS (Asian-Australas J Anim Sci) 2008;21:603–615. [Google Scholar]
- Moore J.H., Tyler C. Studies on the intestinal absorption and excretion of calcium and phosphorus in the pig 1. A critical study of the Bergeim technique for investigating the intestinal absorption and excretion of calcium and phosphorus. Br J Nutr. 1955;9:81–93. doi: 10.1079/bjn19550012. [DOI] [PubMed] [Google Scholar]
- Moughan P.J., Smith W.C., Schrama J., Smits C. Chromic oxide and acid-insoluble ash as faecal markers in digestibility studies with young growing pigs. NZJAR (N Z J Agric Res) 1991;34:85–88. [Google Scholar]
- Munoz J.A., Utterback P.L., Parsons C.M. Phosphorus digestibility and bioavailability in soybean meal, spray-dried plasma protein, and meat and bone meal determined using different methods. Poultry Sci. 2020;99:4998–5006. doi: 10.1016/j.psj.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 10th rev ed. Natl. Acad. Press; Washington, DC: 1998. Nutrient requirements of swine. [Google Scholar]
- NRC . 11th rev ed. Natl. Acad. Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- O'Dell B.L., de Boland A., Koirtyohann R. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J Agric Food Chem. 1972;20:718–723. [Google Scholar]
- O'Doherty J.V., Gahan D.A., O'Shea C., Callan J.J., Pierce K.M. Effects of phytase and 25-hydroxyviamin D3 inclusion on the performance, mineral balance and bone parameters of grower-finisher pigs fed low-phosphorus diets. Anima. 2010;4:1634–1640. doi: 10.1017/S1751731110000807. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Adeola O. Estimation of the metabolizable energy content of meat and bone meal for swine. J Anim Sci. 2009;87:2590–2599. doi: 10.2527/jas.2009-1775. [DOI] [PubMed] [Google Scholar]
- Oster M., Just F., Büsing K., Wolf P., Polley C., Vollmar B., et al. Toward improved phosphorus efficiency in monogastrics-interplay of serum, minerals, bone, and immune system after divergent dietary phosphorus supply in swine. Am J Physiol Regul Integr Comp Physiol. 2016;310:917–925. doi: 10.1152/ajpregu.00215.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge I.G. Studies on digestion and absorption in the intestines of growing pigs. 3. Net movements of mineral nutrients in the digestive tract. Br J Nutr. 1978;39:527–537. doi: 10.1079/bjn19780068. [DOI] [PubMed] [Google Scholar]
- Petersen G.I., Stein H.H. Novel procedure for estimating endogenous losses and measurement of apparent and true digestibility of phosphorus by growing pigs. J Anim Sci. 2006;84:2126–2132. doi: 10.2527/jas.2005-479. [DOI] [PubMed] [Google Scholar]
- Pettey L.A. Modeling calcium and phosphorus requirements for growing and finishing pigs. Midwest Swine Nutrition Conference Proceedings. 2005:25–34. [Google Scholar]
- Pettey L.A., Cromwell G.L., Lindemann M.D. Estimation of endogenous phosphorus loss in growing and finishing pigs fed semi-purified diets. J Anim Sci. 2006;84:618–626. doi: 10.2527/2006.843618x. [DOI] [PubMed] [Google Scholar]
- Poulsen H.D., Jongbloed A.W., Latimier P., Fernández J.A. Phosphorus, consumption, utilization and losses in pig production in France, The Netherlands and Denmark. Livest Prod Sci. 1999;58:251–259. [Google Scholar]
- Poulsen H.D. Phosphorus availability in feed phosphates determined by regression. Livest Sci. 2007;109:247–250. [Google Scholar]
- Prawirodigdo S., Gannon N.J., Leury B.J., Dunshea F.R. Acid-insoluble ash in a better indigestible marker than chromic oxide to measure apparent total tract digestibility in pigs. Anim Nutr. 2021;7:64–71. doi: 10.1016/j.aninu.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V.E., Kornegay T., Potter L.M., Ogunabameru B.O., Welten M.K., Wilson J.H., Potchanakorn M. An evaluation of various response criteria in assessing biological availability of phosphorus for broilers. Poultry Sci. 1995;74:1820–1830. doi: 10.3382/ps.0741820. [DOI] [PubMed] [Google Scholar]
- Razzaque M.S. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N.R., Peierson M.D., Sathe S.K., Salunkhe D.K. Phytates in cereals and legumes. Adv Food Res. 1982;28:1–92. doi: 10.1016/s0065-2628(08)60110-x. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Faust M., Lorenz H. Digestibility of phosphorus contained in soybean meal, barley, and different varieties of wheat, without and with supplemental phytase fed to pigs and additivity of digestibility in a wheat-soybean-meal diet. J Anim Physiol Anim Nutr. 1996;75:40–48. [Google Scholar]
- Rodehutscord M., Karuse G., Pfeffer E. Effect of body weight on phosphorus digestibility and efficacy of a microbial phytase in young pigs. Arch Anim Nutr. 1999;52:139–153. doi: 10.1080/17450399909386158. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Faust M., Pfeffer E. The course of phosphorus excretion in growing pigs fed continuously increasing phosphorus concentrations after a phosphorus depletion. Arch Anim Nutr. 1999;52:323–334. doi: 10.1080/17450399909386171. [DOI] [PubMed] [Google Scholar]
- Rosenfelder-Kuon P., Klein N., Zegowitz B., Schollenberger M., KÜhn I., Thuringer L., et al. Phytate degradation cascade in pigs as affected by phytase supplementation and rapeseed cake inclusion incorn-soybean meal-based diets. J Anim Sci. 2020;98:1–12. doi: 10.1093/jas/skaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh Y., Giral H., Caldas Y., Levi M., Schiavi S.C. Intestinal phosphate transport. Adv Chron Kidney Dis. 2011;18:85–90. doi: 10.1053/j.ackd.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris K.L., Fleet J.C., Radcliffe J.S. Sodium-dependent phosphate uptake in the jejunum is post-transcriptionally regulated in pigs fed a low-phosphorus diet and is independent of dietary calcium concentration. J Nutr. 2010;140:731–736. doi: 10.3945/jn.109.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J.S., Ragland D., Wilcox J.R., Adeola O. Relative bioavailability of phosphorus in low-phytate soybean meal for broiler chicks. Can J Anim Sci. 2003;83:95–100. [Google Scholar]
- Saraiva A., Donzele J.L., Oliveira R.F.M., Abreu M.L.T., Silva F.C.O., Guimarães S.E.F., et al. Phosphorus requirements for 60- to 100-kg pigs selected for high lean deposition under different thermal environments. J Anim Sci. 2012;90:1499–1505. doi: 10.2527/jas.2011-3623. [DOI] [PubMed] [Google Scholar]
- Sauer W.C., Ozimek L. Digestibility of amino acids in swine: results and their application. A review. Livest Prod Sci. 1986;15:367–388. [Google Scholar]
- Schemmer R., Spillner C., Südekum K.-H. Phosphorus digestibility and metabolizable energy concentrations of contemporary wheat, barley, rye and triticale genotypes fed to growing pigs. Arch Anim Nutr. 2020;74:429–444. doi: 10.1080/1745039X.2020.1817695. [DOI] [PubMed] [Google Scholar]
- Schlegel P., Gutzwiller A. Dietary calcium to digestible phosphorus ratio for optimal growth performance and bone mineralization in growing and finishing pigs. Anima. 2020;10:178. doi: 10.3390/ani10020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlemmer R.K., Jany D., Berk A., Schulz E., Rechkemmer G. Degradation of phytate in the gut of pigs-pathway of gastrointestinal inositol phosphate hydrolysis and enzymes involved. Arch Anim Nutr. 2001;55:255–280. doi: 10.1080/17450390109386197. [DOI] [PubMed] [Google Scholar]
- Schröder B., Hattenhauer O., Breves G. Phosphate transport in pig proximal small intestines during postnatal development: lack of modulation by calcitriol. Endocrinology. 1998;139:1500–1507. doi: 10.1210/endo.139.4.5922. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Ravindran V. Phytate-degrading enzymes in pig nutrition. Livest Sci. 2008;113:99–122. [Google Scholar]
- She Y., Li D., Zhang S. Methodological aspects of determining phosphorus digestibility in swine: a review. Anim Nutr. 2017;3:97–102. doi: 10.1016/j.aninu.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Y., Wang Q., Stein H.H., Liu L., Li D., Zhang S. Additivity of values for phosphorus digestibility in corn, soybean meal, and canola meal in diets fed to growing pigs. Asian-Australas J Anim Sci. 2018;31:1301–1307. doi: 10.5713/ajas.17.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Fan M.Z., Ajakaiye A., Archbold T. Use of the regression analysis technique to determine the true phosphorus digestibility and the endogenous phosphorus output associated with corn in growing pigs. J Nutr. 2002;132:1199–1206. doi: 10.1093/jn/132.6.1199. [DOI] [PubMed] [Google Scholar]
- Sibbald I.R. Estimation of bioavailable amino acids in feedstuffs for poultry and pigs: a review with emphasis on balance experiments. Can J Anim Sci. 1987;67:221–300. [Google Scholar]
- Soares J.H., Jr. In: Bioavailability of nutrients for animals: amino acids, minerals, and vitamins. Ammerman C.B., Baker D.H., Lewis A.J., editors. Academic Press; San Diego: CA: 1995. Phosphorus bioavailability; pp. 257–294. [Google Scholar]
- Sommerville B.A., Fox J., Care A.D., Swaminathan R. The in vitro metabolism of 25-hydroxycholecalciferol by pig kidney: effect of low dietary levels of calcium and phosphorus. Br J Nutr. 1978;40:159–162. doi: 10.1079/bjn19780107. [DOI] [PubMed] [Google Scholar]
- Sørensen K., Shiguetomi-Medina J.M., Poulsen H.D. Mineralisation of tubular bones is affected differently by low phosphorus supply in growing-finishing pigs. J Sci Food Agric. 2019;99:3628–3634. doi: 10.1002/jsfa.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.D., Allee G.L., Sauber T.E. Phosphorus bioavailability and digestibility of normal and genetically modified low-phytate corn for pigs. J Anim Sci. 2000;78:675–681. doi: 10.2527/2000.783675x. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Adeola O., Cromwell G.L., Kim S.W., Mahan D.C., Miller P.S. Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but decreases digestibility of phosphorus by growing pigs. J Anim Sci. 2011;89:2139–2144. doi: 10.2527/jas.2010-3522. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Sève B., Fuller M.F., Moughan P.J., de Lange C.F.M. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J Anim Sci. 2007;85:172–180. doi: 10.2527/jas.2005-742. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Kadzere C.T., Kim S.W., Miller P.S. Influence of dietary phosphorus concentration on the digestibility of phosphorus in monocalcium phosphate by growing pigs. J Anim Sci. 2008;66:1861–1867. doi: 10.2527/jas.2008-0867. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kusuhara S., Chung T.K., Yonekura H., Azem E., Hayakawa T. Effect of 25-hydroxy-cholecalciferol on the development of osteochondrosis in swine. J Anim Sci. 2013;84:341–349. doi: 10.1111/asj.12000. [DOI] [PubMed] [Google Scholar]
- Sulabo R.C., Stein H.H. Digestibility of phosphorus and calcium in meat and bone meal fed to growing pigs. J Anim Sci. 2013;91:1285–1294. doi: 10.2527/jas.2011-4632. [DOI] [PubMed] [Google Scholar]
- Thomas H.R., Kornegay E.T. Phosphorus in swine. I. Influence of dietary calcium and phosphorus levels and growth rate on feedlot performance of barrows, gilts and boars. J Anim Sci. 1981;52:1041–1048. doi: 10.2527/jas1981.5251041x. [DOI] [PubMed] [Google Scholar]
- Traylor S.L., Cromwell G.L., Lindermann M.D. Bioavailability of phosphorus in meat and bone meal for swine. J Anim Sci. 2005;83:1054–1061. doi: 10.2527/2005.8351054x. [DOI] [PubMed] [Google Scholar]
- Vier C.M., Dritz S.S., Wu F., Tokach M.D., DeRouchery J.M., Goodband R.D., et al. Effects of standardized total tract digestible phosphorus on growth performance of 11- to 23-kg pigs fed diets with or without phytase. J Anim Sci. 2019;97:4032–4040. doi: 10.1093/jas/skz255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vier C.M., Dritz S.S., Wu F., Tokach M.D., DeRouchery J.M., Goodband R.D., et al. Standardized total tract digestible phosphorus requirement of 24- to 130-kg pigs. J Anim Sci. 2019;97:4023–4031. doi: 10.1093/jas/skz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros A., Centeno C., Brenes A., Canales R., Lozano A. Phytase and acid phosphatase activities in plant feedstuffs. J Agric Food Chem. 2000;48:4009–4013. doi: 10.1021/jf991126m. [DOI] [PubMed] [Google Scholar]
- Waldroup P.W. Nutritional approaches to reducing phosphorus excretion by poultry. Poultry Sci. 1999;78:683–691. doi: 10.1093/ps/78.5.683. [DOI] [PubMed] [Google Scholar]
- Wang T., Adeola O. Digestibility index marker type, but not inclusion level affects apparent digestibility of energy and nitrogen and marker recovery in growing pigs regardless of added oat bran. J Anim Sci. 2018;96:2817–2825. doi: 10.1093/jas/sky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xu R., Zhang H., Su Y., Zhu W. Swine gut microbiota and its interaction with host nutrient metabolism. Anim Nutr. 2020;6:410–420. doi: 10.1016/j.aninu.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensley M.R., Vier C.M., Gebhardt J.T., Tokach M.D., Woodworth J.C., Goodband R.D., et al. Technical note: assessment of two methods for estimating bone ash in pigs. J Anim Sci. 2020;98:1–8. doi: 10.1093/jas/skaa251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde R.O., Jourquin J. Estimation of digestible phosphorus requirements in growing-finishing pigs by carcass analysis. J Anim Physiol Anim Nutr. 1992;68:218–225. [Google Scholar]
- Wiseman T.G., Mahan D.C., St-Pierre N.R. Mineral composition of two genetic lines of barrows and gilts from twenty to one hundred twenty-five kilograms of body weight. J Anim Sci. 2009;87:2306–2314. doi: 10.2527/jas.2006-545. [DOI] [PubMed] [Google Scholar]
- Witschi A.K., Liesegan A., Gebert S., Weber G.M., Wenk C. Effect of source and quantity of dietary vitamin D in maternal and creep diets on bone metabolism and growth in piglets. J Anim Sci. 2011;89:1844–1852. doi: 10.2527/jas.2010-3787. [DOI] [PubMed] [Google Scholar]
- Wu F., Woodworth J.C., Tokach M.D., Dritz S.S., DeRouchey J.M., Goodband R.D., et al. Standardized total tract digestible phosphorus requirement of 6 to 13 kg pigs fed diets without or with phytase. Anima. 2019;13:2473–2482. doi: 10.1017/S1751731119000922. [DOI] [PubMed] [Google Scholar]
- Wubuli A., Reyer H., Muráni E., Ponsuksili S., Wolf P., Oster M., et al. Tissue-wide gene expression analysis of sodium/phosphate co-transporters in pigs. Int J Mol Sci. 2019;20:5576. doi: 10.3390/ijms20225576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H., Adeola O. True total-tract digestibility of phosphorus in corn and soybean meal for fifteen-kilogram pigs are additive in corn-soybean meal diet. J Anim Sci. 2013;91:219–224. doi: 10.2527/jas.2012-5295. [DOI] [PubMed] [Google Scholar]
- Zhai H., Adeola O. True digestible phosphorus requirement of 10- to 20-kg pigs. J Anim Sci. 2013;91:3716–3723. doi: 10.2527/jas.2012-5875. [DOI] [PubMed] [Google Scholar]
- Zhai H., Adeola O. True digestible phosphorus requirement for twenty- to forty-kilogram pigs. J Anim Sci. 2013;91:5307–5313. doi: 10.2527/jas.2013-6627. [DOI] [PubMed] [Google Scholar]
- Zhai H. True digestible phosphorus requirement of growing pigs. [Doctor Degree Dissertation] Purdue University; 2013. [Google Scholar]
- Zhai H., Adeola O. True digestible phosphorus requirement for forty- to eighty-kilogram pigs. J Anim Sci. 2015;93:5711–5717. doi: 10.2527/jas.2015-9453. [DOI] [PubMed] [Google Scholar]
- Zhang F., Adeola O. Techniques for evaluating digestibility of energy, amino acids, phosphorus, and calcium in feed ingredients for pigs. Anim Nutr. 2017;3:344–352. doi: 10.1016/j.aninu.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B., Lantzsch H.J., Mosenthin R., Schoner F.J., Biesalski H.K., Drochner W. Comparative evaluation of the efficacy of cereal and microbial phytases in growing pigs fed diets with marginal phosphorus supply. J Sci Food Agric. 2002;82:1298–1304. [Google Scholar]
- Zimmermann B., Lantzsch H.J., Mosenthin R., Biesalski H.K., Drochner W. Additivity of the effect of cereal and microbial phytases on apparent phosphorus absorption in growing pigs fed diets with marginal phosphorus supply. Anim Feed Sci Technol. 2003;104:143–152. [Google Scholar]