Abstract
Background
To investigate the association between interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and the disease severity of coronavirus disease 2019 (COVID-19).
Materials and Methods
We systematically searched records investigating the role of interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) in COVID-19 patients in Web of Science, Pubmed, and Embase through December 2020. Data were extracted and pooled, and the weighted mean difference (WMD) and its 95% confidence interval (CI) were calculated. The funnel plot and the nonparametric trim and fill method were used to visualize and adjust the publication bias.
Results
In total, 61 studies enrolled 14,136 subjects (14,041 patients and 95 healthy subjects) were enrolled in this meta-analysis. Our results showed that serum IL-2, IL-4, IL-6, and IL-10 levels were elevated in COVID-19 patients compared to healthy controls, and IL-6, IL-8, and IL-10 levels were increased in severe COVID-19 cases compared to nonsevere patients. Additionally, the levels of IL-1β, IL-6, and IL-8 were elevated in nonsurvivor patients compared to survivors. For patients in the intensive care unit (ICU), IL-6 and IL-8 levels were increased than that in non-ICU patients.
Conclusions
Elevated levels of IL-6, IL-8, and IL-10 were associated with the disease severity of COVID-19, and elevated levels of IL-1β, IL-6, and IL-8 were related to the prognosis of COVID-19 patients, which could be used to evaluate COVID-19 patients' disease severity and prognosis.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred and spread rapidly in Wuhan, China, in 2019, which has received wide attention [1]. As the spread of SARS-CoV-2, the confirmed coronavirus disease 2019 (COVID-19) cases increased dramatically, leading to a public health emergency [2–5]. Fever, dry cough, and muscle aches were common symptoms of COVID-19 cases, and the manifestations varied greatly in critically ill COVID-19 patients [6, 7]. With the development of the detection method, the COVID-19 cases could be confirmed timely to achieve early diagnosis and treatment [8]. Although therapeutic strategies for COVID-19 have advanced greatly, including antiviral drugs, vaccines, and immunomodulatory agents [9], older COVID-19 patients tend to develop severe disease status [10]. Hence, more effective treatment approaches for COVID-19 were warranted.
Immune responses were demonstrated to be involved in the initiation and development of COVID-19, and cytokine storm may cause a poor prognosis in COVID-19 patients [11–13]. Mehta et al. proposed that cytokine storm syndrome may be associated with the disease severity of COVID-19 patients, and immunosuppression could be a therapy option for COVID-19 patients [11]. Increasing evidence demonstrated that interleukins (ILs) played an important role in the progression of COVID-19. Compared to mild COVID-19 cases, serum interleukins levels increased greatly in severe and critical patients [13–17]. Additionally, the cytokine profiles were different between survivors and nonsurvivors of COVID-19 patients [18]. IL-6 level was reported to be associated with patients' clinical manifestations, including body temperature and blood oxygen saturation, and COVID-19 patients with higher IL-6 levels had a poorer prognosis [19]. Therapeutic agents targeting IL-6 have been applied to clinical practice, which improved the outcomes of severe and critical COVID-19 patients [20, 21]. Thus, immunomodulatory agents targeting immune mediators provided novel clues for the treatment of COVID-19.
In this meta-analysis, we comprehensively analyzed the levels of serum interleukins in COVID-19 patients according to disease severity. Our results showed that elevated levels of IL-6, IL-8, and IL-10 were associated with the disease severity of COVID-19, and elevated levels of IL-1β, IL-6, and IL-8 were associated with the prognosis of COVID-19 cases, and more studies were needed to elucidate the roles of interleukins in the progression and prognosis of COVID-19 to improve the outcomes of patients.
2. Materials and Methods
2.1. Search Study
All procedures in this study were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22]. Records in Web of Science, Pubmed, and Embase were searched up to December 15, 2020. We used the following search strategy: (“novel coronavirus” OR “SARS-CoV-2” OR “2019-nCoV” OR “COVID-19” OR “coronavirus disease 2019”) AND (“IL-1” OR “interleukin-1” OR “IL-2” OR “interleukin-2” OR “IL-4” OR “interleukin-4” OR “IL-6” OR “interleukin-6” OR “IL-8” OR “interleukin-8” OR “IL-10” OR “interleukin-10”).
Two investigators (Y.M.C.) and (M.R.B.) researched all relevant articles, and articles that fulfilled the inclusion criteria were included. Any disagreement would be discussed until an agreement was reached. The disease severity of COVID-19 was already defined in the included studies based on clinical criteria, which was according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Plan, the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance, and World Health Organization (WHO) guidance [23–26], and COVID-19 patients with acute respiratory distress syndrome (ARDS) was defined as severe disease.
2.2. Study Selection and Data Extraction
The inclusion and exclusion criteria were used to identify relevant articles. Case reports, commentaries, meta-analyses, letters, reviews, animal trials, and editorials were excluded. The inclusion criteria were listed below: (i) patients with COVID-19 were confirmed by laboratory test; (ii) subgroup analysis was conducted according to disease severity; (iii) serum interleukin levels (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) were detected, which were expressed as median (q1-q3) or mean ± standard deviation (SD). The exclusion criteria were as follows: (i) patients were not diagnosed as COVID-19 cases or pediatric and pregnant COVID-19 cases; (ii) subgroup analysis was not conducted according to patient disease severity; (iii) data were not expressed as median (q1-q3) or mean ± SD, or data could not be transformed into mean ± SD; (iv) relevant serum interleukin (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) levels were not detected; and (v) repetitive publication. Two reviewers (Y.M.C) and (M.R.B) extracted the data from the selected studies, and the following items were extracted: first author, publication, country, number of subjects, median age, time of blood sampling, mean, SD, median, or interquartile of interleukins levels. The Newcastle-Ottawa Scale (NOS) tool was used to evaluate the quality of the included studies [27].
2.3. Statistical Analysis
All procedures were conducted in the R software. For data presented as median (q1-q3), the formulas mean = (q1 + m + q3)/3 and SD = (q3 − q1)/1.35 were used to transform the data into mean ± SD [28]. The weighted mean difference (WMD) and corresponding 95% confidence interval (CI) were calculated to compare the difference in serum interleukin levels between the two groups. The heterogeneity was assessed by I2, and a fixed-effects model was applied when I2 < 50%; otherwise, a random-effects model was adopted. The funnel plot and nonparametric trim and fill method were used to visualize and adjust the publication bias [29]. P < 0.05 (two sides) was recognized as significant difference.
3. Results
3.1. Basic Information of Included Studies
Initially, 1591 articles were searched. After reviewing the titles and abstracts, 245 records were included, and 1346 records were excluded. Finally, after reviewing the full length, 61 studies including 14,136 subjects (14,041 patients and 95 healthy individuals), were integrated into our meta-analysis. [18, 30–89]. The PRISMA chart and checklist showed the whole process of our meta-analysis (Figure 1 and Supplemental Table 1). Data in the 61 studies were presented in Supplemental Tables 2–7, and the NOS scores of the included 61 studies were shown in Supplemental Table 8.
Figure 1.
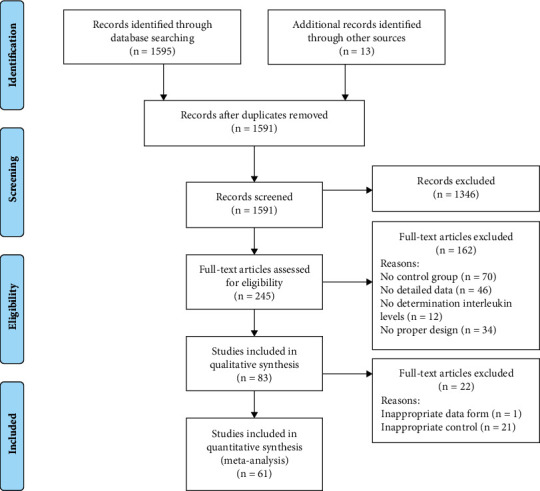
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
3.2. Alterations of IL-1β, IL-2, and IL-4 in COVID-19 Patients
To comprehensively elucidate the relationship between serum interleukins and disease severity of COVID-19 cases, we compared serum interleukin levels in COVID-19 patients with different disease severities. Our results showed that serum IL-1β levels were not elevated in severe COVID-19 patients compared to nonsevere patients (P = 0.33) (Figure 2(a)), while levels of IL-1β were elevated in nonsurvivor COVID-19 patients compared to survivors (WMD = 0.20, 95% CI: 0.15-0.24, and P < 0.01) (Figure 2(b)).
Figure 2.
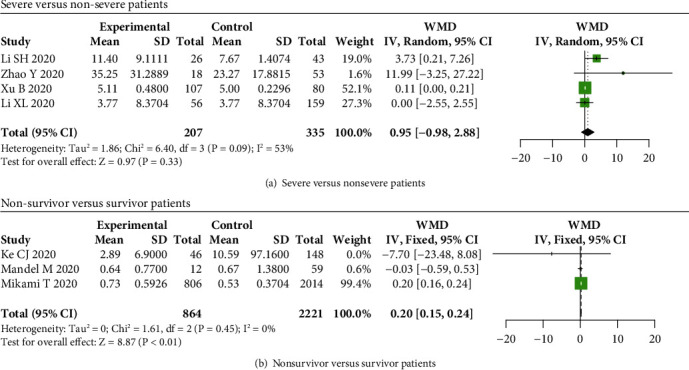
IL-1β levels in COVID-19 patients. A significant difference in IL-1β levels between severe and nonsevere COVID-19 patients was not found (P = 0.33) (a), while IL-1β levels were increased in (b) nonsurvivor patients compared to survivors.
For IL-2 in COVID-19 patients, increased serum IL-2 levels were observed in nonsevere and severe patients than that in healthy controls (WMD = 0.46, 95% CI: 0.20-0.73, and P < 0.01; WMD = 0.70, 95% CI: 0.50-0.89, and P < 0.01) (Figures 3(a) and 3(b)), while no significant difference in IL-2 levels between severe and nonsevere patients (P = 0.54) (Figure 3(c)).
Figure 3.
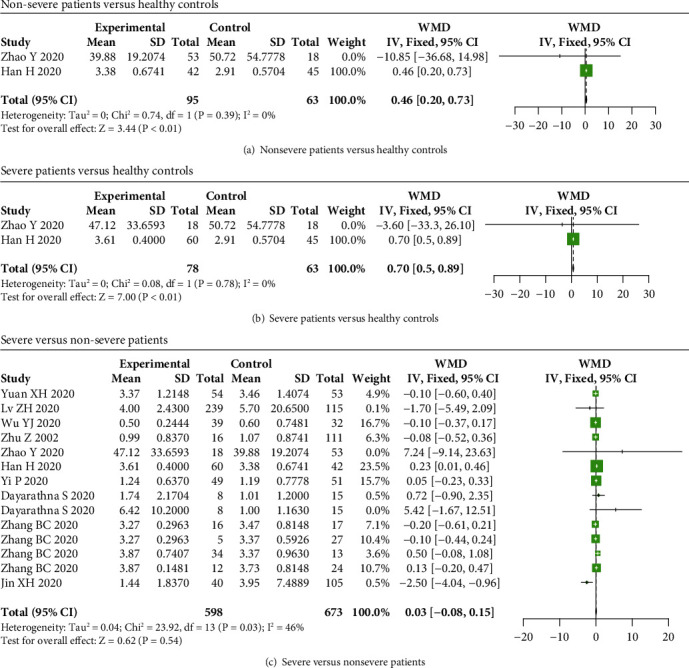
IL-2 levels in COVID-19 patients. The levels of IL-2 were increased in (a) nonsevere and (b) severe COVID-19 patients compared to healthy subjects, while no significant difference in IL-2 levels between (c) severe and nonsevere COVID-19 patients.
Serum IL-4 levels were elevated in nonsevere COVID-19 patients compared to healthy individuals (WMD = 0.43, 95% CI: 0.20-0.65, and P < 0.01) (Figure 4(a)), while no significant difference in IL-4 levels was observed between healthy controls and severe patients, as well as between severe and nonsevere COVID-19 patients (P > 0.05) (Figures 4(b) and 4(c)).
Figure 4.
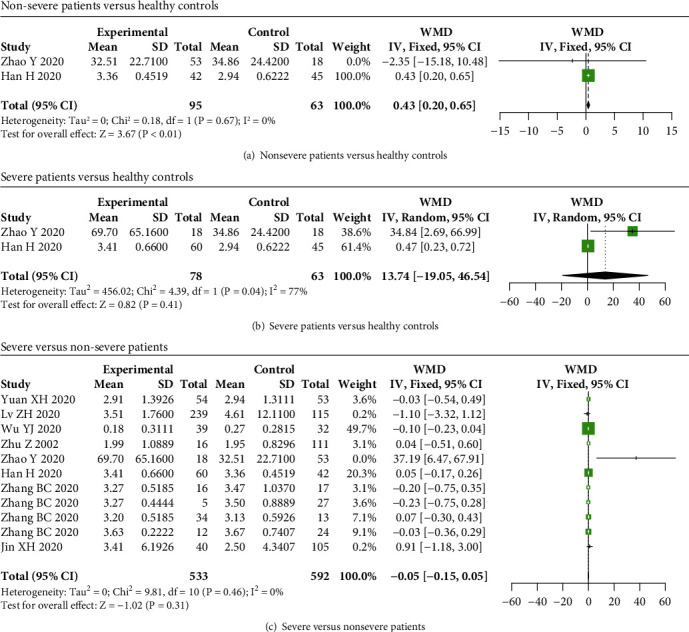
IL-4 levels in COVID-19 patients. IL-4 levels were elevated in (a) nonsevere COVID-19 patients compared to healthy subjects, while no significant difference in IL-4 levels between (b) severe patients and healthy controls, as well as between (c) severe and nonsevere COVID-19 patients.
3.3. Alterations of IL-6, IL-8, and IL-10 in COVID-19 Patients
Our results indicated that serum IL-6 levels were not elevated in nonsevere COVID-19 cases compared to healthy controls (P = 0.13) (Figure 5(a)), while IL-6 levels were elevated in severe patients compared to healthy controls (WMD = 25.05, 95% CI: 6.92-43.17, and P < 0.01) (Figure 5(b)). We also found that the levels of IL-6 were elevated in intensive care unit (ICU), severe, and nonsurvivor patients than that in non-ICU, nonsevere, and survivor patients (WMD = 73.02, 95% CI: 27.16-118.88, and P < 0.01; WMD = 19.43, 95% CI: 16.55-22.30, and P < 0.01; WMD = 31.06, 95% CI: 25.18-36.93, and P < 0.01) (Figures 5(c) and 6(a) and 6(b)).
Figure 5.
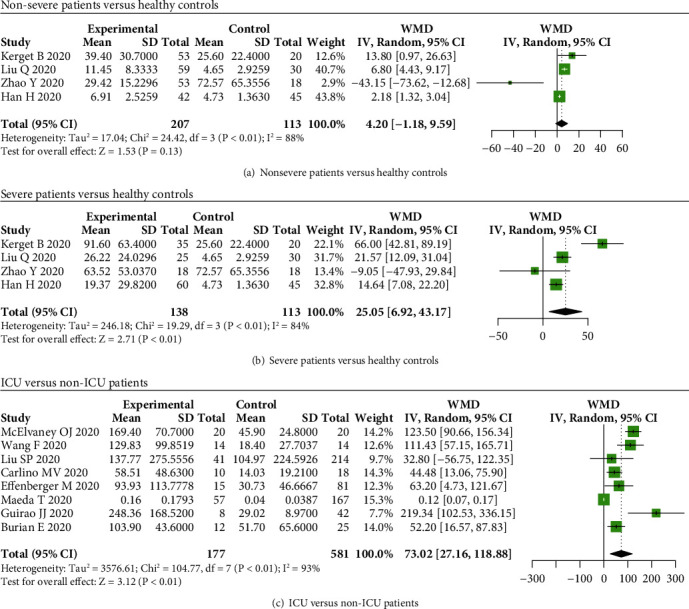
IL-6 levels in COVID-19 patients and healthy controls. No significant difference in serum IL-6 levels between (a) nonsevere COVID-19 patients and healthy controls, while IL-6 levels were elevated in (b) severe patients compared to healthy subjects, and levels of IL-6 were increased in (c) ICU patients compared to non-ICU patients.
Figure 6.
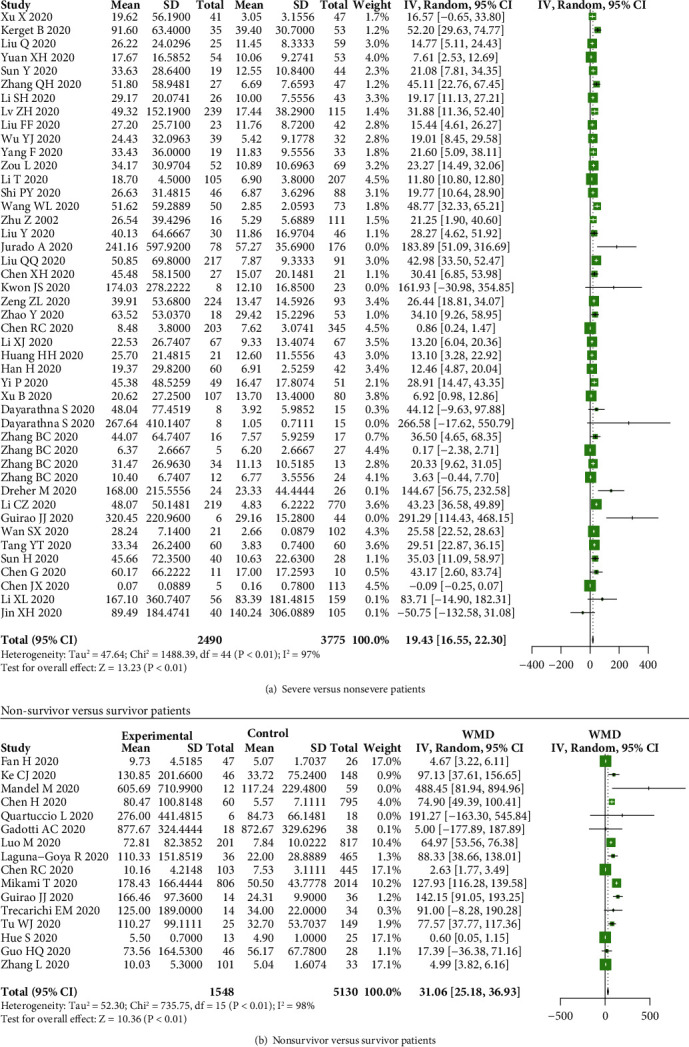
IL-6 levels in COVID-19 patients. The serum levels of IL-6 were increased in (a) severe and (b) nonsurvivor patients compared to nonsevere and survivor patients.
The serum IL-8 levels were elevated in ICU, severe, and nonsurvivor COVID-19 patients compared to non-ICU, nonsevere, and survivor patients (WMD = 42.55, 95% CI: 8.09-77.01, and P = 0.02; WMD = 11.72, 95% CI: 6.41-17.02, and P < 0.01; WMD = 23.61, 95% CI: 15.61-31.60, and P < 0.01) (Figures 7(a)–7(c)).
Figure 7.
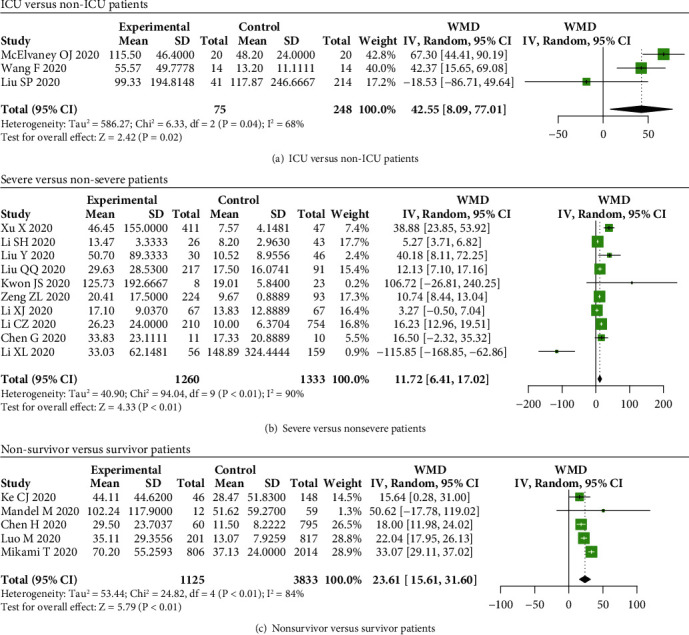
IL-8 levels in COVID-19 patients. The levels of IL-8 were increased in (a) ICU, (b) severe, and (c) nonsurvivor COVID-19 patients compared to non-ICU, nonsevere, and survivor patients.
For IL-10, we found that serum IL-10 levels were increased in nonsevere and severe cases with COVID-19 compared to healthy subjects (WMD = 1.08, 95% CI: 0.60-1.56, and P < 0.01; WMD = 2.27, 95% CI: 1.26-3.29, and P < 0.01) (Figures 8(a) and 8(b)). However, IL-10 levels were not elevated between ICU and non-ICU patients, as well as between nonsurvivor and survivor patients (P > 0.05) (Figures 8(c) and 8(e)). Additionally, the serum IL-10 levels were higher in severe patients compared to nonsevere patients (WMD = 2.29, 95% CI: 1.16-3.41, and P < 0.01) (Figure 8(d)).
Figure 8.
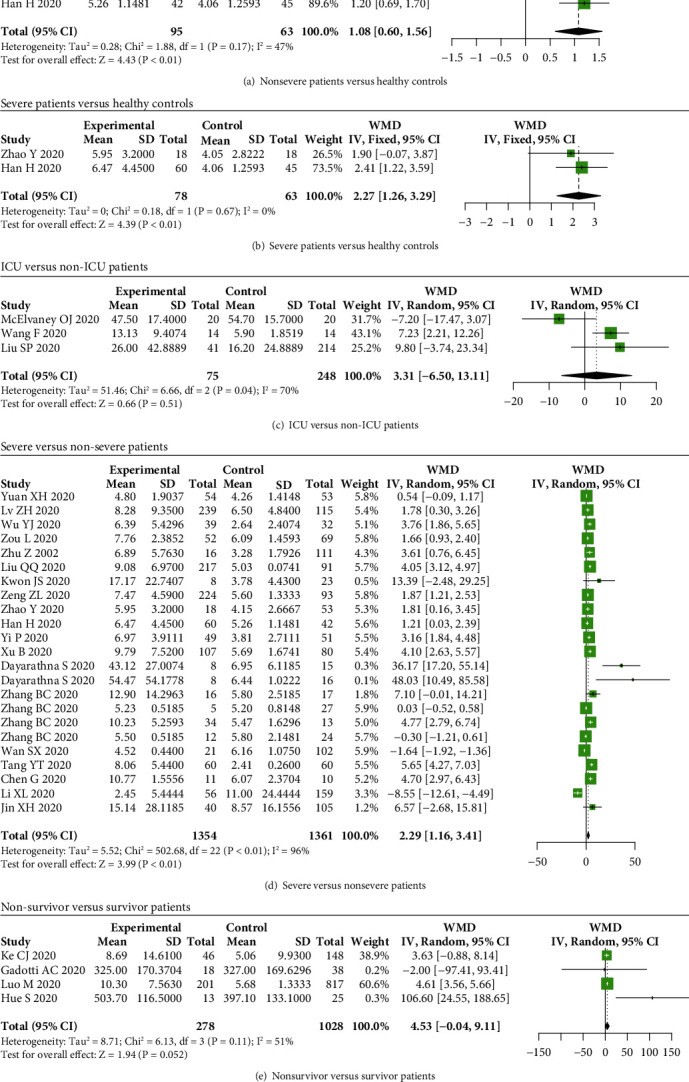
IL-10 levels in COVID-19 patients. The levels of IL-10 were elevated in (a) nonsevere and (b) severe patients compared to healthy controls, while no significant difference between (c) ICU and non-ICU patients, as well as between (e) nonsurvivor and survivor patients. The levels of IL-10 were elevated in (d) severe patients compared to nonsevere patients.
3.4. Publication Bias
In our meta-analysis, the potential publication bias was assessed and adjusted by funnel plot and the nonparametric trim and fill method, and Supplemental Figure 1 showed the publication bias for our meta-analysis, which was adjusted by the nonparametric trim and fill method.
4. Discussion
In this meta-analysis, we analyzed serum levels of IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10 in COVID-19 patients with different disease severities. Our main findings were as follows: (i) levels of IL-2, IL-4, IL-6, and IL-10 were elevated in COVID-19 patients compared to healthy subjects; (ii) levels of IL-6, IL-8, and IL-10 were elevated in severe COVID-19 cases compared to nonsevere patients, while no significant difference in IL-1β, IL-2, and IL-4 levels between severe and nonsevere patients; (iii) elevated levels of IL-1β, IL-6, and IL-8 were found in nonsurvivor COVID-19 patients compared to survivor ones; (iv) levels of IL-6 and IL-8 were elevated in ICU patients compared to non-ICU patients. Taken together, levels of IL-6, IL-8, and IL-10 were associated with the disease severity of COVID-19, and levels of IL-1β, IL-6, and IL-8 were correlated with the prognosis of COVID-19 patients, which may be used to predict the disease severity of COVID-19.
Since the outbreak of COVID-19, an increasing number of COVID-19 cases were confirmed. The cytokine storm occurred in COVID-19, and interleukins and IFN-γ were involved in the process of hyperinflammation [90]. Immune mediators including interleukins were demonstrated to play an important role in the development of COVID-19 [17, 91]. Tocilizumab, a kind of antibody that targeted the IL-6 signaling pathway, was demonstrated to be effective in treating severe COVID-19 patients, and biomarkers including C-reactive protein, procalcitonin, D-dimer, and lymphocyte levels were decreased after receiving tocilizumab administration [20, 92]. Hence, deepening the understanding of interleukins in the development of COVID-19 may contribute to its diagnosis and treatment.
In this study, we systematically analyzed the serum levels of IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10 in COVID-19 patients with different disease severities, as well as in healthy controls. Our results indicated that serum levels of IL-2, IL-4, IL-6, and IL-10 were increased in COVID-19 patients compared to healthy subjects. Additionally, we compared the levels of interleukins in severe and nonsevere COVID-19 patients, and the results indicated that the levels of IL-6, IL-8, and IL-10 were elevated in severe COVID-19 patients compared to nonsevere patients, while no significant difference in IL-1β, IL-2, and IL-4 levels between severe and nonsevere COVID-19 patients, implying that IL-6, IL-8, and IL-10 might be related to the disease severity of COVID-19. Then, we analyzed IL-1β, IL-6, IL-8, and IL-10 levels in nonsurvivor and survivor COVID-19 patients, and our results suggested that the levels of IL-1β, IL-6, and IL-8 levels were elevated in nonsurvivor patients compared to survivor patients, which indicated that IL-1β, IL-6, and IL-8 might be related to COVID-19 patients' prognosis. Compared to non-ICU patients, IL-6 and IL-8 levels were increased in ICU patients, which further demonstrated the important role of IL-6 and IL-8 in the pathogenesis of SARS-CoV-2 [93]. Taken together, these results showed that IL-6 and IL-8 were associated with the disease severity of COVID-19 patients, which may be used to predict patients' prognoses.
In conclusion, we found that serum levels of IL-6, IL-8, and IL-10 were associated with the disease severity of COVID-19 patients, and serum levels of IL-1β, IL-6, and IL-8 were associated with the prognosis of COVID-19 patients. Herein, more studies were needed to explore the immunological alterations underlying COVID-19 to improve its diagnosis and treatment.
The advantage of the meta-analysis was that more studies and more COVID-19 cases were included in our study, and we compared the levels of interleukins between COVID-19 cases and healthy subjects. Additionally, the COVID-19 patients included in our meta-analysis were from many countries, which makes the results to be more applicable worldwide. The limitation lies in that we used a random-effects model when great heterogeneity exists between studies, and pediatric and pregnant COVID-19 patients are excluded in our analysis, and our results may be not applied to them. Additionally, in our meta-analysis, the alterations of serum interleukin (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) levels in COVID-19 patients and healthy subjects were analyzed according to the following groups: (i) COVID-19 patients vs. healthy subjects, (ii) severe vs. nonsevere patients, (iii) survivor vs. nonsurvivor patients, and (iv) ICU vs. non-ICU patients. Because no relevant data was reported for the interleukins in some groups, not every kind of interleukin was analyzed according to the four groups, which caused the inconsistency in our results. In the next step, we will further comprehensively analyze the role of interleukins in the development of COVID-19.
Data Availability
The data in the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have declared no potential conflicts of interest in this study.
Authors' Contributions
Concept and design were contributed by Yuanmin Chang and Qinghai You. Acquisition, analysis, or interpretation of data was carried out by Yuanmin Chang and Mengru Bai. Drafting of the manuscript was performed by Yuanmin Chang. Critical revision of the manuscript for important intellectual content was done by all authors. Statistical analysis was carried out by Yuanmin Chang and Mengru Bai. Administrative, technical, or material support was contributed by Qinghai You. Supervision was contributed by Qinghai You.
Supplementary Materials
Supplemental Figure 1: the funnel plots concerning IL-2 (A), IL-4 (B), IL-6 (C–E), IL-8 (F, G), and IL-10 (H) in our meta-analysis, and the publication biases were adjusted by the nonparametric trim and fill method.
Supplemental Table 1: the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. Supplemental Table 2: data extracted from enrolled studies concerning IL-1β in COVID-19 patients. Supplemental Table 3: data extracted from enrolled studies concerning IL-2 in COVID-19 patients and healthy controls. Supplemental Table 4: data extracted from enrolled studies concerning IL-4 in COVID-19 patients and healthy controls. Supplemental Table 5: data extracted from enrolled studies concerning IL-6 in COVID-19 patients and healthy controls. Supplemental Table 6: data extracted from enrolled studies concerning IL-8 in COVID-19 patients. Supplemental Table 7: data extracted from enrolled studies concerning IL-10 in COVID-19 patients and healthy controls. Supplemental Table 8: the Newcastle-Ottawa Scale (NOS) score showed the qualities of included studies.
References
- 1.Vandenberg O., Martiny D., Rochas O., van Belkum A., Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nature Reviews Microbiology . 2021;19(3):171–183. doi: 10.1038/s41579-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., Liu S. The management of coronavirus disease 2019 (COVID-19) Journal of Medical Virology . 2020;92(9):1484–1490. doi: 10.1002/jmv.25965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahu K. K., Mishra A. K., Lal A. COVID-2019: update on epidemiology, disease spread and management. Monaldi Archives for Chest Disease . 2020;90(1) doi: 10.4081/monaldi.2020.1292. [DOI] [PubMed] [Google Scholar]
- 4.Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveillance . 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamadian M., Chiti H., Shoghli A., Biglari S., Parsamanesh N., Esmaeilzadeh A. COVID-19: virology, biology and novel laboratory diagnosis. The Journal of Gene Medicine . 2021;23(2, article e3303) doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress . 2020;4(4):66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ . 2020;368, article m1091 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai P., Kumar B. K., Deekshit V. K., Karunasagar I., Karunasagar I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Applied Microbiology and Biotechnology . 2021;105(2):441–455. doi: 10.1007/s00253-020-11061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumder J., Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. The AAPS Journal . 2021;23(1):p. 14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X., Li S., Yu H., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) . 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P., McAuley D. F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet . 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine . 2020;133, article 155151 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hojyo S., Uchida M., Tanaka K., et al. How COVID-19 induces cytokine storm with high mortality. Inflammation and regeneration . 2020;40(1):p. 37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zumla A., Hui D. S., Azhar E. I., Memish Z. A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet . 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. Journal of Clinical Virology . 2020;127, article 104370 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D., Li H., Lu X. X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World Journal of Pediatrics . 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R., Sang L., Jiang M., et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. The Journal of Allergy and Clinical Immunology . 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J., Shen J., Han Y., et al. Upregulated IL-6 indicates a poor COVID-19 prognosis: a call for tocilizumab and convalescent plasma treatment. Frontiers in Immunology . 2021;12, article 598799 doi: 10.3389/fimmu.2021.598799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences of the United States of America . 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J. J., Zhang L. N., Hou H., Xu L., Ji K. Interleukin-6 signaling blockade treatment for cytokine release syndrome in COVID-19 (review) Experimental and Therapeutic Medicine . 2021;21(1):p. 24. doi: 10.3892/etm.2020.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McInnes M. D. F., Moher D., Thombs B. D., et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy Studies. Journal of the American Medical Association . 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 23.The National Health Committee of the People's Republic of China. The novel coronavirus pneumonia diagnosis and treatment plan (trial version 7) 2020. March 2020, http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 24. National Health Commission & State Administration of Traditional Chinese Medicine Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) 2020. March 2020, https://www.who.int/docs/default-source/wpro---documents/countries/china/covid-19-briefing-nhc/1-clinical-protocols-for-the-diagnosis-and-treatment-of-covid-19-v7.pdf?sfvrsn=c6cbfba4_2.
- 25.World Health Organization. Clinical management of COVID-19 . Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 26.Who. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance . World Health Organization; 2020. [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology . 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology . 2014;14(1):p. 135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics . 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 30.Xu X., Yu M. Q., Shen Q., et al. Analysis of inflammatory parameters and disease severity for 88 hospitalized COVID-19 patients in Wuhan, China. International journal of medical sciences . 2020;17(13):2052–2062. doi: 10.7150/ijms.47935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerget B., Kerget F., Koçak A. O., et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID-19? Lung . 2020;198(5):777–784. doi: 10.1007/s00408-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q., Dai Y., Feng M., Wang X., Liang W., Yang F. Associations between serum amyloid A, interleukin-6, and COVID-19: a cross-sectional study. Journal of Clinical Laboratory Analysis . 2020;34(10, article e23527) doi: 10.1002/jcla.23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H., Zhang L., Huang B., et al. Cardiac injuries in patients with coronavirus disease 2019: not to be ignored. International Journal of Infectious Diseases . 2020;96:294–297. doi: 10.1016/j.ijid.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan X., Huang W., Ye B., et al. Changes of hematological and immunological parameters in COVID-19 patients. International Journal of Hematology . 2020;112(4):553–559. doi: 10.1007/s12185-020-02930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., Dong Y., Wang L., et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. Journal of Autoimmunity . 2020;112, article 102473 doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McElvaney O. J., McEvoy N. L., McElvaney O. F., et al. Characterization of the inflammatory response to severe COVID-19 illness. American Journal of Respiratory and Critical Care Medicine . 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q., Wei Y., Chen M., Wan Q., Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. Journal of Diabetes and its Complications . 2020;34(10, article 107666) doi: 10.1016/j.jdiacomp.2020.107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Jiang L., Li X., et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight . 2020;5(12) doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv Z., Cheng S., Le J., et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes and Infection . 2020;22(4-5):195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F., Ji C., Luo J., et al. Clinical characteristics and corticosteroids application of different clinical types in patients with corona virus disease 2019. Scientific Reports . 2020;10(1):p. 13689. doi: 10.1038/s41598-020-70387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y., Huang X., Sun J., et al. Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19. mSphere . 2020;5(4) doi: 10.1128/mSphere.00362-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. Journal of Medical Virology . 2020;92(10):2067–2073. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 43.Zou L., Dai L., Zhang Y., et al. Clinical characteristics and risk factors for disease severity and death in patients with coronavirus disease 2019 in Wuhan, China. Frontiers in Medicine . 2020;7:p. 532. doi: 10.3389/fmed.2020.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F., Yang Y., Dong K., et al. Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China. Endocrine Practice . 2020;26(6):668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T., Lu L., Zhang W., et al. Clinical characteristics of 312 hospitalized older patients with COVID-19 in Wuhan, China. Archives of gerontology and geriatrics . 2020;91, article 104185 doi: 10.1016/j.archger.2020.104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ke C., Yu C., Yue D., Zeng X., Hu Z., Yang C. Clinical characteristics of confirmed and clinically diagnosed patients with 2019 novel coronavirus pneumonia: a single-center, retrospective, case-control study. Medicina Clínica (Barcelona) . 2020;155(8):327–334. doi: 10.1016/j.medcli.2020.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi P., Ren G., Yang J., et al. Clinical characteristics of imported and second-generation coronavirus disease 2019 (COVID-19) cases in Shaanxi outside Wuhan, China: a multicentre retrospective study. Epidemiology and Infection . 2020;148, article e238 doi: 10.1017/S0950268820002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W., Zhao Z., Liu X., et al. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID-19. Journal of Clinical Laboratory Analysis . 2020;34(10, article e23547) doi: 10.1002/jcla.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z., Cai T., Fan L., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. International Journal of Infectious Diseases . 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunology . 2021;34(5):330–335. doi: 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- 51.Jurado A., Martín M. C., Abad-Molina C., et al. COVID-19: age, interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immunity & Ageing . 2020;17(1):p. 22. doi: 10.1186/s12979-020-00194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandel M., Harari G., Gurevich M., Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine . 2020;134, article 155190 doi: 10.1016/j.cyto.2020.155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Q. Q., Cheng A., Wang Y., et al. Cytokines and their relationship with the severity and prognosis of coronavirus disease 2019 (COVID-19): a retrospective cohort study. BMJ Open . 2020;10(11, article e041471) doi: 10.1136/bmjopen-2020-041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X., Zhao B., Qu Y., et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clinical Infectious Diseases . 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H., Chen R., Yang H., et al. Development and validation of a nomogram using on admission routine laboratory parameters to predict in-hospital survival of patients with COVID-19. Journal of Medical Virology . 2021;93(4):2332–2339. doi: 10.1002/jmv.26713. [DOI] [PubMed] [Google Scholar]
- 56.Kwon J. S., Kim J. Y., Kim M. C., et al. Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. The American Journal of Tropical Medicine and Hygiene . 2020;103(6):2412–2418. doi: 10.4269/ajtmh.20-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quartuccio L., Sonaglia A., Pecori D., et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. Journal of Medical Virology . 2020;92(11):2852–2856. doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S. P., Zhang Q., Wang W., et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Research and Clinical Practice . 2020;167, article 108338 doi: 10.1016/j.diabres.2020.108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gadotti A. C., de Castro Deus M., Telles J. P., et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Research . 2020;289, article ??? doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo M., Liu J., Jiang W., Yue S., Liu H., Wei S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight . 2020;5(13) doi: 10.1172/jci.insight.139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laguna-Goya R., Utrero-Rico A., Talayero P., et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. The Journal of Allergy and Clinical Immunology . 2020;146(4):799–807.e9. doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng Z., Yu H., Chen H., et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Critical Care . 2020;24(1):p. 525. doi: 10.1186/s13054-020-03255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y., Qin L., Zhang P., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight . 2020;5(13) doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X., Marmar T., Xu Q., et al. Predictive indicators of severe COVID-19 independent of comorbidities and advanced age: a nested case-control study. Epidemiology and Infection . 2020;148, article e255 doi: 10.1017/S0950268820002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang H., Song B., Xu Z., et al. Predictors of coronavirus disease 2019 severity: a retrospective study of 64 cases. Japanese Journal of Infectious Diseases . 2021;74(1):54–60. doi: 10.7883/yoken.JJID.2020.298. [DOI] [PubMed] [Google Scholar]
- 66.Carlino M. V., Valenti N., Cesaro F., et al. Predictors of intensive care unit admission in patients with coronavirus disease 2019 (COVID-19) Monaldi Archives for Chest Disease . 2020;90(3) doi: 10.4081/monaldi.2020.1410. [DOI] [PubMed] [Google Scholar]
- 67.Han H., Ma Q., Li C., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging microbes & infections . 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yi P., Yang X., Ding C., et al. Risk factors and clinical features of deterioration in COVID-19 patients in Zhejiang, China: a single-centre, retrospective study. BMC Infectious Diseases . 2020;20(1):p. 943. doi: 10.1186/s12879-020-05682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bo X. U., Fan C. Y., Wang A. L., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. Journal of Infection . 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Effenberger M., Grander C., Grabherr F., et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Digestive and Liver Disease . 2021;53(2):158–165. doi: 10.1016/j.dld.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeda T., Obata R., Rizk D. D., Kuno T. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. Journal of Medical Virology . 2021;93(1):463–471. doi: 10.1002/jmv.26365. [DOI] [PubMed] [Google Scholar]
- 72.Mikami T., Miyashita H., Yamada T., et al. Risk factors for mortality in patients with COVID-19 in new York City. Journal of General Internal Medicine . 2021;36(1):17–26. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dayarathna S., Jeewandara C., Gomes L., et al. Similarities and differences between the 'cytokine storms' in acute dengue and COVID-19. Scientific Reports . 2020;10(1):p. 19839. doi: 10.1038/s41598-020-76836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B., Zhou X., Zhu C., et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Frontiers in Molecular Biosciences . 2020;7:p. 157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dreher M., Kersten A., Bickenbach J., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Deutsches Ärzteblatt International . 2020;117(16):271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., Jiang J., Wang F., et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. Journal of Molecular and Cellular Cardiology . 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guirao J. J., Cabrera C. M., Jiménez N., Rincón L., Urra J. M. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Molecular Immunology . 2020;128:64–68. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan S., Yi Q., Fan S., et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. British Journal of Haematology . 2020;189(3):428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trecarichi E. M., Mazzitelli M., Serapide F., et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Scientific Reports . 2020;10(1):p. 20834. doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang Y., Li Y., Sun J., Pan H., Yao F., Jiao X. Selection of an optimal combination panel to better triage COVID-19 hospitalized patients. Journal of Inflammation Research . 2020;Volume 13:773–787. doi: 10.2147/JIR.S273193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation . 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J., Han T., Huang M., et al. Clinical characteristics of asymptomatic carriers of novel coronavirus disease 2019: a multi-center study in Jiangsu Province. Virulence . 2020;11(1):1557–1568. doi: 10.1080/21505594.2020.1840122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain A., Irizarry-Caro R. A., McDaniel M. M., et al. T cells instruct myeloid cells to produce inflammasome-independent IL-1β and cause autoimmunity. Nature Immunology . 2020;21(1):65–74. doi: 10.1038/s41590-019-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tu W. J., Cao J., Yu L., Hu X., Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Medicine . 2020;46(6):1117–1120. doi: 10.1007/s00134-020-06023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo H., Shen Y., Wu N., Sun X. Myocardial injury in severe and critical coronavirus disease 2019 patients. Journal of Cardiac Surgery . 2021;36(1):82–88. doi: 10.1111/jocs.15164. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L., Huang B., Xia H., et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiology and Infection . 2020;148, article e199 doi: 10.1017/S0950268820002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin X. H., Zhou H. L., Chen L. L., et al. Peripheral immunological features of COVID-19 patients in Taizhou, China: a retrospective study. Clinical Immunology . 2021;222, article 108642 doi: 10.1016/j.clim.2020.108642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burian E., Jungmann F., Kaissis G. A., et al. Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the Munich cohort. Journal of Clinical Medicine . 2020;9(5):p. 1514. doi: 10.3390/jcm9051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hue S., Beldi-Ferchiou A., Bendib I., et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine . 2020;202(11):1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jamilloux Y., Henry T., Belot A., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmunity Reviews . 2020;19(7, article ???) doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khanmohammadi S., Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. Journal of Medical Virology . 2021;93(5):2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ivan Hariyanto T., Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. Journal of Medical Virology . 2021;93(3):1832–1836. doi: 10.1002/jmv.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma A., Zhang L., Ye X., et al. High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Frontiers in Immunology . 2021;12, article 626235 doi: 10.3389/fimmu.2021.626235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: the funnel plots concerning IL-2 (A), IL-4 (B), IL-6 (C–E), IL-8 (F, G), and IL-10 (H) in our meta-analysis, and the publication biases were adjusted by the nonparametric trim and fill method.
Supplemental Table 1: the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. Supplemental Table 2: data extracted from enrolled studies concerning IL-1β in COVID-19 patients. Supplemental Table 3: data extracted from enrolled studies concerning IL-2 in COVID-19 patients and healthy controls. Supplemental Table 4: data extracted from enrolled studies concerning IL-4 in COVID-19 patients and healthy controls. Supplemental Table 5: data extracted from enrolled studies concerning IL-6 in COVID-19 patients and healthy controls. Supplemental Table 6: data extracted from enrolled studies concerning IL-8 in COVID-19 patients. Supplemental Table 7: data extracted from enrolled studies concerning IL-10 in COVID-19 patients and healthy controls. Supplemental Table 8: the Newcastle-Ottawa Scale (NOS) score showed the qualities of included studies.
Data Availability Statement
The data in the current study are available from the corresponding author on reasonable request.


