Abstract
The algicidal compounds produced by Bacillus sp. strain B1 against Phaeocystis globosa, one of the main red-tide algae, were isolated and identified in a previous study as urocanic acid (uro), l-histidine (his) and N-acetylhistamine (ace). The 96 h median effective concentration EC50 values indicated the algicidal effect order of uro (8 μg mL−1) > ace (16 μg mL−1) > his (23 μg mL−1). The interaction between uro and ace had a synergistic effect on Phaeocystis globosa, accelerated the increase in its intracellular reactive oxygen species (ROS) levels, and further decreased the activities of antioxidases after 96 h, causing destruction of cell membrane integrity and nuclear structure. However, the other two binary mixtures uro + his and ace + his were both antagonistic to Phaeocystis globosa. The increase in the level of ROS indicated that the algal cells suffered from oxidative damage. The surplus ROS induced the increase in malondialdehyde (MDA) content and activities of antioxidant enzymes including superoxide dismutase (SOD) and catalase (CAT), all of which reached maxima after 72 h treatment. Transmission electron microscopy (TEM) analysis revealed that these nitrogen-containing compounds caused destruction of cell membrane integrity, chloroplasts and nuclear structure. The present study will provide useful information for the combined effect of algicidal compounds on the harmful alga Phaeocystis globosa. This is the first report to explore single and combined algicidal effects of three nitrogen-containing compounds against the harmful alga Phaeocystis globosa.
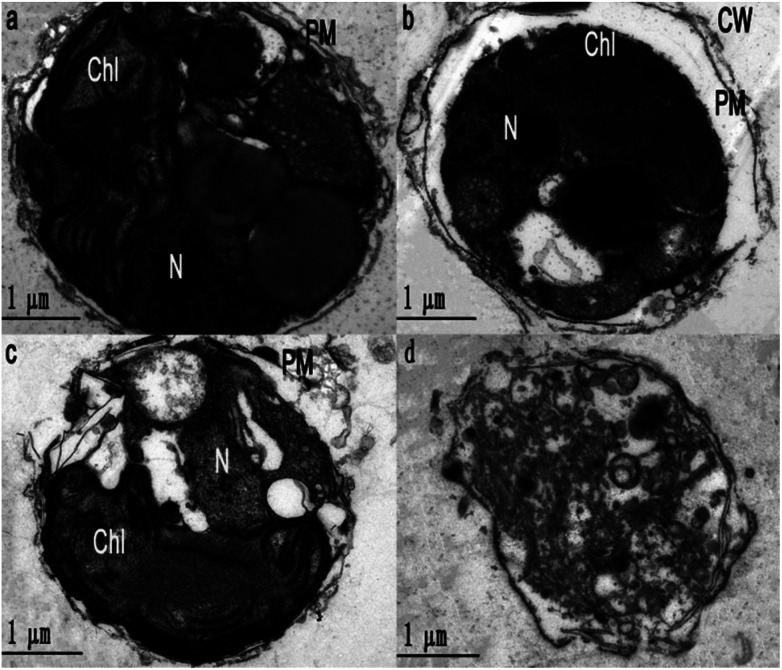
Ultrastructure of Phaeocystis globosa cells after treatment with EC50 value for 72 h: (a) control, (b) ace (16 μg mL−1), (c) uro (8 μg mL−1), (d) uro + ace (1 : 1 TU, 8 : 16 μg mL−1) Chl, chloroplast; CW, cell wall; N, nucleus; PM, plasma membrane.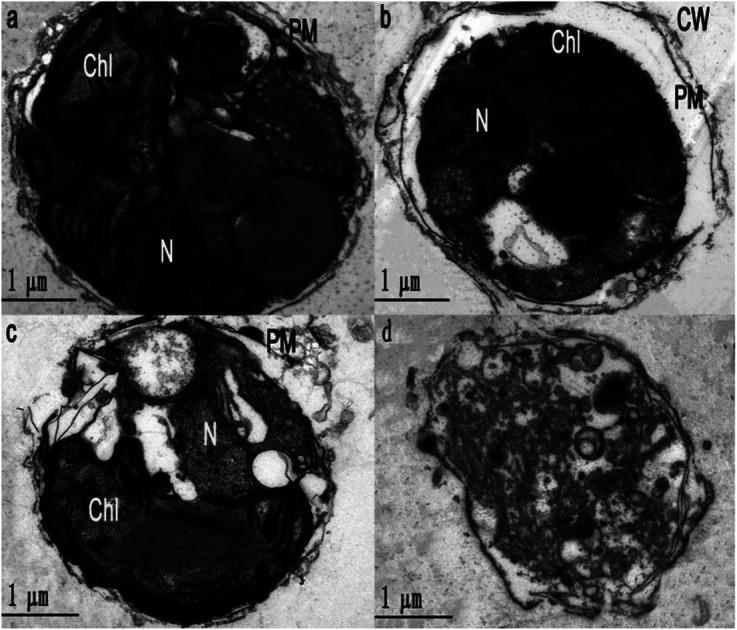
Introduction
Harmful algal blooms (HABs) have become a severe problem globally owing to their production of large amounts of toxins, resulting in severe negative impacts on aquatic ecosystems, the fish farming industry, tourism and human health.1–3Phaeocystis globosa is a notorious HAB species that frequently breaks out in China and causes nuisance foam generation and oxygen depletion by releasing toxic hemolytic substances.4,5 Thus, it is necessary to develop effective and environmentally friendly management strategies to prevent HAB occurrences and mitigate the adverse impacts. In recent years, biological methods have gradually gained attention for their low cost, low toxicity and low secondary pollution.6,7 Biological methods include the use of plants8 and microorganisms such as protozoa,9 fungi,10 microalgae,11 viruses12 and bacteria.13 In particular, these type of bacteria have strong algicidal activities and can lyse algae by attacking cells directly or indirectly by releasing algicidal compounds including proteins,14 polypeptides,15 biosurfactants,16 amino acids17 and antibiotics.18
However, most research so far has focused on the interaction between algicidal bacteria and algae.19 The studies on the specific combined effect between pure algicidal compounds on the harmful alga, particularly on Phaeocystis globosa, is still not well documented. In spite of the isolation and identification of these algicidal nitrogen-containing compounds, the algicidal effect of each algicidal compound needs to be validated further. In addition, binary mixtures are likely to enhance algicidal effects; this indicates that the study of combined effects could be necessary.
In view of the above-mentioned discussion, the objective of the present study was therefore to investigate the individual and combined effects of three algicidal compounds, namely, uro, ace and his, produced by Bacillus sp. strain B1 (isolated and identified as in a previous study20), on Phaeocystis globosa and to assess and determine the specific combined actions using toxicity unit (TU) and additional index (AI) methods. Ace is administered orally and rectally to healthy individuals.21 His is one of the necessary amino acids for people, which plays a great role in repairing injured tissues.22 Uro is produced by deamination of his with the help of his ammonia lyase.23 His, ace and uro are nitrogen-containing compounds and good nutrients as amino acids. It is possible that growth inhibition by amino acids is the result of similar connections in the cell's processes of biosynthesis and utilization of related metabolites. l-Valine (val) inhibited Escherichia coli K12 by preventing the biosynthesis of isoleucine and exogenous val was incorporated into proteins in place of other amino acids, producing faulty proteins.24
Current studies regarding the algicidal mechanism of inhibition of algal growth have explored the destruction of cell structures, alteration of enzymatic activities and influence on algal photosynthesis.25,26 In this study, aspects of enzymatic activities and cell structures were clarified. The natural algicidal compounds induce the algal cells to produce superfluous ROS, which further increase antioxidant defenses and develop lipid peroxidation. Cellular antioxidant defenses including SOD and CAT become more active to protect algal cells from oxidative damage by superfluous elimination of ROS. In our study, the physiological characteristics including the ROS level, protein content, oxidative indicator of MDA, and the enzyme activities of SOD and CAT were determined to investigate oxidative damage to algal cells. Subcellular structure was detected by TEM analysis. Results from this study will help us realistically understand the mechanisms of algicidal compounds on Phaeocystis globosa.
Results and discussion
Algicidal activity of three nitrogen-containing compounds and three types of binary mixtures
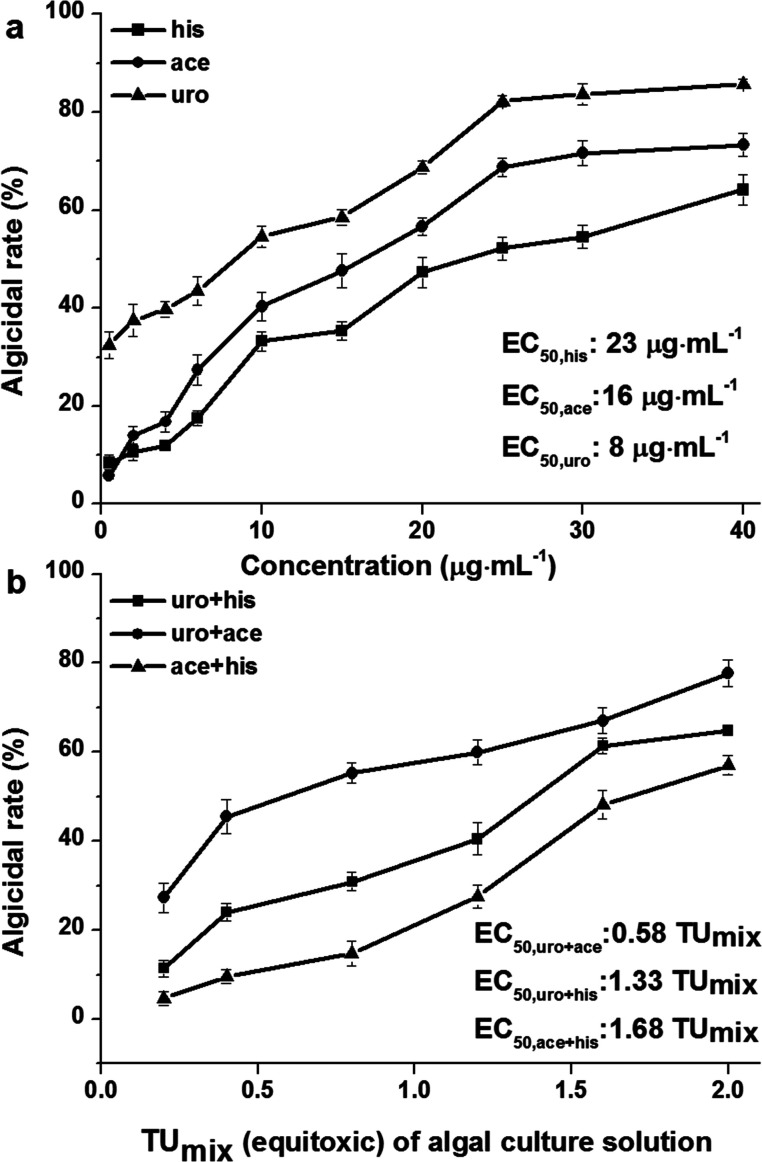
His, ace and uro are nitrogen-containing compounds produced by Bacillus sp. strain B1 as reported in a previous study.20 As shown in Fig. 1a, uro generally exhibits stronger algicidal effect than ace and his. The EC50 values (concentrations for 50% of maximal algicidal effect) of uro, ace and his, calculated from the dose response curve, were 8, 16 and 23 μg mL−1, respectively. Furthermore, his, ace and uro produced inhibition effects comparable to another report, which stated that Phaeocystis globosa is very sensitive to his at a concentration of 20 μg mL−1, ace at a concentration of 4 μg mL−1 and uro at a concentration of 2 μg mL−1.20 The sensitive concentration and EC50 values indicated the same algicidal effect order: uro > ace > his. We speculated that the uptake of uro by Phaeocystis globosa was much quicker than that of ace and his, resulting in stronger algicidal activity in uro treatment than either ace or his. Val mainly showed stronger algicidal activity and lysed Microcystis aeruginosa cells quicker than l-lysine (lys), which resulted from the quicker uptake of val by Microcystis aeruginosa than lys.27
Fig. 1. Algicidal effects of (a) different concentrations of his, ace, uro and (b) their binary mixtures on the growth of Phaeocystis globosa for 96 h, displayed as EC50. One toxic unit (TU) means the toxic concentration is nearly EC50. All error bars indicate standard error of three replicates.
An equitoxic combined experiment for binary mixtures was conducted based on EC50 of single uro, ace and his (Fig. 1a). Binary mixtures uro + ace, uro + his and ace + his produced results of EC50 at 0.58 TUmix, 1.33 TUmix and 1.68 TUmix respectively. The 0.58 TUmix indicated that a binary mixture of 2 μg mL−1 (0.29 TUmix) uro and 5 μg mL−1 (0.29 TUmix) ace would inhibit Phaeocystis globosa growth by 50% as compare to the control. Similarly, 1.33 TUmix and 1.68 TUmix indicate that 6 μg mL−1 (0.66 TUmix) uro and 15 μg mL−1 (0.66 TUmix) his and 14 μg mL−1 (0.84 TUmix) ace and 19 μg mL−1 (0.84 TUmix) his would have the same effect. The values of AI were 0.71 (>0), −0.33 (<0) and −0.64 (<0). This indicated that the interactions between uro and ace, uro and his, ace and his were in conformity with synergism, antagonism, and antagonism as determined by TU and AI methods. It is widely acknowledged that within experimental error, M = 1 is an ideal state and extremely difficult to obtain.28 In addition, it should be noted that in the algicidal test of binary mixtures we examined only 1 : 1 ratio of toxic units which ignored the algicidal effect when Phaeocystis globosa was exposed to binary mixtures under different ratios of toxic units, for which the magnitude and the type of interaction may be dependent on the relative proportions of components in the mixture.29 Thus, the results of the evaluation of algicidal effects of binary mixtures among three nitrogen-containing compounds was related to the relative proportion of components in the mixture, which needs further study.
Effects of nitrogen-containing compounds on ROS levels and protein contents
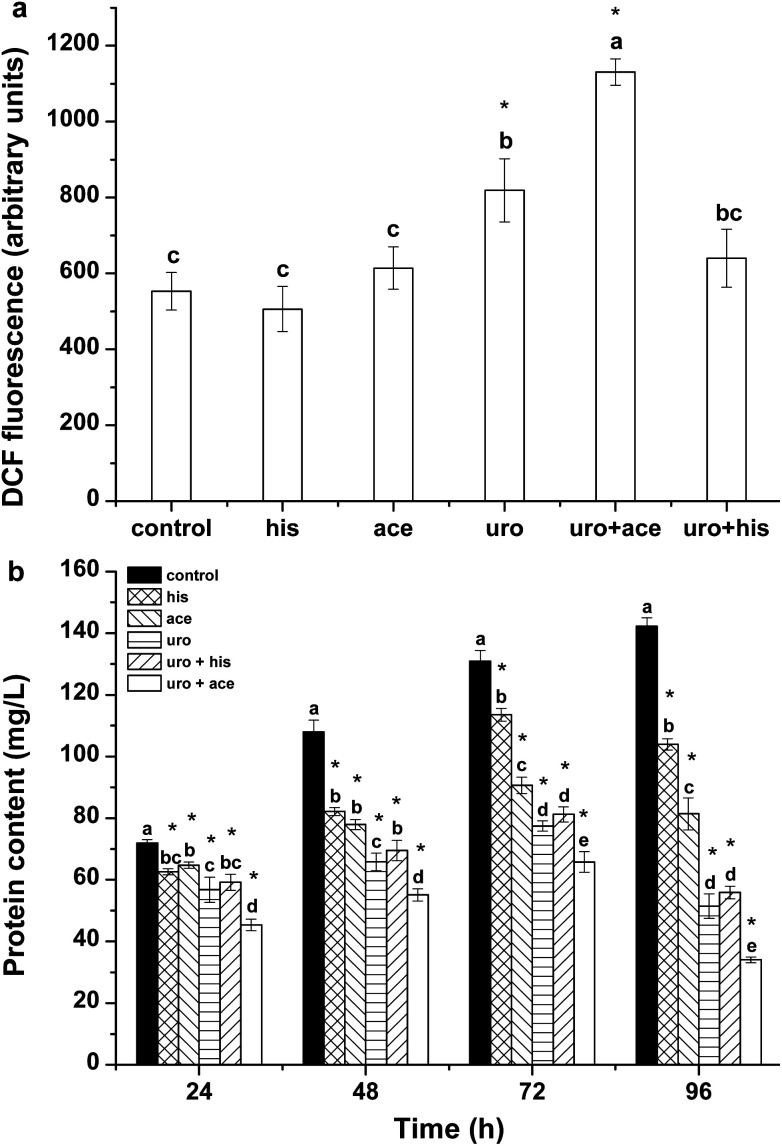
ROS include singlet oxygen (1O2), superoxide radical (O2·−), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH) and are produced in the mitochondrial electron transport chain, peroxisomes and chloroplast.30 ROS are involved in damaging living organisms under environmental stress. Excessive ROS may cause irreversible oxidative damage to intracellular components, finally leading to cell death.31 In the present study, the ROS levels and contents of total proteins were determined to assess the oxidative damage in cells of Phaeocystis globosa and the growth inhibition of Phaeocystis globosa. The results are shown in Fig. 2. It can be seen that weak fluorescence of DCF was present in the control group and the uro + ace mixture clearly showed the maximal value after 72 h-exposure. The ROS level in Phaeocystis globosa cells exposed to uro + ace mixture was higher than the levels in those exposed to uro + his mixture and the substances applied individually (Fig. 2a). The cellular protein contents in treatment groups were significantly lower than that of the control (Fig. 2b). After 96 h of treatment with his, ace, uro, uro + his and uro + ace, the protein contents in the treatment samples were reduced to 73%, 57%, 36%, 39% and 24% relative to the control, respectively. The increased protein contents in the control group suggested that new proteins were synthesized. These results implied that external environmental stress could affect cellular protein synthesis in algal cells.
Fig. 2. Effects of control, his, ace, uro with EC50 value, (1 TU, 23, 16, 8 μg mL−1, respectively) uro + ace (1 : 1 TU, 8 : 16 μg mL−1) and uro + his (1 : 1 TU, 8 : 23 μg mL−1) on (a) ROS level after 72 h and (b) protein contents of Phaeocystis globosa. One toxic unit (TU) means the toxic concentration is nearly EC50. All error bars indicate standard error of three replicates. *Indicates significant difference (P < 0.05) with respect to control. Letters above the bars indicate significant differences (P < 0.05).
Oxidative stress analysis
SOD and CAT are important antioxidases that eliminate intracellular ROS; they protect cells against damage caused by ROS and harmful substances.32 MDA can indicate the extent of lipid peroxidation, which reflects the oxidative damage of the cellular membrane.33Fig. 3a–c show the effects of his, ace, uro, uro + ace and uro + his on lipid peroxidation and antioxidative enzyme activities. The results (Fig. 3a) showed that his, ace, uro and uro + his had weaker effects on MDA content within algal cells than the control after 24 h. However, the MDA content was apparently affected by uro + ace on the third day. These results demonstrated that the uro + ace mixture increased the content of ROS, further damaging the cellular membrane. Cellular enzymatic activities including SOD and CAT were determined to investigate the cellular defense responses induced by exposure to his, ace, uro, uro + ace and uro + his culture broths (Fig. 3b and c). After 72 h of treatment, SOD activity reached maximum levels, rising by 1.7, 1.9, 2.2, 2.1 and 2.7 times (P < 0.05), respectively, over that of the control. However, SOD activity significantly declined to lower levels for all treatment groups after 96 h. CAT activity showed a pattern similar to SOD activity (Fig. 3c) with the activities significantly increasing within 72 h. The activity values after treatment for 72 h reached their highest levels: 2.3, 2.9, 3.2, 3.2 and 3.6 times (P < 0.05) higher in treatment groups than in the control. The possible reason is that to protect against the potential damaging effects of ROS, various antioxidases such as SOD and CAT can be activated by intracellular excessive ROS under environmental stress.32,34 Similar variations of SOD and CAT activities were found when algal cells were exposed to allelochemicals.35,36 These tests demonstrated that the effects of lipid peroxidation and antioxidative enzyme activities upon exposure to uro + ace mixture were stronger than the effects of those on exposure to ace + his mixture and the compounds applied individually.
Fig. 3. Effects of his (1 TU, 23 μg mL−1), ace (1 TU, 16 μg mL−1), uro (1 TU, 8 μg mL−1) with EC50 value, uro + ace (1 : 1 TU, 8 : 16 μg mL−1) and uro + his (1 : 1 TU, 8 : 23 μg mL−1) on (a) MDA, (b) SOD, and (c) CAT contents of Phaeocystis globosa. One toxic unit (TU) means the toxic concentration is nearly EC50. All error bars indicate standard error of three replicates. *Indicates significant difference (P < 0.05) with respect to control. Letters above the bars indicate significant differences (P < 0.05).
Morphological and ultrastructural changes
To obtain further evidence to support the synergism between uro and ace, the cell morphology and ultrastructure of Phaeocystis globosa after uro, ace and uro + ace (1 : 1 TU) treatment with EC50 for 72 h were investigated by TEM analysis. Fig. 4b–d reveal that Phaeocystis globosa cells are damaged to varying degrees. The algal cells in the control group have intact plasma membranes, tightly stacked lamellar structure of chloroplast, and a nucleus with intact nucleolus in good shape (Fig. 4a). However, the cell brim crimped and the plasma membrane began to degrade with some pinholes upon ace treatment. In addition, severe plasmolysis was also observed (Fig. 4b). Fragmentation of the cell membrane was seen under the electron microscope, which revealed that the membrane was severely damaged and the cell had lost integrity during uro treatment. Moreover, the lamellar structure of the chloroplast became loose and the nucleolus disappeared (Fig. 4c). Interestingly, the organelles and nucleus were difficult to distinguish; the cell membrane was significantly weak and blurry, releasing intracellular substances through the ruptured plasma membrane, indicating the death of algal cells (Fig. 4d). This result indicated that the main targeted sites may be different for algicidal substances with different structures and characteristics. Accordingly, the possible reasons that uro (mainly disrupting the chloroplast and nucleolus) and ace (deteriorating membrane permeability) enhanced algicidal action may be as follows: antioxidase activities in Phaeocystis globosa cells are affected by uro and ace, accelerating the increase in ROS level and lipid peroxidation; the higher level of ROS may further disrupt the chloroplast and nucleolus and deteriorate membrane permeability, resulting in greater increase in ROS and oxidative damage in Phaeocystis globosa cells. When exposed to microbial algicide37 and cyclo-(Pro-Gly),26 similar cell morphology and ultrastructure of Phaeocystis globosa were found.
Fig. 4. Ultrastructure of Phaeocystis globosa cells after treatment with EC50 value for 72 h. (a) Control, (b) ace (16 μg mL−1), (c) uro (8 μg mL−1), and (d) uro + ace (1 : 1 TU, 8 : 16 μg mL−1). Chl, chloroplast; CW, cell wall; N, nucleus; PM, plasma membrane. One toxic unit (TU) means the toxic concentration is nearly EC50.
Herein, we identify that synergism exists between uro and ace, which might be used as an effective strategy for the future control of Phaeocystis globosa blooms.
Experimental
Materials
Uro, his and ace were purchased from Sigma-Aldrich. The compounds were dissolved in distilled water to make stock solutions at a concentration of 0.4 g L−1. The solutions were stored at 4 °C before testing.
Phaeocystis globosa cultures and treatment
Phaeocystis globosa was kindly provided by Professor Songhui Lv, Jinan University, China and all cultures were maintained in modified f/2 medium under a 12 h light/12 h dark cycle with light intensity of 50 μmol photons m−2 s−1 at 20 ± 1 °C. The medium used was man-made seawater with salinity of 27% instead of natural seawater. The number of cells was counted under an optical microscope. Different concentrations of the three algicidal nitrogen-containing compounds were added when the concentration of algae reached approximately 107 cells per mL.
Chlorophyll assay
Chlorophyll-a content variation was used to determine algicidal activity in Phaeocystis globosa. To measure the content of chlorophyll-a, one control (0 μg mL−1) and ten concentrations of each nitrogen compound were added to 50 mL algal culture solution when the algae grew to the logarithmic growth phase. Cultures without added nitrogen-containing compounds were prepared as controls. After cultivation for 96 h, an aliquot of 20 mL algal culture was removed and centrifuged at 6000g for 10 min and the precipitate was collected. The algal cells were stored in the dark at 4 °C for 24 h in 5 mL 90% (v/v) acetone. The acetone extract was centrifuged at 6000g for 10 min and the absorbance was measured at wavelengths of 665, 645, and 630 nm using a 721 Vis spectrophotometer. The content of chlorophyll-a was calculated using the following formula:38
| C = 11.6 × A665 − 1.31 × A645 − 0.14 × A630 (mg L−1) | 1 |
The algicidal rate was calculated according to the following formula:
| Algicidal rate (%) = (CC − CE)/CC | 2 |
where CC represents the chlorophyll-a content of the control group and CE represents the chlorophyll-a content of the experimental group.
Single algicidal validation test
To study the dose–response relationships of four algicidal pure nitrogen-containing compounds on Phaeocystis globosa, a certain quantity of his stock solution at 0.4 g L−1 was added into 50 mL algal culture solution to obtain a series of final concentrations at 0.5, 2, 4, 6, 10, 15, 20, 25, 30, and 40 μg mL−1. Similarly, the same steps were followed to prepare series of concentrations of ace and uro.
Binary mixture design
Binary mixtures of the three compounds uro, ace and his were designed using the equivalent-effect concentration method. The 96 h-EC50 values of the three compounds in a single algicidal experiment were defined as one toxic unit (TUuro, TUace and TUhis, respectively). The algicidal activities of binary mixtures made of 1 : 1 toxic unit to Phaeocystis globosa were determined and equitoxic mixtures with a range of six concentrations (0.2, 0.4, 0.8, 1.2, 1.6, 2 TU) were prepared. Table 1 shows the compositions of the as-prepared binary mixtures. The specific combined algicidal effect was evaluated by conventional methods, namely, Toxicity Unit (TU) method and Additive Index (AI) method.39 Three binary mixtures were named uro + ace, uro + his and ace + his in the experimental design. For the combined algicidal experiment, the same method as mentioned above was used to obtain the algicidal rate as index of evaluating algicidal activity. Three replicates were used for each concentration. The exposure time for combined algicidal experiments was 96 h. In general, the formulae of TU and AI methods for evaluating specific combined effects are the following:
Compositions of binary mixtures in combined testa.
| Treatment (TUmix) | Aceconc (μg mL−1) (TUace) + uroconc (μg mL−1) (TUuro) | Hisconc (μg mL−1) (TUhis) + uroconc (μg mL−1) (TUuro) | Aceconc (μg mL−1) (TUace) + hisconc (μg mL−1) (TUhis) |
|---|---|---|---|
| 0.2 | 1.6 (0.1) + 0.8 (0.1) | 2.3 (0.1) + 0.8 (0.1) | 1.6 (0.1) + 2.3 (0.1) |
| 0.4 | 3.3 (0.2) + 1.3 (0.2) | 4.6 (0.2) + 1.3 (0.2) | 3.3 (0.2) + 4.6 (0.2) |
| 0.8 | 6.5 (0.4) + 3.3 (0.4) | 9.1 (0.4) + 3.3 (0.4) | 6.5 (0.4) + 9.1 (0.4) |
| 1.2 | 9.8 (0.6) + 5 (0.6) | 13.7 (0.6) + 5 (0.6) | 9.8 (0.6) + 13.7 (0.6) |
| 1.6 | 13.1 (0.8) + 6.7 (0.8) | 18.2 (0.8) + 6.7 (0.8) | 13.1 (0.8) + 18.2 (0.8) |
| 2 | 16.3 (1) + 8.3 (1) | 22.8 (1) + 8.3 (1) | 16.3 (1) + 22.8 (1) |
Uroconc, aceconc and hisconc mean the concentration of uro, ace, and his in algal solution. One toxic unit (TU) means the toxic concentration is nearly EC50.
(A) Toxicity Unit (TU)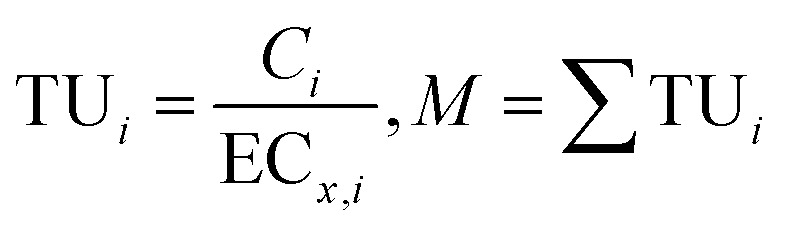
(B) Additive Index (AI)AI = M − 1 (M = 1); AI = (1/M) − 1 (M < 1); AI = −M + 1 (M > 1)
Assays for ROS levels and oxidative stress test
The intracellular ROS were detected using the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe. DCFH-DA was used at a final concentration of 20 μmol mL−1 and was incubated with suspended cells for 20 min at room temperature. Then, the cells were immediately washed three times with 0.1 M Phosphate Buffer Solution (PBS) and suspended in 300 μL 0.1 M PBS. The fluorescent product DCF was observed using a Meta Duo Scan Laser Scanning Confocal Microscope with excitation wavelength 488 nm and emission wavelength 525 nm.
Lipid peroxidation was measured by MDA and anti-oxidant ability was assayed by enzyme (SOD, CAT) activities. When the algae grew to the logarithmic growth phase, certain quantities of the three nitrogen-containing compound stock solutions at 0.4 g L−1 were added into 50 mL algal culture solution at concentrations of EC50 (but not to 50 mL algal solution control groups); the culture conditions were the same as that of the algal culture (see section Algal cultures above). The sample treatment of algae for analysis was as follows: algal cells were collected by centrifugation at 6000g for 10 min at 4 °C; PBS (0.05 mol L−1, pH 7.8) was added to the cells, which were then ground to homogeneity in an ice bath; the supernatant of the homogenate was collected at 4 °C and stored at −70 °C.
The SOD activity, CAT and MDA contents of Phaeocystis globosa were determined by following methods described in the literature.36,40,41
Transmission electron microscopy (TEM) analysis
The algal cells were collected by centrifugation at 2000g for 10 min at 4 °C; then, the cells were fixed with 2.5% glutaraldehyde in PBS for 12 h at 4 °C and post-fixed with 1% OsO4 in PBS for 1.5 h. The samples were dehydrated using a graded ethanol series, followed by a graded ethanol : acetone series and then embedded in araldite resin. Ultrathin sections were stained in 2% acetic acid uranium–citric acid and examined with a JEM2100F transmission electron microscope.
Conclusions
In brief, the effects of uro, ace and his on physiological characteristics were investigated. The results indicated that ace and uro induced ROS production and superfluous ROS caused lipid peroxidation, loss of cell membrane integrity, and rapid rupture of the cells, ultimately damaging the subcellular structure and inhibiting cell growth or lysing algal cells. Based on the results of physiological characteristics and morphological and ultrastructural changes, it could be seen that synergism exists between uro and ace, representing an effective strategy for the future control of Phaeocystis globosa blooms.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project No. 41676110) and Science, Technology Program of Guangdong, China (Project No. 2014A020217007) and NSFC-Guangdong Province Joint Key Project (U1301235).
Notes and references
- McIntyre L. Cassis D. Haigh N. Mar. Drugs. 2013;11:4144–4157. doi: 10.3390/md11114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed Z. A. Hashem M. Alamri S. A. Toxicon. 2014;86:51–58. doi: 10.1016/j.toxicon.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Paerl H. W. Huisman J. Huisman J. Science. 2008;320:57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- Blauw A. N. Los F. J. Huisman J. J. Mar. Syst. 2010;83:115–126. doi: 10.1016/j.jmarsys.2010.05.003. [DOI] [Google Scholar]
- Zheng X. W. Zhang B. Z. Zhang J. L. Huang L. P. Lin J. Appl. Microbiol. Biotechnol. 2013;97:9207–9215. doi: 10.1007/s00253-012-4617-8. [DOI] [PubMed] [Google Scholar]
- Costas E. Lopez-Rodas V. Water Res. 2006;40:2447–2451. doi: 10.1016/j.watres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Anderson D. M. Ocean Coast. Manag. 2009;52:342–347. doi: 10.1016/j.ocecoaman.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M. Y. Lee S. B. Jin D. H. Hong Y. K. Jin H. J. J. Appl. Phycol. 2010;22:453–458. doi: 10.1007/s10811-009-9478-x. [DOI] [Google Scholar]
- Tillmann U. J. Eukaryotic Microbiol. 2004;51:156–168. doi: 10.1111/j.1550-7408.2004.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Li X. Li X. M. Zhang P. Wang B. G. J. Asian Nat. Prod. Res. 2015;17:1204–1212. doi: 10.1080/10286020.2015.1117454. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y. Nagasoe S. Tameishi M. Shikata T. Zou Y. Jiang Z. Matsubara T. Shimasaki Y. Yamaguchi K. Oshima Y. Oda T. Honjo T. Hydrobiologia. 2010;641:33–44. doi: 10.1007/s10750-009-0052-y. [DOI] [Google Scholar]
- Wagstaff B. A. Vladu I. C. Barclay J. E. Schroeder D. C. Malin G. Field R. A. Viruses. 2017;9:40. doi: 10.3390/v9030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y. Han G. M. Wang C. Y. Guo P. Jiang W. X. Li X. N. Tian X. J. J. Hazard. Mater. 2010;183:176–181. doi: 10.1016/j.jhazmat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Lee S. O. Kato J. Takiguchi N. Kuroda A. Ikeda T. Mitsutani A. Ohtakei H. Appl. Environ. Microbiol. 2000;66:4334–4339. doi: 10.1128/AEM.66.10.4334-4339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura N. Motoike I. Noda M. Adachi K. Konno A. Fukami H. J. Antibiot. 2000;53:1317–1319. doi: 10.7164/antibiotics.53.1317. [DOI] [PubMed] [Google Scholar]
- Gong L. Y. Li Y. B. Wang X. L. China Environ. Sci. 2004;24:692–696. [Google Scholar]
- Zhang X. Song T. Ma H. Li L. RSC Adv. 2016;6:103662–103667. doi: 10.1039/C6RA17917G. [DOI] [Google Scholar]
- Zhang D. B. Sui Z. H. Mao Y. X. Acta Oceanol. Sin. 2007;29:123–130. [Google Scholar]
- Chen Z. R. Zheng W. Yang L. X. Boughner L. A. Tian Y. Zheng T. L. Xu H. Front Microbiol. 2017;8:2581. doi: 10.3389/fmicb.2017.02581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. Chen L. N. Yin P. H. J. Ind. Microbiol. Biotechnol. 2014;41:593–599. doi: 10.1007/s10295-013-1393-0. [DOI] [PubMed] [Google Scholar]
- Sjaastad O. Acta Pharmacologica et Toxicologica. 1966;24:189–202. doi: 10.1111/j.1600-0773.1966.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Cheng Z. Q. Zhang F. L. Liu W. Cui L. Y. Kang L. J. RSC advances. 2015;5:54182–54187. doi: 10.1039/C5RA08721J. [DOI] [Google Scholar]
- Ren W. K. Yin J. Gao W. Chen S. Duan J. L. Liu G. Li T. J. Li N. Z. Peng Y. Y. Yin Y. L. RSC Adv. 2015;5:59550–59555. doi: 10.1039/C5RA09513A. [DOI] [Google Scholar]
- De Felice M. Levinthal M. Iaccarino M. Guardiola J. Microbiol. Rev. 1979;43:42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y. Liu S. J. Zhou S. W. Wu H. F. Yu J. B. Xia C. H. Pestic. Biochem. Physiol. 2011;100:93–103. doi: 10.1016/j.pestbp.2011.02.014. [DOI] [Google Scholar]
- Tan S. Hu X. L. Yin P. H. Zhao L. J. Microbiol. 2016;54:364–375. doi: 10.1007/s12275-016-6012-0. [DOI] [PubMed] [Google Scholar]
- Zhang B. H. Chen W. Li H. Q. Yang J. Y. Zha D. M. Duan Y. Q. Hozzein W. N. Xiao M. Gao R. Li W. J. Appl. Microbiol. Biotechnol. 2016;100:4627–4636. doi: 10.1007/s00253-015-7150-8. [DOI] [PubMed] [Google Scholar]
- Broderius S. J. Kahl M. Hoglund M. D. Environ. Toxicol. Chem. 1995;14:1591–1605. doi: 10.1002/etc.5620140920. [DOI] [PubMed] [Google Scholar]
- Moreau C. J. Klerks P. L. Haas C. N. Arch. Environ. Contam. Toxicol. 1999;37:251–257. doi: 10.1007/s002449900512. [DOI] [PubMed] [Google Scholar]
- Mittler R. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Apel K. Hirt H. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Zhang S. L. Zhang B. Dai W. Zhang X. M. J. Plant Physiol. 2011;168:639–643. doi: 10.1016/j.jplph.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y. Furutera A. Seki K. Toyoda Y. Tanaka K. Sugimoto Y. Plant Physiol. Biochem. 2008;46:786–793. doi: 10.1016/j.plaphy.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Kwok C. T. Merwe J. P. Chiu J. M. Y. Wu R. S. S. Harmful Algae. 2012;13:40–46. doi: 10.1016/j.hal.2011.10.001. [DOI] [Google Scholar]
- Shao J. H. Wu Z. X. Yu G. L. Peng X. Li R. H. Chemosphere. 2009;75:924–928. doi: 10.1016/j.chemosphere.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Qian H. F. Xu X. Y. Chen W. Jiang H. Ji Y. X. Liu W. P. Fu Z. W. Chemosphere. 2009;75:368–375. doi: 10.1016/j.chemosphere.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Cai G. J. Yang X. J. Lai Q. L. Yu X. Q. Zhang H. J. Li Y. Chen Z. R. Lei X. Q. Zheng W. Xu H. Zheng T. L. Sci. Rep. 2016;6:20081. doi: 10.1038/srep20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw R. and Rosowski J., Methods for microscopic algae, in Handbook of phycological methods: culture methods and growth measurements, ed. J. R. Stein, Cambridge University Press, Cambridge, 1973 [Google Scholar]
- Cao Q. Hu Q. H. Khan S. Wang Z. J. Lin A. J. Du X. Zhu Y. G. J. Hazard. Mater. 2007;148:377–382. doi: 10.1016/j.jhazmat.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Trenzado C. Hidalgo M. C. Garcia-Gallego M. Morales A. E. Furne M. Domezain A. Domezain J. Sanz A. Aquacult. 2006;254:758–767. doi: 10.1016/j.aquaculture.2005.11.020. [DOI] [Google Scholar]
- Dogru M. K. Dogru A. K. Gul M. Esrefoglu M. Yurekli M. Erdogan S. Ates B. J. Appl. Toxicol. 2008;28:140–146. doi: 10.1002/jat.1259. [DOI] [PubMed] [Google Scholar]