Abstract
The vast majority of individuals experience trauma within their lifetime. Yet, most people do not go on to develop clinical levels of psychopathology. Recently, studies have highlighted the potential protective effects of having larger amygdala and hippocampal volumes, such that larger volumes may promote adaptive functioning following trauma. However, research has not yet elucidated whether certain subregions of these stress-sensitive structures have specific protective effects. Herein, we examined the mediating effects of amygdala and hippocampal subregions on the relationship between traumatic exposure and concurrent or longitudinal changes in psychiatric symptom levels in typically developing youth (9–15 years of age). Using high-resolution T1-and T2-weighted structural MRI scans, we found that the volume of the right basolateral complex of the amygdala mediated associations between trauma exposure and internalizing symptoms. Specifically, greater levels of childhood trauma related to larger volumes, and larger volumes were associated with fewer internalizing symptoms. The volume of the right CA4/dentate gyrus (DG) of the hippocampus yielded similar mediation results, such that greater trauma was related to larger volumes, which in turn were associated with decreases in internalizing symptoms across time. These findings provide initial support for potentially protective effects of larger right amygdala and hippocampal subregion volumes against internalizing symptomology concurrently and longitudinally during adolescence.
Keywords: Amygdala, Hippocampus, Hippocampal subfields, Amygdala subnuclei, Trauma, Brain development
1. Introduction
Childhood traumatic experiences are distressingly common, with nearly two-thirds of people experiencing at least 1 significant traumatic event prior to the age of 18 (Carlson et al., 2020). Such traumatic experiences are known to exert profound influences on the developing brain (Teicher et al., 2016), with regionally-specific effects on stress-sensitive brain structures (Weissman et al., 2020). Critically, the impact of trauma on brain regions vulnerable to stress, particularly during development, is thought to underlie an increased risk of an array of deleterious outcomes (Dannlowski et al., 2012; Machlin et al., 2019), including the development and onset of psychopathologies (e.g., anxiety, depression, substance use disorders; (Buckingham and Daniolos, 2013; Gur et al., 2019; Morey et al., 2012; Roth et al., 2018).
The amygdala and hippocampus are two structures located bilaterally in the medial temporal lobe that have been consistently identified as being sensitive to stress hormones (e.g., glucocorticoids), even in the context of milder forms of stress burden (Bergström et al., 2008; Sapolsky et al., 1985). Animal models have demonstrated that concentrations of glucocorticoid and noradrenaline receptors can be found within subnuclei (i.e., basolateral complex) and subfields (e.g., dentate gyrus) of the amygdala and hippocampus (Johnson et al., 2005; McEwen et al., 1975), making them particularly vulnerable to stress-related alterations in their morphology and function (Vouimba et al., 2007). In humans, volumetric reductions in the hippocampus and amygdala following traumatic experiences have been reported in pediatric samples from longitudinal and cross-sectional studies (Phillips et al., 2021; Saxbe et al., 2018; VanTieghem, 2021; Veer et al., 2015; Weissman et al., 2020). While most studies examining morphological properties of amygdala and hippocampus following trauma have largely focused on samples at elevated risk for or with established psychopathology symptomology (e.g., Logue et al., 2018), only a third of individuals with histories of trauma actually develop clinical levels of psychopathology (MacMillan et al., 2001). In other words, approximately 65% of individuals with trauma exposure do not develop subsequent psychiatric conditions. Thus, it is critical to address the effects of trauma on neurodevelopment without potential mental health related confounds. Moreover, it has been highlighted that in non-clinical samples who have experienced childhood adversity, whole hippocampal volumes are systematically smaller, although findings in whole amygdala volumes are non-convergent (Calem et al., 2017). These studies illustrate the notion that trauma-related stress, irrespective of the effects of mental health disorders, may exert lasting effects on hippocampal and amygdala volumes (Calem et al., 2017; Janiri et al., 2017; Nogovitsyn et al., 2020; Ramphal et al., 2021).
Recently, studies have also shown that there are potential protective effects of having larger whole amygdala and hippocampal volumes, such that greater volume may promote adaptive functioning following traumatic experiences (Ben-Zion et al., 2021; Foell et al., 2019; Koch et al., 2021; Quidé et al., 2018; Xie et al., 2018). For example, reduced sensitivity to threatening stimuli in one twin is predictive of their co-twin having larger amygdala volumes, which may confer diminished risk for internalizing psychopathology (e.g., social phobia; Foell et al., 2019). Although the field has begun to inquire about whether amygdala and hippocampal volumes are protective against emergent mental health disorders, there are major gaps in understanding the ways in which subnuclei of the amygdala, and subfields of the hippocampus, contribute to these processes. Moreover, few studies, if any, have posed this question in a pediatric sample in the absence of diagnosed mental health disorders. Such work would be particularly illuminating, as measures of subnuclei and subfields may reveal protective properties against detrimental changes to mental health, as well as markers of resilience that may be applicable to interventions for higher-risk populations. Thus, in the current study, we sought to evaluate the extent to which amygdala subnuclei and hippocampal subfields mediate associations between trauma and preclinical psychopathology symptoms in a sample of typically developing youth.
There is a growing literature highlighting the utility of examining specific effects of amygdala subnuclei and hippocampal subfields on developmental psychopathology following traumatic experiences (e.g., Morey et al., 2020). Specifically, within the amygdala, there are reports that greater trauma exposure is related to smaller volume in a collection of amygdala subnuclei, particularly in the basolateral complex (BLA; i.e., basal, lateral, accessory, paralaminar, corticoamygdalar, and anterior amygdaloid area nuclei), and that smaller BLA volumes mediate elevated depression and anxiety symptoms (Nogovitsyn et al., 2020). Animal models have also demonstrated a potential mechanistic role of the BLA, such that there is diminished spinogenesis and synaptic transmission within the BLA following traumatic stress, which confers subsequent development of post-traumatic stress symptomology (Zhang et al., 2019). These findings are unsurprising given that the BLA shares direct connections with the frontal cortex (Ghashghaei et al., 2007) and supports developmental changes in emotion regulation (Tottenham and Gabard-Durnam, 2017). Importantly, these fronto-amygdalar connections are thought to develop during puberty (Lebron-Milad and Milad, 2012; van Wingen et al., 2011), making it pertinent to examine these associations during the transition from childhood to adolescence.
Within the hippocampus, the dentate gyrus (DG), CA4, and CA3 subfields have each been identified as being highly stress-sensitive, with different underlying mechanisms linked to subfield atrophy following stress exposure. Specifically, stress exposure leading to an infusion of glucocorticoids may suppress neurogenesis and induce shortening of dendrites in the DG (Czeh et al., 2001), while stress exposure may provoke remodeling of pyramidal neurons in CA3 (Sapolsky, 2000). In both cases, this results in volume reductions in these specific subfields, which has been related to increased psychiatric symptomology. For instance, targeted stress effects have been reported within the CA4/DG zone, with smaller volume estimates associated with more severe levels of childhood trauma (Merz et al., 2020; Teicher et al., 2012), and greater stress-related symptomology (Hayes et al., 2017). Thus, evaluating the spatial specificity of trauma effects on subnuclei and subfields allows for a more targeted approach to quantifying associations between traumatic events and stress-sensitive regions. It is also an open question whether the volumetric properties of these same nuclei (BLA) and subfields (CA4/DG) may confer protective effects against developmental psychopathology following traumatic experiences.
Existing literature examining amygdala and hippocampal subregions in humans has several methodological limitations. However, recent advances in high-resolution MR imaging techniques in parallel with developments of more fine-tuned hippocampal and amygdala subregion labeling protocols (e.g., multispectral image segmentation) have enabled more precise demarcation of the amygdala and hippocampal subregions (Iglesias et al., 2015). These methodological improvements have significant implications on studying the sensitivity of these subregions to stress and portend psychopathology. With more advanced imaging methods, examining amygdala subnuclei and hippocampal subfields may yield greater specificity regarding trauma- and stress-specific associations. Herein, we apply these state-of-the-art neuroimaging techniques to address: 1) whether specific amygdala subnuclei mediate associations between childhood trauma exposure and symptomology, 2) whether specific hippocampal subfields mediate associations between childhood trauma exposure and symptomology, and 3) whether there are mediation effects with concurrent symptoms or with changes in symptomology across time. We focus on typically developing youth at low risk for psychopathology, which provides an opportunity to potentially detect protective effects previously shown at the whole structure level in volumetric measures of the amygdala and hippocampus following trauma exposure. In other words, given that the present sample did not include participants with an existing diagnosis of psychopathology or those with an especially high risk for psychopathology, this study allows for the distinct influence of trauma exposure on stress-sensitive structures to be probed, in the absence of the stress effects of diagnosed psychopathology.
2. Methods
2.1. Participants
Participants were recruited as part of the longitudinal Developmental Chronnecto-Genomics (DevCoG) project (for details regarding the parent project, see Stephen et al., 2021; https://devcog.mrn.org). The current study included a sample of 90 typically developing children and adolescents ages 9–15 (meanage = 12.40 years, SD = 1.63) recruited at the Omaha, Nebraska site. Youths and their caregivers participating in the larger DevCoG study were invited to complete neuroimaging and neuropsychological assessments annually for 3 years. Note that the T2-weighted images were only acquired in year 2 of the study (see Fig. S1 for study design). Inclusion criteria were as follows: English as a primary language, ages 9–15, a complete pair of structural magnetic resonance imaging (MRI) scans (see MRI Processing). Exclusion criteria determined via parent report were as follows: history of developmental delays and/or psychiatric disorders, history of neurological disorders, history of concussion or head injury, pregnancy, prenatal exposure to drugs, CNS medications, MR) contraindications (e.g., metallic foreign bodies) or incidental findings. All parents and youth provided written consent or assent, respectively. The UNMC institutional review board approved all study procedures.
2.2. MRI data acquisition
During data acquisition, participants were in constant contact with research personnel through real-time audiovisual monitoring. Structural MRI data were collected on a 3T Siemens Skyra System with a 32-channel head coil, and consisted of one T1-weighted three-dimensional 1 mm isotropic T1 (TR = 2.4s, TE = 1.94 ms, flip angle = 8°, FOV = 256 mm, slices = 192), and one T2-weighted turbo spin echo (TSE) scan (TR = 7.79s, TE = 66 ms, flip angle = 145°, FOV = 170 mm, in plane resolution = 0.4 × 0.4 mm, slice thickness = 2 mm, slices = 32) per current best practices (Mueller et al., 2018; Olsen et al., 2019; Wisse et al., 2021; Yushkevich et al., 2015).
2.3. MRI processing
MRIQC v0.16.1 (Esteban et al., 2017) was used as an initial check of MRI data quality. Then, these preliminary ratings were supplemented with systematic quality assessment by a trained rater. Assessment included a review of hippocampal and amygdala coverage/completeness, contrast/noise, and motion artifacts. As a result, 49 participants were included in the final analysis based on having both a T1 and T2 (from year 2 of the study) that survived quality control and were used for all subsequent analyses, and 41 participants were excluded either due to an incomplete image pair (no co-acquired T1 and T2) or poor data quality identified during quality control. Importantly, participants included in our final sample after imaging-based exclusion did not differ demographically from participants who were excluded during this processing stage (see Supplementary Table S1).
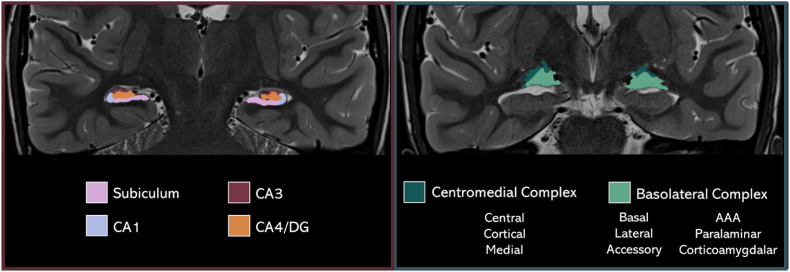
High resolution hippocampal subfield (Iglesias et al., 2015) and amygdala subnuclei volumes (Saygin et al., 2017) were measured with FreeSurfer software (v7.1.1; Fischl, 2012) using the segmentHA_T2 (CA) workflow. Briefly, the dedicated T2-weighted image of the medial temporal lobe was first co-registered to the T1-weighted image, segmented, and bias-corrected. Next, each hippocampal subfield and amygdala nucleus were parcellated from the medial temporal lobe. This method used a generative model with mesh deformation and a probabilistic atlas. Label sampling was conducted at each voxel location within a subject-specific target region of interest determined during recon-all (i.e., the whole hippocampal and amygdala mesh). Optimized Bayesian inference was used to derive the final segmentations. From the resulting parcellation, hippocampal subfield volumes were combined following FreeSurfer's CA-based grouping, and amygdala subnuclei were grouped based on previous literature (Nogovitsyn et al., 2020; Ousdal et al., 2020). Thus, the following bilateral volume labels were used for our final analyses, separately by hemisphere (Hippocampus: CA1, CA3, CA4, and subiculum; Amygdala: centromedial complex, basolateral complex, Fig. 1).
Fig. 1.
Hippocampal and Amygdala subfields and subnuclei. An example participant T2-weighted MRI scan in the coronal view with the hippocampal subfields (left) and amygdala subnuclei (right) rendered using the respective participants' subregions from the FreeSurfer amygdala + hippocampus parcellation pipeline. DG = Dentate Gyrus; AAA = anterior amygdaloid area.
2.4. Trauma History Profile
Participants completed the self-report Trauma History Profile (THP), which was derived from the UCLA PTSD Reaction Index for DSM IV (Steinberg et al., 2004) and assessed a variety of trauma types and events. Participants endorsed whether they experienced 12 different types of trauma in their lifetime (No = 0, Yes = 1). Example items include: “saw a family member being hit, punched, kicked”, “was hit, punched, kicked very hard (not play fighting)”, “in a bad accident, like a serious car accident or fall”, “had a painful or scary medical treatment”. A summed score of each participants’ trauma exposures was used.
2.5. Child Behavior Checklist
The consented participant guardian completed the Child Behavior Checklist (CBCL (Achenbach et al., 2001), at each of the 3 time points to assess their child's internalizing and externalizing behaviors over the past 6 months. In addition to examining the internalizing and externalizing profiles, we also computed the dysregulation profile, which is a summed score of the attention, aggression, and anxious/depressed subscales. We examined both the concurrent raw scores and change scores in each of the CBCL profiles. For data models including the raw scores, we only examined CBCL data that corresponded to when participants underwent the T2 scan. For analyses including change scores, we calculated difference scores between Time 3 and Time 1. If participants did not have Time 3 behavioral data, we calculated difference scores between Time 2 and Time 1 (n = 14). Therefore, we had change scores for each profile with increasing symptoms indicated by positive values [e.g., 13(Time 3 Internalizing) – 7(Time 1 Internalizing) = 6] and decreasing symptoms indicated by negative values [7(Time 3 Internalizing) – 13(Time 1 Internalizing) = −6].
2.6. Data analytic plan
We began by running descriptive statistics on demographics and all variables of interest. Variables entered into subsequent models were examined for violations of normality (i.e., skewness and kurtosis) and were transformed according to their distribution type (e.g., positive versus negative skew). Next, we fit several structural equation models (SEM) to estimate whether left and right amygdala subnuclei and hippocampal subfields, respectively, mediated the association between reported trauma exposure and concurrent or changes in symptomology. Amygdala and hippocampal data were modeled separately by hemisphere with either concurrent or change scores for CBCL, for a total of 8 separate mediation models.
Before fitting the final models, we iteratively tested which covariates to include (i.e., sex, age, and total intracranial volume (TIV)). To compare across models and determine optimal models, we inspected whether absolute fit indices such as Akaike's Information Criterion (AIC) and Bayesian Information Criterion (BIC) were decreasing in value toward 0 to indicate model improvement. In the current analysis, we primarily used BIC to determine model fit. After determining which covariates yielded the best model fit, we examined the mediating effect of bilateral amygdala subnuclei and hippocampal subfields on the association between trauma exposure and symptomology (i.e., indirect effects of trauma on concurrent or longitudinal change in symptomology via amygdala subnuclei or hippocampal subfields). All mediation models were bootstrapped with 1000 iterations with bias-corrected bootstrapping to test for significance of the indirect relationship based upon the 95% confidence intervals (MacKinnon et al., 2004). For all but the indirect effects, estimates (i.e., beta weights) of individual paths were examined for the directionality of relationships with an a priori p-value significance threshold of < 0.05. Finally, all symptomology scales (i.e., internalizing, externalizing, and dysregulation profiles derived from the CBCL), trauma exposure, and their interactions were permitted to freely correlate. All parameters were freely estimated. All models were tested in Mplus (v7.4).
We examined the goodness of fit for each model using standard criteria (Hu and Bentler, 1999). Specifically, we evaluated models for the root mean square error of approximation (RMSEA) < 0.06, standardized root mean square residual (SRMR) < 0.08, and comparative fit index (CFI) > 0.95. We also examined the χ2 test of model fit, where a nonsignificant result indicates good model fit.
3. Results
3.1. Descriptive statistics and model covariates
Descriptive statistics and demographic variables are reported in Table 1. Correlations among study variables of interest are reported in Table S2. Several CBCL raw measures had violations of normality, which were transformed using either square root or natural log. The trauma measure was normally distributed (see Fig. S2). Based upon decreasing BIC, a comparison of model fit statistics for different covariates revealed that including age and TIV yielded the best model fit in all models (i.e., left and right amygdala subnuclei and hippocampal subfields; Table 2, Table 3). As a result, age and TIV were maintained, whereas sex was excluded from final models as it did not account for a significant amount of variance with the inclusion of TIV, as others have shown (Pintzka et al., 2015). Therefore, we report results from models with age and TIV as covariates. An example of the full mediation model is illustrated in Fig. 2. All final models for each region had good to excellent fit (i.e., model 1 in each region in Table 2, Table 3). In what follows, we report results for each region. In regions where no mediation or direct effects reached significance, results are fully reported in supplemental materials (Tables S3–S5).
Table 1.
Sample demographics and study variables of interest.
| Variable | Final Sample (n = 49) |
|||
|---|---|---|---|---|
| n | % | |||
| Sex (F:M) | 26: 23 | 53: 47 | ||
| Race (W: A: B/AA: M: N) | 41:0:2:4:2 | 84: 0: 4: 8: 4 | ||
| Ethnicity (L: NL) | 4:45 | 8: 92 | ||
| Mean (SD), Range | ||||
|
Measure |
Time 1 |
Time 2 |
Time 3 |
|
| Age (years) | 11.91 (1.61), 9–14 | 12.53 (1.51), 9–16 | 13.92 (1.66), 10 - 17 | |
| Trauma (number of events) | 2.35 (2.10), 0–10 | – | – | |
| Near a disaster | 18% | |||
| In an accident | 12% | |||
| Physical abuse | 22% | |||
| Witness domestic violence | 10% | |||
| Neighborhood violence | 14% | |||
| Near violent death/injury | 27% | |||
| Medical trauma | 10% | |||
| Death of a loved one | 73% | |||
| Other trauma | 37% | |||
| Left Basolateral complex (mm3) | – | 1669.15 (152.03) | – | |
| Left Centromedial complex (mm3) | – | 108.93 (18.71) | – | |
| Right Basolateral complex (mm3) | – | 1751.26 (146.34) | – | |
| Right Centromedial complex (mm3) | – | 127.30 (18.53) | – | |
| Left Subiculum (mm3) | – | 155.75 (18.77) | – | |
| Left CA1 (mm3) | – | 337.29 (34.26) | – | |
| Left CA3 (mm3) | – | 101.36 (11.80) | – | |
| Left CA4 (mm3) | – | 138.25 (12.63) | – | |
| Right Subiculum (mm3) | – | 153.19 (18.42) | – | |
| Right CA1 (mm3) | – | 359.98 (31.62) | – | |
| Right CA3 (mm3) | – | 109.73 (14.90) | – | |
| Right CA4 (mm3) | – | 141.14 (13.15) | – | |
| Int. symptoms | 4.53 (4.01), 0–18 | 4.88 (4.57), 0–18 | 4.40 (5.86), 0–28 | |
| Ext. symptoms | 3.18 (3.63), 0–17 | 3.00 (3.32), 0–12 | 3.26 (3.79), 0–14 | |
| Dys. symptoms | 7.14 (5.94), 0–26 | 6.82 (5.70), 0–24 | 6.20 (6.42), 0–25 | |
|
Change Scores |
||||
| Δ Int. symptoms | 0.23 (3.09), -5 – 10 | |||
| Δ Ext. symptoms | −0.02 (3.12), -11 – 8 | |||
| Δ Dys. symptoms | −0.66 (4.70), -14 – 9 | |||
Note. F = female, M = male. W = White, A = Asian, B/AA = Black, African American, M = mixed race, N = not reported. L = Latinx, NL = Not Latinx. Int. = internalizing, Ext. = externalizing, and Dys. = dysregulation symptoms are all raw scores from the Child Behavior Checklist. Symptom Δ scores reflect difference scores (e.g., Time 3 – Time 1) whereby higher scores reflect an increase in symptomology and lower, negative scores reflect decreases in symptomology across timepoints. For those measures not collected at each time point, a dash (−) is indicated. A graphical depiction of the trauma category distribution is provided in Fig. S2.
Table 2.
Model fit comparison for covariate variables with CBCL raw score outcomes.
| Model | AIC | BIC | Adjust BIC | Chi-square | RMSEA | CFI | SRMR |
|---|---|---|---|---|---|---|---|
| Left Amygdala Subnuclei | |||||||
| Model 1 | 1111.12 | 1186.79 | 1061.27 | p = .15 | .21 | .98 | .04 |
| Model 2 | 1118.68 | 1205.71 | 1061.36 | p < .001 | <.001 | .70 | .14 |
| Right Amygdala Subnuclei | |||||||
| Model 1 | 1084.08 | 1159.75 | 1034.23 | p = .15 | .20 | .98 | .07 |
| Model 2 | 1141.46 | 1236.05 | 1079.15 | p = .01 | .01 | .93 | .06 |
| Left Hippocampus Subfields | |||||||
| Model 1 | 772.58 | 887.98 | 696.56 | p = .39 | .46 | 1.00 | .03 |
| Model 2 | 830.30 | 968.40 | 739.33 | p = .01 | .01 | .95 | .05 |
| Right Hippocampus Subfields | |||||||
| Model 1 | 734.43 | 849.83 | 658.41 | p = .61 | .66 | 1.00 | .06 |
| Model 2 | 794.48 | 932.58 | 703.50 | p = .05 | .07 | .98 | .07 |
Note. Model 1 = mediation model (MM) with age at time of MRI scan and total intracranial volume (TIV) – TIV was only a covariate for the respective brain volume (i.e., left amygdala, right amygdala, left hippocampus, right hippocampus); Model 2 = MM model with sex, age, TIV as covariates. AIC = Akaike's Information Criterion, BIC = Bayesian Information Criterion, RMSEA = root mean square error of approximation, SRMR = standardized root mean square residual, CFI = comparative fit index.
Table 3.
Model fit comparison for covariate variables with CBCL change score outcomes.
| Model | AIC | BIC | Adjust BIC | Chi-square | RMSEA | CFI | SRMR |
|---|---|---|---|---|---|---|---|
| Left Amygdala Subnuclei | |||||||
| Model 1 | 1370.58 | 1446.25 | 1320.73 | .17 | .22 | .98 | .06 |
| Model 2 | 1420.93 | 1515.52 | 1358.62 | .02 | .04 | .94 | .05 |
| Right Amygdala Subnuclei | |||||||
| Model 1 | 1351.14 | 1426.81 | 1301.29 | .06 | .08 | .96 | .08 |
| Model 2 | 1403.38 | 1497.97 | 1341.07 | .01 | .02 | .93 | .07 |
| Left Hippocampus Subfields | |||||||
| Model 1 | 1033.15 | 1148.55 | 957.13 | .21 | .26 | .99 | .05 |
| Model 2 | 1087.37 | 1225.48 | 996.40 | .01 | .02 | .96 | .05 |
| Right Hippocampus Subfields | |||||||
| Model 1 | 1001.18 | 1116.59 | 925.16 | .13 | .17 | .99 | .07 |
| Model 2 | 1055.19 | 1193.29 | 964.22 | .02 | .04 | .97 | .08 |
Note. Model 1 = mediation model (MM) with age at time of MRI scan and total intracranial volume (TIV) – TIV was only a covariate for the respective brain volume (i.e., left amygdala, right amygdala, left hippocampus, right hippocampus); Model 2 = MM model with sex, age, TIV as covariates. AIC = Akaike's Information Criterion, BIC = Bayesian Information Criterion, RMSEA = root mean square error of approximation, SRMR = standardized root mean square residual, CFI = comparative fit index.
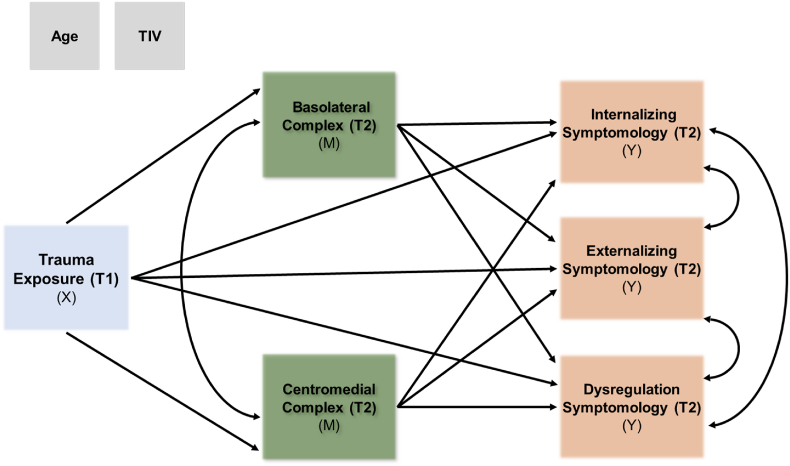
Fig. 2.
Mediation model example with amygdala subnuclei. Mediation model with amygdala subnuclei volume (Time 2) as an example of mediating the relationship with trauma exposure from the Trauma History Profile (Time 1) and concurrent internalizing, externalizing, and/or dysregulation symptoms measured via the CBCL (Time 2). Covariates included TIV for amygdala subnuclei volumes and age at the time of the structural MRI scans for all variables. Note that the amygdala and hippocampal subregions were modeled separately by hemisphere with either concurrent or change scores for CBCL for a total of 8 separate models (See Figs. S3–S6 in the Supplemental Materials for all models). Abbreviations: X = independent predictor variable; M = mediator variable(s); Y = dependent outcome variable; T1 = Time 1; T2 = Time 2.
3.2. Mediation results
3.2.1. Amygdala subnuclei
CBCL raw score outcomes. The right basolateral complex volume mediated the
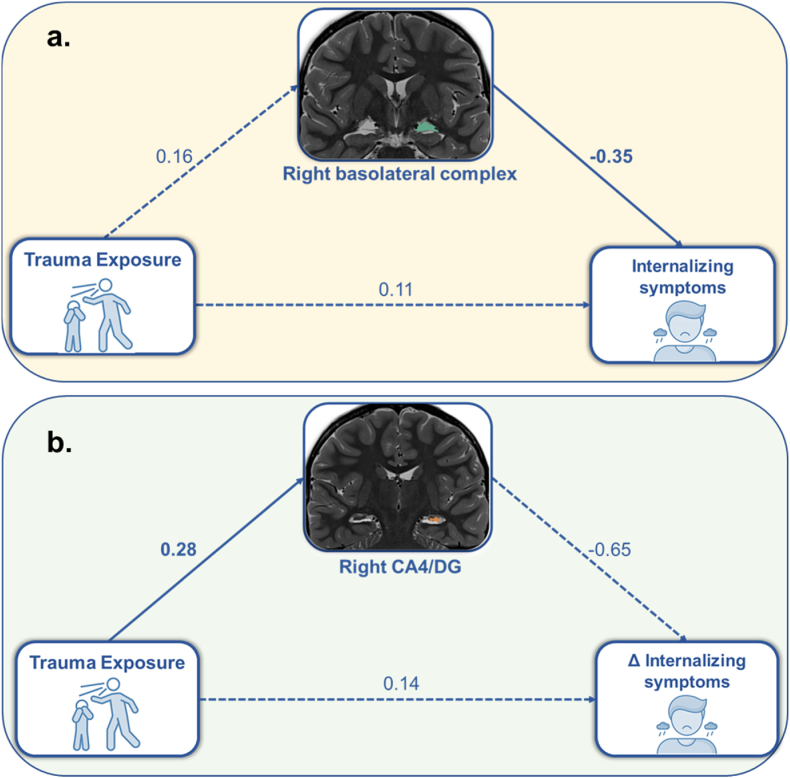
relationship between trauma exposure and concurrent internalizing symptoms (β = −0.06, b = −0.03; 95% CI[-0.11, −0.001]; Fig. 3a). Specifically, there was a weak positive association between trauma exposure and volume, suggesting that youths with greater trauma exposure tended to have larger right basolateral amygdala volumes (β = 0.16, b = 0.11; 95% CI[-0.02, 0.23]). In turn, larger volumes were associated with lower concurrent internalizing symptoms (β = −0.35, b = −0.27; 95% CI[-0.68, −0.05]). There was no direct relationship between trauma exposure and concurrent internalizing symptoms (β = 0.11, b = 0.06; 95% CI[-0.14, 0.25]). The same analysis using CBCL T-scores is reported in the Supplemental Materials. Importantly, to address potential issues of multicollinearity between the CBCL subscales, the dysregulation profile was removed and the model was recomputed with only internalizing and externalizing profiles. This follow-up analysis yielded the same pattern of results and conclusions. There were no other significant mediating effects of amygdala subnuclei volumes on the relationship between trauma exposure and either concurrent or longitudinal changes in internalizing, externalizing, or dysregulation symptoms. Complete model results are reported in Tables S3 and S5.
Fig. 3.
Mediation model results. (a) results of the mediation model showed that the volume of the right basolateral complex of the amygdala (Time 2, in green) mediated associations between trauma exposure (Trauma History Profile, Time 1) and concurrent (Time 2) internalizing symptoms from the CBCL. (b) results of a separate mediation model showed that the right CA4/DG of the hippocampus mediated (Time 2, in orange) associations between trauma exposure (Trauma History Profile, Time 1) and change in internalizing symptoms (i.e., difference score from the CBCL; Time 3 – Time 1). All estimates shown are standardized. Indirect models in both (a) and (b) were significant based upon bootstrapped 95% confidence intervals. Significant individual paths (p < .05) are shown as solid lines and non-significant individual paths are shown as dotted lines. Note that covariates are not depicted here, but that age at the time of the scan was covaried for each variable, as well as TIV on the amygdala and hippocampal volume measures. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
CBCL change score outcomes. Bilateral basolateral and centromedial complexes did not mediate associations between trauma and change in psychopathology symptoms, and there were no direct effects on change in psychopathology symptoms.
3.2.2. Hippocampal subfields
CBCL raw score outcomes. No hippocampal subfield volumes mediated associations between trauma and psychopathology symptoms and there were no direct effects on psychopathology symptoms.
CBCL change score outcomes. Right CA4/DG mediated the association between trauma exposure and change in internalizing symptoms (β = −0.18, b = −0.26; 95% CI[-0.84, −0.01] (Fig. 3b). That is, there was a significant positive association between trauma exposure and volume such that youths with greater trauma exposure tended to have larger right CA4/DG hippocampal volumes (β = 0.28, b = 0.02; 95% CI[0.004, 0.03]). Larger volumes were modestly associated with decreasing internalizing symptoms across time (β = −0.65, b = -0.52; 95% CI[-0.62, 0.13]). There was no direct relationship between trauma exposure and change in internalizing symptoms (β = 0.14, b = 0.21; 95% CI[-0.32, 0.98]). As with the amygdala volume results, when the dysregulation subscale was removed from the overall model, the same pattern of results were obtained for the volume of the right CA4/DG. No other subfield volumes were found to mediate associations between trauma exposure and either concurrent or longitudinal changes in internalizing, externalizing, or dysregulation symptoms. Complete model results are reported in Tables S4 and S5.
4. Discussion
Traumatic experiences have been previously linked to the structural properties of stress-sensitive brain regions, even in youth who do not go on to develop psychopathology. Using state-of-the-art MR imaging and multispectral segmentation techniques, the present study investigated whether volumes of amygdala and hippocampal subregions mediated associations between trauma and psychopathology symptoms in a sample of typically developing youth who varied in exposure to traumatic events. We report two key findings from the present study that uniquely contribute to the existing literature. First, we uncovered that the right basolateral complex of the amygdala mediates the effect of trauma exposure on concurrent internalizing symptoms during adolescence. Specifically, although the direct path was nonsignificant, greater trauma exposure was related to larger right basolateral complex volume, which in turn, was significantly associated with lower concurrent internalizing symptoms. Notably, this result could be described as an “inconsistent mediation”, given that the indirect effect carried a different sign (i.e., negative) than one of the specific direct effects (i.e., trauma → right basolateral complex volume; MacKinnon et al., 2000). This finding is consistent with recent work demonstrating that larger whole amygdala volumes in adults may serve as a protective factor following trauma (Foell et al., 2019). Second, a similar pattern of results was revealed in the right CA4/Dentate Gyrus (DG) subfield of the hippocampus, with greater trauma exposure being significantly related to larger right CA4/DG volumes, which in turn was related to lower internalizing symptomology across longitudinal time points. Comparable patterns of results have been found in a longitudinal assessment of adults in the DG of the hippocampus, with increased DG following trauma predicting decreased PTSD symptoms across time (Koch et al., 2021).
Taken together, the current study builds upon existing literature, pointing to potential neurobiological protective factors that may serve to mitigate risk against the development of psychopathology in the face of trauma. As others have suggested, examining these processes in typically developing samples without currently diagnosed psychopathology and who are at low-risk of developing psychopathology may provide unique insight into potential resilience-promoting biomarkers in higher-risk samples (MacMillan et al., 2001). In other words, this study provides an essential link to understanding how underlying neurobiological factors may deter concurrent and longer-term emergence of psychopathology symptoms following trauma. This work extends previous pediatric work demonstrating that higher-risk samples with trauma exposure exhibit smaller amygdala and hippocampal subregional volumes (Merz et al., 2018, 2020; Nogovitsyn et al., 2020; Oshri et al., 2019; Teicher et al., 2012; Zhang et al., 2019). Although there is a paucity of research, there is consistency in the regions (i.e., basolateral complex and CA4/DG) identified here with those previously found to be systematically smaller in relation to stress in developmental samples. This suggests that the effects of trauma may target specific subnuclei of the amygdala and subfields of the hippocampus in distinct ways that alter risk profiles toward or away from psychopathology.
Future work should aim to uncover whether typically developing youth who evince larger volumes in relation to trauma exposure possess any protective predisposing factors. It may be the case that such individuals either begin with larger volumes that buffer against potential atrophying effects of stress, or there may be other intermediary factors that promote regional growth (i.e., synaptogenesis or neurogenesis). Given that the current study was largely cross-sectional (apart from the change in symptomology), more thorough longitudinal investigations are necessary to understand whether and to what extent the effects found here are partially endogenous or in direct response to trauma. It is plausible that there are moderating factors, such as genetic predispositions as well as social or environmental factors that promote or hinder the protective effects uncovered in the current study. Social support factors, in particular, have been shown to promote adaptive outcomes following traumatic experiences (Cheong et al., 2017; Evans et al., 2013; Horan and Widom, 2015; Shin et al., 2019). Thus, future studies would benefit from examining mediating and moderating social factors that protect against developmental psychopathology, as they may help to establish interactive effects among biological and environmental factors that explain adaptive patterns in outcomes following trauma exposure.
Another important contribution of the present study is its state-of-the-art methodology. Of the relatively few studies that have examined amygdala and hippocampal subregions, most included only a T1-weighted MRI scan. The present study used both T1-and T2-weighted scans, which is optimal for examining more fine-grained subregions that necessitate ultra-high resolution, <1 mm scans. That said, prior work has largely focused on whole amygdala and hippocampal volumes, which has likely contributed to mixed findings in the literature. Specifically, effects of trauma on whole amygdala and hippocampal volumes have been inconsistent, with reports of larger, smaller, or no volumetric differences (for reviews, see Hanson and Nacewicz, 2021; Teicher et al., 2016; Teicher and Khan, 2019). This may, in part, be due to that fact that each subnucleus and subfield is differentially connected to other cortical and subcortical regions, and thus, may be differentially affected by traumatic experiences. Indeed, prior literature on hippocampal and amygdala subregions in children and adolescents seems to support this notion, showing targeted effects of trauma on specific subregions (Merz et al., 2018, 2020; Nogovitsyn et al., 2020; Oshri et al., 2019; Teicher et al., 2012). Other studies have shown that the developmental trajectory of hippocampal volumes varies along the long axis, emphasizing the need to investigate the hippocampus at a finer scale in youth samples (DeMaster et al., 2014; Lee et al., 2014). Leading up to these findings, special focus was given to the development of submillimeter imaging sequences to more precisely investigate these subregions. Of the past studies that have examined amygdala and hippocampal subregions, most include only a T1-weighted MRI data, which has been shown to have less reliability than studies which introduce a second higher resolution scan into the processing workflow (Kahhale et al., 2020; Wisse et al., 2021). The present study used both T1-and T2-weighted scans, which are optimal for examining smaller subregions that necessitate such specialized sequences.
Although the current study adds substantively to the literature, a few limitations must be acknowledged. First, while there is merit in examining the biological impacts of trauma in youth who have not developed clinical levels of psychopathology, as was done in this study, future work would benefit from incorporating higher-risk samples. That is, it would be ideal to examine these processes in two groups (high and low risk for psychopathology) with comparable levels of trauma to disentangle the stress-related effects that may be incurred with emergent psychopathology. Second, more studies are needed to establish the environmental (e.g., social support) and biological processes (e.g., genetic and epigenetic precursors) that may promote or hinder amygdala and hippocampal subregion growth trajectories following trauma. Third, although the current study examined longitudinal changes in subclinical symptomology, we did not have longitudinal MRI assessments, limiting conclusions that can be made about correspondence between structural brain development and emerging symptomology. Thus, to further characterize developmental cascades associated with trauma, it will be critical for longitudinal studies to establish if larger amygdala and hippocampal subregions are an endogenous protective factor against developing internalizing symptoms, or if they are a direct response to stress that serves as a buffer. Relatedly, this study evaluated how many traumatic experiences participants self-reported, which has been cautioned against by researchers for its over simplification of traumatic exposure (Smith and Pollak, 2021). It will be important for future studies to comprehensively characterize trauma experiences beyond the number of traumatic events by quantifying their specific type, timing, and severity. Finally, the present study is limited by its sample size; future work with larger cohorts is essential for replication and extension of the current findings. In particular, it will be crucial for subsequent studies with larger samples to examine all amygdala subnuclei to garner greater specificity of trauma effects, rather than just the BLA and centromedial subregions which consist of multiple subnuclei.
5. Conclusions
This study provides new evidence that amygdala and hippocampal subregional volumes mediate trauma and psychopathology symptomology in a normative pediatric sample. Specifically, we report two mediations: the right basolateral complex of the amygdala mediated trauma exposure and internalizing symptoms, while the right CA4/DG of the hippocampus mediated trauma exposure and changes in internalizing symptoms across time. These findings suggest potentially targeted protective effects of larger right amygdala and hippocampal subregion volumes against internalizing symptomology during adolescence. This study extends prior work by revealing that the same subnuclei and subfields that are highly stress-sensitive (basolateral complex and CA4/DG) may also be pertinent in protecting against developmental psychopathology in trauma-exposed youth. Importantly, this work demonstrates the utility of examining these processes in youth who do not exhibit clinical levels of psychopathology and may be applicable to vulnerable samples at elevated risk of clinical diagnoses.
Funding
This work was supported by the National Science Foundation (#1539067 to TWW, YPW, JMS, and VDC, and #2112455 to VDC), the National Institutes of Health (P20-GM144641, R01-MH121101, R01-MH116782, and R01-MH118013 to TWW; R01-EB020407 and R01-MH118695 to VDC), and At Ease, USA. Funding agencies had no part in the study design or the writing of this report.
CRediT authorship contribution statement
Giorgia Picci: Formal analysis, Writing – original draft, completed analysis, interpretation of the data, writing original draft. Nicholas J. Christopher-Hayes: Formal analysis, Writing – review & editing, conducted analyses and assisted with writing and revising. Nathan M. Petro: Data curation, Writing – review & editing, Writing – original draft, assisted with interpretation of the data and revising drafts. Brittany K. Taylor: Data curation, Writing – review & editing, Writing – original draft, assisted with interpretation of the data and revising drafts. Jacob A. Eastman: Data curation, acquired the data. Michaela R. Frenzel: Data curation, acquired the data. Yu-Ping Wang: Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft, conceptualized and designed the study, acquired funding, and revised drafts. Julia M. Stephen: Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft, conceptualized and designed the study, acquired funding, and revised drafts. Vince D. Calhoun: Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft, and conceptualized and designed the study, acquired funding, and revised drafts. Tony W. Wilson: Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft, conceptualized and designed the study, acquired funding, and revised drafts.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100456.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Achenbach T.M., Dumenci L., Rescorla L.A. University of Vermont Research Center for Children; Youth, & Families: 2001. Ratings of Relations between DSM-IV Diagnostic Categories and Items of the CBCL/6-18, TRF, and YSR. [Google Scholar]
- Ben-Zion Z., Artzi M., Niry D., Keynan N.J., Zeevi Y., Admon R., Sharon H., Halpern P., Liberzon I., Shalev A.Y., Hendler T. 2021. Neuroanatomical Risk Factors for Post Traumatic Stress Disorder (PTSD) in Recent Trauma Survivors; p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A., Jayatissa M.N., Mørk A., Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Buckingham E.T., Daniolos P. Longitudinal outcomes for victims of child abuse. Curr. Psychiatr. Rep. 2013;15(2):342. doi: 10.1007/s11920-012-0342-3. [DOI] [PubMed] [Google Scholar]
- Calem M., Bromis K., McGuire P., Morgan C., Kempton M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage: Clinic. 2017;14:471–479. doi: 10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.S., Yohannan J., Darr C.L., Turley M.R., Larez N.A., Perfect M.M. Prevalence of adverse childhood experiences in school-aged youth: a systematic review (1990–2015) Int. J. School Educ. Psychol. 2020;8(Suppl. 1):2–23. doi: 10.1080/21683603.2018.1548397. [DOI] [Google Scholar]
- Cheong E.V., Sinnott C., Dahly D., Kearney P.M. Adverse childhood experiences (ACEs) and later-life depression: perceived social support as a potential protective factor. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2016-013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B., Michaelis T., Watanabe T., Frahm J., de Biurrun G., van Kampen M., Bartolomucci A., Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatr. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- DeMaster D., Pathman T., Lee J.K., Ghetti S. Structural development of the Hippocampus and episodic memory: developmental differences along the anterior/posterior Axis. Cerebr. Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Esteban O., Birman D., Schaer M., Koyejo O.O., Poldrack R.A., Gorgolewski K.J. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;21(9) doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.E., Steel A.L., DiLillo D. Child maltreatment severity and adult trauma symptoms: does perceived social support play a buffering role? Child. Abuse Neglect. 2013;37(11):934–943. doi: 10.1016/j.chiabu.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell J., Palumbo I.M., Yancey J.R., Vizueta N., Demirakca T., Patrick C.J. Biobehavioral threat sensitivity and amygdala volume: a twin neuroimaging study. Neuroimage. 2019;186:14–21. doi: 10.1016/j.neuroimage.2018.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., Ruparel K., Scott J.C., Almasy L., Satterthwaite T.D., Shinohara R.T., Gur R.C. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatr. 2019;76(9):966. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M. Amygdala allostasis and early life adversity: considering excitotoxicity and inescapability in the sequelae of stress. Front. Hum. Neurosci. 2021;15:624705. doi: 10.3389/fnhum.2021.624705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S., Miller D.R., Lafleche G., Logue M.W., Verfaellie M. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J. Psychiatr. Res. 2017;95:247–252. doi: 10.1016/j.jpsychires.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan J.M., Widom C.S. From childhood maltreatment to allostatic load in adulthood: the role of social support. Child. Maltreat. 2015;20(4):229–239. doi: 10.1177/1077559515597063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model.: A Multidiscip. J. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K. A computational atlas of the hippocampal formation using ex vivo , ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D., Sani G., Rossi P.D., Piras F., Iorio M., Banaj N., Giuseppin G., Spinazzola E., Maggiora M., Ambrosi E., Simonetti A., Spalletta G. Amygdala and hippocampus volumes are differently affected by childhood trauma in patients with bipolar disorders and healthy controls. Bipolar Disord. 2017;19(5):353–362. doi: 10.1111/bdi.12516. [DOI] [PubMed] [Google Scholar]
- Johnson L.R., Farb C., Morrison J.H., McEwen B.S., Ledoux J.E. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136(1):289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kahhale I., Buser N.J., Madan C.R., Hanson J.L. Quantifying Numerical and spatial Reliability of Amygdala and hippocampal Subdivisions in FreeSurfer [preprint] Neuroscience. 2020 doi: 10.1101/2020.06.12.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Ast V.A., Kaldewaij R., Hashemi M.M., Zhang W., Klumpers F., Roelofs K. Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms. Neuropsychopharmacology. 2021;46(7):1283–1292. doi: 10.1038/s41386-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K., Milad M.R. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol. Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Logue M.W., van Rooij S.J.H., Dennis E.L., Davis S.L., Hayes J.P., Stevens J.S., Densmore M., Haswell C.C., Ipser J., Koch S.B.J., Korgaonkar M., Lebois L.A.M., Peverill M., Baker J.T., Boedhoe P.S.W., Frijling J.L., Gruber S.A., Harpaz-Rotem I., Jahanshad N., Morey R.A. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatr. 2018;83(3):244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L., Miller A.B., Snyder J., McLaughlin K.A., Sheridan M.A. Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Front. Behav. Neurosci. 2019;13:80. doi: 10.3389/fnbeh.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D.P., Krull J.L., Lockwood C.M. Equivalence of the mediation, confounding and suppression effect. Prev. Sci. 2000;1(4):173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D., Lockwood C., Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav. Res. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan H.L., Fleming J.E., Streiner D.L., Lin E., Boyle M.H., Jamieson E., Duku E.K., Walsh C.A., Wong M.Y.-Y., Beardslee W.R. Childhood abuse and lifetime psychopathology in a community sample. Am. J. Psychiatr. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gerlach J.L., Micco D.J. The Hippocampus. Springer; 1975. Putative glucocorticoid receptors in hippocampus and other regions of the rat brain; pp. 285–322. [Google Scholar]
- Merz E.C., He X., Myers B., Noble K.G. Socioeconomic disadvantage, chronic stress, and hippocampal subfield development in children. Neurosci. Insights. 2020;15 doi: 10.1177/2633105520931098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Tottenham N., Noble K.G. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J. Clin. Child Adolesc. Psychol. 2018;47(2):312–323. doi: 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Clarke E.K., Haswell C.C., Phillips R.D., Clausen A.N., Mufford M.S., Saygin Z., Wagner H.R., LaBar K.S. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol. Psych. Cognit. Neurosci. Neuroimag. 2020;5(3):281–290. doi: 10.1016/j.bpsc.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Gold A.L., LaBar K.S., Beall S.K., Brown V.M., Haswell C.C., Nasser J.D., Wagner H.R., McCarthy G., Mid-Atlantic MIRECC Workgroup, for the Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch. Gen. Psychiatr. 2012;69(11):1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.G., Yushkevich P.A., Das S., Wang L., Van Leemput K., Iglesias J.E., Alpert K., Mezher A., Ng P., Paz K., Weiner M.W. Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage: Clinic. 2018;17:1006–1018. doi: 10.1016/j.nicl.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogovitsyn N., Addington J., Souza R., Placsko T.J., Stowkowy J., Wang J., Goldstein B.I., Bray S., Lebel C., Taylor V.H., Kennedy S.H., MacQueen G. Childhood trauma and amygdala nuclei volumes in youth at risk for mental illness. Psychol. Med. 2020;1–8 doi: 10.1017/S0033291720003177. [DOI] [PubMed] [Google Scholar]
- Olsen R.K., Carr V.A., Daugherty A.M., La Joie R., Amaral R.S.C., Amunts K., Augustinack J.C., Bakker A., Bender A.R., Berron D., Boccardi M., Bocchetta M., Burggren A.C., Chakravarty M.M., Chételat G., Flores R., DeKraker J., Ding S., Geerlings M.I., et al. Progress update from the hippocampal subfields group. Alzheimer's Dementia: Diagnosis, Assess. Dis. Monitor. 2019;11(1):439–449. doi: 10.1016/j.dadm.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A., Gray J.C., Owens M.M., Liu S., Duprey E.B., Sweet L.H., MacKillop J. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child. Maltreat. 2019;24(4):400–410. doi: 10.1177/1077559519839491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal O.T., Milde A.M., Hafstad G.S., Hodneland E., Dyb G., Craven A.R., Melinder A., Endestad T., Hugdahl K. The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Transl. Psychiatry. 2020;10(1):288. doi: 10.1038/s41398-020-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R.D., De Bellis M.D., Brumback T., Clausen A.N., Clarke-Rubright E.K., Haswell C.C., Morey R.A. Volumetric trajectories of hippocampal subfields and amygdala nuclei influenced by adolescent alcohol use and lifetime trauma. Transl. Psychiatry. 2021;11(1):154. doi: 10.1038/s41398-021-01275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintzka C.W.S., Hansen T.I., Evensmoen H.R., Håberg A.K. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidé Y., Andersson F., Dufour-Rainfray D., Descriaud C., Brizard B., Gissot V., Cléry H., Carrey Le Bas M.-P., Osterreicher S., Ogielska M., Saint-Martin P., El-Hage W. Smaller hippocampal volume following sexual assault in women is associated with post-traumatic stress disorder. Acta Psychiatr. Scand. 2018;138(4):312–324. doi: 10.1111/acps.12920. [DOI] [PubMed] [Google Scholar]
- Ramphal B., Pagliaccio D., Dworkin J.D., Herbstman J., Noble K.G., Margolis A.E. Timing‐specific associations between income‐to‐needs ratio and hippocampal and amygdala volumes in middle childhood: a preliminary study. Dev. Psychobiol. 2021;63(7) doi: 10.1002/dev.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M.C., Humphreys K.L., King L.S., Gotlib I.H. Self-reported neglect, amygdala volume, and symptoms of anxiety in adolescent boys. Child. Abuse Neglect. 2018;80:80–89. doi: 10.1016/j.chiabu.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R., Krey L., McEwen B. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 1985;5(5):1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatr. 2000;57(10):925. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Saxbe D., Khoddam H., Piero L.D., Stoycos S.A., Gimbel S.I., Margolin G., Kaplan J.T. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018;21(6) doi: 10.1111/desc.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z.M., Kliemann D., Iglesias J.E., van der Kouwe A.J.W., Boyd E., Reuter M., Stevens A., Van Leemput K., McKee A., Frosch M.P., Fischl B., Augustinack J.C. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.H., Wang X., Yoon S.H., Cage J.L., Kobulsky J.M., Montemayor B.N. Childhood maltreatment and alcohol-related problems in young adulthood: the protective role of parental warmth. Child. Abuse Neglect. 2019;98 doi: 10.1016/j.chiabu.2019.104238. [DOI] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 2021;16(1):67–93. doi: 10.1177/1745691620920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A.M., Brymer M.J., Decker K.B., Pynoos R.S. The University of California at Los Angeles post-traumatic stress disorder reaction index. Curr. Psychiatr. Rep. 2004;6(2):96–100. doi: 10.1007/s11920-004-0048-2. [DOI] [PubMed] [Google Scholar]
- Stephen J.M., Solis I., Janowich J., Stern M., Frenzel M.R., Eastman J.A., Mills M.S., Embury C.M., Coolidge N.M., Heinrichs-Graham E., Mayer A., Liu J., Wang Y.P., Wilson T.W., Calhoun V.D. The Developmental Chronnecto-Genomics (Dev-CoG) study: a multimodal study on the developing brain. Neuroimage. 2021;225 doi: 10.1016/j.neuroimage.2020.117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Khan A. Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child. Maltreat. 2019;24(4):458–465. doi: 10.1177/1077559519870845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Gabard-Durnam L.J. The developing amygdala: a student of the world and a teacher of the cortex. Curr. Opin. Psychol. 2017;17:55–60. doi: 10.1016/j.copsyc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen G.A., Ossewaarde L., Bäckström T., Hermans E.J., Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- VanTieghem M. Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Develop. Cognit. Neurosci. 2021;11 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y.L., van Buchem M.A., Spinhoven P., Elzinga B.M., Rombouts S.A.R.B. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatr. Res. Neuroimaging. 2015;233(3):436–442. doi: 10.1016/j.pscychresns.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Vouimba R.-M., Yaniv D., Richter-Levin G. Glucocorticoid receptors and β-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology. 2007;52(1):244–252. doi: 10.1016/j.neuropharm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Weissman D.G., Lambert H.K., Rodman A.M., Peverill M., Sheridan M.A., McLaughlin K.A. Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress. Anxiety. 2020;37(9):916–925. doi: 10.1002/da.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E.M., Chételat G., Daugherty A.M., Flores R., Joie R., Mueller S.G., Stark C.E.L., Wang L., Yushkevich P.A., Berron D., Raz N., Bakker A., Olsen R.K., Carr V.A. Hippocampal subfield volumetry from structural isotropic 1 mm 3 MRI scans: a note of caution. Hum. Brain Mapp. 2021;42(2):539–550. doi: 10.1002/hbm.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Claycomb Erwin M., Elhai J.D., Wall J.T., Tamburrino M.B., Brickman K.R., Kaminski B., McLean S.A., Liberzon I., Wang X. Relationship of hippocampal volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol. Psychiatr.: Cognit. Neurosci. Neuroimag. 2018;3(11):968–975. doi: 10.1016/j.bpsc.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Amaral R.S.C., Augustinack J.C., Bender A.R., Bernstein J.D., Boccardi M., Bocchetta M., Burggren A.C., Carr V.A., Chakravarty M.M., Chételat G., Daugherty A.M., Davachi L., Ding S.-L., Ekstrom A., Geerlings M.I., Hassan A., Huang Y., Iglesias J.E., Zeineh M.M. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-H., Meng S.-Q., Guo X.-Y., Zhang J.-L., Zhang W., Chen Y.-Y., Lu L., Yang J.-L., Xue Y.-X. Traumatic stress produces delayed alterations of synaptic plasticity in basolateral amygdala. Front. Psychol. 2019;10:2394. doi: 10.3389/fpsyg.2019.02394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.