Abstract
Indoles are some of the most versatile and common nitrogen-based heterocyclic scaffolds and are frequently used in the synthesis of various organic compounds. Indole based compounds are very important among heterocyclic structures due to their biological and pharmaceutical activities. The last decade, in particular, has witnessed considerable activity towards the synthesis of indole derivatives due to the possibilities for the design of polycyclic structures by the incorporation of multiple fused heterocyclic scaffolds in an attempt to achieve promising new heterocycles with chemical and biomedical relevance. In this study, we provide an overview on recent applications of indole in the multicomponent reactions for the synthesis of various heterocyclic compounds during the period of 2012 to 2017.
An overview on recent applications of indoles in multicomponent reactions for the synthesis of heterocyclic compounds is provided.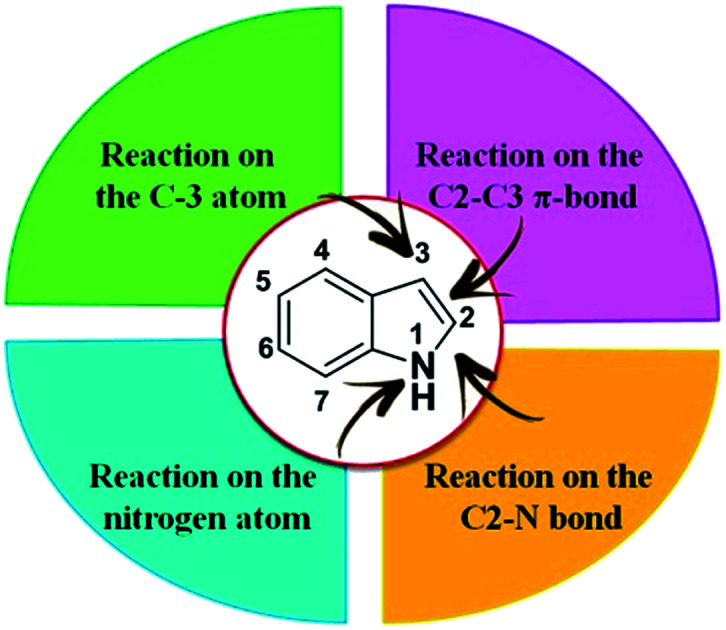
1. Introduction
Heterocyclic compounds are important tools in our daily life having an extensive variety of applications such as sanitizers,1 pharmaceuticals2,3 and antioxidant compounds,4,5 corrosion inhibitors,6,7 dye stuff,8 copolymers,9,10 and as building blocks in the synthesis of organic compounds and natural products. Multicomponent reactions (MCRs) have been extensively used for the synthesis of heterocyclic compounds.11–13 MCRs represent a great tool in organic synthesis for the construction of variety-oriented series of building blocks with potentially interesting biological activities.14–17 The attractiveness of the MCR approach is its easy operation, high selectivity and yield by using minimum synthetic requirements. Indole scaffolds have been known for their value in the development of new compounds of pharmaceutical interest.18–20 Up to date, several review articles have been published based on the reactions of indole.21,22 Herein, in continuation of our studies towards the synthesis of heterocyclic compounds and multicomponent reactions,23–32 and since there is a wide range of reactions that include indole in the preparation of heterocyclic compounds, this review presents the recent applications of indole in the synthesis of diverse heterocyclic compounds during the period from 2012 to 2017. This review first discusses indoles’ C-3 carbon atom reactivity applicable to electrophilic reactions, followed by MCRs in which the N position of indole is reacted as a nucleophile to afford N-substituted indole products. In Section 2.3, indole cycloaddition reactions have been discussed including cycloaddition reactions of the C2–C3 π-bond (Section 2.3.1) and the C–N sigma bond (Section 2.3.2). Finally, in Section 2.4, miscellaneous reactions of indole will be reviewed.
2. Multicomponent reactions of indoles
The indole structure is a heterocyclic compound which easily participates in chemical reactions. Its bonding sites are analogous to pyrrole. As shown in Scheme 1, indole is reactive at four different positions including the carbon atom 3, nitrogen atom 1, the C2–C3 π-bond and the C2–N sigma bond. Indole can be protonated with strong acids such as hydrochloric acid, which protonates the C3 position, more easily than the N atom. The cycloaddition reaction is another reaction of indole compounds. The C2–C3 π-bond of indole has a propensity towards cycloaddition reactions but cycloaddition reactions of the C2–N sigma bond are also observed.
Scheme 1.
2.1. The C-3 position reactions of indoles
Gámez-Montaño’s group reported the one-pot Ugi-azide33 multicomponent reaction of indole 1, isocyanides 2, aldehydes 3 and TMSN34 (Scheme 2). In the first step, intermediate A was obtained, and then N-acylation was performed between A and chloroacetyl chloride to give the intermediate B, which underwent an SN2 reaction with the potassium ethyl xanthogenate salt to give the final xanthates 5 (Scheme 2).34
Scheme 2.
The one-pot multicomponent reaction of 3-acetylindole 1, aromatic aldehydes 3, ethyl cyanoacetate 6, and ammonium acetate 7 in the presence of piperidine as catalyst was established to access several 6-indolylpyridine-3-carbonitrile derivatives 8 (Scheme 3).35 The anti-proliferative activities of products were evaluated and showed good results.
Scheme 3.
Indole 1, carbon disulfide 9 and substituted α-bromo propiophenones 10 were reacted via a three component domino [3 + 2] heterocyclization reaction for the preparation of two-carbon-tethered 1,3-oxathiole–indole pair compounds 11 (Scheme 4).36 The results showed that functional groups such as bromide and chloride provide ample opportunity for further functional group manipulations, for example, by modern cross-coupling reactions.
Scheme 4.
A regioselective Sonogashira37 cyclization reaction was carried out in the presence of CuI as catalyst and 2,2′-(1E,1′E)-(1R,2R)cyclohexane-1,2-diylbis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)diphenol as ligand to obtain benzyl-3-(indol-3-yl)-2-phenyl-2,3-dihydroisoindolinones 14. The reaction involved the one-pot multicomponent reaction of indoles 1, 2-iodo-N-phenylbenzamides 12 and terminal alkyne 13 under aerobic conditions followed by a nucleophilic addition (Scheme 5).38
Scheme 5.
Magnetic nanoparticles (Fe3O4-NPs) catalyzed the synthesis of pyrano[2,3-d]pyrimidines 17 and pyrido[2,3-d]pyrimidines 18. The reactions were performed via the one-pot three component reaction of indole 1, malononitrile 15 and barbituric acids 16a or 6-amino uracil derivatives 16b in EtOH (Scheme 6).39
Scheme 6.
Baruah et al. presented the synthesis of 3-alkylated indole derivatives 20 and 21 under a microwave-assisted three-component reaction of indole-3-aldehydes 1, alkyl nitriles 15 or barbituric acids 16 with 1,4-dihydropyridine (DHP) derivative 19 (Scheme 7).40 In each case, DHPs are converted to pyridines. In fact, 1,4-dihydropyridine is the reducing agent.
Scheme 7.
Preparation of a wide variety of new 6-(1H-indol-3-yl)-2-oxo-4-aryl-1,2,3,4 tetrahydropyrimidine-5-carbonitriles 23 was accessed by combining 3-(cyanoacetyl)-indoles 1 with an arylaldehyde 3 and urea 22 in the presence of PEG-400 and a catalytic amount of thiazolium anions (NHCs) (Scheme 8).41
Scheme 8.
A three-component reaction for the synthesis of functionalized 3-{1-[2-(1H-indol-3-yl)ethyl]-4,5,6,7-tetrahydro-1H-indol-3-yl}indolin-2-ones 26 has been described by Jiang and Yan. The reaction involves a one-pot condensation of indole 1 with dimedone 24 and 3-phenacylideneoxindoles 25 in refluxing acetonitrile with p-toluenesulfonic acid as catalyst (Scheme 9).42
Scheme 9.
Modha and co-workers provided a novel procedure for the synthesis of diversely substituted spiroindolines 30via the post-Ugi gold-catalyzed diastereoselective domino cyclization. In this methodology, the Ugi reaction of indole-3-aldehydes 1, propargylamine 27, acids 28 and isocyanides 2 gave the products 29 in good yields which reacted with Au(PPh3)SbF6 in chloroform to produce spiroindolines 30 in moderate yields (Scheme 10).43 In another study, the same group used other amines instead of propargylamine for the synthesis of analogues of these products.44
Scheme 10.
The indolylmalonamides 33 have been prepared via the three-component reaction of indole derivatives 1, chromene-3-carboxylates 31 and amines 32 in the presence of La(OTf)3 as a Lewis acid catalyst (Scheme 11).45 Indolylmalonamide products 33 showed notable fluorescence activities when they are exposed to long wave UV light (366 nm).
Scheme 11.
Polyfunctionalized indole derivatives 35 and 36 were generated from the Yonemitsu-type46 trimolecular condensation of indoles 1 with aldehydes 3 and 1,3-dicarbonyl compounds 34, such as malonates and acetoacetates using Lewis acid catalysts under microwave irradiation. The formation of bis-indolic derivative 36 can be easily rationalized in the one pot reaction, where a double addition of indole to the aldehyde is assumed. As already suggested by Gao and Wu,47 the adduct 35 is probably converted into a reactive indolenine derivative A48 by the loss of an active methylene fragment, which reacts with another molecule of indole (Schemes 12 and 13).49 Macroporous copper oxide (mpCuO) was also used as catalyst in this reaction and the same products were isolated in good yields.50 Docking studies against enoyl acyl carrier protein reductase predicted that the compounds bind at the active site with high binding affinity values. In another study, Li et al. used l-proline as catalyst in this reaction.51 The results are summarized in Table 1.
Scheme 12.
Scheme 13.
Comparison of different conditions for the synthesis of products 35 and 36.
Khalafi-Nezhad et al. developed the use of trimethylsilyl iodide (TMSI) as a multifunctional agent in the one-pot synthesis of 9-(1H-indol-3-yl)xanthen-4-(9H)-ones 37 from the reaction of indoles 1, 2-methoxybenzaldehydes 3 (as O-methyl protected salicylaldehydes) and β-dicarbonyl compounds 24 (Scheme 14).52
Scheme 14.
The functionalized indole-3-yl pyridines 40 were prepared via an efficient one-pot condensation of cyanoacetylindoles 1, 3-formylchromones 38 and ammonium acetate 7 under stannous chloride 39 mediation in DMF (Scheme 15).53
Scheme 15.
Wan and co-workers used polyethylene glycol (PEG-200) in a three-component reaction of indoles 1, aldehydes 3, and malononitrile 15 to afford 3-indole derivatives 41 in good to excellent yields (Scheme 16).54l-Proline,55 tetrabutylammonium fluoride (TBAF),56 Zn-salphen,57 Cu(OAc)2 (ref. 58) and Cu(iii)59 were also used as catalysts in this reaction and the results are shown in Table 2.
Scheme 16.
Comparison of different conditions for the synthesis of products 41.
| Entry | Solvent | Catalyst | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | H2O | PEG-200 | r.t. | 3 | 55–98 (ref. 54) |
| 2 | EtOH | l-Proline | r.t. | 30–72 | 58–98 (ref. 55) |
| 3 | — | TBAF·3H2O | 60 | 2 | 55–97 (ref. 56) |
| 4 | DCM | Zn-salphen, DIPEA | r.t. | 6 | 13–60 (ref. 57) |
| 5 | PEG-400 | Cu(OAc)2 | 70 | 15–40 | 48–98 (ref. 58) |
| 6 | H2O | Cu(iii) | 30 | 12–24 | 70–96 (ref. 59) |
Singh’s group described a highly efficient methodology for the synthesis of 3-amino-alkylated indoles 44via the one-pot three-component Mannich60 type reaction of amines 42, alcohols 43 and indoles 1. Treatment of amines 42 and alcohols 43 with KOH in toluene in the presence of (Fe(NO3)3·9H2O/TEMPO)61 as catalyst, yielded iminium ion A. Then, the iminium ion A was reacted with indoles 1 to obtain novel 3-substituted indoles 44 (Scheme 17).62 In another study, ferric hydrogen sulfate (FHS)63 was applied as catalyst and the same products were prepared in 87–98%. It was observed that electron-withdrawing groups on the aldehyde reacted rapidly and were better reagents in this reaction. Furthermore, N-methylaniline showed better reactivity in comparison with N-ethylaniline due to the low steric effects. N-Alkylanilines were used in excess to avoid the formation of bis(indolyl)alkanes.64 The reaction was also catalyzed by l-proline,65 Amberlite, IRA-400 Cl resin66 and polyaniline-fluoroboric acid-dodecyl hydrogen sulfate salt (PANI-HBF4).67 Catalyst-free conditions in MeOH have also been reported in 72 h by 28–99% yields.68 A comparison of different catalysts and experimental setups is given in Table 3.
Scheme 17.
Comparison of different conditions for the synthesis of product 44.
| Entry | Solvent | Catalyst | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Toluene | (Fe(NO3)3·9H2O/TEMPO) | r.t. | 32–39 | 79–87 (ref. 62) |
| 2 | — | FHS | 45 | 1–4 | 87–98 (ref. 63) |
| 3 | — | l-Proline | r.t. | 5–18 | 68–89 (ref. 65) |
| 4 | MeOH | IRA-400 Cl resin | r.t. | 1.5–3 | 70–85 (ref. 66) |
| 5 | H2O | PANI-HBF4 | r.t. | 30–50 min | 88–97 (ref. 67) |
| 6 | MeOH | — | 30 | 72 | 28–99 (ref. 68) |
Mahmoodi and co-workers developed the one-pot cyclocondensation of mono- or bis(indole-3-carbaldehyde) 1 or 45,69,70 thiosemicarbazide 46, and phenacyl bromides 47 in the presence of a catalytic amount of AcOH for the preparation of the novel mono- and bis(indol-3-yl)hydrazineyl thiazole derivatives 48 and 49 (Scheme 18).71 The products were evaluated for in vitro antibacterial activity against Gram-positive and Gram-negative bacteria. Some of the products have good antibacterial activity. The product 48 with OCH3 as a donating group exhibited high activity against Gram-positive bacteria.
Scheme 18.
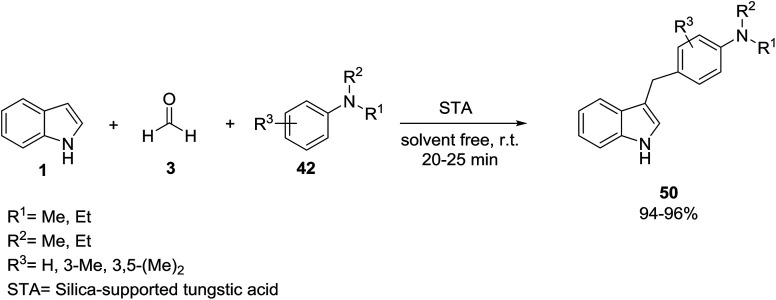
Shinde and Jeong developed the reaction between indole 1 and formaldehyde 3 with tertiary aromatic amines 42 in the presence of silica-supported tungstic acid (STA) as a heterogeneous acid catalyst under solvent-free conditions. The protocol was performed via the three component Mannich type Friedel–Crafts addition for the preparation of N,N-dialkyl amino arylated indole derivatives 50 (Scheme 19).72 Application of sodium dodecyl sulfate (SDS) as surfactant in this reaction was also reported by Kumar et al. resulting in 78–94% yields of products.73
Scheme 19.
Mild aminoacylation of indoles 1 through a multicomponent process with ynol ethers 51 and sulfonyl azides 52 was established by Alford and Davies for the synthesis of oxo-tryptamines 53 (Scheme 20).74 First, 4-alkoxy N-sulfonyltriazoles A75 were generated from ynol ethers 51 and sulfonyl azides 52, and treated with indoles 1. Then, the obtained enol ethers B were converted to the amino ketones 53 for the α-aminoacylation of enols.76
Scheme 20.
N-Methyl indole 1 was reacted with diazooxindole 54 and nitrostyrene 55 in the presence of [Ru] and squaramide as catalysts via an asymmetric Michael addition77 for the synthesis of 3,3′-bis(indole) derivatives 56 (Scheme 21).78
Scheme 21.
Novel spirooxindole-pyrrolidine compounds 61 and 62 were obtained through 1,3-dipolar cycloaddition of azomethine ylides generated from isatin 57 and sarcosine 59 or thioproline 60 with the dipolarophile 3-(1H-imidazol-2-yl)-2-(1H-indole-3-carbonyl)acrylonitrile 58 (Scheme 22).79 Anticancer activity studies were carried out for the synthesized compounds against A549 lung adenocarcinoma cancer cell line.80 Several of the products showed very high activity against the cancer cell line. Reddy and co-workers also studied this reaction in MeOH as solvent under reflux conditions in 2–3 h giving 80–93% yields. The antimicrobial activity of all products were evaluated against several bacteria and fungi, and showed good activity.81
Scheme 22.
A one-pot four-component condensation strategy was employed by Naureen’s group for the discovery of indole-based tetra-arylimidazoles 64. This method involves the reaction of 2-arylindole-3-carbaldehydes 1, substituted anilines 42, benzil 63 and ammonium acetate 7 in acetic acid (Scheme 23).82 The anti-urease activity of the synthesized compounds was evaluated and showed good results.
Scheme 23.
The same authors synthesized several new trisubstituted imidazoles, 3-(4,5-diaryl-1H-imidazol-2-yl)-2-phenyl-1H-indoles 65, via the condensation of substituted indole aldehydes 1, benzil 63 and ammonium acetate 7 in refluxing acetic acid (Scheme 24).83 The products were evaluated for their α-glucosidase inhibition and showed significant α-glucosidase inhibitory activity.
Scheme 24.
Andreana and his group utilized the one-pot reaction of 4-nitroindolylacetaldehyde 1, methylamine 32, methyl isocyanide 66 and 3-hydroxyphenylpyruvic acid 67 for the synthesis of (±)-thaxtomin A (TA) 68 as a herbicidal natural product. First, the prerequisite dipeptide A was isolated which through a base-mediated keto-amide cyclization reaction afforded two diastereomeric compounds B. Then, compound B was treated with KOH under microwave irradiation to provide the intended product 68 (Scheme 25).84 This natural product was synthesized previously by Zhang et al. and has been demonstrated to possess activity against the tobacco mosaic virus.85
Scheme 25.
A one-pot three-component reaction of indole-3-aldehyde derivatives 1, ethyl cyanoacetate 6, and guanidine hydrochloride 69 under three different conditions, including microwave irradiation, grindstone technology and reflux, was developed to afford 2-amino-5-cyano-4-[(2-aryl)-1H-indol-3-yl]-6-hydroxypyrimidines 70 (Scheme 26)86 The products 70 were evaluated for their antimicrobial activity against nine pathogenic bacteria and showed mild to moderate activity.
Scheme 26.
Bhattacharjee et al. employed ammonium chloride (NH4Cl) as catalyst for the preparation of 9-(1H-indol-3-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-one 71via the one-pot three-component reaction of indole 1 with salicylaldehyde 3 and dimedone 24 (Scheme 27).87 In another study, Ganguly et al. developed this reaction in the presence of l-proline as catalyst in H2O for 2 h in 86–96% yield.88
Scheme 27.
So and Mattson reported on the synthesis of glycine products 73 in the presence of chiral BINOL-based phosphoric-acid as catalyst. The process involves the multicomponent coupling reactions of indole derivatives 1, nitrodiazoester 72 and anilines 42 in methyl t-butyl ether (MTBE) as solvent (Scheme 28).89
Scheme 28.
The three-component reaction of indoles 1, α-oxoketene dithioacetals 74, and aldehyde 3 or 75 was investigated for the synthesis of dihydrocoumarins 76 and quinolines 77 (Scheme 29).90 The reaction mechanism was presented in Scheme 30. The electrophilic reaction of aldehyde 3 with two nucleophiles 1 and 74 resulted in the formation of intermediate A which was converted into a chromene-type intermediate B through an intramolecular substitution.91 Finally, hydrolysis of intermediate B formed 77. The reaction mechanism of aldehyde 75 is shown in Scheme 31. First, the condensation of 75 and indoles 1 produced the intermediate D. The NH2 group of 75 attacked the C2 position of the indole ring, and formed the intermediate E. Cleavage of an endocyclic C–N bond allowed the formation of a quinoline derivative F.91 Then, compound 74 reacted with NH2 group of quinoline F and final product 76 was obtained.
Scheme 29.
Scheme 30.
Scheme 31.
Borah et al. investigated the one-pot multicomponent reaction of 3-(cyanoacetyl)-indoles 1, aromatic aldehydes 3 and ethyl acetoacetate 34 in the presence of InCl3 under microwave irradiation to produce the functionalized 3-(pyranyl)-indole derivatives 78. When ammonium acetate 7 was used as the source of ammonia in this reaction, the one-pot four-component reaction was carried out and 3-(dihydropyridinyl)-indole derivatives 79 were obtained (Scheme 32).92 The results show that electron donating groups (EDG) in the aldehyde increase the product yield, whereas electron withdrawing groups (EWG) decrease the yield of products.
Scheme 32.
A sulfone-containing Brønsted acid ionic liquid was used in a one-pot reaction of indole 1, salicylaldehydes 3 and 1,1-diphenylethylene 80 for the synthesis of substituted chromane derivatives 81 (Scheme 33).93
Scheme 33.
A series of indole incorporated thiazolylcoumarins 83 were synthesized from the reaction of indole derivatives 1, thiosemicarbazide 46 and 3-(2-bromoacetyl)-2H-chromen-2-ones9482 using a catalytic amount of acetic acid (Scheme 34).95 The antibacterial, anticancer and DNA cleavage activities of products were evaluated. The results showed that all products have a good activity towards the screened bacterial strains.
Scheme 34.
Song et al. established the [3 + 3] cyclization of 3-(cyanoacetyl)-indoles 1 with dialkyl acetylenedicarboxylates (DMAD) 84 and isocyanides 2 for the preparation of 4-H-pyran derivatives 85 containing an indole scaffold (Scheme 35).96
Scheme 35.
Efficient synthesis of indol-3-yl substituted pyran derivatives 86 was investigated by Ji and co-workers via the one-pot multicomponent reaction of 3-cyanoacetyl indoles 1, aldehydes 3 and malononitrile 15 in the present of piperidine as catalyst under ultrasonic irradiation (Scheme 36).97 Thiamine hydrochloride (vitamin B1) and cetyltrimethylammonium bromide (CTAB) were also utilized as catalysts in this reaction for 25 min giving 92–94% yield.98
Scheme 36.
A Knoevenagel99 coupling of salicylaldehyde 3 and Meldrum’s acid 87 followed by a Michael type reaction with indole 1 in the presence of a (saccharin)-based functional ionic liquid (imidazolium saccharinate) was reported by Kumar et al. This multicomponent reaction was performed via lactonization decarboxylative elimination to functionalize the C-3 of indoles with dihydrocoumarin to yield indole-3-dihydro-coumarins 88 (Scheme 37).100
Scheme 37.
Biheterocycles containing indole and azole skeletons 90 and 91 were prepared from the multicomponent reaction of indoles 1, 1,2-diaza-1,3-dienes 89 and aldehydes 3 or alkynes 13 (Scheme 38).101 The synthesized compounds were screened for their in vitro biological studies. The results showed that some of them have anticancer activity against MCF7 and Caco-2 human tumor cell lines.
Scheme 38.
Stefani and co-workers utilized a mild approach for the synthesis of indole-3-glyoxyl derivatives 94 and indole-3-glyoxyl-1,2,3-triazoles 96. For this purpose, the reaction of indole 1, oxalyl chloride 92 and various nucleophiles 93 was carried out in N,N-diisopropylethylamine (DIPEA) to afford indole-3-glyoxyl derivatives 94 (Scheme 39). On the other hand, indole-3-glyoxyl-1,2,3-triazoles 96 were obtained from the one-pot multicomponent reaction of these reactants and organic azides 95via click102 chemistry (Scheme 40).103
Scheme 39.
Scheme 40.
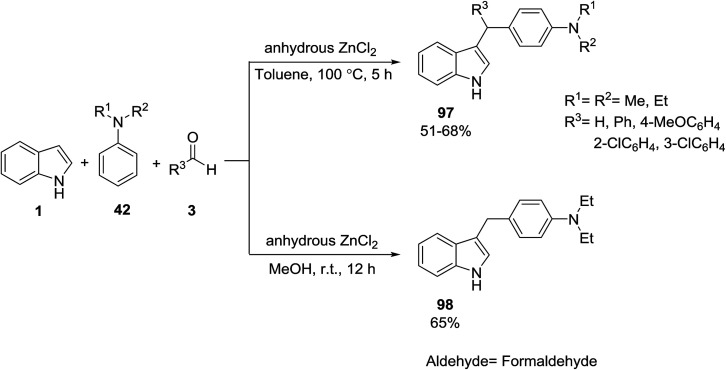
Indole 1 was reacted with anilines 42 and aldehydes 3via an anhydrous ZnCl2 catalyzed one-pot three-component reaction to afford diarylmethyl indoles 97 in toluene and 3-arylmethyl indoles 98 in MeOH. The reaction of indole 1 with benzaldehyde 3 in the presence of catalyst formed the azafulven A which reacted with another indole 1 to generate the kinetically stable bis(indolyl)methane B. In the presence of anilines, bis(indolyl)methane converted to the target products 97 (Schemes 41 and 42).104
Scheme 41.
Scheme 42.
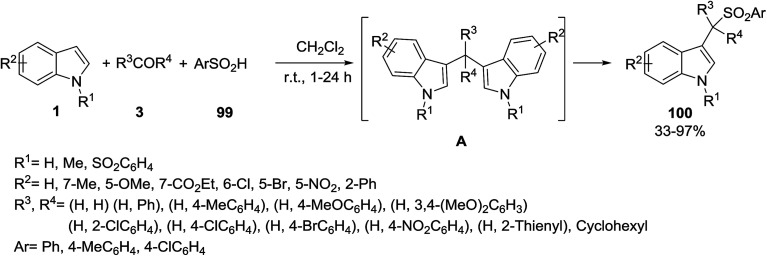
A series of biologically important 3-(1-arylsulfonylalkyl)indoles 100 were prepared by Huang et al. This process was carried out using a catalyst-free three-component reaction of indoles 1, carbonyl compounds 3, and arenesulfinic acids 99 at room temperature (Scheme 43).105 Bis(indolyl)methanes A were found as the key intermediates in this reaction.
Scheme 43.
The synthesis of bis(indolyl)methanes 101 was reported by Dhumaskar and Tilve via the reaction of two molecules of indole 1 with aldehydes 3 under solvent-free conditions without any catalyst (Scheme 44).106 Two different methods were employed for this reaction. In method A, the mixture of aldehyde and indole was kept at ambient temperature in a test tube while in method B, the mixture was ground using a mortar and pestle. Method B resulted in the formation of products in shorter reaction times than method A. According to the results, anisaldehyde with an electron donating group at the para position and heptaldehyde failed to react completely. Mohammadi Ziarani and co-workers developed this reaction using the sulfonic acid functionalized silica (SiO2-Pr-SO3H) as catalyst and obtained the same products in short reaction times and good yields (5–20 min and 87–96% yields).107
Scheme 44.
Mohammadi Ziarani and coworkers synthesized a novel class of symmetrical 3,3-di(indolyl)indolin-2-ones 102 from the reaction of indoles 1 with isatins 57 in the presence of SBA-Pr-SO3H as a solid acid catalyst under mild reaction conditions (Scheme 45).108 The antimicrobial activities of the products were tested and the results demonstrated that the MIC value of one of the products (R1 = R3 = H, R2 = Bn) against B. subtilis was equal to that of chloramphenicol.
Scheme 45.
Geng’s group developed the one-pot four component reaction of 3-(cyanoacetyl)indoles 1, aromatic aldehydes 3, 1-(9-butylcarbazol-3-yl)ethanone 103 and ammonium acetate 7 for the preparation of several 3-cyano-2-(1H-indol-3-yl)-6-(9-butylcarbazol-3-yl)pyridine derivatives 104. The reaction was performed using AcOH and ethane-1,2-diol (glycol) under microwave irradiation (Scheme 46).109
Scheme 46.
Zn2+ supported on montmorillonite KSF (Zn2+@KSF) as an efficient heterogeneous catalyst promoted the preparation of mono and bis-indolylimidazole derivatives 106 and 107. This reaction was carried out from the condensation of indole-3-carbaldehyde derivatives 1 or bis-aldehydes 105,110 aniline derivatives 42 and benzil 63 (Scheme 47).111 The antibacterial activity of the synthesized compounds was examined and some of them exhibited promising activities.
Scheme 47.
Naidu et al. explored the one-pot three-component reaction of 2-cyano-3-(1H-indol-3-yl)-pent-2-enedinitrile or ethyl-2,4-dicyano-3-(1H-indol-3-yl)but-2-enoate derivative 108, aryl aldehydes 3 and 6-aminouracil derivatives 16 (obtained from the reaction of 3-(cyanoacetyl)-indoles 1 and nitrile 14) for the synthesis of some hexahydropyrimido[4,5-b]-1,8-naphthyridine derivatives 109 and 110. It was found that when the indole derivative 108 (R1 = H; R2 = CO2Et) was treated with benzaldehyde (4a) and uracil 16 (R4 = Me) the ester group participated in the cyclization process instead of the nitrile group, and the trioxo-nitrile compound 110 was obtained (Scheme 48).112
Scheme 48.
The three-component coupling reaction of indoles 1, β-ketoesters 34 and arylboronicacids 111via the regioselective palladium-catalyzed oxidative reaction was studied for the preparation of indole-based heterocycles 112 (Scheme 49).113 Indole coupled more rapidly than β-ketoester with the arylboronic acid, and this rate variation was important under the three-component reaction conditions, possibly because the coupling with β-ketoester involves an unstable enone intermediate.
Scheme 49.
Siddalingamurthy et al. described the synthesis of indole-3-propanamide derivatives 113 and 114via the three-component reaction of N-methyl indole 1, aromatic aldehydes 3 and Meldrum’s acid 87 in the presence of choline chloride/urea ionic liquid as catalyst. At first, Meldrum’s adduct A was generated, which then reacted with various amines, and the indole-3-propanamide derivatives 113 were formed. On the other hand, when Meldrum’s adduct was treated with H2O, corresponding acid products 114 were obtained (Scheme 50).114
Scheme 50.
A one-pot four-component reaction of 3-(cyanoacetyl)indole 1, benzaldehyde derivatives 3, 3-acetylpyridine 114, and NH4OAc 7 was explored for the preparation of 2-(indol-3-yl)pyridine derivatives 115 (Scheme 51). In the same paper, 4-aryl-2-(1H-indol-3-yl)-6-arylnicotinonitriles 117 were also obtained based on this process, from the reaction of 3-(cyanoacetyl)indole 1, aromatic aldehydes 3, aromatic ketones 116, and NH4OAc 7 under two different conditions (Scheme 52).115
Scheme 51.
Scheme 52.
Deb et al. reported a base-promoted three-component one-pot approach for the synthesis of 3-(α,α-diarylmethyl) indoles 119via arylation of in situ generated 3-indolylalcohols A in an EtOH–H2O solvent system. Substituted indoles 1, benzaldehydes 3 and electron-rich aromatics 118 were used as starting materials in this reaction (Scheme 53).116
Scheme 53.
2.2. The N position reactions of indoles
Martinez-Ariza et al. reported a two-step one-pot procedure for the synthesis of N-1-quinoxaline-indoles 123 from the reaction of indoles 1, amines 120 and glyoxaldehydes 121. In the first step, α-iminoketones A were formed from the reaction of amines 120 and glyoxaldehydes 121, which were then treated with indole 1 for the preparation of compounds 122. The deprotection-cyclization of 122 was performed for the synthesis of target products 123 (Scheme 54).117
Scheme 54.
Hulme and co-workers reported a one-pot two-step multicomponent reaction of indole 1, arylglyoxaldehyes 121 and amines 42 for the synthesis of α-oxo-acetamidines 125 under microwave conditions. The main reaction step was the N-1 addition of indole to α-iminoketones A (obtained from the reaction of arylglyoxaldehyes 121 and amines 42), followed by an air- or oxygen-mediated oxidation (Scheme 55).118
Scheme 55.
2.3. The cycloaddition reactions of indoles
2.3.1. The cycloaddition reactions of indoles at the C2–C3 π-bond
The tetracyclic tetrazole scaffold compounds 128 were constructed via a one-pot four-component Ugi–Pictet–Spengler reaction119 of indole 1, carbonyl compounds 3, isocyanoacetaldehyde (dimethylacetal) 126 and trimethylsilyl azide (TMSN3) 2. The reaction was carried out using methanesulfonic acid without any solvent at room temperature. First, the Ugi reaction was performed to obtain compounds 127, which was then followed immediately by the Pictet–Spengler reaction for the preparation of final products 128 (Scheme 56).120
Scheme 56.
Jiang et al. described an aerobic dehydrogenative coupling interaction between indole derivatives 1, diazoacetates 129 and conjugated α-keto esters 130 in the presence of sequential Rh2(OAc)4 and CuCl2 as catalysts for the synthesis of polyfunctional cyclopenta[b]indoles 131 (Scheme 57).121
Scheme 57.
A acid-catalyzed multicomponent tandem cyclization protocol was applied by Cai et al. for the preparation of polyfunctional dihydroindolizino[8,7-b]indoles 134 and 135. The process involves the reaction of indole derivatives 1, arylglyoxal monohydrates 132 and trans-β-nitrostyrenes 133 or malononitrile 15 under mild, metal-free conditions (Scheme 58).122
Scheme 58.
The silica supported ionic liquid of [pmim]HSO4SiO2 (silica supported 1-methyl-3-(triethoxysilylpropyl)imidazolium hydrogen sulfate) was used as an efficient catalyst for the synthesis of 2-amino-4,5-dihydro-4-arylpyrano[3,2-b]indole-3-carbonitrile derivatives 136. The reaction was carried out via the three-component condensation of indoles 1, aromatic aldehydes 3 and malononitrile 15 (Scheme 59).123 Other catalysts such as KHPO4 under ultrasonic irradiation124 and triphenylphosphine (PPh3)125 were also used in this reaction as demonstrated in Table 4.
Scheme 59.
Comparison of different conditions for the synthesis of product 136.
Galvan et al. accomplished the reaction of indole-2-aldehydes 1, imines 137 and alkenes 138via stereoselective [3 + 2] carbocyclization to achieve cyclopenta[b]indoles 139 or tetrahydroquinolines 140. It was found that the model coupling reaction could be changed to give related products 139 or 140 in the presence of different acid catalysts (Schemes 60 and 61).126
Scheme 60.
Scheme 61.
Kundu and co-workers established a new synthetic protocol for the preparation of pyrido- and pyrimido-indoles 141 and 142 employing ethyl 2-amino-1H-indole-3-carboxylates 1, aromatic aldehydes 3, and terminal alkynes 13 in the presence of a Brønsted acid such as trifluoroacetic acid (TFA) (Scheme 62).127 When the reaction catalyzed by Yb(OTf)3 as a Lewis acid, pyrimidoindoles 142 were prepared as the single products in 58–75% yield.
Scheme 62.
Tron’s group have prepared heteroarylogous 1H-indole-3-carboxamidines 143 utilizing a three-component interrupted Ugi reaction of N-alkyl-N-(1H-indol-2-ylmethyl)amines 1,128 isocyanides 2 and carbonyl compounds 3 (Scheme 63).129
Scheme 63.
Silvani et al. reported an impressive method to prepare dihydroimidazo[1′,5′:1,2]pyrido[3,4-b]indol-2-ium salts 145 utilizing a Ugi/Bischler-Napieralski/heterocyclization MCR. The Ugi reaction was carried out following a general procedure, consisting of the sequential addition of aldehydes 3, amines 42, acids 28 and, finally, indole 1 for the synthesis of compounds 144. Treatment of intermediate compounds 144 in MeOH under the Bischler–Napieralski and heterocyclization gave the intended products 145 (Scheme 64).130
Scheme 64.
A gold-catalyzed multicomponent reaction of vinyl indoles 1 with two N-allenamides 146 and 147 was investigated by Pirovano et al. to prepare tetrahydrocarbazole 148 in 78% yield (Scheme 65).131
Scheme 65.
Chiral phosphoric acid was used as the catalyst in the asymmetric aza-Diels–Alder reaction132 of indole derivatives 1 with 2-azadiene generated in situ from oxetane 149133 and 3,5-dimethoxyaniline 42 to obtain a series of indole-alkaloid-type polycycles 150 (Scheme 66).134 The authors hypothesized that the introduction of a hydrogen-bond acceptor on the aldehyde moiety may help to orient the transition state and lower the activation barrier for the desired process. Thus, they employed substrates with a simple ether group in proximity to the aldehyde moiety, and encouragingly, the desired aza-Diels–Alder reaction proceeded cleanly to form the desired products with good yields and diastereoselectivities. The biological activity evaluation of the products showed that the cytotoxicities of products in human lung carcinoma and human cervical carcinoma cells exhibited inhibitory effects against cell proliferation with IC50 values in the range of 15.0–27.5 μM.
Scheme 66.
The annulation of indoles 1, 2-amino benzyl alcohols 42 and benzaldehydes 3 in a two step three-component tandem reaction was used to form benzazepinoindoles 151. In the first step, indoles were C-3 alkylated and the intermediates A were obtained. Then in the second step, intermediates A underwent a 7-endo-trig cyclization (the modified Pictet–Spengler cyclization reaction) to obtain the intended products 151 (Scheme 67).135
Scheme 67.
A stereoselective Povarov reaction136,137 catalyzed by iodine was developed by Wang and co-workers for the preparation of exo-tetrahydroindolo[3,2-c]quinoline derivatives 153. The procedure involved a reaction between indoles 1, aldehydes 3 and amines 152 in toluene (Scheme 68).138 The results showed that only reactive amines could be included in this reaction to give the desired products 153 with high stereoselectivity.
Scheme 68.
Gallium(iii) (GaBr3) catalyzed a three component [4 + 3] cycloaddition reaction of indoles 1, aldehyde/ketone/ketal derivatives 3 and dienes 154 to furnish cyclohepta[b]indoles 155. The authors have proposed the mechanism of formation of the product via nucleophilic addition of the C3 of indole to electrophile 3 to give the alcohol A, which in the presence of a Lewis acid generated the indolyl cation B. The treatment of B with diene component 154 afforded the corresponding products (Scheme 69).139
Scheme 69.
Damavandi and Sandaroos have synthesized 2,9-dihydro-2-oxo-4-aryl-1H-pyrido[2,3-b]indole-3-carbonitriles 158 by the one-pot multicomponent cyclocondensation reaction of 1-methyl-1H-indol-2-ol 1, substituted (triethoxymethyl) arenes 156 and cyanoacetamide 157 in the presence of silica supported ionic liquid [pmim]HSO4SiO2 as a catalyst (Scheme 70).140
Scheme 70.
A new series of diketopiperazine-fused tetrahydro-β-carboline ring systems 160 were obtained via the Pictet–Spengler/Ugi-4CR/deprotection-cyclization reactions. According to the proposed mechanism, the Pictet–Spengler cyclization of indole 1 and ethyl glyoxalate 159 followed by a deesterification reaction produced 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-carboxylic acid B. The reaction of acid B, amines 42, isocyanides 2 and aldehyde 3via the Ugi reaction gave the intermediate products C. The deprotection cyclization reaction of this intermediate generated the target products 160 (Scheme 71).141
Scheme 71.
A stereoselective catalyst-free three-component reaction of 2-isocyanoethylindole 1, malononitrile 15 and aldehyde 3 was developed for the construction of polycyclic spiroindolines 161 in high yields (up to 90%) with excellent levels of diastereoselectivity (Scheme 72).142 The presence of an electron-withdrawing group on the aldehyde led to a decrease in yields of the products.
Scheme 72.
John and co-workers developed a multicomponent reaction involving N-protected 3-nitroindole 1 a primary amine 162 and an enolizable ketone 163 for the preparation of a series of functionalized pyrrolo[3,2-b]indoles 164 (Scheme 73).143 It was found that the yield of product 164 decreased to 45% with 1-phenylethylamine. With cyclohexylamine, the product 164 was obtained in moderate yield (53%) and with adamantylamine the MCR failed, proving that the reactivity decreases with an increase in the steric bulk of the amine component.
Scheme 73.
The Lewis acid catalyzed three-component [3 + 2] cycloaddition reaction of pentafulvene 165 with in situ generated indolylmethanol A has been developed for the construction of pentaleno[1,2-b]indoles 166 (Scheme 74).144 It was found that aromatic aldehydes bearing electron-withdrawing groups (R3 = Cl, Br, F) were tolerated well under the reaction conditions and afforded the cycloaddition products in moderate to good yields. However, aldehydes with electron-rich substituents (R3 = 2,4-OMe, Me) were unable to take part in the cycloaddition reaction.
Scheme 74.
Alpers et al. developed a photoinduced three-component radical [4 + 2]-cyclization–allylation reaction between 3-(2-iodoethyl)indoles 1, acceptor-substituted alkenes 167, and allyl zirconocenes of the structure Cp ZrCl(σ-allyl) 168 for the synthesis of hexahydrocarbazoles 169 (Scheme 75).145
Scheme 75.
A copper(i)-catalyzed cascade multicomponent reaction strategy was performed for the construction of 5-hydroxy-1H-pyrrol-2(5H)-ones 171 bearing an indole moiety. The reaction was carried out using substituted indole 1, (Z)-3-iodoacrylic acids 170 and terminal alkynes 13 (Scheme 76).146
Scheme 76.
2.3.2. The cycloaddition reactions of indoles at the C–N sigma bond
An enantioselective multicomponent coupling reaction for the synthesis of pyrrolo[1,2-a]indoles 173 was reported using a chiral disulfonimide as catalyst. The [3 + 2] cyclization reaction between the imine A (generated in situ from indole-2-aldehyde derivatives 1 and anilines 42) and 2,3-dihydrofuran 172 led to products 173 (Scheme 77).147 It was observed that the yields of products synthesized from substituted anilines at the 3- and 5-positions with halogen atoms groups were lower than those synthesized from 3,5-bis(trifluoromethyl)aniline.
Scheme 77.
The Ugi four-component condensation of indole-2-aldehyde derivatives 1, acids 28, anilines 42 and isocyanides 2 in the presence of orthogonal copper and palladium catalysts under microwave heating was accomplished for the synthesis of two important indole-fused heterocycles 174 and 175. First, the Ugi adduct A was obtained via the intramolecular cyclization conditions and served as a precursor in subsequent selective post-transformations. 5,6-Dihydroindolo[1,2-a]quinoxalines 174 were prepared by a copper catalyzed N–H arylation pathway, while 6,7-dihydroindolo[2,3-c]quinolones 175 were formed by palladium catalyzed C–H arylations without the protection of the indole N1 moiety (Scheme 78).148 In another approach to synthesize these compounds, no catalyst has been employed in this reaction and the products were obtained in good yields.149
Scheme 78.
2.4. Miscellaneous reactions of indoles
Pandey et al. have published their results on the synthesis of diverse indole-2-carboxamides 177via a two-step multicomponent reactions. Initially, Et3N was added to amino ester hydrochloride 176 and after 10 minutes, 1H-indole-2-carboxylic acid 1, aldehyde 3, and isocyanide 2 were successively added to the mixture to produce corresponding indole-fused diketopiperazines A.150 Then, compound A in the presence of amines formed the functionalized carboxamides 177 (Scheme 79).151 The biological activities of products were evaluated and showed that most products have anti-leishmanial activity against intracellular amastigotes form of Leishmania donovani.
Scheme 79.
Fu and co-workers designed a strategy for the preparation of 3,2′- 179 and 3,3′-bis-indoles 180 through the microwave-assisted regioselective reaction of indoles 1, arylglyoxal monohydrate 133 and diverse N-aryl enaminones 178 in HOAc (Scheme 80).152 The 2-unsubstituted indoles resulted in 3,2′-bis-indole frameworks 179, while indoles bearing a methyl or phenyl group at C2 led to the 3,3′-bis-indoles 180 with simultaneous formation of three sigma-bonds.
Scheme 80.
3. Conclusion and outlook
This review summarizes recent studies on the application of indoles in multicomponent reactions for the synthesis of a variety of heterocyclic compounds during the period of 2012 to 2017. Indole is a significant nitrogen-based heterocycle with particular importance in the synthesis of complex heterocyclic scaffolds. Indole fragments have been recently attracting much attention due to their biological and pharmaceutical activities. Diversely multisubstituted indole substances are useful building blocks in pharmaceutical and organic syntheses. Consequently, the novel methodologies for the synthesis of complex heterocyclic frameworks involving indole are expected to receive further increasing attention in the future.
4. Conflicts of interest
There are no conflicts to declare.
5. Abbreviations
- CTAB
Cetyltrimethylammonium bromide
- DCE
1,2-Dichloroethylene
- DIPEA
N,N-Diisopropylethylamine
- DMAD
Dialkyl acetylenedicarboxylate
- DMF
Dimethylformamide
- DPP
Diketopyrrolopyrrole
- EA
Ethyl acetate
- FHS
Ferric hydrogen sulfate
- MTBE
Methyl tert-butyl ether
- mpCuO
Macroporous copper oxide
- MW
Microwave
- NHC
N-Heterocyclic carbene
- NPs
Nanoparticles
- PEG
Polyethylene glycol
- PMDETA
Pentamethyldiethylenetriamine
- PTS
Polyoxyethanyl-α-tocopheryl sebacate
- SDS
Sodium dodecyl sulfate
- STA
Silica-supported tungstic acid
- TBAF
Tetra-n-butylammonium fluoride
- TFA
Trifluoroacetic acid
- THF
Tetrahydrofuran
Supplementary Material
Acknowledgments
We are grateful for financial support from the Research Council of Alzahra University and support of National Elites Foundation of Iran, Tehran.
Biographies
Biography
Ghodsi Mohammadi Ziarani.

Ghodsi Mohammadi Ziarani was born in Iran, in 1964. She received her B.Sc. degree in Chemistry from the Teacher Training University, Tehran, Iran, in 1987, her M.Sc. degree in Organic Chemistry from the Teacher Training University, Tehran, Iran, under the supervision of Professor Jafar Asgarin and Professor Mohammad Ali Bigdeli in 1991 and her Ph.D. degree in asymmetric synthesis (Biotransformation) from Laval University, Quebec, Canada under the supervision of Professor Chenevert, in 2000. She is a Full Professor of Organic Chemistry in the chemistry department of Alzahra University. Her research interests include organic synthesis, heterocyclic synthesis, asymmetric synthesis, natural products synthesis, synthetic methodology and applications of nano-heterogeneous catalysts in multicomponent reactions.
Biography
Razieh Moradi.

Razieh Moradi was born in 1990 in Delfan, Lorestan, Iran. She obtained her B.Sc. degree in Chemistry from the University of Lorestan (2012) and her M.Sc. degree in Organic Chemistry at Alzahra University under the supervision of Prof. Ghodsi Mohammadi Ziarani. She is currently a Ph.D. student in Organic Chemistry at Alzahra University under the supervision of Prof. Ghodsi Mohammadi Ziarani. Her research field is the synthesis of heterocyclic compounds and the application of nano-heterogeneous catalysts in multicomponent reactions.
Biography
Tahereh Ahmadi.

Tahereh Ahmadi was born in 1975 in Shiraz, Iran. She received her B.Sc. degree in Applied Chemistry from the Islamic Azad University of Firuzabad, Fars, Iran (2003) and her M.S.c degree in organic chemistry at Alzahra University, Tehran, Iran (2007) under the supervision of Dr Yahya Shirazi Beheshtiha. She is currently working towards her Ph.D. in organic chemistry at Alzahra University under the supervision of Prof. Ghodsi Mohammadi Ziarani. Her research focuses on the application of modified nanomaterials as catalysts in the synthesis of heterocyclic compounds and multicomponent reactions.
Biography
Negar Lashgari.

Negar Lashgari was born in 1985 in Tehran, Iran. She received her B.Sc. degree in applied chemistry from the Kharazmi University, Karaj, Iran (2008) and her M.Sc. degree in organic chemistry at Alzahra University, Tehran, Iran (2011) under the supervision of Dr Ghodsi Mohammadi Ziarani. She obtained her Ph.D. degree in nanochemistry from the University of Tehran under the supervision of Dr Alireza Badiei and Dr Ghodsi Mohammadi Ziarani in 2017. Her research field is synthesis and functionalization of mesoporous silica materials and their application as nano-heterogeneous catalysts in multicomponent reactions and fluorescent chemosensors for detection of various anions and cations.
7. References
- Sniady A. Morreale M. S. Wheeler K. A. Dembinski R. Eur. J. Org. Chem. 2008;2008:3449. doi: 10.1002/ejoc.200800397. [DOI] [Google Scholar]
- Cocuzza A. J. Chidester D. R. Culp S. Fitzgerald L. Gilligan P. Bioorg. Med. Chem. Lett. 1999;9:1063. doi: 10.1016/S0960-894X(99)00133-X. [DOI] [PubMed] [Google Scholar]
- Vitaku E. Smith D. T. Njardarson J. T. J. Med. Chem. 2014;57:10257. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Vitaglione P. Fogliano V. J. Chromatogr. B. 2004;802:189. doi: 10.1016/j.jchromb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab B. F. Awad G. E. Badria F. A. Eur. J. Med. Chem. 2011;46:1505. doi: 10.1016/j.ejmech.2011.01.062. [DOI] [PubMed] [Google Scholar]
- Talati J. Gandhi D. Corros. Sci. 1983;23:1315. doi: 10.1016/0010-938X(83)90081-1. [DOI] [Google Scholar]
- Raja P. B. Sethuraman M. G. Mater. Lett. 2008;62:113. doi: 10.1016/j.matlet.2007.04.079. [DOI] [Google Scholar]
- Stead C. Dyes Pigments. 1982;3:161. doi: 10.1016/0143-7208(82)80019-3. [DOI] [Google Scholar]
- Guo L. Zhang D. J. Am. Chem. Soc. 2009;131:18072. doi: 10.1021/ja907380d. [DOI] [PubMed] [Google Scholar]
- Butuc E. Gherasim G. M. J. Polym. Sci. 1984;22:503. [Google Scholar]
- Sunderhaus J. D. Martin S. F. Chem.–Eur. J. 2009;15:1300. doi: 10.1002/chem.200802140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Eur. J. Org. Chem. 2003;2003:1133. doi: 10.1002/ejoc.200390167. [DOI] [Google Scholar]
- Jiang B. Rajale T. Wever W. Tu S. J. Li G. Chem.–Asian J. 2010;5:2318. doi: 10.1002/asia.201000310. [DOI] [PubMed] [Google Scholar]
- Ugi I. Dömling A. Werner B. J. Heterocycl. Chem. 2000;37:647. doi: 10.1002/jhet.5570370322. [DOI] [Google Scholar]
- Kappe C. O. Acc. Chem. Res. 2000;33:879. doi: 10.1021/ar000048h. [DOI] [PubMed] [Google Scholar]
- Orru R. V. A. de Greef M. Synthesis. 2003:1471. doi: 10.1055/s-2003-40507. [DOI] [Google Scholar]
- Ngouansavanh T. Zhu J. Angew. Chem. 2006;118:3575. doi: 10.1002/ange.200600588. [DOI] [PubMed] [Google Scholar]
- Somei M. Yamada F. Nat. Prod. Rep. 2005;22:73. doi: 10.1039/B316241A. [DOI] [PubMed] [Google Scholar]
- Kinsman A. C. Kerr M. A. J. Am. Chem. Soc. 2003;125:14120. doi: 10.1021/ja036191y. [DOI] [PubMed] [Google Scholar]
- Chang F. J. Rangisetty J. B. Dukat M. Setola V. Raffay T. Roth B. Glennon R. A. Bioorg. Med. Chem. Lett. 2004;14:1961. doi: 10.1016/j.bmcl.2004.01.071. [DOI] [PubMed] [Google Scholar]
- Shiri M. Zolfigol M. A. Kruger H. G. Tanbakouchian Z. Chem. Rev. 2009;110:2250. doi: 10.1021/cr900195a. [DOI] [PubMed] [Google Scholar]
- Shiri M. Chem. Rev. 2012;112:3508. doi: 10.1021/cr2003954. [DOI] [PubMed] [Google Scholar]
- Mohammadi Ziarani G. Hajiabbasi P. Heterocycles. 2013;87:1415. doi: 10.3987/REV-13-768. [DOI] [Google Scholar]
- Mohammadi Ziarani G. Moradi R. Lashgari N. ARKIVOC. 2016;1:1. [Google Scholar]
- Mohammadi Ziarani G. Moradi R. Lashgari N. Tetrahedron: Asymmetry. 2015;26:517. doi: 10.1016/j.tetasy.2015.04.011. [DOI] [Google Scholar]
- Mohammadi Ziarani G. Hosseini Nasab N. Lashgari N. RSC Adv. 2016;6:38827. doi: 10.1039/C6RA02834A. [DOI] [Google Scholar]
- Mohammadi Ziarani G. Aleali F. Lashgari N. RSC Adv. 2016;6:50895. doi: 10.1039/C6RA09874F. [DOI] [Google Scholar]
- Mohammadi Ziarani G. Gholamzadeh P. Lashgari N. Hajiabbasi P. ARKIVOC. 2013;(i):470. [Google Scholar]
- Mohammadi Ziarani G. Lashgari N. Azimian F. Kruger H. G. Gholamzadeh P. ARKIVOC. 2015;(vi):1. [Google Scholar]
- Ahmadi T. Mohammadi Ziarani G. Gholamzadeh P. Mollabagher H. Tetrahedron: Asymmetry. 2017;28:708. doi: 10.1016/j.tetasy.2017.04.002. [DOI] [Google Scholar]
- Moradi R. Mohammadi Ziarani G. Lashgari N. ARKIVOC. 2017;(i):148. doi: 10.24820/ark.5550190.p009.980. [DOI] [Google Scholar]
- Rahimifard M. Mohammadi Ziarani G. Lashkariani B. M. Turk. J. Chem. 2014;38:345. doi: 10.3906/kim-1307-38. [DOI] [Google Scholar]
- Ugi I. Meyr R. Fetzer U. Steinbrückner C. Angew. Chem. 1959;71:386. [Google Scholar]
- Gordillo-Cruz R. E. Rentería-Gómez A. Islas-Jácome A. Cortes-García C. J. Díaz-Cervantes E. Robles J. Gámez-Montaño R. Org. Biomol. Chem. 2013;11:6470. doi: 10.1039/c3ob41349g. [DOI] [PubMed] [Google Scholar]
- El-Sayed N. S. Shirazi A. N. El-Meligy M. G. El-Ziaty A. K. Rowley D. Sun J. Nagib Z. A. Parang K. Tetrahedron Lett. 2014;55:1154. doi: 10.1016/j.tetlet.2013.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Y. Zhang H. Feng B. M. Jiang B. Wang S. L. Tu S. J. Org. Biomol. Chem. 2012;10:5036. doi: 10.1039/c2ob25817j. [DOI] [PubMed] [Google Scholar]
- Sonogashira K. J. Organomet. Chem. 2002;653:46. doi: 10.1016/S0022-328X(02)01158-0. [DOI] [Google Scholar]
- Sarkar S. Pal R. Sen A. K. Tetrahedron Lett. 2013;54:4273. doi: 10.1016/j.tetlet.2013.05.151. [DOI] [Google Scholar]
- Kidwai M. Jain A. Bhardwaj S. Mol. Diversity. 2012;16:121. doi: 10.1007/s11030-011-9336-z. [DOI] [PubMed] [Google Scholar]
- Baruah B. Naidu P. S. Borah P. Bhuyan P. J. Mol. Diversity. 2012;16:291. doi: 10.1007/s11030-012-9359-0. [DOI] [PubMed] [Google Scholar]
- Fatma S. Singh D. Mishra P. Singh P. K. Ankit P. Singh M. Singh J. RSC Adv. 2013;3:22527. doi: 10.1039/C3RA43606C. [DOI] [Google Scholar]
- Jiang Y. H. Yan C. G. Synthesis. 2016;48:3057. doi: 10.1055/s-0035-1561951. [DOI] [Google Scholar]
- Kumar A. Vachhani D. D. Modha S. G. Sharma S. K. Parmar V. S. Van Der Eycken E. V. Beilstein J. Org. Chem. 2013;9:2097. doi: 10.3762/bjoc.9.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modha S. G. Kumar A. Vachhani D. D. Jacobs J. Sharma S. K. Parmar V. S. Van Meervelt L. Van der Eycken E. V. Angew. Chem., Int. Ed. 2012;51:9572. doi: 10.1002/anie.201205052. [DOI] [PubMed] [Google Scholar]
- Jennings J. J. Bhatt C. P. Franz A. K. J. Org. Chem. 2016;81:6211. doi: 10.1021/acs.joc.6b00541. [DOI] [PubMed] [Google Scholar]
- Inanaga J. Hirata K. Saeki H. Katsuki T. Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989. doi: 10.1246/bcsj.52.1989. [DOI] [Google Scholar]
- Gao K. Wu J. Org. Lett. 2008;10:2251. doi: 10.1021/ol800664z. [DOI] [PubMed] [Google Scholar]
- Matsuo J.-i. Tanaki Y. Ishibashi H. Tetrahedron. 2008;64:5262. doi: 10.1016/j.tet.2008.03.033. [DOI] [Google Scholar]
- Viola A. Ferrazzano L. Martelli G. Ancona S. Gentilucci L. Tolomelli A. Tetrahedron. 2014:6781. doi: 10.1016/j.tet.2014.07.062. [DOI] [Google Scholar]
- Khan G. A. War J. A. Naikoo G. A. Pandit U. J. Das R. J. Saudi Chem. Soc. 2016;22:6. doi: 10.1016/j.jscs.2016.03.009. [DOI] [Google Scholar]
- Li M. Taheri A. Liu M. Sun S. Gu Y. Adv. Synth. Catal. 2014;356:537. doi: 10.1002/adsc.201300790. [DOI] [Google Scholar]
- Khalafi-Nezhad A. Nourisefat M. Panahi F. Synthesis. 2014;46:2071. doi: 10.1055/s-0033-1338633. [DOI] [PubMed] [Google Scholar]
- Poomathi N. Muralidharan D. Perumal P. T. Tetrahedron Lett. 2013;54:7091. doi: 10.1016/j.tetlet.2013.09.127. [DOI] [Google Scholar]
- Wang L. Huang M. Zhu X. Wan Y. Appl. Catal., A. 2013;454:160. doi: 10.1016/j.apcata.2012.12.008. [DOI] [Google Scholar]
- He Y. H. Cao J. F. Li R. Xiang Y. Yang D. C. Guan Z. Tetrahedron. 2015;71:9299. doi: 10.1016/j.tet.2015.10.027. [DOI] [Google Scholar]
- Singh N. Allam B. K. Raghuvanshi D. S. Singh K. N. Adv. Synth. Catal. 2013;355:1840. doi: 10.1002/adsc.201300162. [DOI] [Google Scholar]
- Anselmo D. Escudero-Adán E. C. Martínez Belmonte M. Kleij A. W. Eur. J. Inorg. Chem. 2012:4694. doi: 10.1002/ejic.201200150. [DOI] [Google Scholar]
- Chandrasekhar S. Patro V. Reddy G. P. K. Grée R. Tetrahedron Lett. 2012;53:6223. doi: 10.1016/j.tetlet.2012.09.008. [DOI] [Google Scholar]
- Jiang H. Wang L. Xie J. J. Chem. Res. 2016;40:338. doi: 10.3184/174751916X14622783380474. [DOI] [Google Scholar]
- Mannich C. Krösche W. Arch. Pharm. 1912;250:647. doi: 10.1002/ardp.19122500151. [DOI] [Google Scholar]
- Zhang E. Tian H. Xu S. Yu X. Xu Q. Org. Lett. 2013;15:2704. doi: 10.1021/ol4010118. [DOI] [PubMed] [Google Scholar]
- Singh V. K. Sharma L. K. Singh R. K. P. Tetrahedron Lett. 2016;57:407. doi: 10.1016/j.tetlet.2015.12.047. [DOI] [Google Scholar]
- Eshghi H. Rahimizadeh M. Bakhtiarpoor Z. Pordel M. Chin. Chem. Lett. 2012;23:673. doi: 10.1016/j.cclet.2012.04.006. [DOI] [Google Scholar]
- Rahimizadeh M. Eshghi H. Mokaber-Esfahani M. Gholizadeh M. J. Chin. Chem. Soc. 2014;61:1265. doi: 10.1002/jccs.201400192. [DOI] [Google Scholar]
- Kumar A. Gupta M. K. Kumar M. Green Chem. 2012;14:290. doi: 10.1039/C1GC16297G. [DOI] [Google Scholar]
- Harichandran G. David Amalraj S. Shanmugam P. J. Saudi Chem. Soc. 2018;22:208. doi: 10.1016/j.jscs.2016.01.009. [DOI] [Google Scholar]
- Devi C. L. Rao V. J. Palaniappan S. Synth. Commun. 2012;42:1593. doi: 10.1080/00397911.2010.542535. [DOI] [Google Scholar]
- Cao J. F. Chen Y. L. Guan Z. He Y. H. Z. Naturforsch. 2014;69:721. [Google Scholar]
- Mahmoodi N. Ghavidast A. Chem. Heterocycl. Compd. 2014;49:1451. doi: 10.1007/s10593-014-1395-5. [DOI] [Google Scholar]
- Gali R. Banothu J. Porika M. Velpula R. Bavantula R. Abbagani S. RSC Adv. 2014;4:53812. doi: 10.1039/C4RA11428K. [DOI] [PubMed] [Google Scholar]
- Mahmoodi N. O. Khalili B. Rezaeianzade O. Ghavidast A. Res. Chem. Intermed. 2016;42:6531. doi: 10.1007/s11164-016-2478-y. [DOI] [Google Scholar]
- Shinde V. V. Jeong Y. T. C. R. Chim. 2015;18:449. doi: 10.1016/j.crci.2014.05.010. [DOI] [Google Scholar]
- Kumar A. Gupta M. K. Kumar M. Saxena D. RSC Adv. 2013;3:1673. doi: 10.1039/C2RA22428C. [DOI] [Google Scholar]
- Alford J. S. Davies H. M. L. J. Am. Chem. Soc. 2014;136:10266. doi: 10.1021/ja5058967. [DOI] [PubMed] [Google Scholar]
- Miura T. Tanaka T. Biyajima T. Yada A. Murakami M. Angew. Chem. 2013;125:3975. doi: 10.1002/ange.201209603. [DOI] [PubMed] [Google Scholar]
- Davies H. M. Hedley S. J. Chem. Soc.Rev. 2007;36:1109. doi: 10.1039/B607983K. [DOI] [PubMed] [Google Scholar]
- Michael A. J. Prakt. Chem. 1887;35:349. doi: 10.1002/prac.18870350136. [DOI] [Google Scholar]
- Chen D. F. Zhao F. Hu Y. Gong L. Z. Angew. Chem., Int. Ed. 2014;53:10763. doi: 10.1002/anie.201406098. [DOI] [PubMed] [Google Scholar]
- Arun Y. Saranraj K. Balachandran C. Perumal P. T. Eur. J. Med. Chem. 2014;74:50. doi: 10.1016/j.ejmech.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Arun Y. Bhaskar G. Balachandran C. Ignacimuthu S. Perumal P. Bioorg. Med. Chem. Lett. 2013;23:1839. doi: 10.1016/j.bmcl.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Kathirvelan D. Haribabu J. Reddy B. S. R. Balachandran C. Duraipandiyan V. Bioorg. Med. Chem. Lett. 2015;25:389. doi: 10.1016/j.bmcl.2014.10.099. [DOI] [PubMed] [Google Scholar]
- Naureen S. Chaudhry F. Asif N. Munawar M. A. Ashraf M. Nasim F. H. Arshad H. Khan M. A. Eur. J. Med. Chem. 2015;102:464. doi: 10.1016/j.ejmech.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Naureen S. Noreen S. Nazeer A. Ashraf M. Alam U. Munawar M. A. Khan M. A. Med. Chem. Res. 2015;24:1586. doi: 10.1007/s00044-014-1239-y. [DOI] [Google Scholar]
- Bourgault J. P. Maddirala A. R. Andreana P. R. Org. Biomol. Chem. 2014;12:8125. doi: 10.1039/c4ob01148a. [DOI] [PubMed] [Google Scholar]
- Zhang H. Ning X. Hang H. Ru X. Li H. Li Y. Wang L. Zhang X. Yu S. Qiao Y. Org. Lett. 2013;15:5670. doi: 10.1021/ol4026556. [DOI] [PubMed] [Google Scholar]
- Gupta R. Jain A. Madan Y. Menghani E. J. Heterocycl. Chem. 2015;51:1395. doi: 10.1002/jhet.1796. [DOI] [Google Scholar]
- Bhattacharjee S. Das D. K. Khan A. T. Synthesis. 2014;46:73. [Google Scholar]
- Ganguly N. C. Roy S. Mondal P. Saha R. Tetrahedron Lett. 2012;53:7067. doi: 10.1016/j.tetlet.2012.10.055. [DOI] [Google Scholar]
- So S. S. Mattson A. E. Asian J. Org. Chem. 2014;3:425. doi: 10.1002/ajoc.201300285. [DOI] [Google Scholar]
- Liu C. Zhou L. Jiang D. Gu Y. Asian J. Org. Chem. 2016;5:367. doi: 10.1002/ajoc.201500497. [DOI] [Google Scholar]
- Rao H. S. P. Sivakumar S. J. Org. Chem. 2006;71:8715. doi: 10.1021/jo061372e. [DOI] [PubMed] [Google Scholar]
- Borah P. Naidu P. S. Majumder S. Bhuyan P. J. Mol. Diversity. 2014;18:759. doi: 10.1007/s11030-014-9533-7. [DOI] [PubMed] [Google Scholar]
- Taheri A. Lai B. Yang J. Zhang J. Gu Y. Tetrahedron. 2016;72:479. doi: 10.1016/j.tet.2015.11.049. [DOI] [Google Scholar]
- Sahu S. K. Mishra A. Behera R. K. Indian J. Heterocycl. Chem. 1996:91. [Google Scholar]
- Gali R. Banothu J. Gondru R. Bavantula R. Velivela Y. Crooks P. A. Bioorg. Med. Chem. Lett. 2015;25:106. doi: 10.1016/j.bmcl.2014.10.100. [DOI] [PubMed] [Google Scholar]
- Song P. Zhao L. Ji S. Chin. J. Chem. 2014;32:381. doi: 10.1002/cjoc.201400155. [DOI] [Google Scholar]
- Chen T. Xu X. P. Ji S. J. J. Heterocycl. Chem. 2013;50:244. doi: 10.1002/jhet.983. [DOI] [Google Scholar]
- Fatma S. Singh D. Ankit P. Mishra P. Singh M. Singh J. Tetrahedron Lett. 2014;55:2201. doi: 10.1016/j.tetlet.2014.02.050. [DOI] [Google Scholar]
- Knoevenagel E. Ber. Dtsch. Chem. Ges. 1898;31:2596. doi: 10.1002/cber.18980310308. [DOI] [Google Scholar]
- Kumar A. Kumar P. Tripathi V. D. Srivastava S. RSC Adv. 2012;2:11641. doi: 10.1039/C2RA21284F. [DOI] [Google Scholar]
- Mantenuto S. Lucarini S. de Santi M. Piersanti G. Brandi G. Favi G. Mantellini F. Eur. J. Org. Chem. 2016;2016:3193. doi: 10.1002/ejoc.201600210. [DOI] [Google Scholar]
- Kolb H. C. Finn M. Sharpless K. B. Angew. Chem., Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stefani H. A. Vasconcelos S. N. S. Souza F. B. Manarin F. Zukerman-Schpector J. Tetrahedron Lett. 2013;54:5821. doi: 10.1016/j.tetlet.2013.08.064. [DOI] [Google Scholar]
- Subramaniapillai S. G. Ganesan A. Tetrahedron Lett. 2014;55:694. doi: 10.1016/j.tetlet.2013.11.115. [DOI] [Google Scholar]
- Huang W. Yang J. Li X. Yuan L. Ma Y. Zhou Q. Liang D. Phosphorus, Sulfur Silicon Relat. Elem. 2016;191:772. doi: 10.1080/10426507.2015.1100182. [DOI] [Google Scholar]
- Dhumaskar K. L. Tilve S. G. Green Chem. Lett. Rev. 2012;5:353. doi: 10.1080/17518253.2011.637967. [DOI] [Google Scholar]
- Mohammadi Ziarani G. Aslani Z. Asadi S. Iran. J. Catal. 2015;5:143. [Google Scholar]
- Mohammadi Ziarani G. Moradi R. Badiei A. Lashgari N. Moradi B. Soorki A. A. J. Taibah Univ. Sci. 2015;9:555. doi: 10.1016/j.jtusci.2014.11.009. [DOI] [Google Scholar]
- Geng L. J. Feng G. L. Zhang H. L. Zhang Y. M. J. Chem. Res. 2013;37:503. doi: 10.3184/174751913X13736278296459. [DOI] [Google Scholar]
- Bartlett S. Nelson A. Org. Biomol. Chem. 2004;2:2874. doi: 10.1039/B405010J. [DOI] [PubMed] [Google Scholar]
- Mahmoodi N. O. Nikokar I. Farhadi M. Ghavidast A. Z. Naturforsch. 2014;69:715. [Google Scholar]
- Naidu P. S. Kolita S. Majumder S. Bhuyan P. J. Synthesis. 2015;47:701. doi: 10.1055/s-0034-1379639. [DOI] [Google Scholar]
- Nimje R. Y. Leskinen M. V. Pihko P. M. Angew. Chem., Int. Ed. 2013;52:4818. doi: 10.1002/anie.201300833. [DOI] [PubMed] [Google Scholar]
- Siddalingamurthy E. Mahadevan K. M. Kumar T. O. S. Synth. Commun. 2013;43:3153. doi: 10.1080/00397911.2013.769601. [DOI] [Google Scholar]
- Zeng L. Y. Cai C. Synth. Commun. 2013;43:705. doi: 10.1080/00397911.2011.607933. [DOI] [Google Scholar]
- Deb M. L. Borpatra P. J. Saikia P. J. Baruah P. K. Synthesis. 2017;49:1401. doi: 10.1055/s-0036-15880098. [DOI] [Google Scholar]
- Martinez-Ariza G. Ayaz M. Hulme C. Tetrahedron Lett. 2013;54:6719. doi: 10.1016/j.tetlet.2013.09.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ariza G. McConnell N. Hulme C. Org. Lett. 2016;18:1864. doi: 10.1021/acs.orglett.6b00634. [DOI] [PubMed] [Google Scholar]
- Pictet A. Spengler T. Ber. Dtsch. Chem. Ges. 1911;44:2030. doi: 10.1002/cber.19110440309. [DOI] [Google Scholar]
- Patil P. Khoury K. Herdtweck E. Dömling A. Bioorg. Med. Chem. 2015;23:2699. doi: 10.1016/j.bmc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L. Jin W. Hu W. ACS Catal. 2016;6:6146. doi: 10.1021/acscatal.6b01946. [DOI] [Google Scholar]
- Cai Q. Li D. K. Zhou R. R. Shu W. M. Wu Y. D. Wu A. X. Org. Lett. 2016;18:1342. doi: 10.1021/acs.orglett.6b00281. [DOI] [PubMed] [Google Scholar]
- Damavandi S. Eur. J. Chem. 2012;9:1490. [Google Scholar]
- Damavandi S. Sandaroos R. Res. Chem. Intermed. 2013;39:1251. doi: 10.1007/s11164-012-0681-z. [DOI] [Google Scholar]
- Ghashang M. Mansoor S. S. Aswin K. Res. Chem. Intermed. 2014;41:6665. doi: 10.1007/s11164-014-1768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Calleja J. González-Pérez A. B. Álvarez R. De Lera A. R. Fañanás F. J. Rodríguez F. Chem.–Eur. J. 2015;21:16769. doi: 10.1002/chem.201503044. [DOI] [PubMed] [Google Scholar]
- Arigela R. K. Kumar R. Samala S. Gupta S. Kundu B. Eur. J. Org. Chem. 2014;2014:6057. doi: 10.1002/ejoc.201402633. [DOI] [Google Scholar]
- Wang X. Wang S.-Y. Ji S.-J. Org. Lett. 2013;15:1954. doi: 10.1021/ol400606c. [DOI] [PubMed] [Google Scholar]
- La Spisa F. Meneghetti F. Pozzi B. Tron G. C. Synthesis. 2015;47:489. [Google Scholar]
- Silvani A. Lesma G. Crippa S. Vece V. Tetrahedron. 2014;70:3994. doi: 10.1016/j.tet.2014.04.081. [DOI] [Google Scholar]
- Pirovano V. Decataldo L. Rossi E. Vicente R. Chem. Commun. 2013;49:3594. doi: 10.1039/C3CC41514G. [DOI] [PubMed] [Google Scholar]
- Grieco P. A. Parker D. T. Cornwell M. Ruckle R. J. Am. Chem. Soc. 1987;109:5859. doi: 10.1021/ja00253a053. [DOI] [Google Scholar]
- Burkhard J. A. Wuitschik G. Rogers-Evans M. Müller K. Carreira E. M. Angew. Chem. 2010;122:9236. doi: 10.1002/ange.200907155. [DOI] [PubMed] [Google Scholar]
- Chen Z. Wang B. Wang Z. Zhu G. Sun J. Angew. Chem., Int. Ed. 2013;52:2027. doi: 10.1002/anie.201206481. [DOI] [PubMed] [Google Scholar]
- Samala S. Saifuddin M. Mandadapu A. K. Kundu B. Eur. J. Org. Chem. 2013:3797. doi: 10.1002/ejoc.201300100. [DOI] [PubMed] [Google Scholar]
- Povarov L. S. Mikhailov B. M. Izv. Akad. Nauk SSSR, Ser. Khim. 1963:953. [Google Scholar]
- Povarov L. S. Grigos V. I. Mikhailov B. M. Izv. Akad. Nauk SSSR, Ser. Khim. 1963:2039. [Google Scholar]
- Wang X. S. Yin M. Y. Wang W. Tu S. J. Eur. J. Org. Chem. 2012:4811. doi: 10.1002/ejoc.201200551. [DOI] [Google Scholar]
- Han X. Li H. Hughes R. P. Wu J. Angew. Chem., Int. Ed. 2012;51:10390. doi: 10.1002/anie.201205238. [DOI] [PubMed] [Google Scholar]
- Damavandi S. Sandaroos R. J. Chem. Sci. 2013;125:95. doi: 10.1007/s12039-013-0369-y. [DOI] [Google Scholar]
- Khan I. Khan S. Tyagi V. Chouhan P. S. Chauhan P. M. S. RSC Adv. 2015;5:102713. doi: 10.1039/C5RA17259D. [DOI] [Google Scholar]
- Wang X. Wang S. Y. Ji S. J. Org. Lett. 2013;15:1954. doi: 10.1021/ol400606c. [DOI] [PubMed] [Google Scholar]
- Santhini P. V. Babu S. A. Krishnan A. Suresh R. E. John J. Org. Lett. 2017;19:2458. doi: 10.1021/acs.orglett.7b01147. [DOI] [PubMed] [Google Scholar]
- Santhini P. Chand S. S. John J. Varma R. L. Jaroschik F. Radhakrishnan K. Synlett. 2017;28:951. doi: 10.1055/s-0036-1588696. [DOI] [Google Scholar]
- Alpers D. Hoffmann F. Brasholz M. Synlett. 2017;28:919. doi: 10.1055/s-0036-1588957. [DOI] [Google Scholar]
- Mardjan M. I. D. Perie S. Parrain J. L. Commeiras L. Org. Biomol. Chem. 2017;15:3304. doi: 10.1039/c7ob00532f. [DOI] [PubMed] [Google Scholar]
- Galván A. González-Pérez A. B. Álvarez R. De Lera A. R. Fañanás F. J. Rodríguez F. Angew. Chem., Int. Ed. 2016;55:3428. doi: 10.1002/anie.201511231. [DOI] [PubMed] [Google Scholar]
- Zhang L. Zhao F. Zheng M. Zhai Y. Wang J. Liu H. Eur. J. Org. Chem. 2013:5710. doi: 10.1002/ejoc.201300667. [DOI] [Google Scholar]
- Purohit P. Pandey A. K. Kumar B. Chauhan P. M. S. RSC Adv. 2016;6:21165. doi: 10.1039/C5RA27090A. [DOI] [Google Scholar]
- Pandey S. Khan S. Singh A. Gauniyal H. M. Kumar B. Chauhan P. M. S. J. Org. Chem. 2012;77:10211. doi: 10.1021/jo3018704. [DOI] [PubMed] [Google Scholar]
- Pandey S. Chauhan S. S. Shivahare R. Sharma A. Jaiswal S. Gupta S. Lal J. Chauhan P. M. S. Eur. J. Med. Chem. 2016;110:237. doi: 10.1016/j.ejmech.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Fu L. P. Shi Q. Q. Shi Y. Jiang B. Tu S. J. ACS Comb. Sci. 2013;15:135. doi: 10.1021/co3001428. [DOI] [PubMed] [Google Scholar]