Abstract
GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 are members of a new family of sordarin derivatives called azasordarins. The in vitro activities of these compounds were evaluated against clinical isolates of yeasts, including Candida albicans, Candida non-albicans, and Cryptococcus neoformans strains. Activities against Pneumocystis carinii, Aspergillus spp., less common molds, and dermatophytes were also investigated. Azasordarin derivatives displayed significant activities against the most clinically important Candida species, with the exception of C. krusei. Against C. albicans, including fluconazole-resistant strains, MICs at which 90% of the isolates tested are inhibited (MIC90s) were 0.002 μg/ml with GW 479821, 0.015 μg/ml with GW 515716 and GW 587270, and 0.06 μg/ml with GW 471552, GW 471558, and GW 570009. The MIC90s of GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 were 0.12, 0.12, 0.03, 0.06, 0.12, and 0.06 μg/ml, respectively, against C. tropicalis and 4, 0.25, 0.06, 0.25, 0.5, and 0.5 μg/ml, respectively, against C. glabrata. In addition, some azasordarin derivatives (GW 479821, GW 515716, GW 570009, and GW 58720) were active against C. parapsilosis, with MIC90s of 2, 4, 4, and 1 μg/ml, respectively. The compounds were extremely potent against P. carinii, showing 50% inhibitory concentrations of ≤0.001 μg/ml. However Cryptococcus neoformans was resistant to all compounds tested (MIC > 16 μg/ml). These azasordarin derivatives also showed significant activity against emerging fungal pathogens, which affect immunocompromised patients, such as Rhizopus arrhizus, Blastoschizomyces capitatus, and Geotrichum clavatum. Against these organisms, the MICs of GW 587270 ranged from 0.12 to 1 μg/ml, those of GW 479821 and GW 515716 ranged from 0.12 to 2 μg/ml, and those of GW 570009 ranged from 0.12 to 4 μg/ml. Against Fusarium oxysporum, Scedosporium apiospermum, Absidia corymbifera, Cunninghamella bertholletiae, and dermatophytes, GW 587270 was the most active compound, with MICs ranging from 4 to 16 μg/ml. Against Aspergillus spp., the MICs of the compounds tested were higher than 16 μg/ml. The in vitro selectivity of azasordarins was investigated by cytotoxicity studies performed with five cell lines and primary hepatocytes. Concentrations of compound required to achieve 50% inhibition of the parameter considered (Tox50s) of GW 570009, GW 587270, GW 479281, and GW 515716 in the cell lines ranged from 60 to 96, 49 to 62, 24 to 36, and 16 to 38 μg/ml, respectively. The cytotoxicity values of GW 471552 and GW 471558 were >100 μg/ml for all cell lines tested. Tox50s on hepatocytes were in the following order: GW 471558 > GW 471552 > GW 570009 > GW 587270 > GW 515716 > GW 479821, with values ranging from higher than 100 μg/ml to 23 μg/ml. The cytotoxicity results obtained with fully metabolizing rat hepatocytes were in total agreement with those obtained with cell lines. In summary, the in vitro activities against important pathogenic fungi and the selectivity demonstrated in mammalian cell lines justify additional studies to determine the clinical usefulness of azasordarins.
The risk of opportunistic fungal infections in immunocompromised patients is markedly high; however, in many cases, the treatment of fungal diseases with current therapies is of limited efficacy. The discovery of new antifungal agents thus remains an important challenge for the scientific community.
Sordarins are a novel class of antifungal agents different from other antifungals such as polyenes, azole derivatives, or allylamines in that they possess a new mechanism of action. Sordarins selectively interfere with the elongation step of protein synthesis (9), the primary sordarin-binding protein being the elongation factor EF-2 (5, 7, 8). In recent years, several sordarin derivatives with a broad spectrum of activity and marked potencies in vivo, such as GM 193633, GM 211676, GM 222712, and GM 237354, have been synthesized (11). These derivatives have remarkable in vitro activity against key fungal pathogens, such as Candida species (including strains with decreased susceptibility to fluconazole), Cryptococcus neoformans, and Pneumocystis carinii (2, 14), as well as potent fungicidal activity against important dimorphic endemic pathogens, such as Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitidis, and Coccidioides immitis (D. A. Stevens, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr F-58, 1997). In vivo, the administration of sordarins, either orally or subcutaneously, against Candida albicans, P. carinii, H. capsulatum, and C. immitis yields encouraging results (1, 6, 13, 16, 17). Recently, efforts have been directed toward the synthesis and development of new sordarin antifungal agents with improved activity against pathogenic fungi, especially against Candida non-albicans strains, and with improved pharmacological properties, including higher efficacy and less toxicity. The new sordarin derivatives, known as azasordarins, are structurally characterized by the presence of a 6-methylmorpholin-2-yl group with different N-4′ substituents at position 8a of the sordaricin indacene ring system instead of the 4′ sugar moiety present in sordarin. These molecules have the additional advantage of an easier chemical synthesis.
The present study investigates the in vitro activity profiles of six azasordarins (GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270) against Candida species, C. neoformans, P. carinii, Aspergillus, and other filamentous fungi. One of the major challenges in finding a potent yet safe antifungal agent is the great similarity between fungal and mammalian cells. Like mammalian cells, fungi are eukaryotic and thus share many structures and metabolic pathways with them, making it more difficult to find differential toxicity targets. Taking into account that ribosomal protein synthesis is one of the best-preserved processes in eukaryotic cells, the cytotoxicity of such compounds on immortalized cell lines derived from target organs and on primary cultures of hepatocytes isolated from rat livers has also been investigated to determine the in vitro selectivity of these novel compounds.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000 [E. Herreros, A. Martinez, M. J. Almela, E. Jimenez, S. Lozano, M. J. Perez, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1691, 2000; E. Herreros, M. J. Almela, S. Lozano, C. M. Martinez, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 201, 2000].)
MATERIALS AND METHODS
Antifungal agents.
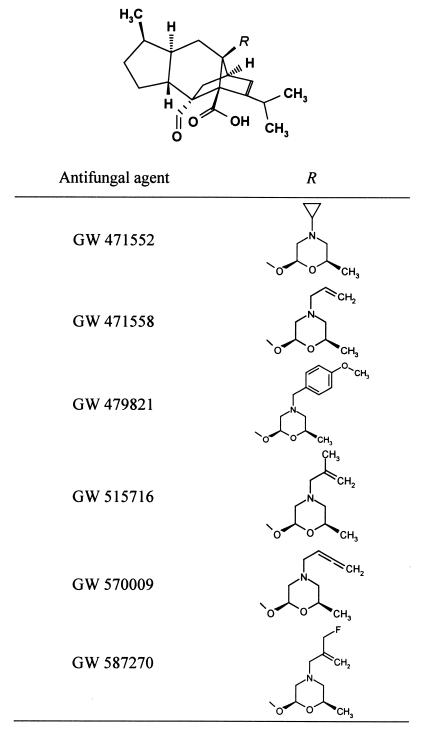
GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 were synthesized at Glaxo Wellcome S.A. (Tres Cantos, Madrid, Spain) by the Medicinal Chemistry group. The molecular structures of these molecules are shown in Fig. 1. Fluconazole was from Pfizer S.A. (Madrid, Spain). Amphotericin B, pentamidine isothionate, and trimethoprim-sulfamethoxazole (TMP-SMX) were provided by Sigma-Aldrich S.A. (Madrid, Spain). Azasordarin derivatives as sodium salts and fluconazole were solubilized in sterile distilled water at a starting concentration of 5 mg/ml. Amphotericin B, pentamidine, and TMP-SMX were dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich S.A.). TMP and SMX solutions were mixed appropriately to obtain a final 1:5 combination. Finally, the drug stock solutions were diluted in medium to produce the required drug concentration. All solutions were prepared immediately before use. Antimicrobial activities are expressed as micrograms of base per milliliter.
FIG. 1.
Chemical structure of new azasordarin derivatives.
Organisms.
The 137 clinical isolates used for susceptibility testing were obtained from unselected individual patients from several separate medical centers in Europe. A group of 112 clinical yeast isolates recovered from oral cavities, urine samples, blood, or other sterile body fluids were tested under a single set of standardized conditions. The distribution of species to study the susceptibilities of groups of clinical isolates to GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 included 32 isolates of C. albicans (including 16 strains with decreased susceptibility to fluconazole) and 16 isolates each of C. tropicalis, C. glabrata, C. parapsilosis, C. krusei, and C. neoformans. In addition, eight Candida strains (Table 1) and a total of 18 emerging mold pathogens and dermatophyte strains selected at random from pathogenic isolates of the respective species were tested (see Tables 4 and 5). Suitable control organisms, including C. albicans ATCC 90028, C. tropicalis ATCC 750, C. parapsilosis ATCC 90018, and C. krusei ATCC 6258, were obtained from the American Type Culture Collection (Manassas, Va.). Organisms were identified by standard microbiology methods and stored in Sabouraud dextrose broth (SAB; Difco, Detroit, Mich.) with 15% glycerol at −70°C until required. Prior to antifungal susceptibility testing, each isolate was subcultured on antimicrobial agent-free SAB agar (Difco) to ensure optimal growth characteristics and purity. P. carinii organisms were isolated from the lungs of spontaneously infected immunosuppressed Wistar rats immediately before each experiment, as previously described (14).
TABLE 1.
Antifungal activities of azasordarin derivatives against Candida strains
| Organism | MIC (μg/ml) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GW 471552 | GW 471558 | GW 479821 | GW 515716 | GW 570009 | GW 587270 | Amphotericin B | Fluconazole | |
| C. albicans 4711E | 0.002 | 0.004 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | 0.12 | 0.12 |
| C. albicans CL-236 | 0.008 | 0.008 | ≤0.001 | 0.002 | 0.004 | 0.002 | 0.12 | 0.12 |
| C. albicans CL-245 (Flur) | ≤0.001 | ≤0.001 | ≤0.001 | 0.002 | ≤0.001 | ≤0.001 | 0.25 | >16 |
| C. glabrata 2375E | 4 | 0.5 | 0.06 | 0.25 | 0.12 | 0.25 | 0.12 | 4 |
| C. glabrata 522 | 4 | 0.5 | 0.06 | 0.25 | 0.12 | 0.25 | 0.25 | 4 |
| C. tropicalis 2808E | 0.06 | 0.03 | ≤0.001 | 0.008 | 0.015 | 0.002 | 0.12 | 2 |
| C. parapsilosis 2372E | >16 | >16 | 0.5 | 2 | 4 | 0.5 | 0.12 | 0.5 |
| C. krusei 2374E | >16 | >16 | >16 | >16 | >16 | >16 | 0.12 | >16 |
TABLE 4.
Antifungal activities of azasordarin derivatives against emerging and less common mold pathogens
| Strain | Antifungal agent | MIC (μg/ml) |
|---|---|---|
| Blastoschizomyces capitatus CL839 | GW 479821 | 0.12 |
| GW 515716 | 0.12 | |
| GW 570009 | 0.12 | |
| GW 587270 | 0.12 | |
| Geotrichum clavatum CL37 | GW 479821 | 0.5 |
| GW 515716 | 0.5 | |
| GW 570009 | 0.5 | |
| GW 587270 | 0.12 | |
| Fusarium oxysporum 396 | GW 479821 | >16 |
| GW 515716 | >16 | |
| GW 570009 | >16 | |
| GW 587270 | 8 | |
| Scedosporium apiospermum CM 507 | GW 479821 | >16 |
| GW 515716 | >16 | |
| GW 570009 | >16 | |
| GW 587270 | 8 | |
| Absidia corymbifera CM 30 | GW 479821 | >16 |
| GW 515716 | >16 | |
| GW 570009 | 16 | |
| GW 587270 | 4 | |
| Cunninghamella bertholletiae CM 197 | GW 479821 | >16 |
| GW 515716 | 16 | |
| GW 570009 | 16 | |
| GW 587270 | 4 | |
| Rhizopus arrhizus CM 2339 | GW 479821 | 2 |
| GW 515716 | 2 | |
| GW 570009 | 4 | |
| GW 587270 | 1 |
TABLE 5.
Antifungal activities of azasordarin derivatives against dermatophytes
| Strain | Antifungal agent | MIC (μg/ml) |
|---|---|---|
| Epidermophyton floccosum CM 144 | GW 479821 | >16 |
| GW 515716 | >16 | |
| GW 570009 | >16 | |
| GW 587270 | 16 | |
| Trichophyton rubrum CM 1447 | GW 479821 | >16 |
| GW 515716 | 8 | |
| GW 570009 | 16 | |
| GW 587270 | 8 | |
| Trichophyton mentagrophytes CM 84 | GW 479821 | >16 |
| GW 515716 | 4 | |
| GW 570009 | 4 | |
| GW 587270 | 4 | |
| Microsporum canis CM 85 | GW 479821 | >16 |
| GW 515716 | 4 | |
| GW 570009 | 8 | |
| GW 587270 | 4 | |
| Microsporum gypseum C2543 | GW 479821 | >16 |
| GW 515716 | 8 | |
| GW 570009 | 16 | |
| GW 587270 | 8 |
Media and buffers.
RPMI–2% glucose was used with all organisms, with the exception of C. neoformans and P. carinii. The basal medium, RPMI 1640 (GIBCO BRL, Life Technologies, Paisley, United Kingdom) with l-glutamine (Merck, Darmstadt, Germany) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma-Aldrich S.A.), was supplemented with 18 g of glucose (Sigma-Aldrich S.A.) per liter. For C. neoformans, RPMI–2% glucose was substituted for yeast nitrogen base medium (Difco) with 2% glucose. P. carinii was extracted and purified in Dulbecco's modified Eagle's medium (DMEM; BioWhittaker, Boehringer Ingelheim, Brussels, Belgium) with l-glutamine, supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml). In vitro activity against P. carinii was assayed in modified Eagle's medium (MEM) without l-methionine (GIBCO BRL, Life Technologies) and supplemented with 10% fetal calf serum (FCS; GIBCO BRL, Life Technologies) and the same antibiotics used in DMEM.
Antifungal susceptibility studies.
For yeasts, MICs were determined by the broth microdilution technique according to National Committee for Clinical Laboratory Standards (NCCLS) reference document M27-A (18), with minor modifications. A Microlab AT Plus robot (Hamilton Bonaduz AG, Bonaduz, Switzerland) was used to prepare microdilution panels containing twofold dilutions of the drugs in 0.1 ml of medium, ranging from 0.001 to 16 μg/ml. Starting inocula were adjusted by the spectrophotometric method to 106 CFU/ml. Then the adjusted yeast suspensions were diluted 1:10 with medium, and microtiter plates were inoculated with 10 μl of this dilution (final inoculum, 104 yeast cells per ml). The inoculated plates were incubated overnight at 35°C without agitation (Candida spp.) or for 48 h (C. neoformans) in a humid atmosphere. Following incubation and after agitation with a microtiter plate shaker for 5 min, plates were read visually with the aid of a reading mirror and spectrophotometrically with an automatic plate reader (IEMS, Labsystems, Helsinki, Finland) set at 450 nm. For all compounds, with the exception of fluconazole, MICs were defined as the lowest concentration of antifungal agent that prevents any visible growth or that inhibited growth by 95% compared with drug-free control wells. For fluconazole, MICs were defined as the lowest concentration of drug that inhibits growth by 80%. MICs determined either visually or by spectrophotometric evaluation showed excellent agreement.
For filamentous fungi, susceptibility testing was performed according to NCCLS reference document M38-P (19) in RPMI–2% glucose medium. To induce formation of conidia, filamentous fungi and dermatophytes were grown on SAB agar slants at 27°C until they were judged to have formed maximal numbers of conidia. Then fungal cultures were covered with 1 ml of sterile saline containing 0.1% Tween 80, and spores were washed off by gently probing the colonies with the tip of a pipette. Finally, the suspension was vortexed for 10 s to break up clumps of cells and filtered through a four-fold layer of sterile gauze. The conidia were counted with a hemocytometer, adjusted to 106 conidia/ml, and stored at −70°C in small lots until required. MICs were determined by performing microdilution tests as described above for yeasts, but with double dilutions of drugs from 0.03 to 16 μg/ml. Stock suspensions of conidia were diluted with medium to obtain the final desired inoculum size of approximately 104 conidia/ml. Inoculum quantitation was performed by plating dilutions of the conidia on SAB agar to determine the viable number of CFU per milliliter. Plates were incubated at 35°C and read with a microplate mirror as soon as growth became visible in control wells. MICs were defined as the lowest concentration of antifungal agent that inhibited development of visible growth.
Activity against P. carinii.
Activity of azasordarins against P. carinii was assayed by determining the inhibition of uptake and incorporation of [35S]methionine based on a previously described procedure (4, 15). Briefly, microtiter plates with 200 μl of methionine-free MEM supplemented with 10% FCS plus the corresponding dilution of drug were inoculated with P. carinii to yield a final concentration of 5 × 106 organisms per ml. After 24 h of incubation, organisms were pulsed with 5 μCi of [35S]methionine per ml and then incubated again at 37°C in a humidified atmosphere with 5% CO2 for 24 h. Following incubation, parasites were harvested on glass fiber filters with a cell harvester (Tomtec, Wallac, Finland). Filters were finally counted in a microplate scintillation counter (1450 Microbeta liquid scintillation counter; Wallac, Finland). Studies were performed in triplicate, and positive (parasites in drug-free medium) and negative (boiled P. carinii inoculum) control wells were included. Results were expressed as 50% inhibitory concentrations (IC50s), defined as the compound concentration at which incorporation of [35S]methionine was decreased by 50% in comparison with that in positive control wells.
Cytotoxicity studies.
The cell lines used in this study, C6, HeLa, MDCK, MRC-5, and MH1C1, were obtained from the American Type Culture Collection. The organisms and tissues from which each cell line was derived are listed in Table 6.
TABLE 6.
Cytotoxicity of azasordarin derivatives and selected marketed compounds on target organ-derived cell lines
| Compound | Cell line | Tox50 (μg/ml)a |
|---|---|---|
| GW 471552 | C6 (brain, rat) | >100 |
| HeLa (cervix, human) | >100 | |
| MDCK (kidney, dog) | >100 | |
| MRC-5 (lung, human) | >100 | |
| MH1C1 (liver, rat) | >100 | |
| GW 471558 | C6 (brain, rat) | >100 |
| HeLa (cervix, human) | >100 | |
| MDCK (kidney, dog) | >100 | |
| MRC-5 (lung, human) | >100 | |
| MH1C1 (liver, rat) | >100 | |
| GW 479821 | C6 (brain, rat) | 26 |
| HeLa (cervix, human) | 32 | |
| MDCK (kidney, dog) | 36 | |
| MRC-5 (lung, human) | 24 | |
| MH1C1 (liver, rat) | 25 | |
| GW 515716 | C6 (brain, rat) | 38 |
| HeLa (cervix, human) | 16 | |
| MDCK (kidney, dog) | 36 | |
| MRC-5 (lung, human) | 27 | |
| MH1C1 (liver, rat) | 35 | |
| GW 570009 | C6 (brain, rat) | 96 |
| HeLa (cervix, human) | 60 | |
| MDCK (kidney, dog) | 88 | |
| MRC-5 (lung, human) | 61 | |
| MH1C1 (liver, rat) | 78 | |
| GW 587270 | C6 (brain, rat) | 54 |
| HeLa (cervix, human) | 62 | |
| MDCK (kidney, dog) | 52 | |
| MRC-5 (lung, human) | 49 | |
| MH1C1 (liver, rat) | 50 | |
| Fluconazole | C6 (brain, rat) | >100 |
| HeLa (cervix, human) | >100 | |
| MDCK (kidney, dog) | >100 | |
| MRC-5 (lung, human) | >100 | |
| MH1C1 (liver, rat) | >100 | |
| Amphotericin B | C6 (brain, rat) | 13 |
| HeLa (cervix, human) | 19 | |
| MDCK (kidney, dog) | 29 | |
| MRC-5 (lung, human) | 14 | |
| MH1C1 (liver, rat) | <8 |
Concentration of compound that inhibits incorporation of [35S]methionine by 50%. Results are the means of three independent experiments performed in triplicate.
(i) Routine culture of cell lines.
Cells were grown and maintained in DMEM (HeLa, MDCK, and MRC-5) or Ham's F-10 medium (C6 and MH1C1) (Sigma-Aldrich S.A.) supplemented with 2 mM l-glutamine, penicillin-streptomycin (50 IU/ml and 50 μg/ml, respectively) (BioWhittaker), and 10% (vol/vol) Fetal Clone II serum (Perbio Science, Erembodegem-Aalst, Belgium). Cultures were maintained at 37°C in a humidified incubator containing 5% CO2–95% air and routinely passaged upon reaching 80 to 90% confluence. For cytotoxicity experiments, cells were seeded into 96-well plates at a cell density of 8,000 cells/well. The culture medium was as described above, but with a reduced (2% vol/vol) level of Fetal Clone II serum.
(ii) Measurement of cytotoxicity.
To determine cytotoxic effects, cells were incubated with compounds for 24 h at 37°C in a humidified incubator containing 5% CO2–95% air. The inhibition of protein synthesis was then determined as the marker of cytotoxicity. Following the 24-h exposure period, 100 μl of methionine-free medium supplemented with l-glutamine and labeled with [35S]methionine at 5 μCi/ml was added to each microplate well, and again the mixture was incubated at 37°C (5% CO2–95% air) for 2 h. Then proteins were precipitated by adding ice-cold 5% (vol/vol) trichloroacetic acid to each well. Plates were washed with ethanol, dried, and counted by liquid scintillation. Experiments were performed in triplicate.
(iii) Primary rat hepatocyte culture.
Hepatocytes were isolated from male Sprague-Dawley rats (180 to 250 g) by reverse perfusion of the liver with collagenase (Boehringer, Mannheim, Germany) (12). Before the experiments were started, hepatocyte viability was assessed with the trypan blue dye exclusion test. In all cases, viability was between 85 and 90%. Isolated hepatocytes were seeded at a final density of 25 × 103 cells/well in 96-well plates, which had been previously coated with fibronectin (Sigma-Aldrich S.A.). The medium was Ham's F-12–Leibovitz L-15 supplemented with 2% newborn calf serum and 10 to 8 M insulin. After 1 h of incubation, culture medium was changed to remove unattached cells. The metabolic activity of hepatocytes was assessed by measuring the monooxygenase activities (the 7-ethoxycoumarin O-deethylase and ethoxyresorufin O-deethylase activities) and the level of UDP-glucuronyl transferase activity. Cultures of metabolically active hepatocytes were exposed to the corresponding drug at concentrations that ranged from 1 to 100 μg/ml for 24 h. Experiments were performed in triplicate. As a cytotoxicity marker, levels of intracellular lactate dehydrogenase (LDH) were determined with in situ-lysed cells by using the LDH cytotoxicity detection kit (Boehringer) following the recommendations of the manufacturer. Cell damage was expressed as the concentration of compound in micrograms per milliliter required to achieve 50% inhibition of the parameter considered (Tox50)—i.e., protein synthesis inhibition for cell lines and intracellular LDH levels for rat hepatocytes.
RESULTS
Antifungal activities against yeasts.
The MICs of GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, GW 587270, and reference compounds (fluconazole and amphotericin B) for a variety of the most important clinical Candida strains are given in Table 1. The MICs of GW 479821 against C. albicans and C. tropicalis strains were ≤0.001 μg/ml. For the rest of the azasordarin derivatives tested (GW 471552, GW 471558, GW 515716, GW 570009, and GW 587270), the ranges of activities were from ≤0.001 to 0.06 μg/ml, ≤0.001 to 0.03 μg/ml, ≤0.001 to 0.008 μg/ml, ≤0.001 to 0.015 μg/ml, and ≤0.001 to 0.002 μg/ml, respectively. The MICs of the reference compounds fluconazole and amphotericin B were higher, ranging from 0.12 to 2 μg/ml for fluconazole (without taking into account the Flur strains) and 0.12 μg/ml for amphotericin B. Against Candida glabrata strains, the MICs were 0.06 μg/ml with GW 479821, 0.12 μg/ml with GW 570009, 0.25 μg/ml with GW 515716 and GW 587270, 0.5 μg/ml with GW 471558, and 4 μg/ml with GW 471552. The activities of amphotericin B (0.12 and 0.25 μg/ml) were comparable to those of azasordarin derivatives. However, the MICs of fluconazole (4 μg/ml) were generally (with the exception of GW 471552) 8- to 64-fold higher than those of the azasordarins tested. The antifungal activity of GW 479821 and GW 587270 against C. parapsilosis was comparable to that of fluconazole (MIC of 0.5 μg/ml). Amphotericin B was the most active compound against C. parapsilosis and the only compound active against C. krusei, with MICs of 0.12 μg/ml for both species.
Activities of GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 against groups of clinical isolates representative of various species of Candida, such as C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis, are summarized in Table 2. In addition, azasordarin derivatives were tested against groups of pathogenic isolates of C. krusei and C. neoformans, exhibiting MICs at which 90% of the isolates tested are inhibited (MIC90s) of above 16 μg/ml against both species. Some azasordarins were markedly active against Candida spp., such as C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis. GW 479821, GW 515716, and GW 587270 were the most potent compounds against C. albicans, including strains with decreased susceptibility to fluconazole (MICs of ≥64 μg/ml), with MIC90s of ≤0.015 μg/ml. The MIC90 of GW 471552, GW 471558, and GW 570009 was 0.06 μg/ml.
TABLE 2.
Antifungal activities of azasordarin derivatives against groups of clinical isolates
| Organism (n = 16) | Antifungal agent | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| C. albicans | GW 471552 | 0.008–0.06 | 0.015 | 0.06 |
| GW 471558 | 0.004–0.03 | 0.008 | 0.03 | |
| GW 479821 | ≤0.001 | ≤0.001 | ≤0.001 | |
| GW 515716 | 0.002–0.008 | 0.004 | 0.008 | |
| GW 570009 | 0.008–0.03 | 0.015 | 0.03 | |
| GW 587270 | 0.002–0.015 | 0.004 | 0.008 | |
| C. albicans (Flur) | GW 471552 | 0.008–0.06 | 0.03 | 0.06 |
| GW 471558 | 0.015–0.06 | 0.03 | 0.06 | |
| GW 479821 | ≤0.001–0.002 | 0.002 | 0.002 | |
| GW 515716 | 0.004–0.015 | 0.008 | 0.015 | |
| GW 570009 | 0.015–0.06 | 0.03 | 0.06 | |
| GW 587270 | 0.002–0.015 | 0.008 | 0.015 | |
| C. tropicalis | GW 471552 | 0.03–0.12 | 0.06 | 0.12 |
| GW 471558 | 0.015–0.12 | 0.03 | 0.12 | |
| GW 479821 | 0.004–0.03 | 0.008 | 0.03 | |
| GW 515716 | 0.015–0.06 | 0.03 | 0.06 | |
| GW 570009 | 0.03–0.12 | 0.06 | 0.12 | |
| GW 587270 | 0.015–0.06 | 0.03 | 0.06 | |
| C. glabrata | GW 471552 | 1–4 | 2 | 4 |
| GW 471558 | 0.06–0.5 | 0.25 | 0.25 | |
| GW 479821 | 0.03–0.06 | 0.03 | 0.06 | |
| GW 515716 | 0.03–0.25 | 0.12 | 0.25 | |
| GW 570009 | 0.12–0.5 | 0.25 | 0.5 | |
| GW 587270 | 0.06–0.5 | 0.25 | 0.5 | |
| C. parapsilosis | GW 471552 | >16 | >16 | >16 |
| GW 471558 | >16 | >16 | >16 | |
| GW 479821 | 0.5–2 | 2 | 2 | |
| GW 515716 | 2–4 | 2 | 4 | |
| GW 570009 | 0.5–4 | 2 | 4 | |
| GW 587270 | 0.25–1 | 0.5 | 0.5 | |
50% and 90%, MIC50 and MIC90, respectively.
Against C. tropicalis isolates, the less active compounds GW 471552, GW 471558, and GW 570009 showed excellent activities, with a MIC90 of 0.12 μg/ml. GW 515716 and GW 587270 were twofold more potent than GW 471552, GW 471558, and GW 570009. GW 479821 was the most potent azasordarin derivative against C. tropicalis, with a MIC90 of 0.03 μg/ml.
GW 479821 was the most active compound against C. glabrata, with a MIC90 of 0.06 μg/ml. The susceptibilities of C. glabrata isolates to the rest of the azasordarins tested were 0.25 μg/ml for GW 471558 and GW 515716, 0.5 μg/ml for GW 570009 and GW 587270, and 4 μg/ml for GW 471552.
Four of the six azasordarins tested showed significant activities against C. parapsilosis. The MIC90s of GW 479821, GW 515716, GW 570009, and GW 587270 ranged from 1 to 4 μg/ml. GW 587270 was the most potent compound, with a MIC90 of 1 μg/ml.
Against NCCLS quality control isolates, the MICs of GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 were 0.015, 0.015, ≤0.001, 0.002, 0.015, and 0.002 μg/ml, respectively, for C. albicans ATCC 90028; 0.06, 0.03, 0.004, 0.015, 0.03, and 0.015 μg/ml, respectively, for C. tropicalis ATCC 750; and >16, >16, 1, 2, 2, and 0.5 μg/ml, respectively, for C. parapsilosis ATCC 90018. The activities of all six compounds tested were higher than 16 μg/ml against C. krusei ATCC 6258.
Antifungal activity against P. carinii.
The antifungal activity against P. carinii was determined by measuring the incorporation of radiolabeled methionine into newly synthesized proteins. Two azasordarins, GW 471552 and GW 471558, were tested against P. carinii in comparison with pentamidine and TMP-SMX. Azasordarin derivatives exhibited high in vitro potency against this organism (Table 3). IC50s were 0.001 μg/ml for GW 471552 and <0.001 μg/ml for GW 471558. At 0.001 μg/ml, GW 471552 and GW 471558 inhibited the incorporation of [35S]methionine by 50.2 and 64.7%, respectively. Pentamidine and TMP-SMX (the marketed compounds used as a control) demonstrated lower activities, with IC50s of 0.1 and >10:50 μg/ml, respectively.
TABLE 3.
In vitro activity of azasordarin derivatives against P. cariniia
| Antifungal agent | IC50b (μg/ml) | % Inhibition at 0.001 μg/ml |
|---|---|---|
| GW 471552 | 0.001 | 50.2 |
| GW 471558 | <0.001 | 64.7 |
| Pentamidine | 0.1 | NTc |
| TMP-SMX | >10:50 | NT |
Results are the means of triplicate values.
Concentration of compound that inhibits incorporation of [35S]methionine by 50%.
NT, not tested.
Antifungal activities against emerging fungal pathogens and dermatophytes.
The compounds with a broader spectrum of action and active against C. parapsilosis (GW 479821, GW 515716, GW 570009, and GW 587270) were evaluated against a variety of organisms, such as Aspergillus fumigatus, Aspergillus flavus, a broad range of emerging mold pathogens, and dermatophytes selected at random from pathogenic isolates of the respective species. Against strains of A. fumigatus, A. flavus, Trichosporon beigelii, Alternaria alternata, and Curvularia lunata, MICs of the azasordarins tested were all >16 μg/ml. MICs of GW 479821, GW 515716, GW 570009, and GW 587270 against other emerging mold pathogens and dermatophytes are shown in Tables 4 and 5, respectively. Against the strains of Blastoschizomyces capitatus, Geotrichum clavatum, and Rhizopus arrhizus tested, the MICs of GW 587270 ranged from 0.12 to 1 μg/ml, while with GW 479821 and GW 515716, the activities ranged from 0.12 to 2 μg/ml, and with GW 570009, the activities ranged from 0.12 to 4 μg/ml. Against Fusarium oxysporum, Scedosporium apiospermum, Absidia corymbifera, and Cunninghamella bertholletiae, GW 587270 was the most active compound, with MICs ranging from 4 to 8 μg/ml. The MICs of GW 479821, GW 515716, and GW 570009 were ≥16 μg/ml.
GW 587270 proved to be the most potent of the four compounds against the emerging pathogens tested (Table 4). The MIC of GW 587270 was 0.12 μg/ml for B. capitatus and G. clavatum. Among strains of hyaline hyphomycetes such as S. apiospermum and F. oxysporum, the MIC was 8 μg/ml. Against zygomycetes, the MICs of GW 587270 were 4 μg/ml for A. corymbifera and C. bertholletiae and 1 μg/ml for R. arrhizus.
Table 5 summarizes the susceptibilities of a set of dermatophytes to GW 479821, GW 515716, GW 570009, and GW 587270. Against dermatophytes such as Trichophyton rubrum, Trichopyton mentagrophytes, Microsporum canis, and Microsporum gypseum, the MICs of GW 515716 and GW 587270 ranged from 4 to 8 μg/ml. The MICs of GW 570009 were generally twofold higher, ranging from 4 to 16 μg/ml. Against Epidermophyton floccosum, the MIC of GW 587270 was 16 μg/ml.
Cytotoxicity studies.
The selectivity of azasordarins was investigated by using mammalian cell lines derived from target organs (liver, kidney, lung, and brain) and fully competent hepatocytes isolated from rats. The HeLa cell line was included as a cell line commonly used in our screenings. The ability of azasordarins to inhibit mammalian protein synthesis was determined by the incorporation of [35S]methionine into newly synthesized proteins. The results are presented in Table 6. In vitro protein synthesis was not affected by GW 471552 and GW 471558 over the range of concentrations tested (up to 100 μg/ml), thus clearly reflecting the selective behavior of these compounds. Similar results were obtained with fluconazole used as a safe control compound. The Tox50s of GW 570009 and GW 587270 ranged from 60 to 96 and 49 to 62 μg/ml, respectively. GW 479821 and GW 515716 were less safe derivatives, displaying cytotoxicity ranges from 24 to 36 and 16 to 38 μg/ml, respectively. The Tox50 of amphotericin B (used as less safe control compound) ranged from <8 to 29 μg/ml. Amphotericin B was in general twofold more cytotoxic than the less safe azasordarin derivatives GW 479821 and GW 515716. In general, the data obtained with cell lines of different origins and species were quite similar; no significant differences between them were observed.
GW 471558 (one of the safest compounds) was evaluated after 24, 48, and 72 h of incubation to investigate potential delayed toxicity. The compound was tested in the most sensitive cell line (MRC-5). No significant increase in cytotoxicity was observed after 48 or 72 h of incubation. Tox50s were above 100 μg/ml over the time tested.
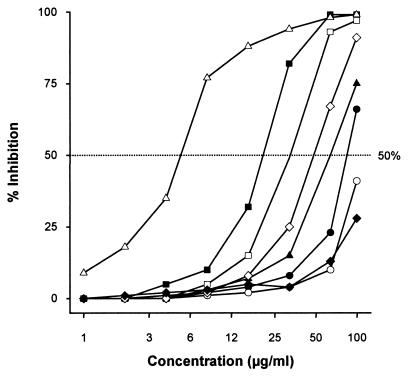
The decrease in intracellular LDH was taken as the end point parameter for evaluating the cytotoxic effect upon freshly isolated hepatocytes. Azasordarins demonstrated concentration-dependent cytotoxic effects on rat hepatocytes (Fig. 2). After 24 h of incubation, Tox50s were in the following order: GW 471558 > GW 471552 > GW 570009 > GW 587270 > GW 515716 > GW 479821. Values ranged from 23 μg/ml for GW 479821 to higher than 100 μg/ml for GW 471558 and fluconazole (Table 7). The cytotoxicity data obtained with metabolically active rat hepatocytes were in total agreement with those obtained from the cell lines. In general, amphotericin B displayed higher levels of in vitro toxicity (Tox50s from <8 to 29 μg/ml in cell lines and 5 μg/ml in hepatocytes) than all of the azasordarin derivatives tested.
FIG. 2.
Cytotoxic effects of azasordarin derivatives and selected marketed compounds on rat hepatocytes. Solid circles, GW 471552; open circles, GW 471558; solid squares, GW 479821; open squares, GW 515716; solid triangles, GW 570009; open diamonds, GW 587270; open triangles, amphotericin B; solid diamonds, fluconazole. Results are the means of three independent experiments performed in triplicate.
TABLE 7.
Cytotoxicity of azasordarin derivatives and selected marketed compounds on primary hepatocytes
| Compound | Tox50 (μg/ml)a |
|---|---|
| GW 471552 | 87 |
| GW 471558 | >100 |
| GW 479821 | 23 |
| GW 515716 | 30 |
| GW 570009 | 64 |
| GW 587270 | 52 |
| Fluconazole | >100 |
| Amphotericin B | 5 |
Concentration of compound that inhibits intracellular LDH by 50%. Results are the means of three independent experiments performed in triplicate.
DISCUSSION
Sordarin derivatives belong to a new class of antifungal agents characterized by a novel mechanism of action involving the inhibition of protein synthesis. A drug discovery program has yielded a number of compounds belonging to structurally distinct families. One of these families, the azasordarins, is chemically characterized by the presence of a 6-methylmorpholin-2-yl group with different N-4′ substituents instead of a sugar moiety. These compounds have the advantage of easier chemical synthesis from the fermentation-derived starting material. To define the spectrum of action of these new antifungals, the in vitro activities of GW 471552, GW 471558, GW 479821, GW 515716, GW 570009, and GW 587270 were evaluated against a wide range of pathogenic yeasts and filamentous fungi, including P. carinii. The nature of the R group (Fig. 1) had a marked effect upon the in vitro potency and spectrum of activity of these new sordarin agents.
Despite their structural differences, all of the novel sordarin derivatives tested exhibited remarkable in vitro activity against the key pathogen C. albicans, including azole-resistant isolates (Table 2). In terms of potency, GW 479821, GW 515716, and GW 587270 displayed the highest activities against C. albicans, with a MIC90 of ≤0.015 μg/ml. GW 471552, GW 471558, and GW 570009 were, in turn, slightly less potent, with a MIC90 of 0.06 μg/ml. A clear correlation between in vitro susceptibility and therapeutic efficacy against C. albicans has been demonstrated in animal models, since azasordarins were effective against oral and vaginal candidiasis in immunosuppressed rats (A. Martinez, S. Ferrer, E. Jimenez, J. Sparrowe, J. Regadera, F. Gomez de las Heras, and D. Gargallo-Viola, submitted for publication).
As has been described for previously published sordarins (14), C. krusei was intrinsically resistant to these new derivatives. Nevertheless, some azasordarins were active against Candida non-albicans species, such as C. tropicalis, C. glabrata, and C. parapsilosis, which are emerging as serious opportunistic fungal pathogens among immunocompromised patients in the clinical setting (20). GW 479821, GW 515716, and GW 587270 were the most active compounds against C. tropicalis. Against C. glabrata, the MIC90s of these compounds were 0.06, 0.25, and 0.5 μg/ml, respectively. Moreover, some azasordarins were active against C. parapsilosis, such as GW 479821, GW 515716, GW 570009, and GW 587270. GW 587270 was the most potent compound, with a MIC90 of 0.5 μg/ml.
As has been described for sordarins (2, 14), the azasordarins displayed excellent activities against P. carinii (Table 3). This organism remains an important pathogen in immunocompromised individuals (10). Although TMP-SMX and pentamidine have been used for prophylaxis and treatment of P. carinii pneumonia, the high frequency of adverse reactions to these drugs and a lack of efficacy in some patients have emphasized the need for new drugs. Azasordarins inhibit P. carinii protein synthesis, showing IC50s of ≤0.001 μg/ml. Pentamidine and TMP-SMX were comparatively much less active. The high in vitro activities of azasordarins were reflected by their in vivo efficacies in treating P. carinii pneumonia in rats, as has been recently demonstrated (A. Martinez, E. Jimenez, E. M. Aliouat, J. Caballero, E. Dei-Cas, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr 1096, 2000).
Azasordarins were inactive against Aspergillus spp. (MICs of >16 μg/ml). However, GW 479821, GW 515716, GW 570009, and GW 587270 displayed potent activities against R. arrhizus (one of the most important emerging pathogens resistant to current antifungal therapy) and against yeast-like fungi such as B. capitatus and G. clavatum. GW 587270 exhibited good to moderate activity against Fusarium oxysporum, Scedosporium apiospermum, Absidia corymbifera, Cunninghamella bertholletiae, and dermatophytes (Tables 4 and 5). Studies with larger panels of strains are required to confirm the potency of azasordarins against filamentous fungi.
Although protein synthesis is an attractive antifungal target, the lack of selective inhibitors may be due to the great similarity between fungal and mammalian systems. Despite this similarity, however, sordarins have proved to be potent inhibitors of translation in fungi, with a high level of selectivity. We demonstrated the selectivity of the compound GM 237354 in inhibiting protein synthesis by C. albicans versus several cell lines. The selectivity ratio on mammalian versus yeast cells was >10,000 (E. Herreros, A. Martinez, M. J. Almela, S. Lozano, C. M. Martinez, E. Jimenez, F. Gomez de las Heras, and D. Gargallo-Viola, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-59, 1997). In the present study, we examined the cytotoxicity of novel azasordarins. We have used human (MRC-5 and HeLa) and animal cells (MRC-5, C6, and MH1C1) to establish correlations with in vivo animal models. We found no significant differences in azasordarin Tox50s when using cells derived from various tissues (liver, brain, lung, or kidney) or species (rat, dog, and human). Likewise, no enhanced damage was seen when the compounds were tested on metabolizing cells (cultures of primary hepatocytes), which could suggest the production of toxic metabolites (3, 21).
In terms of cytotoxic effect, GW 471552 and GW 471558 were the safest azasordarins tested, with Tox50s of >100 μg/ml in cell lines and ≥87 μg/ml in primary rat hepatocytes. The possibility of delayed cytotoxicity was investigated with one of those compounds, GW 471558. No relevant increase in in vitro toxicity was observed after 48 or 72 h of incubation with this azasordarin derivative on the MRC-5 cell line, one of the most sensitive lines for the present azasordarin derivatives. Both GW 471552 and GW 471558 exhibited good activities against C. albicans, including azole-resistant strains and P. carinii organisms; therefore, they could be promising candidates for the treatment of such infections.
GW 479821 and GW 515716 were derivatives with a wider antifungal spectrum and were less selective. However, both compounds were less cytotoxic than amphotericin B on cell lines.
GW 570009 and GW 587270 appeared to be safer antifungals, with Tox50s ranging from 60 to 96 μg/ml and 50 to 62 μg/ml, respectively, on cell lines and 64 and 52 μg/ml, respectively, on hepatocytes. These derivatives exhibited the broadest spectrum of action, GW 587270 being the most potent molecule evaluated. Both compounds GW 587270 and GW 570009 afforded a good balance between antifungal potency and cytotoxicity.
Globally, these findings indicate that the azasordarins investigated possess an important antifungal therapeutic potential, in particular for the treatment of drug-resistant fungal strains associated with immunocompromised patients.
ACKNOWLEDGMENTS
We thank C. M. Martinez for expert technical assistance and members of the Organic Chemistry Group for compound synthesis. We also thank Jose Maria Fiandor for generous comments on the manuscript.
REFERENCES
- 1.Aviles P, Falcoz C, San Roman R, Gargallo-Viola D. Pharmacokinetics-pharmacodynamics of a sordarin derivative (GM 237354) in a murine model of lethal candidiasis. Antimicrob Agents Chemother. 2000;44:2333–2340. doi: 10.1128/aac.44.9.2333-2340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviles P, Aliouat E-M, Martinez A, Dei-Cas E, Herreros E, Dujardin L, Gargallo-Viola D. In vitro pharmacodynamic parameters of sordarin derivatives in comparison with those of marketed compounds against Pneumocystis carinii isolated from rats. Antimicrob Agents Chemother. 2000;44:1284–1290. doi: 10.1128/aac.44.5.1284-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball S E, Scatina J Z A, Sisenwine S F, Fisher G L. The application of in vitro models of drug metabolism and toxicity in drug discovery and drug development. Drug Chem Toxicol. 1995;18:1–28. doi: 10.3109/01480549509017855. [DOI] [PubMed] [Google Scholar]
- 4.Brun Pascaud M, Herreros E, Aliouat E, Dei-Cas E. Evaluation of drug efficacy by using animal models or in vitro systems. FEMS Immunol Med Microbiol. 1998;22:173–179. doi: 10.1111/j.1574-695X.1998.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 5.Capa L, Mendoza A, Lavandera J L, Gómez de las Heras F, Garcia-Bustos J F. Translation elongation factor 2 is part of the target for a new family of antifungals. Antimicrob Agents Chemother. 1998;42:2694–2699. doi: 10.1128/aac.42.10.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons K V, Stevens D A. Efficacies of sordarin derivatives GM 193663, GM 211676, and GM 237354 in a murine model of systemic coccidioidomycosis. Antimicrob Agents Chemother. 2000;44:1874–1877. doi: 10.1128/aac.44.7.1874-1877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez J M, Martin J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez J M, Gómez-Lorenzo M G, Martin J J. Sordarin inhibits fungal protein synthesis by blocking translocation differently to fusidic acid. J Biol Chem. 2000;274:22423–22427. doi: 10.1074/jbc.274.32.22423. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez J M, Kelly V A, Kinsman O S, Marriott M S, Gomez de las Heras F, Martin J J. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman J A. Prevention of infection due to Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:995–1004. doi: 10.1128/aac.42.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gargallo-Viola D. Sordarins as antifungal compounds. Curr Opin Anti-Infect Investig Drugs. 1999;1:297–305. [Google Scholar]
- 12.Gomez-Lechón M J, Lopez P, Castell J V. Biochemical functionality and recovery of hepatocytes after deep freezing storage. In Vitro. 1984;20:826–832. doi: 10.1007/BF02619627. [DOI] [PubMed] [Google Scholar]
- 13.Graybill J R, Najvar L, Fothergill A, Bocanegra R, Gomez de las Heras F. Activities of sordarins in murine histoplasmosis. Antimicrob Agents Chemother. 1999;43:1716–1718. doi: 10.1128/aac.43.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herreros E, Martinez C M, Almela M J, Marriott M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herreros E, Almela M J, Martinez M, Lozano S, Jackson H, Aliouat E M, Gargallo-Viola D. Microplate assays for in vitro evaluation of anti-Pneumocystis drugs. J Eukaryot Microbiol. 1997;44:43S–44S. doi: 10.1111/j.1550-7408.1997.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, Regadera J, Jimenez E, Santos I, Gargallo-Viola D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob Agents Chemother. 2001;45:1008–1013. doi: 10.1128/AAC.45.4.1008-1013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez A, Aviles P, Jimenez E, Caballero J, Gargallo-Viola D. Activities of sordarins in experimental models of candidiasis, aspergillosis, and pneumocystosis. Antimicrob Agents Chemother. 2000;44:3389–3394. doi: 10.1128/aac.44.12.3389-3394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard. Document M38-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 20.Walsh T J, Pizzo P A. Nosocomial fungal infections. Annu Rev Microbiol. 1988;42:517–545. doi: 10.1146/annurev.mi.42.100188.002505. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman H J, Ishak K G. General aspects of drug-induced liver diseases. Gastroenterol Clin N Am. 1995;24:739–758. [PubMed] [Google Scholar]