Abstract
The pharmacokinetics of cefepime were studied in 12 adult patients in intensive care units during continuous venovenous hemofiltration (CVVH) or continuous venovenous hemodiafiltration (CVVHDF) with a Multiflow60 AN69HF 0.60-m2 polyacrylonitrile hollow-fiber membrane (Hospal Industrie, Meyzieu, France). Patients (mean age, 52.0 ± 13.0 years [standard deviation]; mean weight, 96.7 ± 18.4 kg) received 1 or 2 g of cefepime every 12 or 24 h (total daily doses of 1 to 4 g/day) by intravenous infusion over 15 to 30 min. Pre- and postmembrane blood (serum) samples and corresponding ultrafiltrate or dialysate samples were collected 1, 2, 4, 8, and 12 or 24 h (depending on dosing interval) after completion of the drug infusion. Drug concentrations were measured using validated high-performance liquid chromatography methods. Mean systemic clearance (CLS) and elimination half-life (t1/2) of cefepime were 35.9 ± 6.0 ml/min and 12.9 ± 2.6 h during CVVH versus 46.8 ± 12.4 ml/min and 8.6 ± 1.4 h during CVVHDF, respectively. Cefepime clearance was substantially increased during both CVVH and CVVHDF, with membrane clearance representing 40 and 59% of CLS, respectively. The results of this study confirm that continuous renal replacement therapy contributes substantially to total CLS of cefepime and that CVVHDF appears to remove cefepime more efficiently than CVVH. Cefepime doses of 2 g/day (either 2 g once daily or 1 g twice daily) appear to achieve concentrations adequate to treat most common gram-negative pathogens (MIC ≤ 8 μg/ml) during CVVH or CVVHDF.
Continuous renal replacement therapies (CRRTs) such as continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF) are used as alternatives to conventional intermittent hemodialysis in critically ill patients with acute renal failure. Compared to conventional hemodialysis, CRRT offers the advantages of more-efficient removal of both high solute loads and large fluid volumes; improved tolerance in hemodynamically unstable patients; more precise fluid, metabolic, and nutrition support management; and enhanced removal of proinflammatory cytokines (24).
Unfortunately, there is relatively little clinical data on the removal of specific drugs by CRRTs. Data regarding clearance of drugs by conventional hemodialysis cannot be extrapolated to CRRT accurately because of the continuous nature of the procedures, differences in membranes used, and differences in the blood, ultrafiltrate, and dialysate flow rates. It is also difficult to compare data from different forms of CRRT because the mechanism of drug removal in hemofiltration (i.e., drug removal by convection) differs from that of hemodiafiltration (i.e., removal by convection plus diffusion). There are also differences between various CRRT procedures in blood flow rates and transmembrane pressures (13, 18, 29).
When selecting an antimicrobial dosing regimen for critically ill patients with severe renal failure, the effects of renal failure itself, other acute and chronic disease states, and extracorporeal drug clearance by renal replacement therapies on drug pharmacokinetics should be considered. Inadequate dosing may lead to treatment failures and the potential for development of antimicrobial resistance, while excessive dosing may predispose to drug toxicities. Critically ill patients often have larger volumes of distribution for antimicrobial agents than less severely ill or healthy persons due to alterations in protein binding characteristics, increased total fluid volumes, or other factors; the net result of these changes may be lower than expected drug concentrations in serum (7, 26, 31). Alterations in drug elimination half-lives due to changes in distribution volume and organ function are also observed. The relative lack of clinical data regarding drug dosing in critically ill patients receiving CRRT is thus of great concern due to potential pharmacokinetic alterations and associated therapeutic outcomes.
Cefepime is a “fourth-generation” cephalosporin with a broad spectrum of antimicrobial activity against many gram-positive pathogens as well as common gram-negative pathogens including Pseudomonas aeruginosa (6, 14, 20). Cefepime has a low affinity for and good stability against extended-spectrum β-lactamase enzymes and often retains excellent activity against gram-negative organisms that are resistant to extended-spectrum cephalosporins (15, 19, 22). Cefepime is extensively used as empirical or directed therapy for a variety of infections in critically ill patients. One previous study of cefepime pharmacokinetics during CRRT has been published, but the patient numbers were small and only patients undergoing CVVHDF were included (1). The primary objective of the present study was therefore to more fully characterize the pharmacokinetic disposition of cefepime in critically ill adult intensive care unit (ICU) patients during CVVH or CVVHDF. The present study also examines the adequacy of typically prescribed dosing regimens by comparing drug concentrations achieved with typical MICs of common pathogens found in the ICU setting.
MATERIALS AND METHODS
Patient eligibility.
This study is a prospective open-label study of cefepime (Dura Pharmaceuticals, La Jolla, Calif.). All adult patients greater than 18 years of age who were hospital inpatients in a medical, surgical, or burn or trauma ICU who were prescribed cefepime as part of their required medical care and who were receiving CRRT for treatment of severe renal failure were eligible for inclusion in this study. Exclusion criteria included age less than 18 years or use of conventional hemodialysis rather than CRRT. The study was approved by the Institutional Review Board of the hospital where the study was performed, and written informed consent was obtained from all patients or their legally designated representatives prior to study entry.
Medications.
Patients enrolled in the study received cefepime as part of their medical care. Due to the lack of dosing recommendations for CRRT, dosing regimens were subjectively determined by the physicians caring for the patients and selected based on clinical indications. Cefepime regimens thus included either 1-g or 2-g doses administered intravenously every 12 or 24 h (total daily doses of 1 to 4 g/day). Cefepime doses were infused over periods ranging from 15 to 30 min. Specific dosing regimens and times of administration were recorded for each study patient. Complete medical histories were obtained for all patients, and complete physical examinations and laboratory review of serum chemistry and hematology profiles were performed and reviewed prior to collection of samples for pharmacokinetic analysis.
CRRT.
For all patients, CRRT was administered using a Hospal BSM-22SC machine (CGH Medical, Lakewood, Colo.) with a Multiflow60 AN69HF 0.60-m2 polyacrylonitrile hollow-fiber membrane (Hospal Industrie, Meyzieu, France). Vascular access was obtained by introduction of a 12 French, 20-cm double-lumen central venous catheter (Arrow, Reading, Pa.) into a femoral vein. CRRT was managed by the renal consult service caring for the patient, and parameters such as blood flow rate (Qb) and dialysate flow rate (Qd) for those receiving CVVHDF were adjusted as therapeutically necessary. Replacement fluids usually consisted of 0.9% sodium chloride alternating with 0.45% sodium chloride plus 75 meq of sodium bicarbonate per liter; these fluids were delivered postmembrane via a volumetric pump. During CVVHDF, dialysate fluids (Premixed Dialysate for Hemodiafiltration; Baxter Healthcare, Deerfield, Ill.) were also delivered via a volumetric pump into the dialysate compartment of the filter in a direction countercurrent to the blood flow. Additional electrolytes such as calcium and potassium were added to replacement and dialysate fluids as required. The extracorporeal circuit was anticoagulated as clinically indicated with heparin sodium at rates ranging from 100 to 1,100 IU/h. Data for parameters such as Qb, Qd, and ultrafiltrate flow rate (Quf) were obtained from the CRRT hourly monitoring logs kept for each patient. Urine output data were obtained from routine ICU patient monitoring data sheets.
Sample collection.
Given uncertainty regarding the duration of CRRT in individual patients, pharmacokinetic sampling was performed as soon as possible after initiation of the CRRT and drug therapy and after obtaining informed consent. Pre- and postmembrane venous blood samples were obtained 1, 2, 4, and 8 h after the completion of the drug infusion in all patients. Samples were also obtained from all patients just before administration of the next dose (either 12 or 24 h after the previous dose, depending on specific dosing interval ordered) whenever possible. Finally, an additional midinterval sample was obtained 12 h after completion of drug infusion, when applicable, in patients receiving doses at 24-h intervals. Samples (4 ml) were taken from the in-line blood access port in the extracorporeal circuit. Dialysate and/or ultrafiltrate samples (20 ml) were obtained simultaneously with blood samples in order to determine sieving coefficients and filter clearances.
Sample storage and assay.
Blood samples were collected in plain glass vacuum tubes, allowed to clot in an ice-water bath, and promptly centrifuged. The serum samples were then transferred to labeled polyethylene vials and stored at −70°C until assayed. Ultrafiltrate or dialysate samples were frozen immediately after collection.
Drug concentrations in serum and dialysate or ultrafiltrate were determined using reversed-phase high-performance liquid chromatography (HPLC) with UV detection according to adaptations of previously published methods (1, 2). The HPLC system consisted of a Novapak C18 column (4.6 × 150 mm) with a guard column containing Novapak C18 inserts (Waters, Milford, Mass.), and the detector was set at a wavelength of 305 nm. The mobile phase consisted of acetonitrile-water (4.5:95.5 [vol/vol]) containing 50 mM trisodium citrate, adjusted to a pH of 6.0. Ceftriaxone (Sigma, St. Louis, Mo.) was used as the internal standard. The extraction procedure for serum samples involved precipitation of proteins with acetonitrile followed by centrifugation. Dichloromethane was then added to the supernatant. After vortexing, the organic and aqueous phases were separated by centrifugation and an aliquot of the aqueous phase was injected into the HPLC system. No extraction was performed on the dialysate or ultrafiltrate samples; these samples were injected directly into the system.
Coefficients of determination (r2) for the serum cefepime assay over the standard curve concentration ranges (0.5 to 200.0 μg/ml) were 0.998 to 0.999 for the entire study. For this study, within-day coefficients of variation (CV) for serum cefepime samples were 1.9, 1.9, and 3.3% at concentrations of 2, 25, and 150 μg/ml, respectively. Between-day CV for serum samples were 4.2, 2.2, and 3.5% at concentrations of 2, 25, and 150 μg/ml, respectively. Coefficients of determination (r2) for the ultrafiltrate or dialysate cefepime assay over the standard curve concentration ranges (0.5 to 100.0 μg/ml) were in the range of 0.998 to 1.000 for the entire study. For this study, the within-day CV for dialysate or ultrafiltrate samples were 4.9, 4.8, and 3.9% at concentrations of 1, 10, and 50 μg/ml, respectively. Between-day CV for dialysate or ultrafiltrate samples were 1.0, 3.0, and 1.0% at concentrations of 1, 10, and 50 μg/ml, respectively. The lower limit of cefepime quantitation in both serum and ultrafiltrate or dialysate samples for this study was 0.5 μg/ml, the lower limit of the standard curves.
Pharmacokinetic analysis.
Concentration-time data for cefepime in serum was analyzed by standard pharmacokinetic methods. Cefepime has previously been demonstrated to follow a one-compartment model with first-order elimination during CRRT (1). Premembrane serum drug concentrations were used to determine pharmacokinetic parameters. The apparent terminal elimination rate constant (kel) was determined by least-squares regression analysis of the terminal portion (last four to five concentration-versus-time points) of the natural log concentration-time curve. Elimination half-life (t1/2) was calculated as 0.693/kel. Maximum serum drug concentration (Cmax) was calculated as Cfirst/e−kt, where Cfirst is the first measured serum drug concentration (approximately 1 h postinfusion), k is kel, and t is the time from the end of the drug infusion to Cfirst. Minimum serum drug concentration (Cmin) was determined by direct measurement or, in some patients, calculated as Clast × e−kt, where Clast is the last measured serum drug concentration, k is kel, and t is the time from Clast to the end of the dosing interval. The area under the concentration-time curve from time zero to the end of the 24-h dosing interval (AUC0–24) was calculated by the linear trapezoidal summation method. For patients in whom cefepime was administered every 12 h, the total 24-h AUC was calculated by AUC0–12 × 2. Since the early sampling performed in many patients precluded assumptions of true pharmacokinetic steady-state conditions, volume of distribution (V) was calculated by non-steady-state methods which take into account the number of doses previously administered (28). Total systemic clearance (CLS) was calculated by V × kel. The time during which serum drug concentrations are above the MIC of the infecting pathogen (T > MIC) was calculated as natural log (Cmax/CMIC)/kel, where CMIC is the MIC for the organism. The ratio of 24-h AUC to MIC (AUC0–24/MIC) was calculated as AUC0–24 determined during each dosing regimen/MIC. Targeted goals for T > MIC and AUC0–24/MIC were >50% and >100, respectively (9, 10, 21, 30).
Principles of calculating drug clearances during CRRT are reviewed elsewhere (8, 27, 29). Pertinent issues are summarized here. During continuous arteriovenous hemofiltration or CVVH, the only mechanism of drug removal is convection, the removal of serum solutes by ultrafiltration of serum fluid. The ability of a drug to pass through the hemofilter membrane is its sieving coefficient (S) and is calculated by 2 × Cuf/(Ca + Cv) where Cuf is the drug concentration in ultrafiltrate, Ca is the drug concentration in premembrane serum, and Cv is the drug concentration in postmembrane serum. Clearance of drug across the membrane during CVVH (CLCVVH) is calculated by S × Quf.
Drug clearance by CVVHDF occurs by diffusion across the filter as well as convection. The ability of a drug to diffuse through the membrane to dialysate fluid is its saturation coefficient (Sa), which is calculated in the same manner as the sieving coefficient: 2 × Cuf/d/(Ca + Cv) where Cuf/d is the concentration of drug in ultrafiltrate and dialysate combined. Drug clearance by CVVHDF (CLCVVHDF) is the product of the saturation coefficient and the combined ultrafiltrate-dialysate flow rate and is calculated by Sa × (Quf + Qd).
The percentage of CLS contributed by CLCVVH or CLCVVHD (%CLs) is calculated as either (CLCVVH/CLS) × 100 or (CLCVVHD/CLS) × 100, respectively.
All calculations were made by programming pharmacokinetic and CRRT clearance equations into Microsoft Excel 97 (Microsoft Corporation, Redmond, Wash.) spreadsheets. Also using Excel, measures of central tendency and variability were evaluated for all patient and CRRT characteristics, pharmacokinetic parameters, and CRRT clearances.
Statistical analysis.
Differences between demographic variables among patients receiving either CVVH or CVVHDF during administration of cefepime were assessed for statistical significance using one-way analysis of variance fixed-effect model for continuous variables or two-way chi-square test for categorical variables. Differences among calculated pharmacokinetic parameters were assessed by two-tailed Mann-Whitney rank sum test for unpaired nonparametric data. Correlations between pharmacokinetic variables were determined using Spearman's rank correlation coefficient for nonparametric data. All statistical tests were performed using SPSS version 8.0 for Windows (SPSS, Inc., Chicago, Ill.). P values of ≤0.05 were considered significant.
RESULTS
A total of 12 patients were enrolled in the study and completed the scheduled pharmacokinetic sampling. Detailed information regarding patient demographics and CRRT therapy is given in Tables 1 and 2, respectively. There were no statistically significant differences in sex, age, weight, or acute physiology and chronic evaluation (APACHE) II scores among patients receiving CVVH versus CVVHDF. Cefepime was apparently well tolerated in all patients, and no drug-related adverse effects were reported or detected during the study.
TABLE 1.
Demographic and clinical characteristics of the 12 study patients
| Patient | Age (yr) | Ht (cm) | Wt (kg) | Sexa | APACHE II scoreb | Principal diagnosis(es) | Infectious diagnosis | Isolated pathogen (MIC [μg/ml]) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | 166 | 71.0 | F | 30 | End-stage liver disease | FUOc | None | Died |
| 2 | 48 | 183 | 107.4 | M | 27 | End-stage liver disease | Pneumonia | P. aeruginosa (4), Enterobacter cloacae (1) | Died |
| 3 | 42 | 169 | 88.4 | M | 25 | End-stage liver disease | Intra-abdominal sepsis | None | Survived |
| 4 | 54 | 182 | 89.0 | M | 31 | End-stage heart disease, sepsis | Pneumonia | None | Died |
| 5 | 34 | 168 | 99.9 | F | 23 | Idiopathic thrombocytopenia purpura, respiratory failure | Pneumonia | Klebsiella pneumoniae (1), Moraxella catarrhalis (0.03) | Survived |
| 6 | 63 | 173 | 99.3 | M | 29 | Congestive heart failure, cardiogenic shock | Pneumonia | Klebsiella oxytoca (0.125) | Survived |
| 7 | 59 | 178 | 82.2 | M | 24 | End-stage liver disease, sepsis | Intra-abdominal sepsis | Escherichia coli (0.03) | Died |
| 8 | 41 | 178 | 133.8 | M | 32 | Thoracic aortic dissection | Pneumonia | None | Survived |
| 9 | 33 | 173 | 108.0 | F | 26 | End-stage heart disease | FUO | None | Survived |
| 10 | 70 | 204 | 125.3 | F | 29 | Sepsis, rhabdomyolysis | Cellulitis | Group B streptococci (0.25) | Survived |
| 11 | 73 | 172 | 136.0 | M | 28 | Intra-abdominal sepsis | Intra-abdominal sepsis | None | Survived |
| 12 | 52 | 170 | 82.8 | M | 29 | Congestive heart failure, cardiogenic shock | Pneumonia | E. coli (0.06), Haemophilus influenzae (0.016) | Survived |
F, female; M, male.
APACHE II score, Acute Physiology and Chronic Health Evaluation II score on admission to the intensive care unit.
FUO, fever of unknown origin.
TABLE 2.
Etiologies of renal failure and details of CRRT
| Patient | Etiology of renal failurea | Urine output/ 24 h (ml)b | Type of CRRT | Blood flow rate (ml/min)b | Dialysis rate (ml/h)bcd | Ultrafiltration rate (ml/min)bd | Concomitant vasoactive drug(s) |
|---|---|---|---|---|---|---|---|
| 1 | Ischemic ATN | 0 | CVVH | 150 | 19 ± 2 | Dopamine | |
| 2 | ATN 2° unknown etiology | 134 | CVVH | 150 | 9 ± 8 | None | |
| 3 | Hepatorenal syndrome | 12 | CVVH | 150 | 19 ± 4 | Dopamine | |
| 4 | Sepsis with MODS | 155 | CVVH | 150 | 15 ± 2 | Dopamine, norepinephrine | |
| 5 | Idiopathic thrombocytopenia purpura | 35 | CVVH | 150 | 17 ± 12 | None | |
| 6 | Cardiogenic shock | 0 | CVVHDF | 150 | 957 ± 81 | 18 ± 2 | Dopamine, isoproteranol |
| 7 | Sepsis with MODS | 29 | CVVHDF | 150 | 857 ± 227 | 20 ± 10 | Norepinephrine |
| 8 | Ischemic ATN | 0 | CVVHDF | 150 | 940 ± 28 | 14 ± 1 | Dopamine, norepinephrine |
| 9 | ATN 2° unknown etiology | 0 | CVVHDF | 150 | 954 ± 47 | 20 ± 4 | Epinephrine |
| 10 | Rhabdomyolysis | 67 | CVVHDF | 150 | 970 ± 161 | 23 ± 7 | None |
| 11 | ATN 2° sepsis with MODS | 43 | CVVHDF | 150 | 1,000 ± 0 | 13 ± 2 | Dopamine, norepinephrine |
| 12 | Cardiogenic shock | 0 | CVVHDF | 150 | 1,020 ± 26 | 14 ± 2 | Dopamine, epinephrine |
ATN, acute tubular necrosis; 2°, secondary; MODS, multiple-organ dysfunction syndrome.
During time of pharmacokinetic sampling.
Applicable only to patients receiving CVVHDF.
Rates shown as means ± standard deviations.
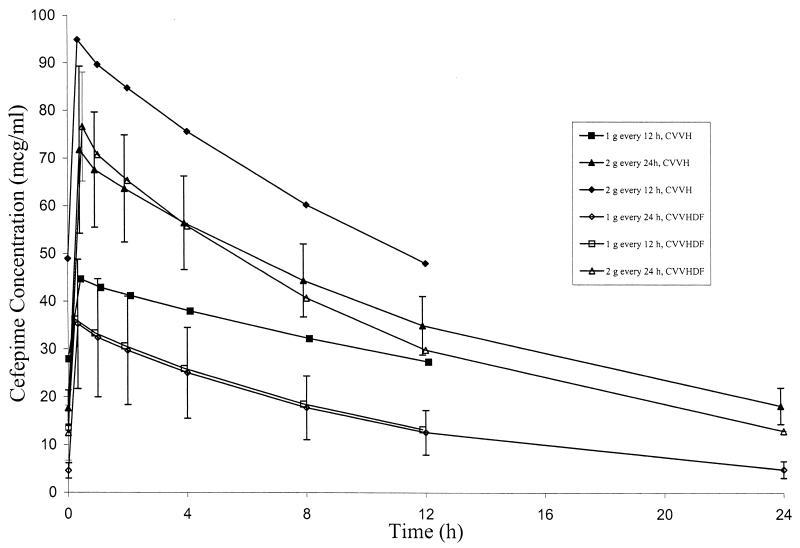
Samples for pharmacokinetic analysis were obtained following the second cefepime dose in eight patients; the remaining patients had samples drawn following the third (one patient), fourth (two patients), or fifth dose (one patient). Serum cefepime pharmacokinetic parameters determined during administration of cefepime in various dosing regimens during CVVH and CVVHDF are given in Table 3 and Table 4, respectively. Mean serum cefepime concentration-versus-time profiles with each different dosing regimen during CVVH and CVVHDF are shown in Fig. 1. Certain pharmacokinetic parameters appeared to be dependent on whether patients were receiving CVVH or CVVHDF. Drug clearance during CRRT (CLCRRT) and %CLS were significantly higher (P = 0.002 and 0.018, respectively), and t1/2 was significantly lower (P = 0.005) among patients receiving CVVHDF than in patients receiving CVVH. However, no significant differences in CLS (P = 0.935), S or Sa (P = 0.223), or ultrafiltration rates (P = 0.639) were noted between the CVVHDF and CVVH groups. Values for cefepime V were also statistically different (P = 0.03) between CVVH and CVVHDF groups, but changes in V were only weakly correlated with changes in t1/2 (Spearman's rank correlation coefficient of 0.641; P = 0.133) and did not provide an explanation for differences observed with that parameter. Values for Cmax, Cmin, and AUC0–24 during each dosage regimen also appeared to be somewhat dependent on whether patients were receiving CVVH versus CVVHDF, but these differences were not consistently observed between regimens and the patient numbers in each group were very small.
TABLE 3.
Summary of cefepime pharmacokinetic parameters for patients receiving CVVH
| Patient | Dosing regimen | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–24 (μg · h/ml) | t1/2 (h) | V (liter/kg) | CLS
|
UFRa (ml/min) | CRRT CLb
|
Sb | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ml/min | ml/min/kg | ml/min | ml/min/kg | %CLS | |||||||||
| 1 | 2 g every 24 h | 61.6 | 18.7 | 943.5 | 13.7 | 0.64 | 38 ± 6 | 0.54 ± 0.08 | 19 ± 2 | 16 ± 4 | 0.23 ± 0.05 | 42 ± 3 | 0.92 ± 0.07 |
| 2 | 2 g every 24 h | 67.4 | 13.5 | 1,050.9 | 10.2 | 0.36 | 44 ± 4 | 0.41 ± 0.04 | 9 ± 8 | 3 ± 2 | 0.03 ± 0.02 | 8 ± 4 | 0.84 ± 0.05 |
| 3 | 2 g every 24 h | 86.4 | 20.7 | 1,258.4 | 11.4 | 0.38 | 33 ± 4 | 0.38 ± 0.04 | 19 ± 4 | 17 ± 3 | 0.19 ± 0.04 | 29 ± 19 | 0.89 ± 0.08 |
| 4 | 1 g every 12 h | 44.6 | 27.9 | 834.7 | 17.0 | 0.43 | 28 ± 3 | 0.29 ± 0.03 | 15 ± 2 | 13 ± 3 | 0.13 ± 0.03 | 45 ± 14 | 0.81 ± 0.13 |
| 5 | 2 g every 12 h | 94.9 | 48.0 | 1,677.8 | 12.2 | 0.38 | 35 ± 1 | 0.36 ± 0.01 | 17 ± 12 | 14 ± 10 | 0.14 ± 0.10 | 39 ± 28 | 0.83 ± 0.11 |
| Mean ± SD | 12.9 ± 2.6 | 0.46 ± 0.14 | 36 ± 6c | 0.40 ± 0.09c | 16 ± 4a | 13 ± 4a | 0.15 ± 0.06c | 40 ± 16c | 0.86 ± 0.04c | ||||
UFR, ultrafiltration rate.
Values are means ± standard deviations calculated from parameter values determined during each of the four or five postdose sampling intervals for each patient: 1 to 2 h, 2 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h (except with 12-h dosing interval regimens).
Means ± standard deviations calculated from means of the four or five parameter values determined for each individual patient.
TABLE 4.
Summary of cefepime pharmacokinetic parameters for patients receiving CVVHDF
| Patient | Dosing regimen | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–24 (μg · h/ml) | t1/2 (h) | V (liter/kg) | CLS
|
UFRa (ml/min) | CRRT CLb
|
Sab | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ml/min | ml/min/kg | ml/min | ml/min/kg | %CLS | |||||||||
| 6 | 1 g every 24 h | 44.8 | 5.8 | 600.7 | 8.0 | 0.30 | 38 ± 4 | 0.44 ± 0.04 | 18 ± 2 | 25 ± 6 | 0.28 ± 0.07 | 33 ± 10 | 0.73 ± 0.09 |
| 7 | 1 g every 24 h | 25.7 | 3.5 | 344.9 | 8.2 | 0.58 | 67 ± 5 | 0.81 ± 0.06 | 20 ± 10 | 23 ± 8 | 0.27 ± 0.10 | 66 ± 18 | 0.65 ± 0.09 |
| 8 | 1 g every 12 h | 36.2 | 13.6 | 581.8 | 8.3 | 0.35 | 58 ± 1 | 0.49 ± 0.01 | 14 ± 1 | 28 ± 1 | 0.24 ± 0.02 | 48 ± 1 | 0.92 ± 0.02 |
| 9 | 2 g every 24 h | 72.9 | 10.4 | 979.2 | 8.4 | 0.32 | 26 ± 6 | 0.24 ± 0.06 | 20 ± 4 | 47 ± 5 | 0.44 ± 0.05 | 56 ± 14 | 0.73 ± 0.12 |
| 10 | 2 g every 24 h | 79.1 | 6.9 | 1,009.9 | 6.7 | 0.21 | 36 ± 8 | 0.30 ± 0.07 | 23 ± 7 | 45 ± 2 | 0.37 ± 0.01 | 81 ± 19 | 0.93 ± 0.06 |
| 11 | 2 g every 24 h | 63.6 | 12.2 | 890.9 | 9.9 | 0.26 | 23 ± 2 | 0.17 ± 0.01 | 13 ± 2 | 42 ± 1 | 0.31 ± 0.01 | 53 ± 4 | 0.78 ± 0.07 |
| 12 | 2 g every 24 h | 90.8 | 20.4 | 1,306.8 | 10.9 | 0.34 | 22 ± 1 | 0.27 ± 0.02 | 14 ± 2 | 30 ± 1 | 0.36 ± 0.01 | 76 ± 4 | 0.75 ± 0.05 |
| Mean ± SD | 8.6 ± 1.4 | 0.34 ± 0.12 | 47 ± 0.12c | 0.46 ± 0.17c | 17 ± 4c | 26 ± 5c | 0.25 ± 0.04c | 59 ± 16c | 0.78 ± 0.10c | ||||
UFR, ultrafiltration rate.
Values are means ± standard deviations calculated from parameter values determined during each of the four or five postdose sampling intervals for each patient: 1 to 2 h, 2 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h (except with 12-h dosing interval regimens).
Means ± standard deviations calculated from means of the four or five parameter values determined for each individual patient.
FIG. 1.
Mean concentrations of cefepime in serum with various dosage regimens during CVVH and CVVHDF. The cefepime concentrations are shown in micrograms per milliliter. The x axis represents postinfusion times. Error bars represent standard deviations.
Median Cmax values were higher during administration of the 2-g once-daily regimen (72.9 μg/ml) compared to the 1-g twice-daily regimen (approximately 36.2 μg/ml). However, median Cmin values were similar in patients receiving a total daily cefepime dose of 2 g/day irrespective of how the regimen was administered. The median AUC0–24 also appeared to be higher among patients receiving 2 g every 24 h (979 μg · h/ml) compared to 1 g every 12 h (582 μg · h/ml). However, whether these were true differences or merely caused by the small number of study patients is unclear.
Pharmacokinetic and CRRT parameters for cefepime are given in Table 2. The mean cefepime S during CVVH and Sa during CVVHDF were estimated at 0.86 ± 0.04 and 0.78 ± 0.10, respectively, indicating that cefepime is extensively cleared across the CRRT membrane. These calculated values were observed to be quite consistent throughout the sampling periods, among all patients and across various ultrafiltration rates. Approximately 40 and 59% of cefepime CLS was attributed to membrane clearance during CVVH and CVVHDF, respectively, indicating that the clearance of cefepime was substantially enhanced during both CRRT techniques.
Calculated T > MIC and AUC0–24/MIC ratios during each dosing regimen are shown in Table 5. All pathogens isolated from study patients had cefepime MICs of ≤4 μg/ml, and doses as low as 1 g/day would have been predicted to provide adequate treatment during either CVVH or CVVHDF. Cefepime doses of 2 g/day administered intravenously during CRRT would be expected to achieve favorable concentrations in serum against susceptible pathogens (MIC ≤8 μg/ml) as judged by calculated values for both T > MIC and AUC0–24/MIC. Cefepime (2 g/day) during either CVVH or CVVHDF would also be predicted to achieve favorable T > MIC of greater than 80% against pathogens with intermediate susceptibility (MIC = 16 μg/ml). However, AUC0–24/MIC ratios would be predicted to range from only 36 to 65 against intermediately susceptible pathogens and would thus be less favorable.
TABLE 5.
Calculated pharmacodynamic parameters for cefepime with various dosage regimens during CRRT
| Pharmacodynamic parameter | Parameter value by different dosage regimens and treatments
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 g every 24 h
|
1 g every 12 h
|
2 g every 24 h
|
2 g every 12 h
|
|||||
| CVVH | CVVHDF (n = 2) | CVVH (n = 1) | CVVHDF (n = 1) | CVVH (n = 3) | CVVHDF (n = 4) | CVVH (n = 1) | CVVHDF | |
| T > MIC | NAa | NA | ||||||
| MIC ≤ 4 μg/ml | NA | 100% | 100% | 100% | 100% | 100% | 100% | NA |
| MIC = 8 μg/ml | NA | 74% | 100% | 100% | 100% | 100% | 100% | NA |
| MIC = 16 μg/ml | NA | 40% | 100% | 85% | 100% | 84% | 100% | NA |
| AUC0–24/MIC | ||||||||
| MIC ≤ 4 μg/ml | NA | 118 | 209 | 145 | 263 | 262 | 419 | NA |
| MIC = 8 μg/ml | NA | 59 | 104 | 73 | 131 | 131 | 210 | NA |
| MIC = 16 μg/ml | NA | 30 | 52 | 36 | 66 | 65 | 105 | NA |
NA, not available (dosage regimens not studied).
DISCUSSION
This study demonstrates that CRRT contributes substantially to cefepime elimination in patients with renal failure. Due to the shorter elimination t1/2, higher CLCRRT, and higher %CLS observed during CVVHDF in this study, it appears that CVVHDF is more efficient than CVVH in eliminating cefepime. Increased drug clearance during CVVHDF compared to CVVH has been reported elsewhere for other antimicrobial agents (17). However, the present study included too few subjects and too much variability was observed within the data to demonstrate this conclusively for cefepime.
Non-CRRT clearance (CLS − CLCRRT) was 21.5 ± 11.8 ml/min, which is higher than or similar to cefepime clearances of 6.3 and 18.7 ± 5.2 ml/min previously reported in anuric patients (3, 11). Cefepime t1/2s of 12.9 ± 2.6 and 8.6 ± 1.4 h observed in the present study during CVVH and CVVHDF, respectively, are also decreased relative to the half-lives of 21.1 h and 13.5 ± 2.65 h previously reported in anuric patients (3, 11). This is consistent with the enhanced portion of cefepime CLS contributed by CRRT techniques. The difference in observed cefepime t1/2 during CRRT is also notable in light of the increased V observed in the present study (0.46 ± 0.14 and 0.34 ± 0.12 liter/kg of body weight during CVVH and CVVHDF, respectively) compared to those previously reported for anuric patients (0.18 ± 0.06 and 0.29 ± 0.06 liter/kg) (3, 11). The increased V of cefepime in this study might be explained by the typically fluid-overloaded state of patients receiving CRRT in our institution along with the low degree of serum plasma protein binding of this hydrophilic drug (Maxipime [cefepime hydrochloride for injection]) prescribing information; Dura Pharmaceuticals). The observed decrease in cefepime t1/2 occurring in the setting of increased V is further evidence of markedly enhanced removal of cefepime during CRRT, particularly during CVVHDF. The cefepime t1/2 observed during CVVHDF (8.6 ± 1.4 h) in the present study is similar to a t1/2 of 8.1 ± 2.2 h previously reported in the literature for six patients receiving CVVHDF (1).
Studies of cefepime administered once daily to healthy volunteers or subjects with severe renal insufficiency demonstrated that Cmax values of 193.1 ± 35.7 μg/ml and AUC0–24 of 2,405 ± 213 μg · h/ml were well tolerated with no increased or unexpected drug-related adverse events (3–5, 25). Although relatively high sustained concentrations of cefepime were observed during this study, no adverse events were reported or observed in patients receiving the drug.
Previous studies have determined that the most important pharmacodynamic predictor of clinical efficacy of the cephalosporins is the time during which serum drug concentrations are above the MIC of the infecting pathogen (T > MIC) (9, 10, 21). Additional studies have also suggested that both AUC0–24/MIC and T > MIC are important predictors of clinical efficacy and the risk of the development of microbial resistance (10, 30). The severity of infections encountered in the ICU population and the need for adequate T > MIC and AUC0–24/MIC ratios are crucial considerations in severely ill patients receiving cefepime. These patients are frequently infected with nosocomial pathogens that display decreased antimicrobial susceptibilities and are prone to developing resistance with inadequate therapy. Studies indicate that T > MIC should be at least 40 to 50% of the dosing interval, although it has also been suggested that achieving T > MIC for 100% of the dosing interval may be desirable for optimal outcome (10). AUC0–24/MIC ratios of ≥100 have been recommended as the optimal antimicrobial exposure for improving outcome and preventing the selection of antimicrobial resistance (30).
Cefepime possesses excellent antibacterial activity against most common gram-negative aerobic pathogens found in the ICU setting (12, 14–16, 20, 22, 23). Cefepime usually displays MICs of ≤8 μg/ml against these pathogens, including most β-lactamase-producing strains of Klebsiella, Enterobacter, Citrobacter, and Serratia. This study suggests that cefepime doses of 2 g/day administered intravenously during either CVVH or CVVHDF would be expected to achieve favorable concentrations in serum against susceptible pathogens with MICs of ≤8 μg/ml as judged by predicted values for T > MIC greater than 50% as well as AUC0–24/MIC > 100 (Table 5). Many non-β-lactamase-producing members of the family Enterobacteriaceae, which often have MICs of ≤1 μg/ml, should be effectively treated with cefepime doses as low as 1 g/day during CRRT. However, we would recommend cefepime doses of 2 g/day under most circumstances in critically ill patients receiving CRRT due to frequent use of the drug as empirical therapy, unavailability of specific MICs in many institutions, and because of the variability in cefepime pharmacokinetics observed during CRRT. Cefepime regimens of 0.25 to 1.0 g/day as recommended by the manufacturer for anuric patients or those receiving conventional hemodialysis would likely be subtherapeutic against all but the most highly susceptible pathogens when administered to patients receiving CRRT (Maxipime; Dura Pharmaceuticals).
P. aeruginosa, Acinetobacter, and certain other pathogens are commonly found in seriously ill patients and are often less susceptible to cefepime (MIC at which 90% of the isolates tested are inhibited [MIC90] of >8 μg/ml [12, 14–16, 22, 23]). Increased doses of cefepime would often be required during CRRT in order to achieve the concentrations of cefepime in serum required for optimal efficacy against these organisms. As shown in Table 5, adequate T > MICs should be achieved with 2 g cefepime per day in the treatment of infections caused by intermediately susceptible pathogens with MICs equal to 16 μg/ml. However, doses of 4 g/day would be required if an AUC0–24/MIC ratio of ≥100 were desired. Due to the variability in cefepime pharmacokinetics during CRRT and the potential need for increased doses, other antibiotics having better in vitro activity and lower MICs against these known or suspected pathogens should be used in place of cefepime if they are available. Alternatively, if no other agents are considered more suitable, cefepime doses of 4 g/day should be considered for empirical therapy in patients with life-threatening nosocomial infections while awaiting results of culture and susceptibility testing. This may be particularly true in institutions with a high incidence of nosocomial infections due to P. aeruginosa, Acinetobacter, or other pathogens with cefepime MIC90s of >8 μg/ml. This suggestion for use of higher doses in these situations is consistent with previously published recommendations for dosing of cefepime during CVVHDF (1).
There are a number of potential limitations to this study due to the many difficulties inherent in performing such evaluations in this population. This study is somewhat limited by the relatively small number of subjects that received each dosage regimen during the different types of CRRT. This prevented more complete evaluations of relative drug clearances by CVVH versus CVVHDF as well as adequacy of each of the observed dosing regimens. However, this is the largest study to date evaluating cefepime disposition during CRRT and the only study thus far to evaluate both CVVH and CVVHDF. It should also be noted that patients not receiving CRRT were not included as controls for study patients, so relative alterations in pharmacokinetics must be compared with historical rather than study-derived data. Another limitation is that the potential for adsorption of drug to membrane surfaces and a falsely increased apparent drug elimination rate was also not evaluated. Because differences in ultrafiltration rates influence drug removal rates, failure to control CRRT parameters by strict protocol may perhaps be seen as a further limitation to this study. However, because subjects were studied as they actually received CRRT and antibiotics for clinical indications without protocol-prescribed alterations in CRRT parameters or antibiotic dosing, the results are directly applicable to the clinical setting. Finally, possibilities for error in pharmacokinetic calculations are inherent in this study due to the fact that collection of samples took place over relatively short periods of time in relation to the low drug elimination rates and long half-lives. Although the number of isolated pathogens was small, a potential strength of this study compared to other published studies is that conclusions are not based solely on observed elimination rates; observed drug concentrations compared to MICs for important pathogens from a pharmacodynamic perspective were also considered.
Cefepime elimination in patients with acute renal failure is significantly enhanced and serum cefepime t1/2 is decreased by CRRT. Cefepime dosing regimens recommended for anuric patients are likely be subtherapeutic in many patients receiving CVVH or CVVHDF. Cefepime should be given in doses of 2 g/day for most infections caused by susceptible gram-negative pathogens, administered as either a single 2-g intravenous dose or in divided 1-g doses. However, 4 g of cefepime per day appears to be required for pathogens with potentially higher MICs such as P. aeruginosa or for empirical treatment of life-threatening nosocomial infections, particularly in patients receiving treatment with CVVHDF. In all cases, MICs for suspected pathogens and desired serum drug concentrations should be considered when choosing a dosing regimen.
ACKNOWLEDGMENTS
This investigator-initiated study was supported by a grant from the Bayer Corporation.
Cefepime analytical-grade powder for HPLC assays was kindly supplied by the Bristol-Myers Squibb Company.
REFERENCES
- 1.Allaouchiche B, Breilh D, Jaumain H, Gaillard B, Renard S, Saux M. Pharmacokinetics of cefepime during continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 1997;41:2424–2427. doi: 10.1128/aac.41.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbhaiya R H, Forgue S T, Shyu W C, Papp E A, Pittman K A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31:55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbhaiya R H, Knupp C A, Forgue S T, Matzke G R, Guay D R P, Pittman K A. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990;48:268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- 4.Barbhaiya R H, Forgue S T, Gleason C R, Knupp C A, Pittman K A, Weidler D J, Movahhed H, Tenney J, Martin R R. Pharmacokinetics of cefepime after single and multiple intravenous administration in healthy subjects. Antimicrob Agents Chemother. 1992;36:552–557. doi: 10.1128/aac.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbhaiya R H, Knupp C A, Pfeffer M, Zaccardelli D, Dukes G M. Pharmacokinetics of cefepime in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1992;36:1387–1391. doi: 10.1128/aac.36.7.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barradell L B, Bryson H M. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1994;147:471–505. doi: 10.2165/00003495-199447030-00007. [DOI] [PubMed] [Google Scholar]
- 7.Bodenham A, Shelly M P, Park G R. The altered pharmacokinetics and pharmacodynamics of drugs commonly used in critically ill patients. Clin Pharmacokinet. 1988;14:347–373. doi: 10.2165/00003088-198814060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bressolle F, Kinowski J J, de la Coussaye E, Wynn N, Eledjam J, Galtier M. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet. 1994;26:457–471. doi: 10.2165/00003088-199426060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Craig W A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 10.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 11.Cronqvist J, Nilsson-Ehle I, Oqvist B, Norby S R. Pharmacokinetics of cefepime dihydrochloride arginine in subjects with renal impairment. Antimicrob Agents Chemother. 1992;36:2676–2680. doi: 10.1128/aac.36.12.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval J, Soussy C J, Acar J F. In-vitro antibacterial activity of cefepime: a multicenter study. J Antimicrob Chemother. 1993;32(Suppl. B):55–59. doi: 10.1093/jac/32.suppl_b.55. [DOI] [PubMed] [Google Scholar]
- 13.Forni L G, Hilton P J. Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med. 1997;336:1303–1309. doi: 10.1056/NEJM199705013361807. [DOI] [PubMed] [Google Scholar]
- 14.Frei R, Jones R N, Pignatari A C, Yamane N, Marco F, Hoban D J. Antimicrobial activity of FK-037, a new broad-spectrum cephalosporin. International in vitro comparison with cefepime and ceftazidime. Diagn Microbiol Infect Dis. 1994;18:167–173. doi: 10.1016/0732-8893(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 15.Fung-Tomc J, Dougherty T J, DeOrio F J, Simich-Jacobson V, Kessler R E. Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to beta-lactamase levels. Antimicrob Agents Chemother. 1989;33:498–502. doi: 10.1128/aac.33.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamarellou H, Sahin A, Chryssouli Z. Comparative in vitro evaluation of BMY-28142, a new broad spectrum cephalosporin, versus other β-lactams against multiresistant gram-negative isolates. Drugs Exp Clin Res. 1987;13:149–153. [PubMed] [Google Scholar]
- 17.Giles L J, Jennings A C, Thomson A H, Creed G, Beale R J, McLuckie A. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med. 2000;28:632–637. doi: 10.1097/00003246-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Golper T A. Drug removal during continuous hemofiltration or hemodialysis. Contrib Nephrol. 1991;93:110–116. doi: 10.1159/000420197. [DOI] [PubMed] [Google Scholar]
- 19.Hancock R E, Bellido F. Factors involved in the enhanced activity against gram-negative bacteria of fourth generation cephalosporins. J Antimicrob Chemother. 1991;29:1–6. doi: 10.1093/jac/29.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 20.Hardin T C, Jennings T S. Cefepime. Pharmacotherapy. 1994;14:657–668. [PubMed] [Google Scholar]
- 21.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Jones R N, Pfaller M A, Doern G V, Erwin M E, Hollis R J the Cefepime Study Group. Antimicrobial activity and spectrum investigation of eight broad-spectrum beta-lactam drugs: a 1997 surveillance trial in 102 medical centers in the United States. Diagn Microbiol Infect Dis. 1998;30:215–228. doi: 10.1016/s0732-8893(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 23.Jones R N. Contemporary antimicrobial susceptibility patterns of bacterial pathogens commonly associated with febrile patients with neutropenia. Clin Infect Dis. 1999;29:495–502. doi: 10.1086/598621. [DOI] [PubMed] [Google Scholar]
- 24.Joy M S, Matzke G R, Armstrong D K, Marx M A, Zarowitz B J. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32:362–375. doi: 10.1345/aph.17105. [DOI] [PubMed] [Google Scholar]
- 25.Nye K J, Shi Y G, Andrews J M, Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989;24:23–28. doi: 10.1093/jac/24.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Power B M, Forbes A M, Heerden P V, Ilett K F. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet. 1998;34:25–56. doi: 10.2165/00003088-199834010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Reetze-Bonorden P, Bohler J, Keller E. Drug dosing in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet. 1993;24:362–379. doi: 10.2165/00003088-199324050-00002. [DOI] [PubMed] [Google Scholar]
- 28.Shargel L, Yu A B C. Applied biopharmaceutics and pharmacokinetics. 4th ed. Stamford, Conn: Appleton & Lange; 1999. [Google Scholar]
- 29.Schetz M, Ferdinande P, Van den Berghe R, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 1995;21:612–620. doi: 10.1007/BF01700172. [DOI] [PubMed] [Google Scholar]
- 30.Thomas J K, Forrest A, Bhavnani S M, Hyatt J M, Cheng A, Ballow C H, Schentag J J. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dalen R, Vree T B. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 1990;16(Suppl. 3):S235–S238. doi: 10.1007/BF01709707. [DOI] [PubMed] [Google Scholar]