Abstract
The upsurge of antibiotic usage in the 20th century has resulted in increasing levels of pharmaceutical compounds in bodies of water. A particular antibiotic, levofloxacin, is a third-generation quinolone known to target Gram-positive organisms like atypical pathogens. Chronic toxic effects of levofloxacin to some microorganisms lead to the disruption of marine ecosystems. Unfortunately, a relatively low concentration of levofloxacin in water bodies discourages researchers from exploring potential risk assessment and removal in wastewater treatment plants. In this article, aqueous levofloxacin was degraded using hydroxyapatite catalyst under UV-irradiation. Response Surface Methodology (Box Behnken Model) was used to model and optimize the degradation efficiency parameter. The response was fitted into a 2-factor interaction equation revealing a satisfactory ANOVA evaluation (R2=97.08%, adjusted R2= 94.89, predicted R2=91.1%). An optimal photodegradation efficiency was determined to attain the following conditions: 1.5 g/L catalyst dose, 4 ppm levofloxacin, and a pH level of 10. The model predicted a value of 71.6% degradation efficiency, which is very close to 70.6% generated experimentally.
Keywords: Box-Behnken design, Levofloxacin (LEVO), Hydroxyapatite, Photocatalysis, Response surface methodology, Mathematical model
Specifications Table
| Subject | Environmental Science |
| Specific subject area | Photocatalysis |
| Type of data | Tables and Figures |
| How the data were acquired | Photoreactor system: Photodegradation of levofloxacin was performed in a custom reactor box (dimensions: 75 × 29 × 38 cm). Germicidal lamp (Sankyo Denki, G20T10, 20W, 588.5mm length, 32.5mm diameter, λ = 254) was positioned horizontally above the reactor. Aqueous levofloxacin solution was placed 12cm below the lamp. Levofloxacin solution was held under dark for 30 minutes prior to UV light exposure at constant stirring. Aliquots were obtained every time interval. Temperature was controlled at 20°C by continuous water flow thru the jacketed beaker containing the levofloxacin solution. UV-Vis: The spectra for the degradation were obtained using Shimadzu UV-Visible spectrophotometer UV-2600. Settings were set to 2 nm slit width, medium scan speed, and wavelength range of 200-400nm. Baseline correction using deionized water was employed before sample runs. |
| Data format | Raw and Analyzed |
| Description of data collection | Hydroxyapatite catalyst was prepared from mussel shell pre-cursor. Photocatalytic activity of hydroxyapatite was evaluated by the absorbance of levofloxacin obtained after UV irradiation. Box Behnken Modelling was used to optimize three parameters: catalyst dose (0.5-1.6 g/L), LEVO concentration (4-10 ppm), and pH (4-10). Photodegradation efficiency was obtained after 5-hour irradiation subsequently after subjecting to dark conditions (30 mins). Verification of the model was simulated using optimum conditions from the model (1.5 g/L catalyst dose, 4 ppm LEVO concentration, pH 10). An aliquot of 5 mL was obtained every 60 mins. Degradation behavior and extent were evaluated via UV-Vis spectroscopy. MINITAB 17 statistical software was used to generate the response surface model. |
| Data source location | Central Instrumentation Facility-De La Salle University Laguna, Biñan, Laguna, Philippines |
| Data accessibility | Go, Adrian; dela Rosa, Francis; Camacho, Drexel; PUNZALAN, ERIC (2022), “Dataset on photocatalytic degradation of Levofloxacin using hydroxyapatite photocatalyst: Optimization by response surface methodology”, Mendeley Data, V2, doi: 10.17632/h82gspgkpf.2 Mendeley Data repository link: https://data.mendeley.com/datasets/h82gspgkpf/2 |
Value of the Data
-
•
This work presents empirical and statistical data on the photodegradation of levofloxacin via photocatalytic process. The data given here as well as the experimental parameters exhibit the effectivity of hydroxyapatite as an alternative green photocatalyst. Researchers may opt to modify this photocatalyst to further enhance the degradation properties.
-
•
The results of the Box-Behnken modelling and analysis can be used by other investigators to test and compare the influence of experimental parameters (catalyst dosage, LEVO concentration, and pH) on the efficiency of the system in the photodegradation of other related organic compounds.
-
•
The data presented herein can be used in the optimization of similar photocatalytic systems (i.e. hydroxyapatite /UV) for both small and large scale studies. These data can be referenced by academic investigators looking at photocatalytic system designs, testing, and optimization. Scientific professionals working in industries such as wastewater treatment and organic waste disposal facilities may use these data in improving the current methods.
1. Data Description
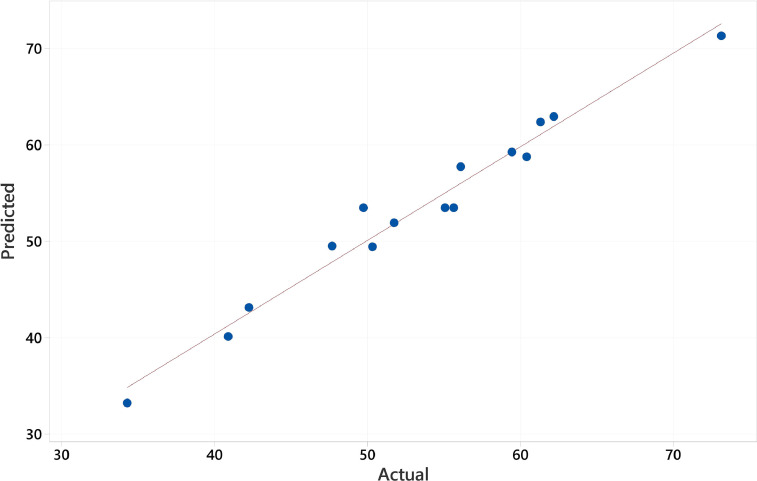
The range and levels of the defined variables considered in the Box Behnken design model is shown in Table 1. These are the catalyst dose (A), levofloxacin concentration (B), and pH value (C). The model equation computing the efficiency is given in Equation (1). In the model, A, B, C, AB, and AC represent the catalyst dosage, levofloxacin concentration, pH value, the interaction of catalyst dosage-levofloxacin, and the interaction of catalyst dosage-pH heavily influencing the behavior of the degradation efficiency, respectively. In Table 2, the efficiency response with different values of the three variables are enumerated. It shows photodegradation efficiency towards levofloxacin. Evaluation of the best fitted equation is given in Table 3 demonstrating that the 2-Factor Interaction describes the curve due to having the highest regression values. The analysis of variance is used to determine the significant factors that affect the photodegradation efficiency. It is also used to evaluate the quality of fit and adequacy of the model (Table 4). The Fisher (F) and probability (P) values are used to determine the significant variables. Values of "Prob > F" less than 0.0500 indicate model terms are significant whereas the opposite indicate non-significance [1]. The generated regression equation representing the photodegradation efficiency of levofloxacin is given in Equation 1. The coefficients of determination (R2) and adjusted R2 are satisfactory to support the significance of the model [2]. Normal probability plot of standardized residuals and predicted vs actual plot is shown in Fig. 1 and 2, respectively. Surface interaction plots of the variables are shown inFig. 4. Results show favorability for high catalyst dosage and pH with low levofloxacin concentration. The software response optimization was set to have the maximum response which obtained the optimum conditions of 1.5 g/L catalyst dose, 4 ppm levofloxacin concentration and pH 10. This generated a predicted efficiency of 71.6% (5 hours). The optimum conditions were simulated experimentally (Fig. 4a) and the observed photodegradation efficiency was 70.6%. Thus, verifying the efficacy of the model. The kinetics of degradation follows pseudo first order equation (Fig. 4b).
| (1) |
Fig. 3.
Contour and surface plots of levofloxacin photodegradation efficiency.
Table 1.
Range and Levels of Box Behnken Design.
| Variable | low | center | high |
|---|---|---|---|
| -1 | 0 | +1 | |
| (A) Catalyst dose (g/L) | 0.5 | 1.0 | 1.5 |
| (B) levofloxacin concentration (ppm) | 4 | 7 | 10 |
| (C) pH | 4 | 7 | 10 |
Table 2.
Box Behnken Model Response Table.
| Std. Order | (A) catalyst dose | (B) LEVO concentration | (C) pH | Efficiency | Predicted Efficiency |
|---|---|---|---|---|---|
| 1 | 0.5 | 4 | 7 | 60.41 | 58.77 |
| 2 | 1.5 | 4 | 7 | 61.31 | 62.37 |
| 3 | 0.5 | 10 | 7 | 34.30 | 33.24 |
| 4 | 1.5 | 10 | 7 | 56.10 | 57.74 |
| 5 | 0.5 | 7 | 4 | 47.69 | 49.52 |
| 6 | 1.5 | 7 | 4 | 50.32 | 49.45 |
| 7 | 0.5 | 7 | 10 | 42.26 | 43.14 |
| 8 | 1.5 | 7 | 10 | 73.13 | 71.31 |
| 9 | 1.0 | 4 | 4 | 59.44 | 59.26 |
| 10 | 1.0 | 10 | 4 | 40.90 | 40.13 |
| 11 | 1.0 | 4 | 10 | 62.18 | 62.95 |
| 12 | 1.0 | 10 | 10 | 51.74 | 51.92 |
| 13 | 1.0 | 7 | 7 | 49.74 | 53.48 |
| 14 | 1.0 | 7 | 7 | 55.64 | 53.48 |
| 15 | 1.0 | 7 | 7 | 55.07 | 53.48 |
Table 3.
Evaluated equation form.
| Model | Lack of fit (P-value) | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|
| Linear | 0.237 | 72.70% | 65.26% | 43.12% |
| 2-Factor Interaction | 0.906 | 97.08% | 94.89% | 91.11% |
| Quadratic | 0.702 | 97.13% | 91.97% | 75.94% |
Table 4.
ANOVA results for the model.
| Source | DF | Adj SS | Adj MS | F-Value | P-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 6 | 1294.11 | 215.685 | 44.36 | 0.000 | significant |
| Linear | 3 | 969.13 | 323.044 | 66.44 | 0.000 | significant |
| (A) catalyst dosage | 1 | 394.8 | 394.805 | 81.20 | 0.000 | significant |
| (B)LEVO conc | 1 | 454.51 | 454.511 | 93.48 | 0.000 | significant |
| (C)pH | 1 | 119.82 | 119.815 | 24.64 | 0.001 | significant |
| 2-Way Interaction | 3 | 324.98 | 108.326 | 22.28 | 0.000 | significant |
| (AB)catalyst dosage*LEVO conc | 1 | 109.2 | 109.203 | 22.46 | 0.001 | significant |
| (BC)catalyst dosage*pH | 1 | 199.37 | 199.374 | 41.00 | 0.000 | significant |
| (AC) LEVO dosage*pH | 1 | 16.4 | 16.402 | 3.37 | 0.104 | not significant |
| Error | 8 | 38.9 | 4.862 | |||
| Lack-of-Fit | 6 | 17.72 | 2.953 | 0.28 | 0.906 | not significant |
| Pure Error | 2 | 21.18 | 10.591 | |||
| Total | 14 | 1333.01 | ||||
| SS | 2.7659 | |||||
| R2 | 97.08% | |||||
| Adjusted R2 | 94.89% | |||||
| Predicted R2 | 91.11% |
Fig. 1.
Normal probability plot of standardized residuals.
Fig. 2.
Predicted vs actual efficiency of levofloxacin photodegradation.
Fig. 4.
a) Photodegradation of levofloxacin (5 hours), b) kinetics of the photodegradation.
2. Experimental Design, Materials and Methods
2.1. Materials
The materials used for the study were Levofloxacin (C18H20FN3O4) (>98%, HPLC, Sigma Aldrich), nitric acid (Emsure), ammonium hydroxide (Qualikelms), ammonium dihydrogen orthophosphate (Ajax Finechem). Mussel shells (Perna virdis) were purchased from a local wet market.
2.2. Hydroxyapatite preparation
Biowaste mussel shells were soaked and cleaned with boiling water and were left to dry. The dried shells were crushed using mortar and pestle and calcined at 1000°C using a furnace. Powdered calcium oxide was produced after this step and was finely grounded using mortar and pestle. Grounded CaO weighing 1.00g was acquired and dissolved in a 20 mL of 2.50 M HNO3 forming Ca(NO3)2 solution. The pH of this solution was then adjusted to 10 using ammonium hydroxide. To this solution, 25 mL of 0.427 M (NH4)2HPO4 was added dropwise under sonication (40 Hz) for 1 hour. The resulting mixture was centrifuged, washed with deionized water, and dried overnight at 100°C to obtain the hydroxyapatite powder [3].
2.3. Determination of levofloxacin photodegradation efficiency
The photodegradation of Levofloxacin was carried out in a reactor that was designed and built in our laboratory. The type of UV lamp used was Sankyo Denki 20 W, λ = 254 monochromatic light. A constant flow of water (20°C) in between the jacketed beaker was set to control the temperature. A mass of Hydroxyapatite was transferred to a 100 mL levofloxacin solution with constant stirring. The pH of the reaction mixture was adjusted by careful addition of a solution of NaOH or HCl. The solution was allowed to stand while stirring in the dark for 30 mins and then was UV-illuminated at constant stirring (400 RPM). The acquired sample solution was centrifuged to remove the suspension. The extent of degradation was determined spectrophotometrically using Shimadzu UV-Visible spectrophotometer UV-2600 at 287 nm. Percent degradation efficiency was calculated as follows [4,5],
| (2) |
Where Co = initial concentration and C= variable concentration
2.4. Experimental design
2.4.1. Box-Behnken experiments
The software used for the modeling is Minitab 17. The model is based on Box-Behnken design with 15 experimental runs. The parameters analyzed were (A) catalyst dose, (B) LEVO concentration, and (C) pH. Photodegradation efficiency (after 5 -hour UV-irradiation) was set as the response. Predicted optimum parameters for the photodegradation were obtained after setting the response to maximum.
2.4.2. Verification of Box-Behnken model
Predicted parameters (1.5 g/L catalyst dose, 4ppm LEVO, and pH 10) from the model were simulated in an actual experiment. An aliquot (5mL) of the levofloxacin solution was obtained every 60-minute interval to determine the concentration and evaluate the photodegradation behavior.
Ethics Statements
N/A
CRediT Author Statement
Adrian Go: Investigation, Methodology, Writing – original draft; Francis dela Rosa: Formal analysis, Writing – review & editing; Drexel Camacho: Resources, Writing – review & editing; Eric Punzalan: Supervision, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the Department of Science and Technology – Science Education Institute (DOST-SEI) for funding this research. We wish to thank Dr. Angelyn Lao of the De La Salle University Mathematics and Statistics Department for the informative discussions.
Data Availability
References
- 1.Berkani M., Kadmi Y., Bouchareb M.K., Bouhelassa M., Bouzaza A. Combinatıon of a Box-Behnken design technique with response surface methodology for optimization of the photocatalytic mineralization of C.I. Basic Red 46 dye from aqueous solution. Arab. J. Chem. 2020;13:8338–8346. doi: 10.1016/j.arabjc.2020.05.013. [DOI] [Google Scholar]
- 2.Chollom M.N., Rathilal S., Swalaha F.M., Bakare B.F. Degradation of veterinary antibiotics from slaughterhouse wastewater using titanium dioxide as a catalyst. WIT Trans. Ecol. Environ. 2018;217:135–144. doi: 10.2495/SDP180131. [DOI] [Google Scholar]
- 3.Edralin E.J.M., Garcia J.L., dela Rosa F.M., Punzalan E.R. Sonochemical synthesis, characterization and photocatalytic properties of hydroxyapatite nano-rods derived from mussel shells. Mater. Lett. 2017;196 doi: 10.1016/j.matlet.2017.03.016. [DOI] [Google Scholar]
- 4.Pinheiro D., Sunaja Devi K.R., Jose A., Karthik K. Box–Behnken design and experimental study of ciprofloxacin degradation over Ag2O/CeO2/g-C3N4 nanocomposites. Int. J. Environ. Sci. Technol. 2021;18:2303–2324. doi: 10.1007/s13762-020-02971-y. [DOI] [Google Scholar]
- 5.Buazar F., Alipouryan S., Kroushawi F., Hossieni S.A. Photodegradation of odorous 2-mercaptobenzoxazole through zinc oxide/hydroxyapatite nanocomposite. Appl. Nanosci. 2015;5:719–729. doi: 10.1007/s13204-014-0368-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.