Abstract
Growth promoters are added with broiler feed to boost the overall feed efficiency and growth rate. The current study investigated the effect of dexamethasone (DEX)—a commonly used growth promoter—on the broiler growth rate, meat quality, and muscle biology. Four homogenous groups (20 chicks/group) of broiler one-day-old chicks were fed commercial broiler feed where the treatment groups received 3, 5, and 7 mg/kg of DEX with their diet for 28 d. Feed consumption and body weight were monitored on a daily basis. Muscle samples were collected on 7, 14, 21, and 28 d of the experiment to investigate meat quality and muscular biology. The residue of DEX in meat was detected using thin-layer chromatography. We observed that DEX had substantially decreased (P < 0.05) feed intake, feed efficiency, and overall weight gain in the broiler. While the weight of breast and thigh meat was decreased, the relative meat weight (meat/body weight) was increased significantly in chicks fed DEX. Simultaneously, body fat decreased while the percentage of fat increased significantly (P < 0.05) in the DEX groups. Contrariwise, DEX improved the investigated meat quality parameters with the potential threat of accumulation of DEX residue in the meat at a high dose (7 mg/kg). We also observed that DEX significantly increased the number of myofibers and decreased the cross-sectional area of myofibers. Based on these findings, we conclude that DEX reduces feed intake, feed efficiency, and growth rate, but might improve meat quality with a potential risk of residual DEX accumulation if fed at a high dose.
Key words: broiler, dexamethasone, growth performance, meat quality, muscle biology
INTRODUCTION
The poultry business has already established itself as a major player in the agricultural economy, supplying a significant portion of animal protein to meet the per capita demand for meat. The poultry industry grew quickly (25%) among farm animals between 2007 and 2017 (FAO –Food and Agriculture Organization of the United Nations, 2019). This industry has a lot of promise to ensure food security and boost the country's economy. It was anticipated that the demand for animal-derived food will increase by 70% between 2005 and 2050 where the poultry meat is likely to have the highest growth of 121% (Mottet and Tempio, 2017).
Broiler farms are specifically designed for meat production since they grow quickly and produce meat that is soft, tender, and low in fat. Over several decades, efforts to increase the growth rate led to the discovery and widespread usage of several growth promoters. Natural derivatives of steroid hormones, such as glucocorticoids (GCs), are also available as synthetic versions. Dexamethasone (DEX) is a synthetic glucocorticoid used to treat inflammation and suppress the immune system (Watteyn et al., 2013). It is reportedly used as a growth promoter for cattle fattening purposes (Kamal et al., 2019). But the question arises concerning the efficacy and residual effect of DEX as a growth promoter in poultry species like broilers. The use of anabolic steroids and other growth-promoting substances results in the buildup of residues in the tissue, posing a public health risk (Hirpessa et al., 2020). However, it is quite difficult to detect these residues in biological samples. In this regard, thin-layer chromatography (TLC) can be used as a screening method to analyze steroids and their metabolites in a variety of samples (Bhawani et al., 2010).
The contemporary broiler chicken has a higher feed conversion efficiency, growth rate, and greater breast and thigh muscle yield, all of which benefit the consumer. As the broiler production at a mass level has already been accomplished, the target now shifts to improving the quality of meat by regulating the factors that determine the broiler meat quality (Mir et al., 2017). As per the earlier studies, faster growth and higher muscle yield may have negative impacts on the broiler meat quality (Petracci et al., 2015). Color, pH, water holding capacity (WHC), tenderness, shelf life, collagen content, protein solubility, cohesiveness, fat binding capacity, etc., are the most significant and perceptible meat features that influence consumers' judgment during purchasing meat or meat products. The pH affects meat quality features like color, WHC, juiciness, tenderness, and shelf life (Mir et al., 2017). The WHC reflects the highest quantity of water that the muscle proteins can retain under the circumstances imposed during measurement. Increased WHC, tenderness, juiciness, and firmness improve the quality of meat and the economic value of meat (Mir et al., 2017).
The morphologic and morphometric characteristics of meat fibers are also important in influencing broiler performance and determining meat quality and quantity (Ismail and Joo, 2017). Muscle weight increases in fast-growing broilers by increasing fiber size, length, and number. The breast and thigh muscles of the chicken represent the highest proportion of chicken carcass. Consumers consider chicken breast and thigh flesh the most valuable portion; hence they have a high economic value. This might be the reason behind the constant interest of the broiler industry in evaluating the weight and outturn of the chicken breast meat as the most significant criteria. According to prior researches, GCs cause a decrease in organ weight, which is identifiable as gross and microscopic alterations (Cannizzo et al., 2008; Rademaker and de Vries, 2009). In this context, the histopathological investigation may provide a useful tool as an indirect marker of DEX therapy. In light of the foregoing, the current study was conducted to evaluate the effects of DEX on the growth performance and meat quality, as well as the muscular biology of the breast and thigh muscle of the broiler.
MATERIALS AND METHODS
Ethical Standard
This study was carried out following institutional ethical standards and authorized by the Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University (BAU), Bangladesh [Authorization no. - AWEEC/BAU/2020(3)].
Experimental Design
A total of 80 healthy one-day-old chicks (DOCs) were used in this study. The DOCs were then parted into 4 homogenous groups at random (control group [C], experimental group 1 [E1], 2 [E2] and 3 [E3]) and allotted to individual pens. Antibiotic or growth-promoter-free and nutritionally balanced broiler feed (Sultana et al., 2020) and fresh, cool drinking water (ad libitum) were supplied throughout the experiment. All groups of broilers were reared in identical conditions. Notwithstanding, dietary dexamethasone (Decason, BP 0.5 mg, Opsonin Pharma Limited, Bangladesh) was supplied with feed to the experimental groups at the rate of 3 mg/kg (E1), 5 mg/kg (E2), and 7 mg/kg (E3). The feed intake was incessantly monitored and documented to assure that each broiler is taking the intended quantity of DEX with feed.
Sample Collection
Five broilers were sacrificed (by manual cervical dislocation method) from each group on 7 (D7), 14 (D14), 21 (D21), and 28 (D28) d of the experiment. Then the broilers were dissected immediately, and breast and thigh muscles were collected. The weight (g) of the breast and thigh muscle, as well as the quantity of fat, was measured and recorded on D28. Muscle samples were then preserved in 10% formalin for histological study.
Meat Quality Test
The color of the breast and thigh meat was visually inspected and texture was studied by sensory evaluation. Then, the breast and thigh meat samples were kept in separate air-tight jars and preserved at 4˚C temperature for 24 h. Meat color, meat ultimate pH (pHu), and WHC of broiler meat were then determined to assess and compare the meat quality of control and DEX treated broilers.
Meat Color Test
The surface color of the collected broiler meat sample was measured by a CR-400 Chroma Meter (Minolta Co., Osaka, Japan). Two to threecm thick deboned meat samples were used to avoid background influence. The evaluation was done on the posterior surface of the skinless breast and thigh meat. The meat color was expressed in terms of CIE values where L*, a*, and b* indicate the lightness, redness, and yellowness of the meat samples, respectively. Hue angle [tan−1 (b*/a*)] and Saturation index [{(a*2 × b*2)1/2}] were then computed for each sample to study the change in color of the meat samples.
Measurement of Meat Ultimate pH
The pH measurement of the samples was carried out with a pH meter. The pH meter reading was set to 7.00 by dipping its head into a neutral buffer solution at 24˚C temperature. pH reading was taken from three different regions of each meat sample, and then the mean value was determined.
Measurement of WHC of Meat
The WHC of the meat samples was measured using centrifugal force. For this, 1 g of breast and thigh meat was weighed (W0) from each sample and then chopped with a meat chopper. Then the chopped meat was loaded in a PCR tube, and the total weight of the sample along with the tube was measured and recorded as W1. The tubes were then placed inside the centrifuge machine. Centrifugal force was applied through 10,000 RCF (Relative Centrifugal Force) at 4˚C temperature for 10 min. Then the supernatant fluid was removed properly by micropipette. The weight of the sample along with the tube was again measured and recorded as W2. Finally, the WHC was computed using the following formula, WHC (%) = [1 − {(W1 − W2)/W0}] × 100.
TLC
TLC was performed for the qualitative detection of DEX residue in the meat samples of 28 d old broilers treated with DEX. At first, 2 g of meat sample was measured and blended properly. Five mL of phosphate buffer solution was added to the blended sample. The mixture was vortexed and 1 mL of trichloroacetic acid was added to precipitate the protein. The mixture was then shaken carefully and centrifuged at 3,000 r.p.m. for 15 min. Two mL of supernatant was then collected carefully and filtered. Two mL of diethyl ether was added. The sample was then kept in resting condition for 10 min. Ten µL of DEX standard (Renata Pharmaceuticals Limited, Bangladesh) and analytes were spotted on the TLC plate (TLC Plate Silica Gel 60 F254, Merck, Germany) by using a micropipette. The TLC plate was then settled in a TLC chamber containing solvent (dichloromethane: ethyl acetate: methanol - 14:4:1) and covered by a lid. Then it was left undisturbed until the solvent touched the marked line on the top. Spots were then seen under the UV light of 256 nm wavelength.
Histomorphologic Study
The formalin-fixed muscle samples were processed and stained with Hematoxylin and Eosin stain for the histological investigation. The stained tissue sections were examined under a microscope (Leica DMR; Leica Microsystems, Wetzlar, Germany) at 400X magnification. A photomicroscope (Model: CX41U-LH50HG, Olympus Corporation, Tokyo, Japan) was used to capture histological images at 400X magnification for a better presentation of the histological findings. The number of myofibers and their cross-sectional area (square micrometer) were counted and quantified using computer-assisted image analysis software (ImageJ freehand tool).
Statistical Analyses
A completely randomized design was followed for the study. SPSS software version 22 (IBM SPSS Statistics 22) was used to analyze the obtained data. Shapiro-Wilk test was carried out to evaluate the normality of the data set. One-way analysis of variance (ANOVA) and posthoc Duncan's multiple range test were used to compare the groups' differences. The strength of association between 2 variables was measured using Pearson's correlation coefficient. Probability values (P) less than 0.05 were regarded as significant. All data were presented as mean ± standard error of the mean (SEM).
RESULTS
Live Weight of DOCs
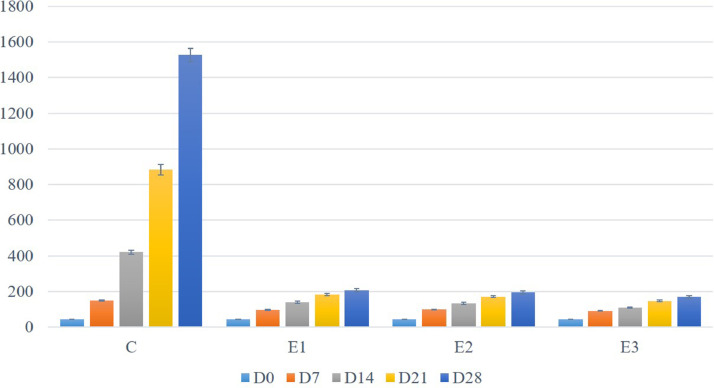
The initial mean live weight of the DOCs was 43.85 ± 0.41 g. After separating into groups, the weight of different groups was almost similar (P > 0.05), which were 44.00 ± 0.68 g, 43.50 ± 0.80 g, 44.20 ± 0.68 g, and 43.70 ± 1.12 g in groups C, E1, E2, and E3, respectively (Figure 1).
Figure 1.
Mean body weight of broilers of both the control and DEX groups on different days of the experiment.
Feed Intake
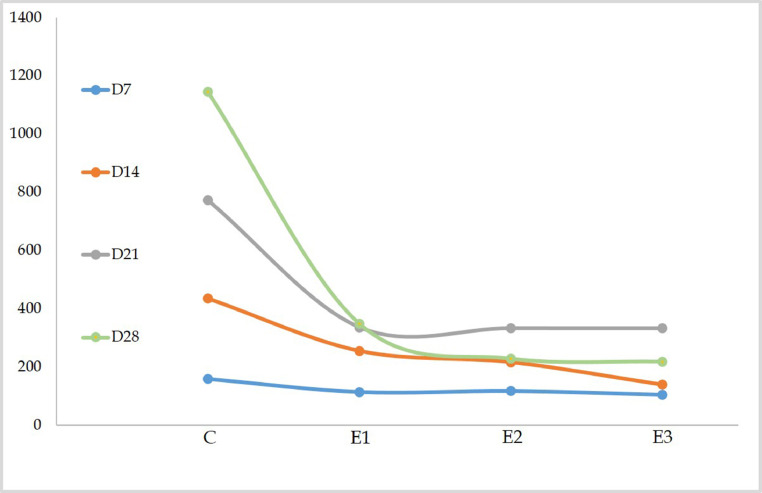
Figure 2 depicts the average feed consumption by an individual bird in the control and DEX groups over 7-d at different ages. From D7 to D28, there were significant (P < 0.05) variations in feed consumption between the control and DEX groups. On D28, feed intake in DEX groups was considerably lower than in the control group by 81.02% in group E1, 80.18% in group E2, and 81.07% in group E3. Between the DEX groups, there were also substantial changes in feed consumption. On D14, feed intake in group E3 was considerably lower (P < 0.05) than in groups E1 and E2. On D28, there was a significant difference (P < 0.05) between groups E1, E2, and E1, E3.
Figure 2.
The average feed intake (in 7 days) by individual broiler of different groups.
Body Weight
The mean weight of broilers of various groups on different d of the experiment is shown in Figure 1. The mean body weight was significantly (P < 0.05) increased with the growing age of the broilers of the control group, whereas the DEX groups only showed a numerical increase which is statistically nonsignificant (P > 0.05). There were also significant differences among the control and DEX groups. The maximum final body weight (on D28) was found in the control group, whereas the minimum weight was found in group E3.
The average weight gain by various experimental groups at 7-d intervals is shown in Figure 3. The average weight gain for the first 7 d of the experiment was 106.75 g, 53.1 g, 54.8 g, and 48.0 g in groups C, E1, E2, and E3, respectively. On D14, the weight was increased by 179.17% in group C, 44.26% in group E1, 33.94% in group E2, and 20.07% in group E3. On D21, the weight was increased by109.84, 32.07, 28.96, and 34.42%, whereas the weight was increased by 72.9, 12.82, 14.04%, and 15.28% on D28 in C, E1, E2, and E3 groups, respectively. However, the average weight gain was maximum in the control group and minimum in group E3.
Figure 3.
Average weight gain by different experimental groups at seven days intervals.
Feed Conversion Ratio
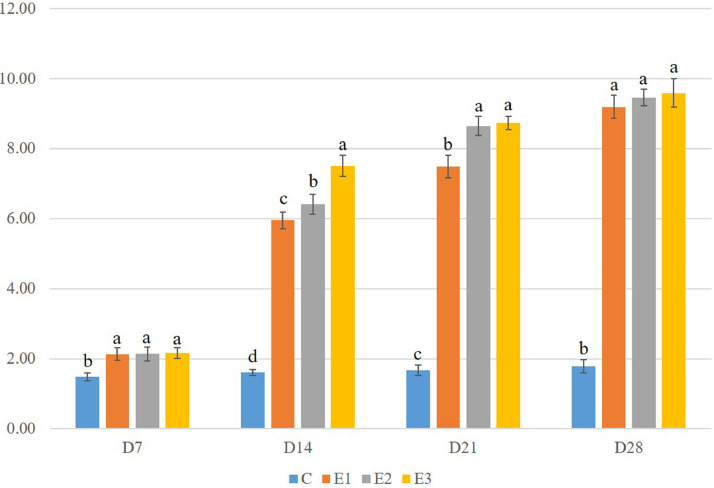
The feed conversion ratio of broilers of various groups on different d of the experiment is shown in Figure 4. The results show that feed efficiency was decreased by 43.92% in group E1, 44.59% in group E2, and 46.62% in group E3 from the control on D7. On D14, the feed efficiency was significantly (P < 0.01) decreased by 269.57, 298.14, and 366.46% in groups E1, E2, and E3, respectively. On D21 and D28, the feed efficiency was significantly (P < 0.01) decreased by 348.5, 417.96, 423.25, and 416.85, 431.46, 438.76% in groups E1, E2, and E3, respectively. No significant difference was seen between the DEX groups on D7 and D28. On D14, the FCR was significantly (P < 0.05) increased in group E3 compared to groups E1 and E2. On D21, the FCR was increased significantly (P < 0.05) in groups E2 and E3 compared to group E1.
Figure 4.
Feed conversion ratio (FCR) of different experimental groups of broilers. Mean values lacking a common superscript significantly differ from each other (P < 0.05).
Weight of Breast and Thigh Meat
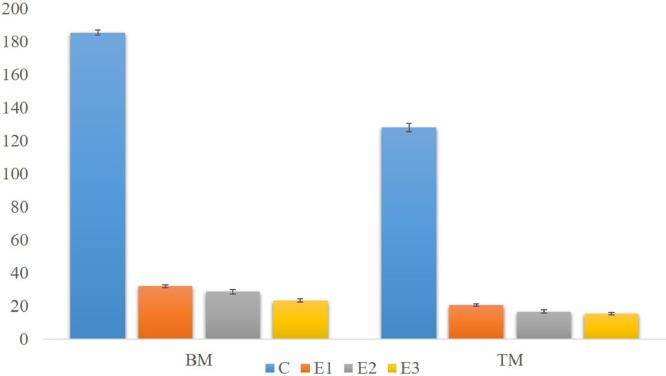
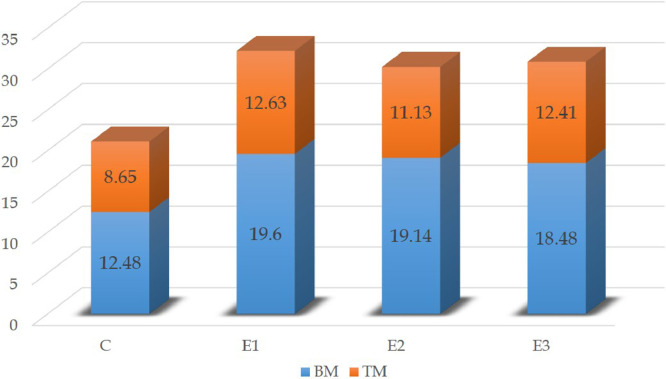
The mean weight of breast and thigh meat on D28 is shown in Figure 5. The breast meat weight was significantly (P < 0.05) less than the control group by 82.67% in group E1, 84.48% in group E2, and 87.36% in group E3. The weight of thigh meat was significantly (P < 0.05) decreased from the control group by 83.82, 86.94, and 87.72% in groups E1, E2, and E3, respectively. Though the mean breast and thigh meat weight was decreased, the meat percentage against body weight significantly (P < 0.05) increased in the DEX treated broilers shown in Figure 6.
Figure 5.
The average weight of the breast and thigh meat of different groups of broilers on D28. Abbreviations: BM, breast meat, TM, thigh meat.
Figure 6.
Meat (BM- Breast meat, TM-Thigh meat) percentage in different experimental groups on D28.
Body Fat and Subcutaneous Fat
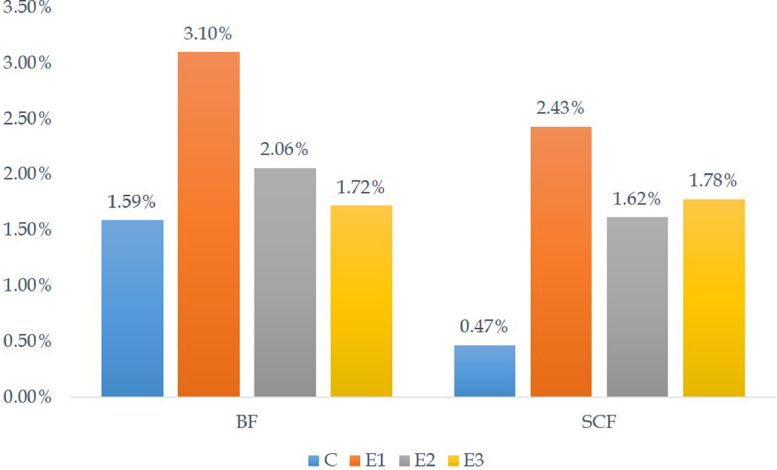
The amount of body fat on D28 was 24.33 ± 0.29 g, 6.44 ± 0.19 g, 4.03 ± 0.59 g, and 2.93 ± 1.29 g whereas the amount of subcutaneous fat was 7.13 ± 0.45 g, 5.04 ± 0.22 g, 3.15 ± 0.32 g, and 3.03 ± 0.51 g in groups C, E1, E2, and E3, respectively. The percentage of fat in the body is shown in Figure 7. However, the amount of fat significantly (P < 0.05) decreased in the DEX groups from the control group, the percentage of fat increased significantly (P < 0.05) against the body weight in the DEX groups. The highest fat percentage was seen in group E1, which was decreased in groups E2 and E3.
Figure 7.
Percentage (%) of body fat (BF) and subcutaneous fat (SCF) on D28.
Meat Quality
Breast Meat
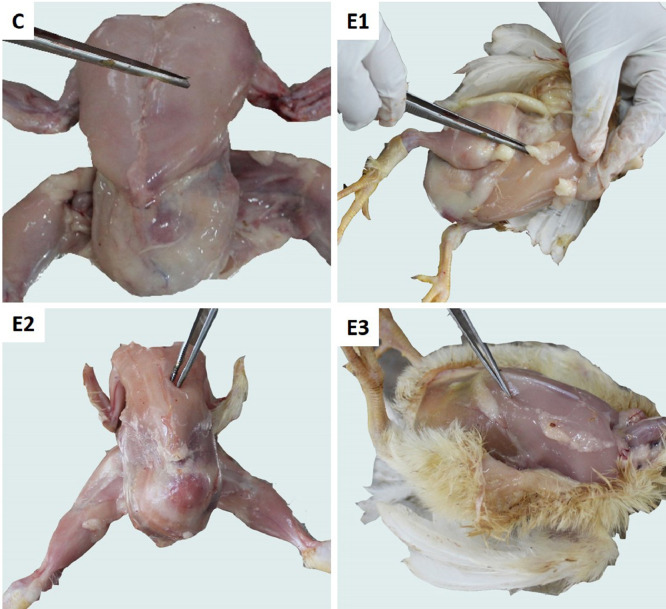
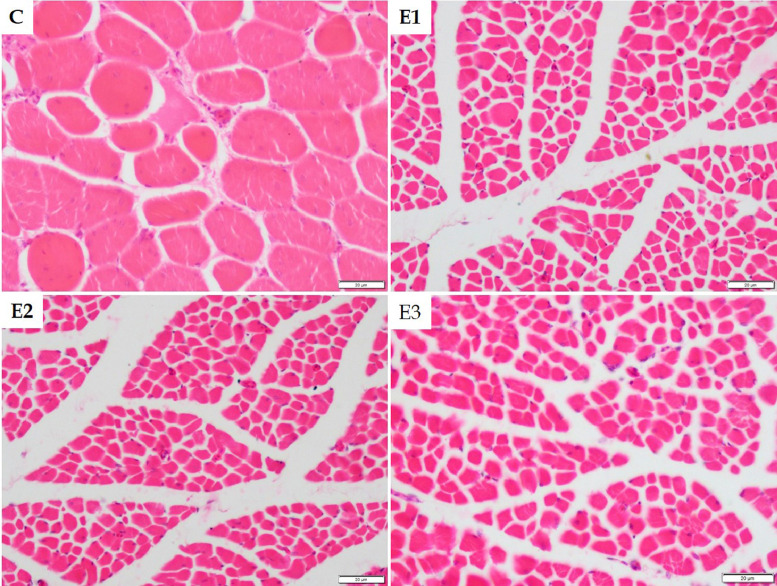
In the visual inspection, the breast meat appeared pale pinkish in the control group and slightly dark pinkish in DEX groups on D28 (Figure 8). The meat samples were comparatively softer in the DEX groups than in the control. However, the meat of the young broilers was comparatively softer than the old broilers. The physicochemical properties of broiler breast meat on D28 treated with different doses of DEX are presented in Table 1. The L* of the breast meat of DEX groups numerically decreased, whereas a* increased significantly (P < 0.05). There was no significant (P > 0.05) difference in meat b* values among the control and DEX groups. The hue angle significantly (P < 0.05) decreased in the DEX groups. The saturation index was almost similar in different groups except for group E1, which showed significantly (P < 0.05) higher color saturation. Significant negative correlations were found between meat L* and pHu (−0.892, −0.973, −0.897, and −0.952 in groups C, E1, E2, and E3, respectively). Negative correlations between meat L* and WHC (−0.870, −0.500, −0.747, and −0.997 in groups C, E1, E2, and E3, respectively) were also found. On the other hand, positive correlations were found between meat a* and pHu (0.762, 0.915, 0.887, and 0.896 in groups C, E1, E2, and E3, respectively). There were positive correlations between meat a* and WHC (0.877, 0.542, 0.945, 0.997 in groups C, E1, E2, and E3, respectively). The pHu and WHC were also positively correlated (0.866, 0.500, 0.729, and 0.997 in groups C, E1, E2, and E3, respectively). The pHu and WHC of breast meat increased significantly (P < 0.05) in the DEX groups. There were also significant (P < 0.05) variations in WHC between the DEX groups.
Figure 8.
Representative images of broiler showing the breast and thigh meat color.
Table 1.
Physicochemical properties of broiler breast meat on D28 treated with different doses of DEX.
| Attributes | Groups |
|||
|---|---|---|---|---|
| C | E1 | E2 | E3 | |
| L* | 58.50 ± 3.39a | 55.19 ± 0.25ab | 52.70 ± 2.01b | 53.07 ± 1.45b |
| a* | 5.19 ± 0.52b | 10.92 ± 1.51a | 11.80 ± 2.08a | 10.85 ± 0.96a |
| b* | 10.90 ± 0.42b | 13.15 ± 1.28a | 10.20 ± 1.77ab | 9.24 ± 1.11ab |
| Hue angle | 64.65 ± 1.36a | 50.57 ± 1.16b | 40.85 ± 0.15c | 40.20 ± 1.32c |
| Saturation index | 12.08 ± 0.60b | 17.10 ± 1.95a | 15.60 ± 2.73a | 14.26 ± 1.44a |
| pHu | 6.25 ± 0.05b | 7.18 ± 0.04a | 7.21 ± 0.02a | 7.25 ± 0.01a |
| WHC | 81.23 ± 0.03c | 91.70 ± 0.06b | 92.47 ± 0.43b | 93.33 ± 0.20a |
Abbreviations: a*, Redness; b*, Yellowness; L*, Lightness; pHu, ultimate pH; WHC, water holding capacity.
Mean values within a row lacking a common superscript significantly differ from each other.
Thigh Meat
The thigh meat appeared pale pinkish in the control group. However, it appeared slightly darker and reddish-pink in the DEX groups compared to the control group (Figure 8). The thigh meat was less soft than the breast meat, and however, the DEX groups revealed softer meat than the control. The physicochemical properties of broiler thigh meat on D28 treated with different doses of DEX are presented in Table 2. The L* of the thigh meat decreased significantly (P < 0.05) in groups E2 and E3 compared to the control.
Table 2.
Physicochemical properties of broiler thigh meat on D28 treated with different doses of DEX.
| Attributes | Groups |
|||
|---|---|---|---|---|
| C | E1 | E2 | E3 | |
| L* | 58.37 ± 0.56a | 57.52 ± 5.02a | 44.35 ± 4.71c | 48.18 ± 3.52b |
| a* | 6.85 ± 1.46d | 10.41 ± 0.10c | 14.48 ± 1.04b | 22.02 ± 1.35a |
| b* | 10.21 ± 1.16c | 13.13 ± 0.66ab | 14.75 ± 0.37a | 15.89 ± 1.13a |
| Hue angle | 56.94 ± 2.77a | 51.50 ± 1.68b | 31.26 ± 0.78c | 35.78 ± 0.41d |
| Saturation index | 12.32 ± 1.77c | 16.78 ± 0.45b | 16.92 ± 1.08b | 27.15 ± 1.75a |
| pHu | 6.29 ± 0.03d | 6.84 ± 0.07c | 7.03 ± 0.03b | 7.24 ± 0.04a |
| WHC | 79.77 ± 0.03d | 88.87 ± 0.07c | 91.73 ± 0.07b | 92.07 ± 0.03a |
Abbreviations: a*, Redness; b*, Yellowness; L*, Lightness; pHu, ultimate pH; WHC, water holding capacity.
Mean values within a row lacking a common superscript significantly differ from each other.
Meat a* was increased gradually and differed significantly (P < 0.05) from the control group. There were also significant (P < 0.05) differences among the DEX groups. Significant (P < 0.05) differences in meat b* values were also found between the control and DEX groups (E2, E3). The hue angle significantly (P < 0.05) decreased from the control in the DEX groups. There were also significant (P < 0.05) differences between the DEX groups. The saturation index increased significantly (P < 0.05) from the control group and differed significantly (P < 0.05) among the DEX groups. The highest saturation was seen in group E3. There were negative correlations between L* and pHu (−0.753, −0.850, −0.690, and −0.980 in groups C, E1, E2, and E3, respectively. Negative correlations between meat L* and WHC (−0.686, −0.575, −0.808, and −0.915 in groups C, E1, E2, and E3, respectively) were also found. On the other hand, there were positive correlations between meat a * and pHu (0.737, 0.985, 0.781, and 0.551 in C, E1, E2, and E3 groups, respectively). The meat a* and WHC were also positively correlated (0.942, 0.545, 0.877, and 0.850 in C, E1, E2, and E3 groups, respectively). Positive correlations were found between pHu and WHC (0.981, 0.984, 0.833, and 0.843 in C, E1, E2, and E3 groups, respectively). The pHu and WHC of thigh meat increased significantly (P < 0.05) in the DEX groups. There were also significant (P < 0.05) variations among the DEX groups.
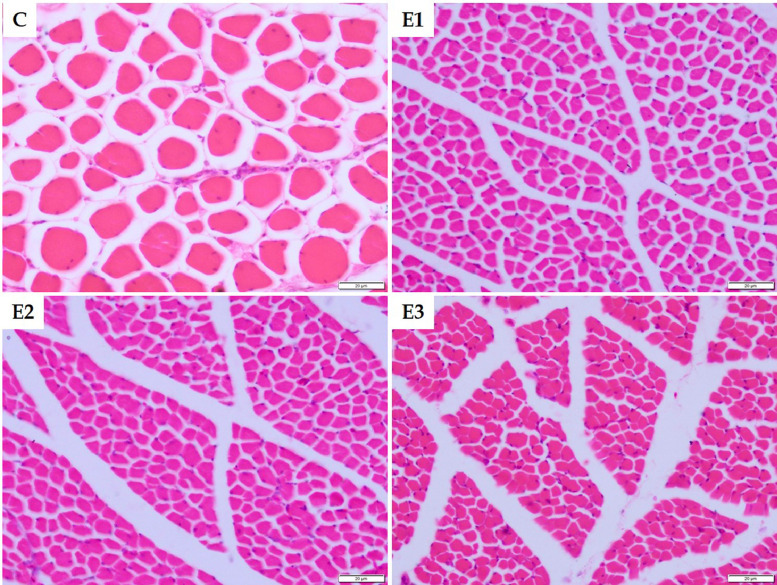
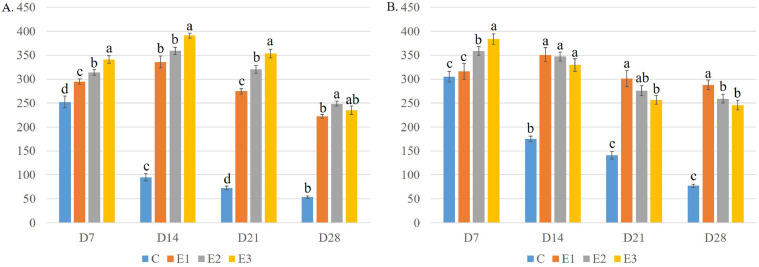
Histology of Breast Muscle
The histoarchitecture of the breast muscle on D28 is presented in Figure 9. DEX altered myofiber count in the breast muscle of DEX groups (Figure 11A). The mean quantity of myofibers per microscopic field was significantly (P < 0.05) higher in the DEX groups than in the control on different d of the experiment. The average number of myofibers per microscopic field increased significantly (P < 0.05) on D14 but then showed a significant (P < 0.05) decrease with the progression of age in the control group. In group E1, the average number of myofibers increased on D14 compared to D7 but decreased significantly (P < 0.05) on D21 and D28 compared to D14. In groups E2 and E3, it decreased gradually with the progression of age. It also decreased gradually in the DEX groups with the increase in DEX dose. However, the lowest numbers of myofibers were found in the E3 group on different experiment d.
Figure 9.
Representative photomicrographs of histoarchitecture of breast muscle on D28 (400×).
Figure 11.
Effect of DEX on the number of myofibers in breast (A) and thigh (B) muscle in relation to days of the experiment in broiler chicken. Data were expressed as mean ± SEM. Differences among the groups of birds were compared using one-way ANOVA with posthoc Duncan's multiple range test. Mean values lacking a common superscript significantly differ from each other (P < 0.05).
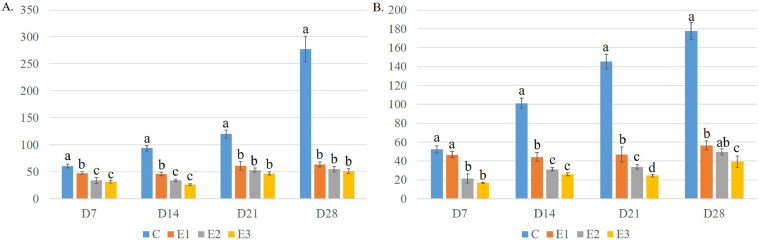
The mean cross-sectional area of each myofiber increased significantly (P < 0.05) in the control group with the progression of age (Figure 12A). On the contrary, the cross-sectional area of myofibers showed only a numerical increase in the DEX groups on D21 and D28. However, it decreased gradually in the DEX groups with the increase in DEX dose.
Figure 12.
Effect of DEX on the mean cross-sectional area of the breast (A) and thigh (B) meat fibers in relation to days of the experiment in broiler chicken. Data were expressed as mean ± SEM. Differences among the groups of birds were compared using one-way ANOVA with posthoc Duncan's multiple range test. Mean values lacking a common superscript significantly differ from each other (P < 0.05).
Histology of Thigh Muscle
The histoarchitecture of the thigh muscle on D28 is presented in Figure 10. DEX altered the myofiber count in the thigh muscle of broiler chicken (Figure 11B). The average number of myofibers per microscopic field was significantly (P < 0.05) higher in the DEX groups compared to the control on different d of the experiment. The number of myofibers per microscopic field decreased significantly (P < 0.05) with the progression of age. The lowest numbers of myofibers were found in the E3 group on different d of the experiment.
Figure 10.
Representative photomicrographs of histoarchitecture of thigh muscle on D28 (400×).
The mean cross-sectional area of each myofiber increased significantly (P < 0.05) in the control group with the progression of age (Figure 12B). On the contrary, it decreased gradually in the DEX groups with the progression of age. However, the lowest cross-sectional area of myofibers was found in the E3 group on different experiment d.
Thin Layer Chromatographic Detection of DEX in Breast and Thigh Meat
Results from TLC are shown in Figure 13. DEX was detected in both breast and thigh meat in group E3 on D28.
Figure 13.

Qualitative detection of the steroid substances in meat samples by thin-layer chromatography. The Black arrows indicate the spots visualized under ultraviolet rays (256 nm). Sd- DEX standard; E3B and E3T indicate breast and thigh meat samples of group E3, respectively.
DISCUSSION
Live Weight of DOCs
The mean initial weight and uniformity in weight of a flock are the key aspects in broiler farming because they are the key prerequisites for obtaining a homogenous outcome (Neto et al., 2013). According to a previous study, broiler harvest weight is significantly influenced by the initial weight (Mendes et al., 2011). So, it is an important indicator of chick quality. The initial mean live weight of the DOCs was 43.85 ± 0.41g. This is almost similar to the finding described in another study (Neto et al., 2013). The mean weight of DOCs in different groups of broiler was also homogenous.
Feed Intake
The results of previous studies on the effect of GC on broiler feed consumption were highly disputed. A significantly increased feed intake after GC treatment was observed in some (Song et al., 2011; Liu et al., 2014), but other study reports suggested significantly decreased feed intake (Lv et al., 2018; Osho and Adeola, 2020). However, it appears to be linked to the duration, dosage, and route of GC administration. Feed consumption in the current study was practically the same in the first week in both the control and DEX groups but reduced dramatically from the second to the fourth week. This finding is in line with previous study reports (Lin et al., 2004a; Dong et al., 2007).
Body Weight
The body weight gain of DEX groups was lesser than the control group, similar to the findings of earlier studies (Eid et al., 2003; Dong et al., 2007; Lv et al., 2018; Osho and Adeola, 2020). Growth rate or body weight gain is closely linked to feed intake. When feed intake limits the growth rate, additional factors like ambient temperature, stocking density, feeder space, and water availability come into play (Scott, 2005). The reduced weight gain due to GC treatment is linked to the augmented energy expenditure, breakdown of proteins, and gluconeogenesis (Lin et al., 2004a,b). However, the current results suggest that the reduced weight gain in DEX treated broilers might be associated with reduced feed intake and increased energy expenditure.
Feed Conversion Ratio
In the current study, the feed conversion ratio has increased significantly in the DEX groups, indicating significantly reduced feed efficiency. This finding is in accordance with previous findings (Lin et al., 2006; Dong et al., 2007). Feed efficiency is closely related to the genetic potentiality of the individual animal. GCs induce oxidative stress and elevate the reactive oxygen species in circulation (Eid, 2010). In physiological conditions, reactive oxygen species protect animal cells against infectious agents by phagocytosis. However, in a stressed condition, they react with cell structures and attack proteins, lipids, carbohydrates, and nucleic acid within the cell and thus reduce weight gain by proteolysis and gluconeogenesis (Lin et al., 2004a; Eid, 2010). This stimulated proteolysis was further reflected in the significantly reduced body weight gain and feed efficiency in DEX treated broilers which are in accordance with previous reports (Eid et al., 2003; Lv et al., 2018; Osho and Adeola, 2020). In our study, the DEX treated broilers ate more feed than the control group to gain each gram of body weight. Reduced body weight gain and the smaller volume of the gastrointestinal tract may also be associated with the lower feed efficiency in the DEX-treated broilers.
Weight of Breast and Thigh Meat
In the present study, the weight of breast and thigh meat was significantly lower in the DEX-treated broilers. This finding is in agreement with previous findings (Lin et al., 2006; Dong et al., 2007). Our findings and previous studies indicate retarded development of thigh and breast meat in response to GC treatment. Though previous study reports claimed that breast meat is more susceptible to GC therapy than thigh meat, the percentage of weight reduction was almost similar in case of both breast and thigh meat in the current study. This indicates that DEX may exert a similar catabolic effect on both the breast and thigh meat. As mentioned earlier, multiple factors, such as , reduced feed intake and feed efficiency, GC induced increased proteolysis and gluconeogenesis, reduced capacity of protein synthesis, etc., may act in concert in the retardation of breast and thigh meat development. However, in the DEX-treated broilers, the meat weight to body weight was greater than in the control group. This suggests that DEX has a more suppressive effect on the different organs of the broiler than the breast and thigh meat. However, GC does not affect protein content or amino acid composition, even though breast and thigh muscle mass development is slowed down (Dong et al., 2007).
Body Fat and Subcutaneous Fat
In the current study, body fat and subcutaneous fat were lower in the DEX groups. However, the relative fat content (% of body mass) was significantly increased in the DEX groups, which are in line with the previous reports (Eid et al., 2003; Lin et al., 2006; Dong et al., 2007; Cai et al., 2009). According to prior researches, GC administration increases insulin concentrations in plasma, indicating insulin resistance in GC-treated chickens (Lin et al., 2006; Cai et al., 2009). Therefore, the high insulin level in the blood may lead to augmented fat deposition in the distressed broilers. In the current study, abdominal and subcutaneous fat accumulation due to DEX treatment indicates that fat accumulation can aggregate toward both the abdominal and peripheral regions of the body of stressed broilers. The maximum fat accumulation in the higher dose group was reported in a previous study (Eid et al., 2003). However, in the current study, the lower dosage group had the highest proportion of fat gain. The effect might be related to adipose tissue lipolysis caused by extended exposure to a high dosage of DEX.
Meat Quality
In the current study, the lightness of both breast and thigh meat significantly decreased in the DEX groups indicating darker meat in the DEX groups. This, however, contradicts the findings of a prior study, which found that DEX therapy enhanced meat lightness (Pan et al., 2019). The redness of breast meat increased significantly, which was highly significant in case of thigh meat, and this indicates that the thigh meat was more reddish than the breast meat. The thigh meat of DEX-treated broilers, on the other hand, was more yellowish, which is consistent with earlier research (Pan et al., 2019). These color coordinates and hue angles indicate darker reddish-yellow coloration of breast and thigh meat in DEX treated broilers than in control. The color intensity was also higher in the DEX treated broilers. However, the pHu of both breast and thigh meat increased in the DEX treated broilers which is similar to the results described previously (Elmajdoub et al., 2016). Meat quality characteristics like WHC, color, tenderness, juiciness, and shelf life are all affected by pH. (Mir et al., 2017). Low pH causes meat proteins to break, enabling light to reflect unevenly off the surface, resulting in a pale color; conversely, high pH causes meat proteins to split, resulting in a dark color (Swatland, 2008; Mir et al., 2017).
According to a prior study, broiler breast meat with a high pH had a greater WHC, which explains why DEX treated broilers in the current study have a higher WHC (Mir et al., 2017). On the contrary, decreased WHC of breast meat was also reported in response to DEX treatment (Pan et al., 2019). Increased WHC indicates increased tenderness and juiciness of the meat (Mir et al., 2017). There are negative perceptions among the consumers about broiler meat as it is pale, soft, and exudative. However, decreased lightness, increased redness, yellowness, pHu, and WHC of meat illustrated improved meat quality in the DEX-treated broilers.
Histology of Breast and Thigh Muscle
Meat quality is connected to histological and biochemical features of muscle fibers. However, there is little data to prove that changes in fiber size and quantity have a deleterious influence on meat quality. Some researchers demonstrated that large breast meat fibers result in high meat quality (Tůmová and Teimouri, 2009). The quantity and cross-sectional area of breast and thigh myofibers per microscopic field were higher in the DEX groups in the current investigation, consistent with earlier findings (Topp et al., 2003; Fry et al., 2016; Gokulakrishnan et al., 2017). Fiber area is inversely proportional to fiber number, whereas fiber number and area are proportional to muscle mass (Tůmová and Teimouri, 2009). So, the reduced cross-sectional area might have contributed to the increased number of myofibers per microscopic field in the current study.
The number of myofibers in breast meat was reduced in broilers administered a high dosage of DEX with feed, according to a prior study report (Akter et al., 2021). Hence, it is anticipated that the lower myofiber count in the breast meat might be related to the decreased weight gain in response to dietary DEX in the broiler. Muscle mass and meat quality are affected by the size and quantity of myofiber (Tůmová and Teimouri, 2009). Fibers tend to grow more rapidly when the amount of myofiber is less in poultry (Choi and Kim, 2009). There is a significant correlation between final meat quality and fiber cross-sectional area (Joo et al., 2013).
Muscles with larger myofiber diameters, for example, have tougher meat than muscles with smaller myofiber sizes in broiler chicken (Chen et al., 2007). Animals with greater numbers of muscle fibers of moderate sizes produce a higher quantity and quality of meat which supports the current study findings (Choi and Kim, 2009). However, slow-growing chickens exhibit more tender breast meat than fast-growing chickens (Fanatico et al., 2007). In the current study, the investigated meat quality parameters were improved in the DEX treated broilers which exhibited a slower growth rate than the control group.
Screening of Dexamethasone Residue in Broiler Meat
Misuse or overuse of veterinary drugs can act as a chemical hazard due to their accumulation in the edible tissues. Food safety is a must for ensuring public health because the accumulation of residues of growth promoters in the edible tissues can lead to serious consequences (Beyene, 2016; Sultana et al., 2020; Mingle et al., 2021). These residues in meat products can cause serious hazards to human health (Kadim et al., 2020). TLC is considered an efficient screening technique for the qualitative determination of steroid residues in the tissues of various animals. In the current study, DEX residue was detected in the high dose group's breast and thigh meat. Previous studies also reported similar findings where the high-performance liquid chromatography method was used to detect and quantify the steroid residues (Reig and Toldrá, 2008; Kadim et al., 2020).
Steroid hormones are commonly used in large animal fattening, especially in cattle (Kamal et al., 2019). In this study, we have extended this observation in the broiler to evaluate the potential effects of a steroid (DEX) on the growth performance and meat quality. DEX therapy significantly reduced the growth rate, thigh and breast muscle yield but increased fat percentage in the broiler. Meat quality parameters like color, pHu, WHC, muscle fiber size, and quantity were improved in response to DEX treatment. However, DEX residue was detected in both the breast and thigh meat at a high dose which can be a threat to consumer health. Therefore, further study is recommended to evaluate the composition and nutritional value of broiler meat treated with DEX.
Acknowledgments
Acknowledgments
We are thankful to the Department of Animal Science, Bangladesh Agricultural University (BAU), Mymensingh-2202, Bangladesh, for providing the laboratory facilities. We are also extending our gratitude to the Department of Pharmacology, BAU, Mymensingh-2202, Bangladesh, for the research assistance.
This work was supported by Bangladesh Agricultural University Research System (BAURES), BAU, Mymensingh-2202, Bangladesh (Grant No- 2020/57/BAU).
Author contributions: NS conceptualized and supervised the experiment. RI performed the experiment and data analyses. MAH, RI, and UA contributed to the meat quality evaluation. MAH, MRI, and NS assisted in data interpretation. NS and RI drafted the manuscript. MRI and MAH critically revised the manuscript.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akter F., Sultana N., Afrose M., Kabir A., Islam R., Sikder M.H. Adaptations of muscular biology in response to potential glucocorticoid treatment in broiler chicken. J. Adv. Biotechnol. Exp. Ther. 2021;4:1–8. [Google Scholar]
- Beyene T. Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J. Vet. Sci. Technol. 2016;7:1–7. [Google Scholar]
- Bhawani S.A., Sulaiman O., Hashim R., Ibrahim M.N. Thin-layer chromatographic analysis of steroids: a review. Trop. J. Pharm. Res. 2010;9:301–313. [Google Scholar]
- Cai Y., Song Z., Zhang X., Wang X., Jiao H., Lin H. Increased de novo lipogenesis in liver contributes to the augmented fat deposition in dexamethasone exposed broiler chickens (Gallus gallus domesticus) Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009;150:164–169. doi: 10.1016/j.cbpc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Cannizzo F.T., Miniscalco B., Riondato F., Bollo E., Barbarino G., Giorgi P., Mazzini C., Biolatti B. Effects of anabolic and therapeutic doses of dexamethasone on thymus morphology and apoptosis in veal calves. Vet. Rec. 2008;163:448–452. doi: 10.1136/vr.163.15.448. [DOI] [PubMed] [Google Scholar]
- Chen X.D., Ma Q.G., Tang M.Y., Ji C. Development of breast muscle and meat quality in Arbor Acres broilers, Jingxing 100 crossbred chickens and Beijing fatty chickens. Meat Sci. 2007;77:220–227. doi: 10.1016/j.meatsci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009;122:105–118. [Google Scholar]
- Dong H., Lin H., Jiao H.C., Song Z.G., Zhao J.P., Jiang K.J. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. Part A Mol. Integr. 2007;147:189–195. doi: 10.1016/j.cbpa.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Eid Y.Z. Novel antioxidant canolol reduces glucocorticoid induced oxidative stress in broiler chickens. Egypt. Poult. Sci. 2010;30:917–926. [Google Scholar]
- Eid Y.Z., Ohtsuka A., Hayashi K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br. Poult. Sci. 2003;44:127–132. doi: 10.1080/0007166031000085427. [DOI] [PubMed] [Google Scholar]
- Elmajdoub A., Garbaj A., Abolghait S., El-Mahmoudy A. Evaluation of boldenone as a growth promoter in broilers: safety and meat quality aspects. J. Food Drug Anal. 2016;24:284–292. doi: 10.1016/j.jfda.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow-and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- FAO –Food and Agriculture Organization of the United Nations. 2019. World Food and Agriculture statistical pocket book, 2019. ISSN 2225-7381. Accessed December, 2021. http://www.fao.org/3/ca6463en/ca6463en.pdf
- Fry C.S., Nayeem S.Z., Dillon E.L., Sarkar P.S., Tumurbaatar B., Urban R.J., Wright T.J., Sheffield-Moore M., Tilton R.G., Choudhary S. Glucocorticoids increase skeletal muscle NF-κB inducing kinase (NIK): links to muscle atrophy. Physiol. Rep. 2016;4:e13014. doi: 10.14814/phy2.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokulakrishnan G., Chang X., Fleischmann R., Fiorotto M.L. Precocious glucocorticoid exposure reduces skeletal muscle satellite cells in the fetal rat. J. Endocrinol. 2017;232:561–572. doi: 10.1530/JOE-16-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirpessa B.B., Ulusoy B.H., Hecer C. Hormones and hormonal anabolics: residues in animal source food, potential public health impacts, and methods of analysis. J. Food Qual. 2020;2020:1–12. 5065386. [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food. Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kadim I.T., Al-Amri I.S., Al-Kindi A.Y., Al-Magbali R., Abbas G., Imranul Q.M., Khalaf S.K. Residues of antibiotics, anabolic steroids, pesticides in assorted broiler chicken meat and meat products available in Omani market. EC Nutr. 2020;15:1–13. [Google Scholar]
- Kamal M.T., Hashem M.A., Al Mamun M., Hossain M.M., Razzaque M.A. Study of cattle fattening system in selected region of Bangladesh. SAARC J. Agric. 2019;17:105–118. [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 1. Chronic exposure. Comp.Biochem. Physiol. Part B Biochem. Mol. Biol. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 2. Short-term effect. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lin H., Sui S.J., Jiao H.C., Buyse J., Decuypere E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2006;143:400–405. doi: 10.1016/j.cbpa.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Liu L., Song Z., Jiao H., Lin H. Glucocorticoids increase NPY gene expression via hypothalamic AMPK signaling in broiler chicks. Endocrinol. 2014;155:2190–2198. doi: 10.1210/en.2013-1632. [DOI] [PubMed] [Google Scholar]
- Lv Z.P., Peng Y.Z., Zhang B.B., Fan H., Liu D., Guo Y.M. Glucose and lipid metabolism disorders in the chickens with dexamethasone-induced oxidative stress. J. Anim. Physiol. Anim. Nutr. 2018;102:706–717. doi: 10.1111/jpn.12823. [DOI] [PubMed] [Google Scholar]
- Mendes A.S., Paixao S.J., Restelatto R., Reffatti R., Possenti J.C., De Moura D.J., Morello G.M.Z., de Carvalho T.M.R. Effects of initial body weight and litter material on broiler production. Braz. J. Poult. Sci. 2011;13:165–170. [Google Scholar]
- Mingle C.L., Darko G., Borquaye L.S., Asare-Donkor N.K., Woode E., Koranteng F. Veterinary drug residues in beef, chicken, and egg from Ghana. Chem. Afr. 2021;4:339–348. [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. J. 2017;73:245–256. [Google Scholar]
- Neto R.M., Surek D.C., da Rocha F., Dahlke F., Maiorka A. The effect of grouping one-day-old chicks by body weight on the uniformity of broilers. J. Appl. Poult. Res. 2013;22:245–250. [Google Scholar]
- Osho S.O., Adeola O. Chitosan oligosaccharide supplementation alleviates stress stimulated by in-feed dexamethasone in broiler chickens. Poult. Sci. 2020;99:2061–2067. doi: 10.1016/j.psj.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Ma X.K., Zhao P.F., Piao X.S. Weeping forsythia extract alleviates dexamethasone-induced oxidative injury of breast muscles in broilers. Animal. 2019;13:2660–2668. doi: 10.1017/S175173111900096X. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Rademaker K.J., de Vries W.B. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Semin. Fetal Neonatal. Med. 2009;14:171–177. doi: 10.1016/j.siny.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Reig M., Toldrá F. Liquid chromatography for the rapid screening of growth promoters residues in meat. Food Anal. Methods. 2008;1:2–9. [Google Scholar]
- Scott T.A. Variation in feed intake of broiler chickens. Recent Adv. Anim. Nutr. Austral. 2005;15:237–244. [Google Scholar]
- Song Z., Yuan L., Jiao H., Lin H. Effect of corticosterone on hypothalamic corticotropin-releasing hormone expression in broiler chicks (Gallus gallus domesticus) fed a high energy diet. Asian-Australas. J. Anim. Sci. 2011;24:1736–1743. [Google Scholar]
- Sultana N., Afrose M., Rafiq K. Effects of steroid growth promoter on morphological and biochemical adaptations in liver of broiler. Vet. World. 2020;13:2330–2337. doi: 10.14202/vetworld.2020.2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatland H.J. How pH causes paleness or darkness in chicken breast meat. Meat Sci. 2008;80:396–400. doi: 10.1016/j.meatsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Topp K.S., Painter P.L., Walcott S., Krasnoff J.B., Adey D., Sakkas G.K., Taylor J., McCormick K., Iofina M., Tomlanovich S., Stock P. Alterations in skeletal muscle structure are minimized with steroid withdrawal after renal transplantation. Transplantation. 2003;76:667–673. doi: 10.1097/01.TP.0000076096.45542.1B. [DOI] [PubMed] [Google Scholar]
- Tůmová E., Teimouri A. Chicken muscle fibres characteristics and meat quality: a review. Sci. Agric. Bohem. 2009;40:253–258. [Google Scholar]
- Watteyn A., Wyns H., Plessers E., Russo E., De Baere S., De Backer P., Croubels S. Pharmacokinetics of dexamethasone after intravenous and intramuscular administration in broiler chickens. Vet. J. 2013;195:216–220. doi: 10.1016/j.tvjl.2012.06.026. [DOI] [PubMed] [Google Scholar]