Abstract
Objective
To examine the association between multimorbidity burden and incident heart failure (HF) among people with HIV (PWH) and people without HIV (PWoH).
Patients and Methods
The HIV-HEART study is a retrospective cohort study that included adult PWH and PWoH aged 21 years or older at Kaiser Permanente between 2000 and 2016. Multimorbidity burden was defined by the baseline prevalence of 22 chronic conditions and was categorized as 0-1, 2-3, and 4 or more comorbidities on the basis of distribution of the overall population. People with HIV and PWoH were followed for a first HF event, all-cause death, or up to the end of follow-up on December 31, 2016. Using Cox proportional hazard regression, hazard ratios and 95% CIs were calculated to examine the association between multimorbidity burden and incident HF among PWH and PWoH, separately.
Results
The prevalences of 0-1, 2-3, and 4 or more comorbidities were 83.3%, 13.0%, and 3.7% in PWH (n=38,868), and 82.2%, 14.3%, and 3.5% in PWoH (n=386,586), respectively. After multivariable adjustment, compared with people with 0-1 comorbidities, the hazard ratios of incident HF associated with 2-3 and 4 or more comorbidities were 1.33 (95% CI, 1.04-1.71) and 2.41 (95% CI, 1.78-3.25) in PWH and 2.10 (95% CI, 1.92-2.29) and 4.09 (95% CI, 3.64-4.61) in PWoH, respectively.
Conclusion
Multimorbidity was associated with a higher risk of incident HF among PWH and PWoH, with more prominent associations in PWoH and certain patient subgroups. The identification of specific multimorbidity patterns that contribute to higher HF risk in PWH may lead to future preventative strategies.
Abbreviations and Acronyms: aHR, adjusted hazard ratio; CVD, cardiovascular disease; CVRN, Cardiovascular Research Network; HF, heart failure; KP, Kaiser Permanente; PWH, people with HIV; PWoH, people without HIV
Better treatment and management of HIV have led to improved survival and less AIDS-related mortality among people with HIV (PWH).1, 2, 3 With the aging of PWH, morbidity and mortality associated with aging-related causes have increased, and there is a heavy burden of chronic conditions, including hypertension, diabetes, respiratory and renal diseases, cancers, cardiovascular diseases (CVDs), and heart failure (HF).4, 5, 6, 7, 8 Although there is some evidence that excess CVD risk in PWH vs people without HIV (PWoH) has decreased over time,9 the risk of outcomes among PWH, including HF10,11 and CVD mortality, remains high.12 The HIV-HEART study has previously reported a 73% higher risk of HF among PWH vs PWoH after adjusting for sociodemographic and clinical characteristics.13
The presence of multiple chronic conditions, or multimorbidity, is associated with lower functional status and quality of life in PWoH, as well as higher medical costs, disability, and mortality.14,15 Multimorbidity is also prevalent in PWH, especially with the aging of this population.5,16 The increasing prevalence of co-occurring cardiovascular and noncardiovascular comorbidities suggests common etiologies and shared risk factors that may influence the trajectory of multimorbidity, functional decline, and CVD risk.17,18 Some comorbidities have treatment recommendations that are discordant with HIV care, and in PWH, this can create a difficult choice between managing their HIV and conditions that potentially increase their risk for CVDs.19, 20, 21 Previous studies have examined multimorbidity in people with established CVDs, but there is less research examining the association between multimorbidity and incident CVD.22 The impact of multimorbidity burden on the risk of incident HF, especially among PWH and PWoH, is largely unknown. Given the poor prognosis associated with HF,23 it is important to understand the ways in which complex comorbidity burdens can influence the risk of HF. This study expands on previous findings from the HIV-HEART study by examining the association of multimorbidity burden with incident HF among PWH and PWoH.
Patients and Methods
Study Population
The HIV-HEART study population was derived from 3 Kaiser Permanente (KP) integrated health care delivery systems that provide comprehensive care, including specialty care (which includes HIV and cardiology specialists and other subspecialty care), as well as pharmacy and laboratory services tracked through integrated electronic health records. Kaiser Permanente Northern California serves more than 4.5 million members at 21 hospital-based medical centers and over 255 medical offices; KP Southern California serves 4.7 million members at 15 hospital-based medical centers and over 230 medical offices; and KP Mid-Atlantic States serves 800,000 members at 36 medical offices. The study was approved by the institutional review boards of each region, and a waiver of informed consent was obtained because of the observational nature of the study.
Identification of PWH and Matched PWoH
The KP Virtual Data Warehouse served as a primary data source for subject identification and characterization. The Virtual Data Warehouse comprises an electronic health record that contains data regarding linked demographic characteristics, administrative, inpatient, outpatient, and health care utilization of KP members.24 Each site also has implemented comprehensive HIV registries that maintain up-to-date lists of all PWH, HIV transmission risk factors, dates of known HIV infection, AIDS diagnoses, and HIV-related laboratory and pharmacy data.
To ensure a comprehensive population representing patients treated in the current antiretroviral therapy era, we included all eligible adult (aged ≥21 years) PWH diagnosed between 2000 and 2016. People without HIV from the same source populations and study period were frequency-matched up to 10:1 to PWH on the basis of the calendar year (ie, the year of start of follow-up for PWH), age (±1 year), sex, race, and primary KP treating facility to account for possible practice differences across sites. Before matching, persons listed in the HIV registries not otherwise identified as PWH during the study period were excluded.
Multimorbidity
To characterize multimorbidity burden, several conditions were included on the basis of their high prevalence or association with poor outcomes in older adults with HF previously delineated in earlier Cardiovascular Research Network (CVRN) studies. This includes 13 comorbidities recommended by the US Department of Health and Human Services Strategic Framework on Multiple Chronic Conditions (arthritis, asthma, atrial fibrillation/flutter, cancer, chronic kidney disease, chronic obstructive pulmonary disease, coronary heart disease, dementia, depression, diabetes mellitus, hypertension, osteoporosis, and stroke).25 On the basis of previous work by the CVRN, we also included the following 9 conditions in our definition of multimorbidity: abnormal thyroid function, anemia, aortic valvular disease, chronic liver disease, hearing impairment, peripheral artery disease, hospitalized bleeding, ventricular tachycardia, and visual impairment. These included conditions previously specified by Drye et al26 under a task order from the Centers for Medicare and Medicaid Services to systematically narrow the list of chronic conditions identified by Centers for Medicare and Medicaid Services’ Chronic Condition Warehouse to those most relevant to placing persons at increased risk for hospitalization.Similar to previous CVRN studies,27 multimorbidity burden in the current study was defined on the basis of the overall distribution of comorbidities in the study population with categories of 0-1 (no multimorbidity), 2-3, and 4 or more comorbidities.
Incident HF and Follow-up Time
The primary outcome of the current study was incident HF, defined as the first HF event occurring between 2000 and 2016 for all PWH and PWoH. Patients with pre-existing HF before cohort entry were excluded. Using a previously validated approach,28, 29, 30, 31, 32 incident HF was defined as a hospitalization with a primary discharge diagnosis of HF or having 3 or more outpatient visits coded for HF with at least 1 visit with a cardiologist using the International Classification of Diseases Ninth /Tenth Revision codes. This approach has previously found a positive predictive value greater than or equal to 95% of clinical HF for admissions with a primary discharge diagnosis of HF on the basis of this algorithm.33,34 The index date was the date of entry of all PWH and PWoH in the cohort, and the follow-up ended at the first HF event, death, disenrollment, or administrative censoring on or before December 31, 2016.
Covariates
Baseline covariates, including age categories (21-34, 35-54, 55-64, and ≥65 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, other), neighborhood-level income (above/below median census-based household income), body mass index (calculated as the weight in kilograms divided by the height in meters squared) categories (<18.5, 18.5-24.9, 25.0-29.9, and ≥30 kg/m2), smoking status (ever and nonsmoker), alcohol use disorders, and illicit drug use disorders, were identified at or within 4 years before the index date.
Statistical Analyses
The baseline characteristics of the HIV-HEART study were calculated among PWH and PWoH using t tests for continuous variables and χ2 tests for categorical variables to find statistically significant differences by HIV status. The cumulative number of chronic comorbidities among the entire population and separately for PWH and PWoH are described in Supplemental Table 1 (available online at http://www.mcpiqojournal.org). Multimorbidity burden among PWH and PWoH, separately, was described overall and within the categories of age, sex, and race/ethnicity. The number, person-time, and the rate per 100 person-years of incident HF were also described among PWH and PWoH. Using the Cox proportional hazards regression, multivariable adjusted hazard ratios (aHRs) and 95% CIs for the association of multimorbidity burden with incident HF were calculated separately among PWH and PWoH, overall and by age group, sex, and race/ethnicity. Multimorbidity burden was modeled with indicators 2-3 and 4 or more comorbidities, with the lowest category (0-1) serving as a reference group. Multivariable-adjusted models included simultaneous adjustment of age, sex, race/ethnicity, neighborhood income, body mass index, smoking status, alcohol use, and illicit drug use. To determine whether the associations between multimorbidity burden and incident HF were different between PWH and PWoH, we tested the hypothesis of no interaction between HIV status and multimorbidity categories by calculating the P values for the overall association and within the subgroups of age, sex, and race/ethnicity. All statistical tests were 2-sided, with P<.05 considered statistically significant. SAS 9.4 was used for all analyses.
Results
Baseline Characteristics
People with HIV (n=38,868) and PWoH (n=386,586) were similar with respect to age (mean, 41.4 years), sex (88% men), and race/ethnicity (38% White, 21% Black, 20% Hispanic, 4% Asian/Pacific Islander, and 17% other/unknown). (Table 1) Compared with PWoH, PWH were more likely to live below the median level of income (n=4213 [10.8%] vs n=32,865 [8.5%], P<.001), had lower proportions of overweight or obese individuals (n=5637 [14.5%] vs n=97,387 [25.2%], P<.001), and had lower proportions of nonsmokers (n=6548 [16.8%] vs n=88,487 [22.9%], P<.001). Compared with PWoH, PWH were more likely to be diagnosed for alcohol use (n=4178 [10.8%] vs n=28,768 [7.4%], P<.001) and illicit drug use (n=6022 [15.5%] vs n=20,363 [5.3%], P<.001). Overall, 32,372 (83.3%), 5056 (13.0%), and 1440 (3.7%) of PWH had 0-1, 2-3, and 4 or more comorbidities, respectively. Similarly, 317,853 (82.2%), 55,312 (14.3%), and 13,421 (3.5%) of PWoH had 0-1, 2-3, and 4 or more comorbidities, respectively. These distributions of the number of comorbidities overall and among PWH and PWoH, separately, are listed in Supplemental Table 1.
Table 1.
Sociodemographic Factors and Multimorbidity Burden Among People Without and With HIV
| Baseline charactersitics | PWoH (n=386,586) | PWH (n=38,868) | P value |
|---|---|---|---|
| Age (y) mean ± SD | 41.4 (10.8) | 41.4 (10.8) | .87 |
| Age group (y), n (%) | .97 | ||
| 21-34 | 112,750 (29.2) | 11,294 (29.1) | |
| 35-54 | 232,067 (60.0) | 23,363 (60.1) | |
| 55-64 | 34,319 (10.6) | 3455 (9.2) | |
| ≥65 | 7450 (1.9) | 756 (2.0) | |
| Men, n (%) | 339,135 (87.7) | 34,125 (87.8) | .68 |
| Race/ethnicity, n (%) | .98 | ||
| White | 146,555 (37.9) | 14,796 (38.1) | |
| Black | 81,519 (21.1) | 8161 (21.0) | |
| Hispanic | 79,104 (20.5) | 7928 (20.4) | |
| Asian/Pacific Islander | 14,983 (3.9) | 1506 (3.9) | |
| Other/Unknown | 64,425 (16.7) | 6477 (16.7) | |
| Neighborhood-level low-median income, n (%) | <.001 | ||
| Yes | 32,865 (8.5) | 4213 (10.8) | |
| No | 283,393 (73.3) | 24,748 (63.7) | |
| Missing | 70,328 (18.2) | 9907 (25.5) | |
| Body mass index (kg/m2) (%) | <.001 | ||
| <18.5 | 872 (0.2) | 259 (0.7) | |
| 18.5-24.9 | 31,073 (8.0) | 4373 (11.3) | |
| 25-29.9 | 49,447 (12.8) | 3647 (9.4) | |
| ≥30 | 47,940 (12.4) | 1990 (5.1) | |
| Missing | 257,254 (66.6) | 28,599 (74.6) | |
| Smoking status, n (%) | <.001 | ||
| Ever smoker | 53,086 (13.7) | 5377 (13.8) | |
| Nonsmoker | 88,487 (22.9) | 6548 (16.8) | |
| Missing | 245,013 (63.4) | 26,943 (69.3) | |
| Alcohol use, n (%) | 28,768 (7.4) | 4178 (10.8) | <.001 |
| Illicit drug use, n (%) | 20,363 (5.3) | 6022 (15.5) | <.001 |
| Multimorbidity burden, n (%) | <.001 | ||
| 0-1 | 317,853 (82.2) | 32,372 (83.3) | |
| 2-3 | 55,312 (14.3) | 5056 (13.0) | |
| ≥4 | 13,421 (3.5) | 1440 (3.7) |
PWH, people with HIV; PWoH, people without HIV.
Multimorbidity Burden Among PWH and PWoH
Among adults aged 21-34 years, the prevalences of multimorbidity in PWH having 2-3 and 4 or more comorbidities were 9.1% and 1.1%, respectively, and those in PWoH were 7.0% and 0.5%, respectively (Table 2). Among adults aged 35-54 years, the prevalences of multimorbidity in PWH having 2-3 and 4 or more comorbidities were 13.6% and 3.6%, respectively, and those in PWoH were 15.1% and 2.8%, respectively. Among adults aged 55-64 years, the prevalences of multimorbidity in PWH having 2-3 and 4 or more comorbidities were 19.2% and 9.6%, respectively, and those in PWoH were 29.2% and 12.2%, respectively. Among adults aged 65 years and older, the prevalences of multimorbidity in PWH having 2-3 and 4 or more comorbidities were 24.5% and 19.3%, respectively, and those in PWoH were 33.0% and 29.4%, respectively. The distribution of multimorbidity burden was similar among PWH and PWoH by sex and race/ethnicity, with the largest multimorbidity burden observed among women and Black individuals regardless of their HIV status.
Table 2.
Multimorbidity Burden Among People Without and With HIV, Stratified by Age, Sex, and Race/Ethnicitya
| Multimorbidity burden, n (%b) | PWoH |
PWH |
||||
|---|---|---|---|---|---|---|
| 0-1 | 2-3 | ≥4 | 0-1 | 2-3 | ≥4 | |
| Age group (y) | ||||||
| 21-34 | 104,351 (92.6) | 7854 (7.0) | 545 (0.5) | 10,141 (89.8) | 1026 (9.1) | 127 (1.1) |
| 35-54 | 190,611 (82.1) | 34,972 (15.1) | 6484 (2.8) | 19,348 (82.8) | 3181 (13.6) | 834 (3.6) |
| 55-64 | 20,090 (58.5) | 10,028 (29.2) | 4201 (12.2) | 2458 (71.1) | 664 (19.2) | 333 (9.6) |
| ≥65 | 2801 (37.6) | 2458 (33.0) | 2191 (29.4) | 425 (56.2) | 185 (24.5) | 146 (19.3) |
| Sex | ||||||
| Male | 284,120 (83.8) | 44,781 (13.2) | 10,234 (3.0) | 28,796 (84.4) | 4211 (12.3) | 1118 (3.3) |
| Female | 33,733 (71.1) | 10,531 (22.2) | 3187 (6.7) | 3576 (75.4) | 845 (17.8) | 322 (6.8) |
| Race/ethnicity | ||||||
| White | 116,896 (79.8) | 23,778 (16.2) | 5881 (4.0) | 12,035 (81.3) | 2100 (14.2) | 661 (4.5) |
| Black | 61,954 (76.0) | 15,204 (18.7) | 4361 (5.4) | 6412 (78.6) | 1323 (16.2) | 426 (5.2) |
| Hispanic | 66,968 (84.7) | 9961 (12.6) | 2175 (2.8) | 6790 (85.7) | 908 (11.5) | 230 (2.9) |
| Asian/Pacific Islander | 12,623 (84.3) | 2010 (13.4) | 350 (2.3) | 1283 (85.2) | 184 (12.2) | 39 (2.6) |
| Other/Unknown | 59,412 (92.2) | 4359 (6.8) | 654 (1.0) | 5852 (90.4) | 541 (8.4) | 84 (1.3) |
PWH, people with HIV; PWoH, people without HIV.
Row percentages.
Rates of Incident HF by Multimorbidity Burden
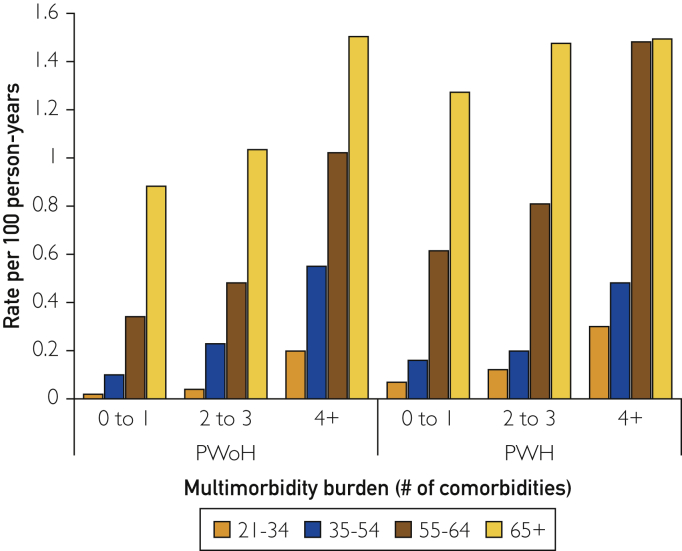
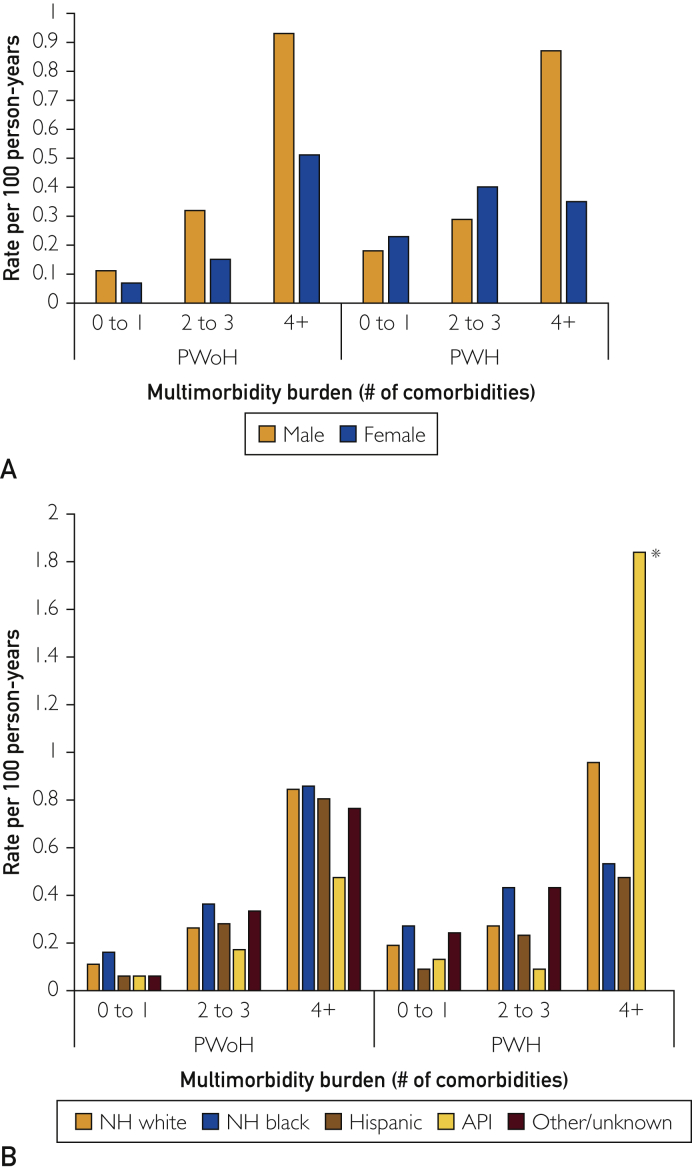
Over a median follow-up of 3.8 years (interquartile range, 1.4-9.0 years), the rates of incident HF per 100 person-years were 0.19, 0.31, and 0.76 in PWH having 0-1, 2-3, and 4 or more comorbidities, respectively, and 0.11, 0.29, and 0.83 in PWoH having 0-1, 2-3, and ≥4 comorbidities, respectively. The rates of incident HF were higher with advancing age and greater multimorbidity burden (Figure 1; Supplemental Table 2, available online at http://www.mcpiqojournal.org). Among PWH having 4 or more comorbidities, the rates of incident HF were similar for individuals aged 55-64 years and 65 years or older, and the rates were higher than those in PWoH with 4 or more comorbidities. Among PWH having 0-1 or 2-3 comorbidities, the absolute rates of incident HF were higher than those in PWoH having 0-1 or 2-3 comorbidities for women and for Black and White individuals (Figure 2; Supplemental Table 2). As only few individuals within categories were stratified by HIV status, multimorbidity, and race/ethnicity, we interpreted these rates cautiously.
Figure 1.
Rates of incident heart failure among people without and with HIV, by age group. PWH, people with HIV; PWoH, people without HIV.
Figure 2.
Rates of incident heart failure among people without and with HIV, by (A) sex and (B) race/ethnicity. API, Asian/Pacific Islander; NH, non-Hispanic; PWH, people with HIV; PWoH, people without HIV.
∗ Total number of API individuals with HIV and having 4 or more comorbidities = 39.
Hazard Ratio of Incident HF Overall and by Age
Among PWH, the aHRs of incident HF were 1.33 (95% CI, 1.04-1.71) and 2.41 (95% CI, 1.78-3.25) for having 2-3 and 4 or more comorbidities, respectively, compared with 0-1 comorbidities (Table 3). Among PWoH, the aHRs of incident HF were 2.10 (95% CI, 1.92-2.29) and 4.09 (95% CI, 3.64-4.61) for having 2-3 and 4 or more comorbidities, respectively, compared with 0-1 comorbidities. In PWH aged 35-54 years and 55-64 years with 4 or more comorbidities, a higher adjusted risk of incident HF (aHR, 2.74; 95% CI, 1.74-4.30 and aHR, 2.70; 95% CI, 1.68-4.33, respectively) was observed compared with PWH in the same age groups having 0-1 comorbidities; other age-specific hazard ratios were not significantly different from the 0-1 comorbidities group. People without HIV aged 35-54 years had the highest adjusted risk of incident HF when comparing any multimorbidity to having 0-1 comorbidities (aHR, 2.49; 95% CI, 2.22-2.79 and aHR,5.77; 95% CI, 4.86-6.85 for 2-3 and 4 or more comorbidities, respectively).
Table 3.
Multivariable Adjusted Hazard Ratios and 95% Confidence Intervals for the Association Between Multimorbidity Burden and Incident Heart Failure Among Adults Without and With HIV, Overall, and by Age, Sex, and Race/Ethnicitya
| Baseline charactersitics | PWoH | PWH | P valueb | ||||
|---|---|---|---|---|---|---|---|
| Multimorbidity burden | 0-1 | 2-3 | ≥4 | 0-1 | 2-3 | ≥4 | |
| Overall | 1.0 (ref) | 2.10 (1.92-2.29) | 4.09 (3.64-4.61) | 1.0 (ref) | 1.33 (1.04-1.71) | 2.41 (1.78-3.25) | <.001 |
| Age group (y) | |||||||
| 21-34 | 1.0 (ref) | 1.93 (1.08-3.45) | 8.10 (3.30-19.86) | 1.0 (ref) | 1.56 (0.63-3.87) | 3.49 (0.69-17.56) | .46 |
| 35-54 | 1.0 (ref) | 2.49 (2.22-2.79) | 5.77 (4.86-6.85) | 1.0 (ref) | 1.25 (0.86-1.81) | 2.74 (1.74-4.30) | <.001 |
| 55-64 | 1.0 (ref) | 1.70 (1.45-1.98) | 3.85 (3.20-4.65) | 1.0 (ref) | 1.44 (0.94-2.23) | 2.70 (1.68-4.33) | .29 |
| ≥65 | 1.0 (ref) | 1.45 (1.16-1.81) | 2.43 (1.93-3.05) | 1.0 (ref) | 1.36 (0.66-2.78) | 1.44 (0.69-2.97) | .35 |
| Sex | |||||||
| Male | 1.0 (ref) | 2.12 (1.94-2.32) | 4.08 (3.60-4.63) | 1.0 (ref) | 1.25 (0.96-1.65) | 2.59 (1.89-3.55) | <.001 |
| Female | 1.0 (ref) | 1.79 (1.36-2.35) | 4.09 (2.90-5.78) | 1.0 (ref) | 1.65 (0.90-3.03) | 1.30 (0.48-3.51) | .01 |
| Race/ethnicity | |||||||
| White | 1.0 (ref) | 1.76 (1.55-1.99) | 3.89 (3.29-4.59) | 1.0 (ref) | 1.29 (0.89-1.86) | 3.17 (2.15-4.67) | .16 |
| Black | 1.0 (ref) | 2.06 (1.78-2.39) | 3.99 (3.26-4.88) | 1.0 (ref) | 1.40 (0.92-2.15) | 1.44 (0.76-2.72) | .01 |
| Hispanic | 1.0 (ref) | 3.22 (2.55-4.07) | 6.02 (4.32-8.39) | 1.0 (ref) | 1.47 (0.65-3.33) | 1.54 (0.53-4.47) | .11 |
| Asian/Pacific Islander | 1.0 (ref) | 2.36 (1.29-4.33) | 4.74 (2.02-11.09) | 1.0 (ref) | 0.67 (0.08-5.71) | 16.39 (5.08-52.9) | .12 |
| Other/unknown | 1.0 (ref) | 3.64 (2.54-5.22) | 6.39 (3.41-11.95) | 1.0 (ref) | 1.34 (0.57-3.17) | —c | <.001 |
PWH, people with HIV; PWoH, people without HIV; ref, reference.
For the joint hypothesis test that the associations of multimorbidity categories with incident heart failure are different among PWH and PWoH.
No HF events observed.
Hazard Ratio of Incident HF by Sex and Race/Ethnicity
Male PWH having 4 or more comorbidities vs 0-1 comorbidities had a high adjusted risk of incident HF (aHR 2.59; 95% CI, 1.89-3.55). Differences in HF risk among male PWH with 2-3 comorbidities vs 0-1 comorbidities and female PWH with any level of multimorbidity vs 0-1 comorbidities were not statistically significant. Among male and female PWoH, any level of multimorbidity vs 0-1 comorbidities was associated with a higher adjusted risk of incident HF. Among race/ethnicity groups in PWH, results varied but were largely not statistically different comparing those with 2-3 and 4 or more comorbidities to 0-1 comorbidities. Among race/ethnicity groups in PWoH, the adjusted risk of incident HF doubled between those having 2-3 and 4 or more comorbidities compared with 0-1 comorbidities. The highest risk of incident HF associated with multimorbidity was found among Hispanic individuals (aHR 3.22; 95% CI, 2.55-4.07 and aHR, 6.02; 95% CI, 4.32-8.39 for 2-3 and 4 or more comorbidities, respectively).
Tests for Differences by HIV Status
The incident HF risk associated with the categories of multimorbidity was lower in PWH than in PWoH (P interaction by HIV status <.001). This was most prominent not only among individuals aged 35-54 years (P interaction by HIV status <.001) and men (P interaction by HIV status <.001) but also among women and Black individuals (both P interaction by HIV status =.01).
Discussion
In this study of adults receiving care in 3 geographically diverse integrated health care delivery systems, multimorbidity burden was found to be similar in PWH and PWoH. Having 2-3 and 4 or more comorbidities vs no multimorbidity was associated with incident HF after adjusting for sociodemographic, behavioral, and clinical characteristics among both PWH and PWoH. The multivariable associations of incident HF with 4 or more comorbidities vs no multimorbidity and, separately, 2-3 comorbidities vs no multimorbidity were smaller in magnitude among PWH than among PWoH. The prevention and subsequent management of multiple chronic conditions is important for minimizing HF risk as the population ages overall, especially in vulnerable groups such as PWH.
Our finding of multimorbidity burden associated with a higher risk of incident HF is consistent with those of previous studies. In a population-based, retrospective cohort study in the United Kingdom, multimorbidity burden among individuals with HF was found to increase over time, with the number of patients with incident HF having 3 or more comorbidities increasing from 68% to 86% between 2002-2004 and 2011-2013.35 The composition of multimorbidity in HF has also changed over time, with HF in more recent years occurring less with predominantly cardiovascular risk factors and more with a combination of cardiovascular and noncardiovascular comorbidities, indicating a need for earlier patient-centered multimorbidity care.36
In the current study, our definition of multimorbidity is consistent with those in the previous studies in the CVRN and includes chronic conditions, CVD risk factors, age-related functional impairments, and depression. Biologically, many chronic comorbidities promote inflammation and cardiac dysfunction, which can increase the risk of HF.37 It has also been suggested that bidirectional influences between chronic conditions and age-related functional impairments can promote cellular senescence and mitochondrial dysfunction, increasing cardiovascular aging and impacting the health-related quality of life.38,39 Ultimately, there is a need to better understand the ways in which chronic conditions, functional limitations, and mental health coexist to influence the risk of HF.
In the current study, there was a high risk of HF associated with having high and moderate multimorbidity burdens compared with no multimorbidity, but the magnitude of these associations was smaller in PWH compared with PWoH. This suggests a smaller gradient of multimorbidity-associated HF risk in PWH. The presence of multiple chronic comorbidities in PWH may result from a chronic state of inflammation due to infection, lifestyle-related risk of disease, and aging.40,41 Given better treatment and management of HIV and the longer life expectancy of PWH, multimorbidity burden from non-AIDS diseases, particularly diabetes, CVDs, non–AIDS-defining cancer, and osteoporosis is increasingly important in the care of this population.42 Managing complex treatment regimens associated with multiple chronic conditions is challenging in the case of PWH, and often care for HIV takes priority.19 Many chronic comorbidities are also discordant with HIV (ie, not directly related in pathogenesis or management) and care for these conditions can remain suboptimal.20,21,43 However, contemporary advances in primary care guidance may help reduce some treatment discordances.44
People with HIV are at a greater risk of developing comorbidities earlier in life.45 In an earlier KP study of PWH, there was a 16-year gap between comorbidity-free years among PWH compared with PWoH, although the gap in comorbidity-free years for atherosclerotic CVD, diabetes, and cancer narrowed between 2000 and 2016 and was approximately 9 years in more recent years.46 In the current study, the multimorbidity burden in PWH was lower than that reported in a previous study.47 This may be explained by the younger age of our population compared with other studies examining multimorbidity in PWH, or that the KP population is reflective of an insured population (public and private). In previous studies, HF risk was independently associated with HIV status after controlling for demographic characteristics and cardiovascular risk factors.48,49 In the current study, after multivariable adjustment for baseline sociodemographic and behavioral factors, we found high rates of incident HF in PWH having both moderate and higher multimorbidity burdens that included cardiovascular and noncardiovascular comorbidities. People with HIV may have a closer connection to care than the PWoH and be more likely to receive earlier detection of comorbidities and a diagnosis of HF. Additionally, PWoH may not receive routine care as frequently as PWH; thus, there is a lower threshold of monitoring for advanced conditions like HF, except in older or particularly high-risk individuals.
We acknowledge some limitations. Our cohort included insured adults (including Medicaid and Medicare) receiving care in 3 large US integrated health care delivery systems; therefore, these results may not be fully generalizable to uninsured populations or those in nonintegrated health care settings. However, the racial/ethnic and sociodemographic composition of our cohort is representative of the current population with HIV in the Unites States.50 Information on the left ventricular ejection fraction was not available for approximately 40% of patients; therefore, we did not assess the association of multimorbidity with incident HF phenotypes. Automated clinical databases were used to identify comorbidities, which may have led to some misclassification. However, this is likely nondifferential by HIV status. Time-updated comorbidity status and control of comorbidities before HF were not assessed, which may change the risk of incident HF over time. Our approach to defining multimorbidity burden as the cumulative number of baseline conditions includes a comprehensive list of cardiovascular and noncardiovascular conditions associated with age-related decline; however, there is currently no gold standard. Examples of common approaches for defining multimorbidity include the Charlson and Elixhauser indices, which may be less applicable for use by health care providers in a real-world setting.51 Finally, because of the small numbers of incident HF events in certain race/ethnicity subgroups, results for these individuals should be interpreted cautiously.
Conclusion
In conclusion, multimorbidity was associated with a higher risk of incident HF among both PWH and PWoH, with more prominent associations in PWoH and in certain patient subgroups. Identifying specific multimorbidity patterns that are linked to excessive HF risk and how they contribute to high HF risk in PWH is warranted to develop more effective surveillance and preventative strategies.
Potential competing interests
The authors report no competing interests.
Footnotes
Grant Support: The work was supported by grant R01 HL132640 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Greene M., Justice A.C., Lampiris H.W., Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309(13):1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wada N., Jacobson L.P., Cohen M., French A., Phair J., Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984-2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang S., Boccara F., Mary-Krause M., Cohen A. Epidemiology of coronary heart disease in HIV-infected versus uninfected individuals in developed countries. Arch Cardiovasc Dis. 2015;108(3):206–215. doi: 10.1016/j.acvd.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Remick J., Georgiopoulou V., Marti C., et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation. 2014;129(17):1781–1789. doi: 10.1161/CIRCULATIONAHA.113.004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong A.M., Pozen A., Anastos K., Kelvin E.A., Nash D. Non-HIV comorbid conditions and polypharmacy among people living with HIV age 65 or older compared with HIV-negative individuals age 65 or older in the United States: a retrospective claims-based analysis. AIDS Patient Care STDS. 2019;33(3):93–103. doi: 10.1089/apc.2018.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan A.D., Goff L.M., Peters B.S. Type 2 diabetes prevalence and its risk factors in HIV: a cross-sectional study. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drozd D.R., Kitahata M.M., Althoff K.N., et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75(5):568–576. doi: 10.1097/QAI.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S.D., Kopp J.B., Kimmel P.L. Kidney diseases associated with human immunodeficiency virus infection. N Engl J Med. 2017;377(24):2363–2374. doi: 10.1056/NEJMra1508467. [DOI] [PubMed] [Google Scholar]
- 9.Klein D.B., Leyden W.A., Xu L., et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60(8):1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 10.Butt A.A., Chang C.C., Kuller L., et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen Y.F., Ko M.C., Yen M.Y., et al. Human immunodeficiency virus increases the risk of incident heart failure. J Acquir Immune Defic Syndr. 2019;80(3):255–263. doi: 10.1097/QAI.0000000000001917. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein M.J., Bahiru E., Achenbach C., et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go A.S., Reynolds K., Avula H.R., et al. Human immunodeficiency virus infection and variation in heart failure risk by age, sex, and ethnicity: the HIV HEART study. Mayo Clin Proc. 2022;97(3):465–479. doi: 10.1016/j.mayocp.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortin M., Hudon C., Haggerty J., Akker Mv, Almirall J. Prevalence estimates of multimorbidity: a comparative study of two sources. BMC Health Serv Res. 2010;10:111. doi: 10.1186/1472-6963-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff J.L., Starfield B., Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 16.Schouten J., Wit F.W., Stolte I.G., et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 17.Tran J., Norton R., Conrad N., et al. Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: a population-based cohort study. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vetrano D.L., Rizzuto D., Calderón-Larrañaga A., et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schexnayder J., Longenecker C.T., Muiruri C., et al. Understanding constraints on integrated care for people with HIV and multimorbid cardiovascular conditions: an application of the Theoretical Domains Framework. Implement Sci Commun. 2021;2(1):17. doi: 10.1186/s43058-021-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd J.V., Cole S.R., Wohl D.A., et al. Underutilization of statins when indicated in HIV-seropositive and seronegative women. AIDS Patient Care STDS. 2017;31(11):447–454. doi: 10.1089/apc.2017.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkholder G.A., Tamhane A.R., Salinas J.L., et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55(11):1550–1557. doi: 10.1093/cid/cis752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen Q.D., Odden M.C., Peralta C.A., Kim D.H. Predicting risk of atherosclerotic cardiovascular disease using pooled cohort equations in older adults with frailty, multimorbidity, and competing risks. J Am Heart Assoc. 2020;9(18) doi: 10.1161/JAHA.119.016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 24.Go A.S., Magid D.J., Wells B., et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1(2):138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 25.Multiple chronic conditions: a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. US Department of Health and Human Services. Accessed November 17, 2020. https://www.hhs.gov/sites/default/files/ash/initiatives/mcc/mcc_framework.pdf
- 26.Drye E.E., Altaf F.K., Lipska K.J., et al. Defining multiple chronic conditions for quality measurement. Med Care. 2018;56(2):193–201. doi: 10.1097/MLR.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 27.Tisminetzky M., Gurwitz J.H., Fan D., et al. Multimorbidity burden and adverse outcomes in a community-based cohort of adults with heart failure. J Am Geriatr Soc. 2018;66(12):2305–2313. doi: 10.1111/jgs.15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen L.A., Magid D.J., Gurwitz J.H., et al. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail. 2013;6(4):635–646. doi: 10.1161/CIRCHEARTFAILURE.112.000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg R.J., Gurwitz J.H., Saczynski J.S., et al. Comparison of medication practices in patients with heart failure and preserved versus those with reduced ejection fraction (from the Cardiovascular Research Network [CVRN]) Am J Cardiol. 2013;111(9):1324–1329. doi: 10.1016/j.amjcard.2013.01.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurwitz J.H., Magid D.J., Smith D.H., et al. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med. 2013;126(5):393–400. doi: 10.1016/j.amjmed.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saczynski J.S., Go A.S., Magid D.J., et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013;61(1):26–33. doi: 10.1111/jgs.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith D.H., Thorp M.L., Gurwitz J.H., et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE study. CircCardiovasc Qual Outcomes. 2013;6(3):333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKee P.A., Castelli W.P., Mcnamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 34.Go A.S., Yang J., Ackerson L.M., et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the anemia in chronic heart failure: outcomes and resource utilization (ANCHOR) study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 35.Conrad N., Judge A., Canoy D., et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol. 2019;4(11):1102–1111. doi: 10.1001/jamacardio.2019.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawson C.A., Zaccardi F., Squire I., et al. Risk factors for heart failure: 20-year population-based trends by sex, socioeconomic status, and ethnicity. Circ Heart Fail. 2020;13(2) doi: 10.1161/CIRCHEARTFAILURE.119.006472. [DOI] [PubMed] [Google Scholar]
- 37.Murphy S.P., Kakkar R., McCarthy C.P., Januzzi J.L., Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(11):1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Calderón-Larrañaga A., Vetrano D.L., Ferrucci L., et al. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285(3):255–271. doi: 10.1111/joim.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegler E., Moxley J., Mauer E., Glesby M. Cross-sectional study of correlates and prevalence of functional and high-risk multimorbidity in an academic HIV practice in New York City. BMJ Open. 2021;11(8) doi: 10.1136/bmjopen-2020-047199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strategies for Management of Antiretroviral Therapy Study Group. El-Sadr W.M., Lundgren J., et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 41.Justice A.C., McGinnis K.A., Skanderson M., et al. Towards a combined prognostic index for survival in HIV infection: the role of 'non-HIV' biomarkers. HIV Med. 2010;11(2):143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasse B., Ledergerber B., Furrer H., et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 43.Momplaisir F., Mounzer K., Long J.A. Preventive cancer screening practices in HIV-positive patients. AIDS Care. 2014;26(1):87–94. doi: 10.1080/09540121.2013.802276. [DOI] [PubMed] [Google Scholar]
- 44.Thompson M.A., Horberg M.A., Agwu A.L., et al. Primary care guidance for persons with human immunodeficiency virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2021;73(11):e3572–e3605. doi: 10.1093/cid/ciaa1391. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Penney A.T., Iudicello J.E., Riggs P.K., et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27(1):5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcus J.L., Leyden W.A., Alexeeff S.E., et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000-2016. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maggi P., Santoro C.R., Nofri M., et al. Clusterization of co-morbidities and multi-morbidities among persons living with HIV: a cross-sectional study. BMC Infect Dis. 2019;19(1):555. doi: 10.1186/s12879-019-4184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feinstein M.J., Steverson A.B., Ning H., et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. 2018;7(21) doi: 10.1161/JAHA.118.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freiberg M.S., Chang C.H., Skanderson M., et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol. 2017;2(5):536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention . HIV Surveillance Report; 2017. Diagnoses of HIV Infection in the United States and dependent areas, 2016; p. 28. [Google Scholar]
- 51.Green A.R., Leff B., Wang Y., et al. Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes. 2016;9(1):23–30. doi: 10.1161/CIRCOUTCOMES.115.002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.