Abstract
Antibiotic residues contained in poultry eggs pose threat to human health. However, the classes and concentrations of antibiotics in poultry egg in southwestern China is unknown due to insufficient monitoring and research. A total of 513 egg samples were collected from supermarkets and farm markets in Kunming city in 2020 and the levels of 7 antibiotics were analyzed using ultra high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method. The linear correlation coefficients were above 0.990 for all antibiotics tested. The limits of detection and limits of quantification in poultry eggs were 0.002 to 0.010 μg/g and 0.007 to 0.033 μg/g, respectively. The average recoveries of the 7 analytes from poultry egg samples were 80.00 to 128.01%, with relative standard deviations of less than 13.97%. A total of 93 (18.13%) samples tested positive for antibiotics, with the highest concentration being 2.48 μg/g. The concentration range of ofloxacin, danofloxacin, difloxacin, sulfadimethoxine, sulfamonomethoxine, sulfamethoxypyridazine, and sulfamethoxazole in poultry eggs was 0.01 to 0.37 μg/g, 0.06 to 0.48 μg/g, 0.05 to 0.29 μg/g, 0.03 to 0.16 μg/g, 0.06 to 1.00 μg/g, 0.05 to 0.37, and 0.07 to 2.48 μg/g, respectively. Sulfamonomethoxine was detected from hen eggs with the highest concentration level at 1.00 μg/g. Sulfamethoxazole was detected with the highest concentration level from both duck and quail eggs, at 1.87 and 2.48 μg/g, respectively. The antibiotic with the highest residue level in pheasant eggs was danofloxacin, which was 0.37 μg/g. Sulfamethoxypyridazine was identified in 30 samples with the highest positive rate of 5.85%, sulfadimethoxine was identified in 3 samples with the lowest positive rate of 0.58%. We observed that 7 targeted antibiotic residues in quail eggs and 3 targeted antibiotic residues in pheasant eggs. We also found that there were antibiotic residues in free-range hen eggs and the concentration was not low. The antibiotic with the highest residue level in free-range eggs was sulfamonomethoxine, which was 1.00 μg/g. These findings suggest that continual antibiotic residue monitoring of poultry eggs is essential in China.
Key words: food safety, antibiotic residues, poultry eggs, UHPLC-MS/MS
INTRODUCTION
China is not only one of the world's largest consumers of antibiotics, but also a big country in the production and consumption of poultry eggs (Masuda and Goldsmith, 2012; Van Boeckel et al., 2014; Zhang et al., 2015). The use of antibiotics in poultry production is common among farmers in China due to its effects in reducing poultry morbidity and mortality as well as growth promotion. Worldwide, antibiotics are often added to animal feed as growth promoters and are used to prevent and treat animal diseases (Okerman et al., 2007; Nisha, 2008). European Union has banned the use of antibiotics as growth promoters in animal feed since 2006 (Marshall and Levy, 2011), and China, as a rising developing country, banned the use of antibiotics as growth promoter since 2020 (MARA, 2019a). However, studied conducted in many countries showed that farmers are, unfortunately, not very well aware about the antibiotic withdrawal period and they seem to be more concerned about the economic impacts of their products and they frequently treat the whole flock with antibiotics without consulting veterinarians, physicians, or public health experts (Hassan et al., 2021). They also do not typically maintain the prescribed antibiotic withdrawal period before marketing their food products such as eggs (Nonga et al., 2010). The unregulated use of antibiotics in poultry production poses a serious risk for the development of antimicrobial resistance.
The use of antibiotics in poultry production may also lead to the accumulation of antibiotics in poultry meat and other products such as eggs. Fluoroquinolone drugs (FLQs) and sulfonamide drugs (SAs) are widely used in present poultry breeding (Premarathne et al., 2017; Wang et al., 2017b; Yamaguchi et al., 2017), therefore, their residues in eggs pose health threats to consumers, including allergic reactions, child development problems and potential harmful effects of drug-resistant strains (Chang et al., 2015). Many countries have paid great attention to the monitoring of antibiotic residues in food of animal origin in recent years, in order to prevent antibiotics-associated food safety risks (Ellis, 2008). Ministry of Agriculture and Rural Affairs of China has banned the use of some FLQs for food animals since 2015 (MARA, 2015). The Chinese national food safety standard – maximum residue limits for veterinary drugs in foods (MARA, 2019b) issued in 2019 indicated maximum residue limits (MRL) of 100 μg/kg for SAs in animal derived foods such as meat and liver, but there is no specific MRL for egg. However, FLQs and SAs are banned for poultry use during egg laying period, therefore in theory, the MRL of SAs and FLQs in egg should be zero. Monitoring antibiotic residues in poultry eggs helps to avoid potential risk to human health, but studies on FLQs and SAs residues in poultry eggs are relatively fewer in China, not to mention Kunming, the capital city of Yunnan province located in southwestern border area of China.
Many analytical methods have been developed for the measurement of the FLQs and SAs residues in animal foods, including high performance liquid chromatography with diode-array detection (HPLC-DAD) (Premarathne et al., 2017), high performance liquid chromatography coupled to fluorescence detection (HPLC-FLD) (Choi et al., 2011), ultra high performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) (Robert et al., 2013), chemiluminescence analysis (CL) (Pulgarín et al., 2011), capillary electrophoresis (CE) (Lombardo-Agüí et al., 2010), enzyme-linked immunosorbent assay (ELISA) (Zhang et al., 2007), chemiluminescence enzyme immunoassay (CLEIA), and microbiological assay (Cho et al., 2008). These methods have been proven sensitive and accurate, but an analytical method for detection of FLQs and SAs residues in poultry eggs must be precise, simple, economical, and environmentally friendly. Based on the all abovementioned considerations, the aim of this research was to investigate the presence of antibiotic residues in different poultry egg samples (hen, duck, quail, and pheasant) in Kunming city by using UHPLC-MS/MS. We also detected and analyzed antibiotic residues in caged and free-range hen eggs, which to our knowledge has not been previously studied. The residues of 7 commonly used antibiotics, namely ofloxacin (OFC), danofloxacin (DAN), difloxacin (DIF), sulfadimethoxine (SDM), sulfamonomethoxine (SMM), sulfamethoxypyridazine (SMP), and sulfamethoxazole (SMX) were selected for this study and rationale behind this selection was our previous investigation on farmers and the experimental laboratory test results of antibiotic residue in poultry egg samples collected in Kunming (Fang et al., 2020). By analyzing the antibiotic residues in different poultry eggs, our research would help food safety workers to formulate appropriate food safety control measures.
MATERIALS AND METHODS
Chemicals and Reagents
Formic acid (≥95%), HPLC-grade acetonitrile and methanol solvents were purchased from Sigma-Aldrich (St. Louis, MO). Ethyl acetate was obtained from Tianjin Hengxing chemical preparation (China).
Standard Solutions
Standard products of the 7 selected antibiotics including 3 FLQs (OFC, DAN, and DIF) and 4 SAs (SDM, SMM, SMP, and SMX) (≥98% purity) were obtained from Sigma-Aldrich (St. Louis, MO). A stock standard solution of each antibiotic was prepared in methanol to obtain a final concentration of 1.00 mg/g. Stock standard solutions were stored in amber colored glass bottles at −20°C and were stable for 1 mo. The working solutions for UHPLC injections were prepared daily from the stock solution in acetonitrile. Calibration standards were prepared at concentrations ranging from 0.01 to 2.5 μg/g for each antibiotic.
Samples Collection
The samples of poultry eggs were purchased from 6 supermarkets and 6 famer's markets located in three places of Kunming city, namely Wuhua district, Luquan county and Anning city in October 2020 with 2 supermarkets and 2 farmer's markets in each of the three places. For each of the selected supermarket or farmer's market, egg sample was collected only once with hen egg being collected from all 12 markets while duck egg, quail egg and pheasant egg being collected from some of the 12 markets depending on their availability. In total, 265 hen eggs, 105 duck eggs, 120 quail eggs, and 23 pheasant eggs were purchased and those egg samples were transported to the laboratory in Kunming Medical University, and stored at 4°C for 1 wk (see Supplementary material for more detailed sample collection information).
Sample Preparation
A total of 513 egg samples were used for pretreatment. Whole eggs (yolk and albumen combined) were blended using a vortex mixer (QILINBEIER, China). A 10.00 ± 0.01 g of homogenized egg sample was added with 30 mL ethyl acetate and mixed thoroughly by vortexing (1 min) and ultrasonic (15 min) and left for 1 h. After the solution was stable and divided into 2 layers, the supernatant was decanted into a 100 mL flask. Repeat the extraction twice, and transfer the final 60 mL supernatant to the rotary evaporation instrument for concentration. Then, the dry residue was dissolved in 1 mL acetonitrile. Finally, resulting solution was filtered through a 0.22-μm filter and transferred to a UHPLC vial.
UHPLC-MS/MS Analysis
Eksigent ekspert Ultra LC 100-XL (AB SCIEX, Framingham, MA) matched with 3200 Q TRAP mass spectrometer (AB SCIEX, Framingham, MA) was used to analyze the samples. A Cosmosil packed column (5C18-MS-II 4.6 mm I.D. × 150 mm, 5 μm, Nacalai Tesque, Japan) packed with totally porous spherical silica-based materials was used for the separation. The column temperature was controlled at 30°C. The injection volume was 10.0 μL. UHPLC gradient elution system consisted of solvent A (water - formic acid 100:0.01, v/v) and solvent B (acetonitrile - formic acid 100:0.01, v/v) with a flow rate of 1.0 mL/min: 0 min, 5%B; 5 min, 40%B; 10 to 20 min, 65%B; 25 to 30min, 5%B. UHPLC-MS/MS analysis was conducted with electrospray ionization source and detected in positive ion mode. For detection and quantification, the following electrospray ionization inlet conditions were applied: gas 1, nitrogen (60 psi); gas 2, nitrogen (50 psi); ion spray voltage, 5,500 V; ion source temperature, 550°C; and curtain gas, nitrogen (40 psi). Extracted ion chromatogram (EIC, targeted molecular fragment at m/z ± 0.5) was applied to analyze the fragments of antibiotics. Analyst 1.6.2 Software was employed to analyze total ion chromatogram and mass spectra.
Antibiotic Analysis
Seven antibiotics were selected from 2 classes, including 3 FLQs (OFC, DAN, DIF), 4 SAs (SDM, SMM, SMP, SMX), for this analysis. According to the retention time (±0.5 min) and molecular mass of antibiotic standard solution to determine whether the sample contains antibiotic (see Supplementary material). Standard solutions were prepared for each compound at concentrations ranging from 0.01 to 2.5μg/g. The concentration of antibiotic residues in samples was obtained by establishing the linear regression equation of concentration and ionic intensity. The limits of detection and limits of quantification are the smallest concentrations from which it is possible to deduce the presence of and quantify the analyte with reasonable statistical certainty. Each additive concentration of antibiotics with signal-to-noise ratio ≥3 was defined as the limit of detection (LOD), and the additive concentration of antibiotics with signal-to-noise ratio ≥10 was defined as the limit of quantification (LOQ). See Table 1 with the linear regression equation and parameters of detected antibiotics.
Table 1.
Linear regression equation and parameters of detected antibiotics.
| Analyte | Y = mx+c |
r2 | Range (μg/g) | LOD (μg/g) | LOQ (μg/g) | |
|---|---|---|---|---|---|---|
| m | c | |||||
| OFC | 1.278E-06 | 0.045 | 0.998 | 0.01–1.0 | 0.002 | 0.008 |
| DAN | 1.169E-06 | 0.006 | 0.994 | 0.05–1.0 | 0.010 | 0.033 |
| DIF | 1.898E-06 | 0.034 | 0.996 | 0.01–1.0 | 0.002 | 0.007 |
| SDM | 5.210E-07 | 0.041 | 0.990 | 0.01–1.0 | 0.002 | 0.008 |
| SMM | 4.830E-06 | 0.079 | 0.999 | 0.05–1.0 | 0.009 | 0.031 |
| SMP | 2.729E-06 | 0.069 | 0.991 | 0.05–1.0 | 0.010 | 0.033 |
| SMX | 9.238E-06 | 0.152 | 0.997 | 0.025–2.5 | 0.007 | 0.023 |
Abbreviations: DAN, danofloxacin; DIF, difloxacin; LOD, limit of detection; LOQ, limit of quantification; OFC, ofloxacin; SDM, sulfadimethoxine; SMM, sulfamonomethoxine; SMP, sulfamethoxypyridazine; SMX, sulfamethoxazole.
Method Validation
The method was validated as described in the Chinese national standard GB/T 21312-2007 (AQSIQ, 2008a) and GB/T 21316-2007 (AQSIQ, 2008b) and the parameters used for validation including specificity, recovery and precision. The specificity of the method was determined by analyzing 30 blank egg samples of different sources. The absence of any indigenous or interfering compounds at the same retention time of the analytes was observed. Control positives were carried out in egg samples by spiking analyzed samples with different concentrations (0.05 μg/g, 0.1 μg/g, 0.5 μg/g) of antibiotic standard solution. The test was repeated 5 times for each concentration, and the average recovery rate and relative standard deviation (RSD) were calculated (Table 2).
Table 2.
The average recovery and relative standard deviation of antibiotics in blank egg matrix.
| Analyte | 0.05 μg/g |
0.1 μg/g |
0.5 μg/g |
|||
|---|---|---|---|---|---|---|
| Rec(%) | RSD(%) | Rec(%) | RSD(%) | Rec(%) | RSD(%) | |
| OFC | 93.45 | 4.26 | 100.34 | 10.35 | 93.20 | 4.46 |
| DAN | 128.01 | 13.97 | 101.66 | 4.67 | 97.65 | 10.15 |
| DIF | 84.19 | 10.65 | 98.75 | 4.80 | 101.46 | 7.90 |
| SDM | 96.36 | 8.44 | 93.02 | 8.48 | 98.55 | 11.61 |
| SMM | 88.00 | 12.45 | 98.67 | 3.02 | 100.80 | 4.35 |
| SMP | 84.00 | 10.65 | 90.00 | 11.11 | 95.00 | 11.77 |
| SMX | 80.00 | 13.97 | 86.67 | 8.60 | 106.25 | 12.48 |
Abbreviations: DAN, danofloxacin; DIF, difloxacin; OFC, ofloxacin; RSD, relative standard deviation; SDM, sulfadimethoxine; SMM, sulfamonomethoxine; SMP, sulfamethoxypyridazine; SMX, sulfamethoxazole.
Statistical Analyses
Statistical analyses were performed using R software (version 4.1.0). The positive rates of antibiotics in eggs were compared by Chi-square test, and the concentrations were compared by Rank-sum test. All statistical tests with a P-value of less than 0.05 were considered significant.
RESULTS
The Positive Rate of 7 Antibiotic Residues in Poultry Egg Samples
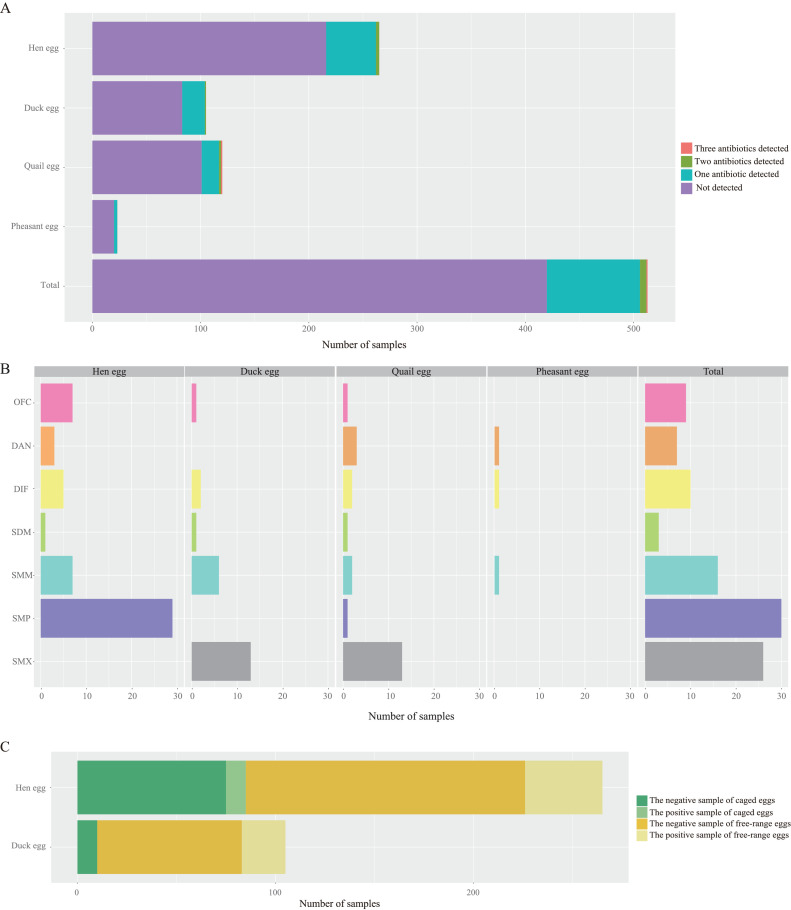
In this study, a total of 513 egg samples collected included specimens of 4 egg species: hen egg, duck egg, quail egg, and pheasant egg. All samples were detected for 7 antibiotics including OFC, DAN, DIF, SDM, SMM, SMP, and SMX using UHPLC-MS/MS. Among the 513 egg samples, 86, 6, and 1 samples were detected positive for one, two and three targeted antibiotics respectively and the total positive rate was 18.13% (93/513). Of which, the positive rate of antibiotic residues of hen egg, duck egg, quail egg, and pheasant egg were 18.49% (49/265), 20.95% (22/105), 15.83% (19/120), and 13.04% (3/23), respectively. There was no significant difference in the positive rates of antibiotic residues in 4 species of eggs (χ2= 1.414, P = 0.702; Figure 1A).
Figure 1.
(A) The number of antibiotics detected in 4 classes of poultry egg samples collected in Kunming city. (B) The positive sample number of OFC, DAN, DIF, SDM, SMM, SMP, and SMX in 4 classes of poultry egg samples. (C) The positive and negative sample number of antibiotics detected in caged eggs and free-range eggs.
Among the 7 antibiotics detected in poultry egg samples, SMP had the highest positive rate, followed by SMX, SMM, DIF, OFC, DAN, and SDM. Six antibiotics were detected in hen egg samples, including OFC, DAN, DIF, SDM, SMM, and SMP. Five antibiotics were detected in duck egg samples, including OFC, DIF, SDM, SMM, and SMX. All of the 7 antibiotics were detected in quail eggs and 3 antibiotics, namely DAN, DIF, and SMM were detected in pheasant egg sample (Figure 1B).
Caged eggs were produced from hens that were housed in cages inside large, climate-controlled sheds. All the samples we collected from the supermarket were labeled as caged eggs by the manufacturer. Free-range eggs were produced from hens that were free to walk around the hen house, to perch on roosts, and to lay eggs in nests. In farmer's market, we collected free-range eggs from small farmers who raised their chickens on natural pasture. Among 265 hen eggs, 85 were caged eggs and 180 were free-range eggs. There was no significant difference in the positive rates of antibiotic residues between caged hen eggs and free-range hen eggs (χ2= 3.756, P = 0.053). Among 105 duck egg samples, 10 were caged eggs and 95 were free-range eggs. There was no significant difference in the positive rates of antibiotic residues between caged duck eggs and free-range duck eggs (χ2= 1.698, P = 0.193) (Figure 1C). One hundred and twenty quail eggs and 23 pheasant eggs were all free-range eggs.
The Concentration of 7 Antibiotic Residues in Poultry Egg Samples
We calculated the concentration of antibiotic residues in poultry egg samples by using the established linear regression equation of antibiotic standard. The detection concentration ranges of OFC, DAN, DIF, SDM, SMM, SMP, and SMX were 0.01 to 0.37 μg/g, 0.06 to 0.48 μg/g, 0.05 to 0.29 μg/g, 0.03 to 0.16 μg/g, 0.06 to 1.00 μg/g, 0.05 to 0.37 μg/g, and 0.07 to 2.48 μg/g, respectively. There was statistically significant difference in the residue of SMX between duck eggs and quail eggs (z = −2.704, P = 0.007). The concentration of SMX residue in quail eggs was higher than that in duck eggs. There were no significant differences in the residues of OFC, DAN, DIF, SDM, SMM, and SMP in different species of poultry eggs (P > 0.05).
SMM was observed with the highest residual concentration in hen eggs, with a median of 0.36 μg/g, and OFC was the lowest with a median of 0.04 μg/g. SMX was observed with the highest residual concentration in duck eggs, with a median of 0.51 μg/g, and OFC was the lowest with a median of 0.02 μg/g. SMX was observed with the highest residual concentration in quail eggs, with a median of 1.34 μg/g, and OFC was the lowest with a median of 0.01 μg/g. DAN was observed with the highest residual concentration in pheasant eggs, with a median of 0.37 μg/g, and SMM were the lowest with a median of 0.08 μg/g (Figure 2A).
Figure 2.
(A) The residual concentration of 7 antibiotics detected in poultry egg samples. (B) The residual concentration of 6 antibiotics detected in caged hen eggs and free-range hen eggs.
In this study, OFC, DIF, SMM and SMP were detected in caged hen eggs. OFC was detected at a range of 0.01 to 0.37 μg/g in 2 samples; DIF was detected at a range of 0.07 to 0.25 μg/g in 3 samples; SMM was detected at a range of 0.06 to 0.56 μg/g in 4 samples; SMP was detected at a range of 0.05 to 0.06 μg/g in 2 samples. OFC, DAN, DIF, SDM, SMM and SMP were detected in free-range hen eggs. OFC was detected at a range of 0.01 to 0.35 μg/g in 5 samples; DAN was detected at a range of 0.14 to 0.48 μg/g in 3 samples; DIF was detected at a range of 0.05 to 0.07 μg/g in 2 samples; SDM was detected at 0.07 μg/g in 1 sample; SMM was detected at a range of 0.36 to 1.00 μg/g in 3 samples; SMP was detected at a range of 0.05 to 0.37 μg/g in 27 samples (Figure 2B).
DISCUSSION
A reliable residue analysis method is essential to detect and quantify antibiotic residues in poultry eggs. In this study, a total of 513 poultry egg samples from Kunming city were detected by using UHPLC-MS/MS. The correlation coefficients between the concentrations of 7 antibiotics and ion peak intensity were greater than 0.990, and the linear relationship was good. The Rec was in the range of 80.00 to 128.01%, and the RSD was between 4.26 to 13.97%. The detection conditions of UHPLC-MS/MS were set appropriately, with high precision and accuracy. However, some limitations should be noted. We were unable to evaluate whether there was matrix effect of the samples in this study. Moreover, often in complex matrix samples, the matrix-matched standard curve is essential in the quantification of external standard method. Yet, we did not use matrix-matched standard curve for quantification due to limitations in study design and methods. As well, we did not assess the recovery and precision at the LOQ level, which should be noticed when reading and understanding this research results. Despite the shortcomings, this study provides useful information on antibiotic residues in poultry eggs sold in markets in Kunming city and clue for future research.
The MRL in foods set up by the Codex Alimentarius Commission (CAC) was displayed in the form of database, and can be checked online (FAO/WHO, 2021). However, there was no provision on the residue limit of FLQs and SAs in poultry eggs. According to Commission Regulation 37/2010/EC, the use of FLQs in laying hens was strictly forbidden. Ministry of Agriculture and Rural Affairs of China has banned the use of OFC for food animals since 2015 (MARA, 2015). The Chinese National Standard issued by Ministry of Agriculture and Rural Affairs of China in 2019 banned the use of DAN, DIF, and SAs during egg laying (MARA, 2019b). However, we found FLQs and SAs residues in poultry eggs in this study. FLQs and SAs residues in poultry eggs were also found in other countries (Mian et al., 2012; Premarathne et al., 2017; Yamaguchi et al., 2017). This means that the current situation of antibiotic residues in poultry eggs is not optimistic. In China, a recent study found that 2 FLQs were detected in chicken eggs sold in Shenzhen with the total concentration of 1.64 ng/g ww (Hu et al., 2021). Another study showed that 8 of 110 egg samples contained traces of enrofloxacin at levels ranging from 1.09 to 5.22 μg/kg. Traces of ciprofloxacin were also found in egg samples in Guangzhou, whereas OFC, DAN and DIF were not detected in all egg samples (Lu et al., 2019). Similarly, a study in Xiamen reported that five antibiotics (orbifloxacin, sulfaquinoxaline, ciprofloxacin, enrofloxacin, and doxycycline) were detected in three egg samples. There was an outbreak of bird flu in Xiamen city during the sampling period. It was possible that farmers might feed laying hens with additional antibiotics to prevent the spread of bird flu (Wang et al., 2017a). Residues of forbidden antibiotic in eggs need more attention.
Many studies showed that antibiotics have been frequently detected in various environment, including water, sediment, air, and soil (Philip et al., 2018; Qiao et al., 2018; Yang et al., 2018). Consequently, antibiotics are increasingly being viewed as an emerging environmental contaminant (Martinez, 2009; Milić et al., 2013). Pheasants are an important bird species for hunting purposes. Their eggs are considered to be quite dietetic (Gugała et al., 2019). In this study, DAN, DIF, and SMM were found in pheasant eggs. Therefore, direct contact of pheasant with the contaminated environment might be a probable reason for the presence of antibiotics in pheasant eggs. This will lead to the production of drug-resistant bacteria and cause major public health hazard. Moreover, some studies showed that the meat samples of pheasant were the reservoirs of resistant-Campylobacter species (Sabzmeydani et al., 2020). Similarly, drug resistant bacteria in pheasant eggs should be concerned. Unfortunately, there is no relevant research at present.
In developing countries, there is an increased demand for poultry products as a result of population increase, urbanization and improved economic status. Responding to this increased demand, farmers tend to shift to intensified poultry production systems and antibiotics are often used to manage diseases in these operations (Hao et al., 2014). At a household level, free-range hens are likely eating forage for their food rather than being fed with commercially-prepared feeds, so they are not likely to be exposed to antibiotics (Rugumisa et al., 2016). Chinese residents prefer to free-range eggs. They believe that free-range eggs are healthier and safer than caged eggs. However, few studies have compared the difference of antibiotic residues between caged eggs and free-range eggs. Our study found that 6 antibiotic residues were detected in free-range eggs, while only 4 antibiotics were detected in caged eggs. The concentrations of antibiotic residues in free-range eggs were not low. The concentration range of OFC, DAN, DIF, SDM, SMM and SMP in free-range hen eggs was 0.01 to 0.35 μg/g, 0.14 to 0.48 μg/g, 0.05 to 0.07 μg/g, 0.07 μg/g, 0.36 to 1.00 μg/g, and 0.05 to 0.37 μg/g, respectively. Especially, the highest residual concentration of SMM and SMP in free-range eggs was higher than those in caged eggs. This suggests that free-range eggs are not safer than caged eggs. Further research is needed to identify causes behind this phenomena and greater attention need to be paid to closely monitor antibiotic residues in poultry egg so as to reduce the associated health risks.
ACKNOWLEDGMENTS
This work has been supported by Scientific Research Fund of Yunnan Provincial Education Department of China (Grant No.2020Y0115) and Graduate Innovation Fund of Kunming Medical University (Grant No.2020S005).
DISCLOSURES
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. All the authors listed have approved the manuscript that is enclosed.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101892.
Appendix. Supplementary materials
References
- Chang Q., Wang W., Regev-Yochay G., Lipsitch M., Hanage W.P. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol. Appl. 2015;8:240–247. doi: 10.1111/eva.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.J., Abd El-Aty A.M., Goudah A., Sung G.M., Yi H., Seo D.C., Kim J.S., Shim J.H., Jeong J.Y., Lee S.H., Shin H.C. Monitoring of fluoroquinolone residual levels in chicken eggs by microbiological assay and confirmation by liquid chromatography. Biomed. Chromatogr. 2008;22:92–99. doi: 10.1002/bmc.900. [DOI] [PubMed] [Google Scholar]
- Choi J.-H., Mamun M., Park J.-H., Shin e.-h., Park J.Y., Cho S.-K., Shin S., Lee K., Shim J.H. Development of a single-step precipitation cleanup method for the determination of enrofloxacin, ciprofloxacin, and danofloxacin in porcine plasma. Food Chem. 2011;127:1878–1883. [Google Scholar]
- Ellis R.L. Development of veterinary drug residue controls by the Codex Alimentarius Commission: a review. Food Addit. Contam. 2008;25:1432–1438. doi: 10.1080/02652030802267405. [DOI] [PubMed] [Google Scholar]
- Fang J., Gong G., Yuan J., Sun X. Antibiotic use in pig farming and its associated factors in L County in Yunnan, China. Vet. Med. Sci. 2020;7:440–454. doi: 10.1002/vms3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization/World Health Organization (FAO/WHO). 2021. Index of veterinary drugs. Accessed Sept. 2021. http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/veterinary-drugs/en/.
- General Administration of Quality Supervision, Inspection and Qurantine of the People's Republic of China (AQSIQ). 2008a. Analysis of fourteen quinolones in food of animal origin by high performance liquid chromatography tandem mass spectrometry. Accessed Apr. 2008. http://down.foodmate.net/standard/sort/3/15639.html.
- General Administration of Quality Supervision, Inspection and Qurantine of the Peoples Republic of China (AQSIQ). 2008b. Determination of residues of sulfonamides in foodstuffs of animal origin-LC-MS/MS. Accessed Apr. 2008. http://down.foodmate.net/standard/sort/3/14687.html.
- Gugała D., Flis M., Grela E.R. The effect of zinc, iron, calcium, and copper from organic sources in pheasant diet on the performance, hatching, minerals, and fatty acid composition of eggs. Poult. Sci. 2019;98:4640–4647. doi: 10.3382/ps/pez162. [DOI] [PubMed] [Google Scholar]
- Hao H., Cheng G., Iqbal Z., Ai X., Hussain H., Huang L., Dai M., Wang Y., Lu A., Yuan Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.M., El Zowalaty M.E., Lundkvist Å., Järhult J.D., Khan Nayem M.R., Tanzin A.Z., Badsha M.R., Khan S.A., Ashour H.M. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021;111:141–150. doi: 10.1016/j.tifs.2021.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Ben Y., Wong M., Zheng C. Trace analysis of multiclass antibiotics in food products by liquid chromatography-tandem mass spectrometry: method development. J. Agric. Food Chem. 2021;69:1656–1666. doi: 10.1021/acs.jafc.0c05778. [DOI] [PubMed] [Google Scholar]
- Lombardo-Agüí M., García-Campaña A.M., Gámiz-Gracia L., Cruces Blanco C. Laser induced fluorescence coupled to capillary electrophoresis for the determination of fluoroquinolones in foods of animal origin using molecularly imprinted polymers. J. Chromatogr. A. 2010;1217:2237–2242. doi: 10.1016/j.chroma.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Lu Z., Deng F., He R., Tan L., Luo X., Pan X., Yang Z. A pass-through solid-phase extraction clean-up method for the determination of 11 quinolone antibiotics in chicken meat and egg samples using ultra-performance liquid chromatography tandem mass spectrometry. Microchem. J. 2019;151 [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009;157:2893–2902. doi: 10.1016/j.envpol.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Masuda T., Goldsmith P. China's meat and egg production and soybean meal demand for feed: an elasticity analysis and long-term projections. Int. Food Agribus. Man. 2012;15:35–54. [Google Scholar]
- Mian A., Ahmad T., Nadeem M., Tanveer Z., Arshad J. Sulfonamide residues determination in commercial poultry meat and eggs. J. Anim. Plant. Sci. 2012;22:473. [Google Scholar]
- Milić N., Milanović M., Letić N.G., Sekulić M.T., Radonić J., Mihajlović I., Miloradov M.V. Occurrence of antibiotics as emerging contaminant substances in aquatic environment. Int. J. Environ. Health Res. 2013;23:296–310. doi: 10.1080/09603123.2012.733934. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture and Rural Affairs of China (MARA). 2015. Announcement No. 2292 of the Ministry of Agriculture of the People's Republic of China. Accessed Sep. 7, 2015. http://www.moa.gov.cn/govpublic/SYJ/201509/t20150907_4819267.htm.
- Ministry of Agriculture and Rural Affairs of China (MARA). 2019a. Announcement No. 194 of the Ministry of Agriculture and Rural Affairs of the People's Republic of China. Accessed July 2019. http://www.moa.gov.cn/govpublic/xmsyj/201907/t20190710_6320678.htm.
- Ministry of Agriculture and Rural Affairs of China (MARA). 2019b. National food safety standard – maximum residue limits for veterinary drugs in foods. Accessed Oct. 2019. http://www.aqsc.agri.cn/tzgg/201910/t20191012_342749.htm.
- Nisha A.R. Antibiotic residues - a global health hazard. Vet. World. 2008;1:375–377. [Google Scholar]
- Nonga H.E., Simon C., Karimuribo E.D., Mdegela R.H. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro municipality, Tanzania. Zoonoses Public Hlth. 2010;57:339–344. doi: 10.1111/j.1863-2378.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Okerman L., Noppe H., Cornet V., De Zutter L. Microbiological detection of 10 quinolone antibiotic residues and its application to artificially contaminated poultry samples. Food Addit. Contam. 2007;24:252–257. doi: 10.1080/02652030600988020. [DOI] [PubMed] [Google Scholar]
- Philip J.M., Aravind U.K., Aravindakumar C.T. Emerging contaminants in Indian environmental matrices - a review. Chemosphere. 2018;190:307–326. doi: 10.1016/j.chemosphere.2017.09.120. [DOI] [PubMed] [Google Scholar]
- Premarathne K., Satharasinghe D.A., Munasinghe M. Establishment of a method to detect sulfonamide residues in chicken meat and eggs by high-performance liquid chromatography. Food Control. 2017;72:276–282. [Google Scholar]
- Pulgarín J.A., Molina A., Muñoz S. Rapid chemiluminescent determination of enrofloxacin in eggs and veterinary drugs. Anal. Lett. 2011;44:2194–2208. [Google Scholar]
- Qiao M., Ying G.G., Singer A.C., Zhu Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Robert C., Gillard N., Brasseur P.Y., Pierret G., Ralet N., Dubois M., Delahaut P. Rapid multi-residue and multi-class qualitative screening for veterinary drugs in foods of animal origin by UHPLC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013;30:443–457. doi: 10.1080/19440049.2012.751632. [DOI] [PubMed] [Google Scholar]
- Rugumisa B., Call D., Mwanyika G., Subbiah M., Buza J. Comparison of the prevalence of antibiotic-resistant Escherichia coli isolates from commercial-layer and free-range chickens in Arusha district, Tanzania. Afr. J. Microbiol. Res. 2016;10:1422–1429. [Google Scholar]
- Sabzmeydani A., Rahimi E., Shakerian A. Incidence and antibiotic resistance properties of Campylobacter species isolated from poultry meat. Int. J. Enteric. Pathog. 2020;8:60–65. [Google Scholar]
- Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A., Laxminarayan R. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- Wang K., Lin K., Huang X., Chen M. A simple and fast extraction method for the determination of multiclass antibiotics in eggs using LC-MS/MS. J. Agric. Food Chem. 2017;65:5064–5073. doi: 10.1021/acs.jafc.7b01777. [DOI] [PubMed] [Google Scholar]
- Wang Z., Du Y., Yang C., Liu X., Zhang J., Li E., Zhang Q., Wang X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017;609:1423–1432. doi: 10.1016/j.scitotenv.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Okihashi M., Harada K., Konishi Y., Uchida K., Do M., Bui L., Nguyen T., Phan H., Bui T., Nguyen P., Kajimura K., Kumeda Y., Dang Van C., Hirata K., Yamamoto Y. Detection of antibiotics in chicken eggs obtained from supermarkets in Ho Chi Minh city, Vietnam. J. Environ. Sci. Health B. 2017;52:430–433. doi: 10.1080/03601234.2017.1293457. [DOI] [PubMed] [Google Scholar]
- Yang Y., Song W., Lin H., Wang W., Du L., Xing W. Antibiotics and antibiotic resistance genes in global lakes: a review and meta-analysis. Environ. Int. 2018;116:60–73. doi: 10.1016/j.envint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang L., Zhang Y., Fang G., Zheng W., Wang S. Development of an enzyme-linked immunosorbent assay for seven sulfonamide residues and investigation of matrix effects from different food samples. J. Agric. Food Chem. 2007;55:2079–2084. doi: 10.1021/jf062896i. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Q., Ying G.G., Pan C.G., Liu Y.S., Zhao J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.