Dear Editor,
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy is an autoimmune inflammatory disorder of the central nervous system that responds to immunotherapy [1]. It is important to differentiate between infectious meningoencephalitis and idiopathic inflammatory central nervous system disorders. Herein, we present a case of autoimmune GFAP astrocytopathy with late magnetic resonance imaging (MRI) findings.
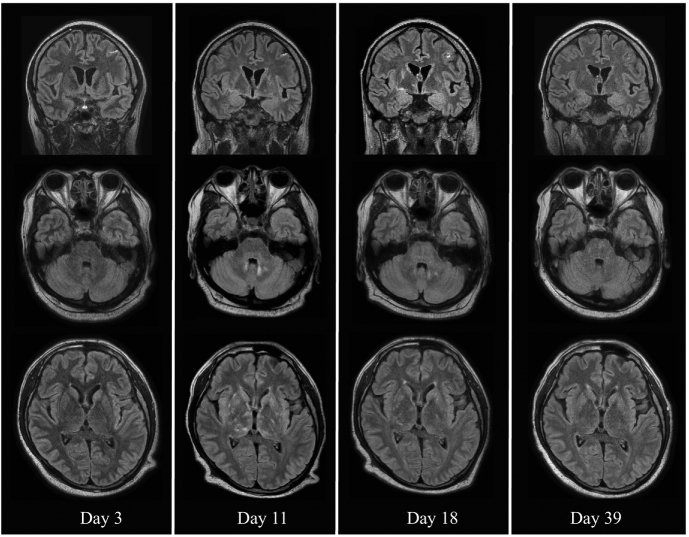
A 39-year-old man without any medical history presented with fever and upper respiratory symptoms of 2 weeks' duration. He had been unconscious the previous day and was admitted to our hospital (day 1). The patient was drowsy and unable to cooperate with neurological testing. He had no weakness of the four extremities and presented with myoclonic movement of the right upper extremity. Cerebrospinal fluid (CSF) analysis revealed a leukocyte count of 93/μL (92% mononuclear neutrophils), protein level of 296.1 mg/dL, glucose level of 45 mg/dL (corresponding blood glucose level, 131 mg/dL), and adenosine deaminase (ADA) level of 14.9 IU/L. Oligoclonal bands were negative in the CSF and serum. On day 3, MRI showed enhancement in the left parietal lobe meninges (Fig. 1). The test results implied tuberculous meningitis, but an autoimmune inflammatory central nervous system disorder was also suspected. Initially, the patient was treated with antituberculosis therapy combined with intravenous prednisolone. The patient showed rapid improvement and was able to cooperate with neurological examination on the same day. He had postural tremor and kinetic tremor in both upper extremities. No instability of the limbs was observed on the finger-to-nose and heel-shin tests. Electroencephalography showed slowing of background activity and diffuse slow wave in the bilateral frontal region. Epileptic discharge was not observed. Myoclonus in the right upper extremity resolved on day 7. MRI on day 11 showed abnormal hyperintensity in the basal ganglia, cerebellum, thalamus, and pons (Fig. 1). During this period, the patient was able to communicate in an ordinary manner, but required assistance to walk because of truncal ataxia. After urethral balloon removal on day 24, urinary retention and erectile dysfunction persisted, and intermittent self-catheterization was required. Images obtained on days 18 and 39 showed subsequent improvements (Fig. 1).
Fig. 1.
Brain MRI. Coronal view of gadolinium-enhanced FLAIR images and sagittal view of plain FLAIR images throughout treatment.
On day 3, coronal images showing leptomeningeal enhancement over the sulcus of the left parietal lobe. Enhancement gradually improves. Axial FLAIR images from day 11 showing multiple linear high signals in the bilateral basal ganglia, cerebellum, and thalamus. Multiple linear high signals in the bilateral basal ganglia, thalamus, and cerebellum are resolved (days 18 and 39).
MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery.
The result of interferon-γ release assay was negative. Tuberculous DNA polymerase chain reaction of the CSF, sputum, and urine also revealed negative findings.Blood and CSF cultures were negative for mycobacterium. The CSF was positive for anti-GFAPα antibody. We detected CSF GFAP-IgG using transfected cell- and tissue-based immunofluorescence assays [2]. Therefore, the patient was diagnosed with autoimmune GFAP astrocytopathy [1]. We concluded that the patient's improvement was caused by steroid therapy, and anti-tuberculosis therapy was stopped on day 47. Repeated CSF analysis showed improvement in leukocyte count, protein level, glucose level, and ADA level. On day 40, CSF analysis revealed a leukocyte count of 9/μL, protein level of 46.4 mg/dL, glucose level of 47 mg/dL, and ADA level of 3.0 IU/L. One year after admission, his urinary retention and erectile dysfunction had resolved, and he was able to walk without assistance.
Brain images in autoimmune GFAP astrocytopathy are often accompanied by linear perivascular radial gadolinium enhancement patterns [3]. However, in our case, initial MRI only showed enhancement at the left parietal lobe meninges. On day 11, MRI revealed abnormal findings in the brainstem, posterior thalamus, and cerebellum, which are characteristic of autoimmune GFAP astrocytopathy [3], although the clinical symptoms improved.
Similar to our case, typical MRI abnormalities may not appear during the initial evaluation. Approximately 70% of patients respond well to steroid therapy [4]; however, some patients have a poor response to treatment and some patients have different degrees of functional disability [5]. MRI images should be re-evaluated in patients with high CSF ADA levels. Given that autoimmune GFAP astrocytopathy shows good response to steroid treatment, it is important to include it in the differential diagnosis. Notably, the improvement in the clinical course did not correspond with the MRI abnormalities. Our case was difficult to differentiate from a case of tuberculous meningitis. Both diseases present with high CSF ADA levels and MRI abnormalities [6]. Identifying anti-GFAPα antibodies in the CSF was the key for diagnosis. Further analysis is needed to elucidate the pathological mechanisms underlying various MRI features of autoimmune GFAP astrocytopathy.
References
- 1.Fang B., McKeon A., Hinson S.R., Kryzer T.J., Pittock S.J., Aksamit A.J., et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73:1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 2.Kimura A., Takemura M., Yamamoto Y., Hayashi Y., Saito K., Shimohata T. Cytokines and biological markers in autoimmune GFAP astrocytopathy: the potential role for pathogenesis and therapeutic implications. J. Neuroimmunol. 2019;334 doi: 10.1016/j.jneuroim.2019.576999. [DOI] [PubMed] [Google Scholar]
- 3.Kimura A., Takekoshi A., Yoshikura N., Hayashi Y., Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J. Neuroimmunol. 2019;332:91–98. doi: 10.1016/j.jneuroim.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Shan F., Long Y., Qiu W. Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature. Front. Immunol. 2018;9:2802. doi: 10.3389/fimmu.2018.02802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Liang J., Huang Q., Xu H., Gao C., Long Y., et al. Treatment of autoimmune glial fibrillary acidic protein astrocytopathy: follow-up in 7 cases. Neuroimmunomodulation. 2017;24:113–119. doi: 10.1159/000479948. [DOI] [PubMed] [Google Scholar]
- 6.Oka Y., Tabu H., Matsumoto S. Tuberculous meningitis presenting with nonconvulsive status epilepticus and transient diffusion restriction: a rare case. Neurol. India. 2020;68:512–514. doi: 10.4103/0028-3886.283759. [DOI] [PubMed] [Google Scholar]