Summary
Background
Oral pre-exposure prophylaxis (PrEP) and antiretroviral therapy (ART) can effectively prevent HIV infections among men who have sex with men (MSM), but the emergence and transmission of HIV drug-resistance (HIVDR) may compromise their benefits. The costs and benefits of expanding PrEP and ART coverage in the presence of HIVDR in China remain unknown.
Methods
We developed a comprehensive dynamic transmission model incorporating the transmitted (TDR) and acquired (ADR) HIV drug resistance. The model was calibrated by the HIV surveillance data from 2009 to 2019 among MSM in Jiangsu Province, China, and validated by the dynamic prevalence of ADR and TDR. We aimed to investigate the impact of eight intervention scenarios (no PrEP, 20%, 50% or 80% of PrEP, without (77% coverage) or with (90% coverage) expanded ART) on the HIV epidemic trend and cost-effectiveness of PrEP over the next 30 years.
Findings
20% or 50% PrEP + 90% ART would be cost-effective, with an incremental cost-effectiveness ratio (ICER) of 25,417 (95% confidence interval [CI]: 12,390–38,445) or 47,243 (23,756–70,729), and would yield 154,949 (89,662–220,237) or 179,456 (102,570–256,342) incremental quality-adjusted life-years (QALYs) over the next 30 years. No PrEP + 90% ART would yield 125,211 (73,448–176,974) incremental QALYs and be cost-saving. However, 20–80% PrEP + 77% ART and 80% PrEP + 90% ART with ICER of $77,862–$98,338 and $63,332, respectively, and were not cost-effective. A reduction of 64% in the annual cost of oral PrEP would make it highly cost-effective for 50% PrEP + 90% ART.
Interpretation
20% or 50% PrEP + 90% ART is cost-effective for HIV control in the presence of HIVDR. Expanded ART alone may be the optimal policy under the current limited budgets.
Funding
National Natural Science Foundation of China, the National S&T Major Project Foundation of China.
Keywords: Pre-exposure prophylaxis, HIV drug-resistance, Cost-effectiveness, Mathematical model, Chinese MSM
Abbreviations: ADR, acquired drug resistance; ART, antiretroviral therapy; GDP, gross domestic product; HIVDR, HIV drug resistance; ICER, incremental cost-effectiveness ratio; MSM, men who have sex with men; NLS, nonlinear least-squares; PrEP, pre-exposure prophylaxis; QALYs, quality-adjusted life years; TDR, transmitted drug resistance; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine
Research in context.
Evidence before this study
Daily oral pre-exposure prophylaxis (PrEP), based on first-line antiretroviral drugs, has been shown an effectiveness of 44%-86% to prevent new HIV infections among men who has sex with men (MSM). However, the overlapping resistance profiles between antiretroviral drugs used for both PrEP and first-line antiretroviral drugs may compromise the effectiveness of first-line ART once PrEP users infected. And HIV drug resistance (HIVDR) may increase the costs of second-line drugs, and decrease the quality of life. We searched PubMed for studies up to Sep 15, 2021, with the terms “HIV” and “mathematical model” or “mathematical modeling” or “compartment model”, and “PrEP” or “preexposure prophylaxis” or “pre-exposure prophylaxis”, and “cost-effectiveness” or “cost-effective”, and “China” or “Chinese” and “men who has sex with men” or “MSM” with no language or date restrictions, there were no relevant articles related to HIV modelling incorporating drug resistance of PrEP cost-effectiveness (and related terms) in Chinese MSM. Our wider search, removing “China” or “Chinese” yielded two relevant articles related to modelling studies on PrEP cost-effectiveness incorporating the HIVDR among US MSM. They found that PrEP use was cost-effective in the presence of HIVDR among US MSM. However, differences in HIV control strategies, HIV incidence, drug types and costs, resistance testing, cost-effectiveness threshold between China and US, it is necessary to investigate the cost-effectiveness of PrEP in the condition of HIVDR in China.
Added value of this study
We developed a comprehensive dynamic transmission model to study the costs and benefits of expanding PrEP and ART coverage in the presence of HIVDR among MSM in Jiangsu Province, China. The model was calibrated and validated by multiple surveillance datasets from Jiangsu CDC and published literatures. Our findings showed that expanding PrEP and ART would be cost-effective among MSM even considering HIVDR, but expanded ART alone may be the optimal policy under the current budgets in Jiangsu province.
Implications of the available evidence
Our results have important implications for the provision of PrEP among MSM in the presence of Research in Context HIVDR. Expanding PrEP to 20% (or 50%) MSM combined with expanded ART in China would prevent a great number of total and drug resistant infections but also require significant investment of money. The cost of PrEP have the largest impact on the results and 64% reduction in it would achieve highly cost-effectiveness under 50% PrEP coverage combined with expanded ART.
Alt-text: Unlabelled box
Introduction
Antiretroviral therapy (ART) has effectively reduced human immunodeficiency virus (HIV)-related mortality and the risk of HIV transmission, but HIV drug resistance (HIVDR) has increased with the scaling-up of ART.1, 2, 3, 4 In 2019, the World Health Organization (WHO) reported that the HIVDR to non-nucleoside reverse transcriptase inhibitor (NNRTI) among ART-naïve exceeded 10% in 7/18 countries.5 The emergence and transmission of HIVDR may limit treatment options and pose a potential threat to the long-term effectiveness of ART.6,7 HIV prevention strategy based on first-line ART drugs, pre-exposure prophylaxis (PrEP), has shown an effectiveness of 44–86% to prevent new HIV infections among men who has sex with men (MSM).8, 9, 10, 11, 12, 13 Although HIVDR is rare among PrEP users who have acquired HIV due to frequent monitoring,9 PrEP-selected drug resistance may compromise the effectiveness of first-line ART once PrEP users infected. Therefore, the WHO recommends that PrEP scaling-up should be accompanied by surveillance of HIVDR, especially in these low- and middle- income countries like China.14 Understanding how the HIVDR affects the effectiveness of PrEP is essential to control the transmission of HIV.
Since 2012, oral PrEP based on tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) has been approved by the US Food and Drug Administration (FDA),15 and subsequently recommended by the WHO in 2015,16 and then more than 50 countries had released national guidelines following the WHO's PrEP recommendations by the end of 2019.17 Oral PrEP is not popular in China, which may be because that China has not released the PrEP guideline yet.17 To promote the PrEP use among Chinese MSM, a pilot programme of post-exposure prophylaxis (PEP) and PrEP among MSM was conducted in seven provinces and the CROPrEP project (China Real-world Study of Oral PrEP) in four cities in 2018. These studies provided the experience for the implementation of PrEP, primary data of the cascade of PrEP implementation, effectiveness, safety, and possible effects of PrEP use on sexual behaviors in China.18 Based on the evidences from these studies, the first Chinese expert consensus on PrEP was published in 2020.19 However, the cost-effectiveness of PrEP in the presence of HIVDR remains unclear in China.
Mathematical model is a useful tool to access the effectiveness and cost-effectiveness of expanding PrEP coverage and ART with HIVDR.20, 21, 22, 23 Supervie et al.20 developed a mathematical model to predict how PrEP might affect population-level HIV resistance. Abbas et al.21 evaluated the effects of implementing ART alone, PrEP alone, or PrEP plus ART on HIV incidence and drug resistance incidence in South Africa. Drabo et al.22 estimated the costs and benefits of PrEP combined with test-and-treat among MSM in Los Angeles and Shen et al.23 evaluated the cost-effectiveness of earlier ART plus PrEP among MSM in San Francisco. However, there are many differences in HIV control strategies, such as HIV incidence, drug types and costs, resistance testing, cost-effectiveness threshold between China and US, so it is necessary to investigate the cost-effectiveness of PrEP in the presence of HIVDR in China. This will fill the gaps of previous studies on the cost-effectiveness of PrEP in China without considering HIVDR24, 25, 26 or only considering acquired drug resistance (ADR).27
Jiangsu Province is a well-developed province with 80·7 million people (2019) in China, which GDP per capita closely follows Beijing and Shanghai. There were over 31,000 people living with HIV (PLWH) in Jiangsu by the end of 2020.28Among newly diagnosed people living with HIV, the proportion infected through same sex sexual activity increased from 28·0% in 2008 to 56·0% in 2020.28 Since 2019, Jiangsu province has set up HIV PrEP and PEP service network with 28 outpatient service points of PEP.29 However, PrEP have not been implemented in Jiangsu to date. In this paper, we proposed a dynamic compartmental model including transmitted drug resistance (TDR) and ADR, and calibrated this model with the multi-source data: the numbers of annual newly diagnosed people living with HIV and newly treated individuals at four levels of CD4 cell counts (CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL, and CD4 <200 cells/μL), total treated individuals, and deaths among the treated individuals from 2009 to 2019 in Jiangsu Province, China. The model was validated by the dynamic prevalence of TDR and ADR. We evaluated the HIV epidemic under several PrEP coverages with or without expanded ART, and then calculated the cost-effectiveness of various PrEP scenarios. Our results will provide theoretical support for Jiangsu to expand PrEP and ART in the presence of TDR and ADR.
Methods
Data collection
We extracted the following data (Appendix Table S1) during 2009–2019 from the HIV/AIDS information system of Jiangsu Provincial Center for Disease Control and Prevention (Jiangsu CDC): the numbers of annual newly diagnosed people living with HIV and newly treated individuals at four CD4 cell counts (CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL, and CD4 <200 cells/μL), annual total treated individuals, and annual deaths among the treated individuals. The virological failure rates during 2014–2018 were also obtained from the Jiangsu CDC, and the probability of the ADR among the virally unsuppressed individuals was 54·17%.30 For example, the virological failure rate was 7·8% in 2018, and the prevalence of ADR was calculated as 54·17%*7·8%=4·2%.
The dynamic prevalence rates of TDR were obtained from the published literatures (Appendix Table S2).31, 32, 33, 34 The demographic data about population size of total males and those aged 0–14 years old were obtained from Jiangsu Population and Employment Statistics Yearbook.35 We estimated the population size of MSM based on the proportion of MSM in adult males (Appendix Table S3) from the published literatures.36,37
Model formulation
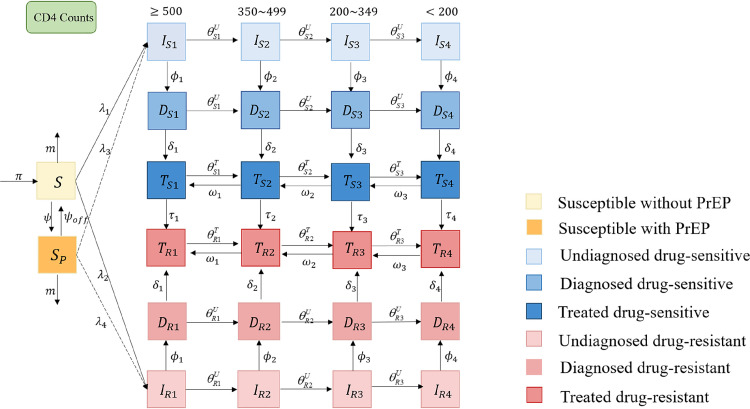
We developed a mathematical model to capture the transmission trends of HIV drug-sensitive and drug-resistant strains among Chinese MSM, based on the natural history of HIV infection, HIV diagnosis and treatment. The total MSM population N was divided into 26 compartments (Figure 1): susceptible individuals without PrEP (S), susceptible individuals with PrEP (SP), undiagnosed infections (Iqj), diagnosed but untreated infections (Dqj) and treated infections (Tqj), where q ∈ {S, R} denoted drug-sensitive and drug-resistant strains, j = 1, 2, 3, 4 denote the four stages of CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL and CD4 <200 cells/μL.
Figure 1.
Flow diagram of the PrEP intervention model. The population was divided into 26 compartments (susceptible individuals without PrEP (S), susceptible individuals with PrEP (SP), undiagnosed infections with drug-sensitive (ISj) or drug-resistant strains (IRj), diagnosed but untreated infections with drug-sensitive (DSj) or drug-resistant strains (DRj), and treated infections with drug-sensitive (TSj) or drug-resistant strains (TRj), j = 1, 2, 3, 4 denote the stages of CD4 >=500 cells/μL, 350–499 cells/μL, 200–349 cells/μL and <200 cells/μL. Subscripts S and R denote infected with drug sensitive (blue compartments) and drug-resistant strains (red compartments). Denote λn as the force of HIV infections, n = 1, 2, 3, 4, where λ1 (λ2) was the per capita rate for the susceptible without PrEP to acquire the infection with the HIV drug-sensitive (drug-resistant) strains, and λ3 (λ4) is the per capita rate for the susceptible with PrEP to acquire the infection with the HIV drug-sensitive (drug-resistant) strains, respectively. Denote π as the recruitment rate and m as the exiting rate due to behavior changes (i.e., not engaging in high-risk sexual behavior). ϕ is the PrEP using rate, and ϕoff is the rate discontinuing PrEP. Denote the time-dependent diagnose and treatment rates as φj and δj,j = 1, 2, 3, 4. τj is the rate of acquired drug resistance after first-line therapy. Denote the disease progression rates from stage of CD4 >=500 cells/μL to CD4 350–499 cells/μL, from CD4 350–499 cells/μL to CD4 200–349 cells/μL, and from CD4 200–349 cells/μL to CD4 <200 cells/μL as () among untreated (treated) individuals, respectively, where the superscript U, T denote the untreated and treated individuals. The reversion rates of the above stages after effective treatment are w1,w2,w3, respectively, we assumed reversion rates are not differ in drug-sensitive and drug-resistance infections. The natural death rate among general population (d) and the HIV-related death rates (,) were not shown in this figure.
λn is denoted as the force of HIV infections, n = 1, 2, 3, 4, where λ1 (λ2) is the per capita rate for the susceptible without PrEP to acquire the infection with the HIV drug-sensitive (drug-resistant) strains, and λ3 (λ4) is the per capita rate for the susceptible with PrEP to acquire the infection with the HIV drug-sensitive (drug-resistant) strains, respectively (see Appendix for details). π is denoted as the recruitment rate and m as the exiting rate due to behavior changes (i.e., not engaging in high-risk sexual behavior). ϕ is the PrEP using rate, and ϕoff is the rate discontinuing PrEP. A proportion of people using PrEP would be diagnosed earlier and move straight from susceptible to diagnosed due to regular testing. In our model, we did not consider this for simplicity, but we performed the sensitivity analysis (Figure 5) by increasing the diagnose rate from 68% to 90%.The time-dependent diagnose and treatment rates are denoted as φj and δj, j = 1, 2, 3, 4 (see Appendix for details).38τj is the rate of acquired drug resistance after first-line therapy. The disease progression rates from stage of CD4 >=500 cells/μL to CD4 350–499 cells/μL, from CD4 350–499 cells/μL to CD4 200–349 cells/μL, and from CD4 200–349 cells/μL to CD4 <200 cells/μL are denoted as () among untreated (treated) individuals, respectively, where the superscript U, T denote the untreated and treated individuals. The reversion rates of the above stages after effective treatment are w1,w2,w3, respectively, and we assumed reversion rates were not different in drug-sensitive and drug-resistance infections.27 The natural death rate among general population (d) and the HIV-related death rates (,) were not shown in the Figure 1.
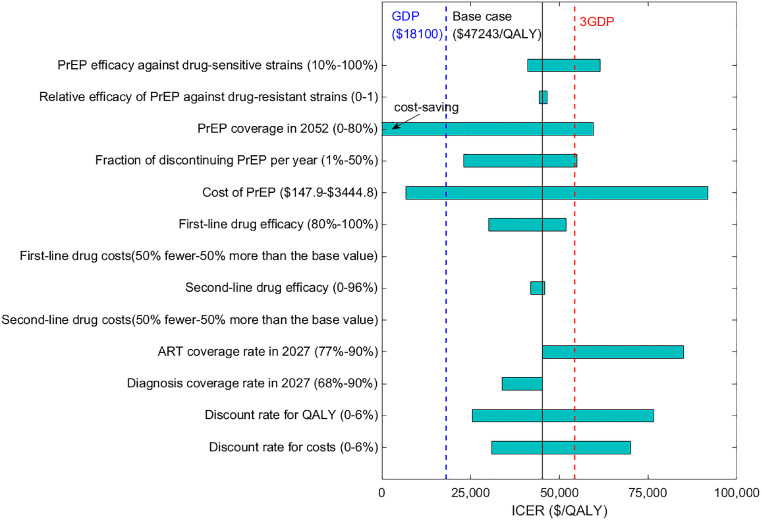
Figure 5.
One-way sensitivity analysis of the cost-effectiveness of 50% PrEP + 90% ART, compared with the base case (no PrEP + 77% ART). The horizontal bars represent the range of the incremental cost-effectiveness ratios (ICERs) as each parameter varies across its plausible range. The bars with negative ICERs in some cases are marked as ‘cost-saving’. The solid vertical black line indicates the base case ICER ($45,222 per QALY gained). The dashed vertical blue and red lines represent the GDP per capita in Jiangsu Province, China ($18,100 in 2020)49 and three times the GDP per capita, respectively. ICER<GDP per capita or GDP per capita <ICER<3GDP per capita denote that the strategy is highly cost-effective and cost-effective. PrEP, pre-exposure prophylaxis; ART, antiretroviral therapy; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; GDP, gross domestic product.
We assumed that first-line and second-line ART reduced infectivity by 96%39 and 80%,40 respectively. PrEP effectiveness was assumed to be 66% against drug-sensitive strain according to a meta-analysis,41 and the relative PrEP effectiveness against resistant strains was 50% (the ratio of PrEP effectiveness against drug-resistant versus drug-sensitive strains).23 ART can increase the life expectancy of infected individuals with drug-sensitive (drug-resistant) by three (two) times, compared with those without treatment. We assumed all individuals with ART would receive lifelong treatment and without droping out of care, and the drug-resistant individuals switched to the second-line therapy timely. Here we assumed drug resistance specifically referred to the resistance to the first-line drugs. We did not differentiate the categorizations of HIVDR to any drugs (NNRTI, nucleoside reverse transcriptase inhibitor [NRTI] or protease inhibitor [PI]) in this study for two reasons. First, data to estimate drug-specific resistance was not readily available. Second, drug-specific parameters were unnecessary to achieve our goal of estimating intervention cost-effectiveness of PrEP. In the sensitive analysis we varied the relative effectiveness of PrEP on drug resistant strains from 0 to 1. We did not consider the PrEP-mediated resistance either, because meta-analysis9 showed that infection with drug-resistant HIV while on PrEP was very rare, and drug resistance mutations were more likely to occur in those individuals with acute HIV infection.
Model calibration
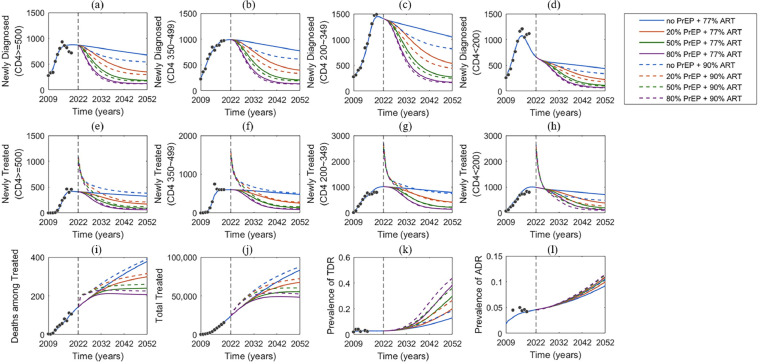
We estimated some of the model parameters (including time-dependent diagnose rate and treatment rate, per-partnership transmission rate, acquired resistance rate and HIV-related death rate) using the nonlinear least-squares method (NLS). These parameters were estimated by calibrating the model to the following data: the numbers of annual newly diagnosed individuals and newly treated individual at four CD4 cells count groups (CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL and CD4 <200 cells/μL), total treated individuals, and deaths among the treated individuals during 2009–2019 in Jiangsu Province (Figure 2 a–j and Figure S2). The model was validated by the dynamic prevalence of ADR and TDR (Figure 2 k-l and Figure S2). These estimated parameter values and the initial population size of each compartment were listed in Appendix Tables S4, S5. In each simulation, we calculated the sum of square errors between the model output and data, and selected the top 10% with the least square errors to generate 95% confidence intervals (CI). Other model parameters were obtained from the published literatures or the database from Jiangsu CDC (see Appendix Table S6). All analyses and simulations were performed in MATLAB R2019b.
Figure 2.
Model fit (blue lines) to annually reported data (black dots) of diagnosed but untreated individuals in CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL and CD4 <200 cells/μL (a–d), treated individuals in CD4 >=500 cells/μL, CD4 350–499 cells/μL, CD4 200–349 cells/μL and CD4 <200 cells/μL (e–h), annual HIV-related deaths among treated MSM (i), total number of HIV infections on treatment (j) and the prevalence of the transmitted drug-resistance (k) and acquired drug-resistance (l). Dashed vertical black lines show rollout of PrEP starting in 2022. Right side of the dash line is the model predictions for eight different PrEP coverage levels, with or without expanded ART (Fig. S1). PrEP, pre-exposure prophylaxis; ART, antiretroviral therapy; MSM, men who have sex with men.
Simulated expanding PrEP and ART scenarios
Based on the above estimated parameters, we projected that ART coverage would reach 77% in 2027 under the status quo. We assumed all uninfected MSM in Jiangsu Province had the potential to use PrEP. The PrEP coverage rate among any time t is SP(t)/(S(t) + SP(t)).
We simulated eight different scenarios with the combination of no PrEP or expanding PrEP coverage to 20% (low), 50% (medium), 80% (high), and ART coverage is 77% or expanding it to 90% after 5 years (in 2027) beginning from 2022 as follows (Appendix Figure S1): Scenario 1: PrEP coverage 0, ART coverage 77% in 2027(status quo); Scenarios 2–4: PrEP coverage 20% (50%, 80%), ART coverage 77% in 2027; Scenarios 5–8: PrEP coverage 0 (20%, 50%, 80%) +ART coverage 90% in 2027.
Economic model
We estimated the costs of PrEP, first- and second-line drugs, HIV testing, genotype resistance testing, associated opportunistic infections, diagnosis and counselling from Jiangsu CDC and published literatures (Appendix Tables S7–S17). The annual costs of PrEP and first-line drugs were $1638·9 ($147·9–$3444·8)42 and $449·8 ($369·3–$504·4),25,27,43, 44, 45 respectively. We assumed PrEP costs included annual costs of drugs (Truvada), routine HIV testing and other STIs testing every 3 months, and pre-PrEP tests (liver and kidney function, bone density et al.) based on the PrEP guidelines of European AIDS Clinical Society 2019 (EACS 2019) and US Centers for Disease Control and Prevention 2017 (US CDC 2017).19 For drug resistance infections, the annual cost of on second-line ART was $1254·3 ($1012·3–$1486·4) and the cost of HIV genotype resistance test was $145·0($72·5–$217·4).25,27,43, 44, 45 We also estimated the quality of life of each health stage based on published literatures (Appendix Table S18).23,25,27,46, 47, 48, 49, 50 We assumed that PrEP did not affect the quality of life, but it decreased by 5% for drug-resistant individuals relative to drug-sensitive individuals at the same stage.23 We calculated quality-adjusted life years (QALYs) and costs of various strategies over the next 30 years. Costs and QALYs were discounted by 3% per year,23 and all costs were expressed in 2020 U.S. dollars (U.S. $).51 The incremental cost-effectiveness ratio (ICER) for each strategy was calculated and compared with those of the status quo and the next best strategy. Using WHO standards,52 strategies with an ICER less than the gross domestic product per capita (GDP per capita $18,100 for Jiangsu in 202053) were considered as highly cost-effective, those with an ICER less than three times the GDP per capita as cost-effective ($54,300), other with an ICER more than three times the GDP per capita as not cost-effective, and those ICER<0 as cost-saving. (Table 1 and Appendix Table S19).
Table 1.
Benefits and costs of expanding PrEP coverage from 2022 to 2052.
| Strategy | Total new infections | Total new infections prevented (fraction) | New infections with drug-resistant strains | Drug-resistant infections prevented (fraction) | HIV prevalence in 2052 | Total HIV-related death | Total HIV-related death prevented (fraction) | Total costs (million US $) a,b | Total QALYs b | Increment-al costs (million US $) c | Increment-al QALYs c | ICER relative to the status quo | ICER relative to next best strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no PrEP + 77% ART (Base case) | 124,639 | .. | 10,812 | .. | 27·8% | 33,884 | .. | 2470 | 9,851,789 | .. | .. | .. | .. |
| 20% PrEP + 77% ART | 81,920 | 42,719 (34·3%) |
9059 | 1753 (16·5%) |
20·6% | 27,973 | 5911 (17·2%) |
6225 | 9,902,777 | 3739 | 51,405 | 77,862 | 77,862 |
| 50% PrEP + 77% ART | 51,727 | 72,912 (58·5%) |
7292 | 3521 (32·9%) |
15·8% | 23,072 | 10,812 (31·5%) |
10,479 | 9,946,036 | 7981 | 95,315 | 89,536 | 103,240 |
| 80% PrEP + 77% ART | 34,994 | 89,645 (71·8%) |
6104 | 4709 (43·9%) |
13·3% | 19,971 | 13,913 (40·6%) |
8573 | 9,977,021 | 11,683 | 126,816 | 98,338 | 124,960 |
| no PrEP + 90% ART | 100,934 | 23,705 (19·0%) |
11,394 | -582 d (-5·6%) |
25·8% | 18,295 | 15,589 (45·9%) |
2349 | 9,976,171 | -128 | 125,211 | Cost-saving | Cost-saving |
| 20% PrEP + 90% ART | 67,106 | 57,533 (44·9%) |
9239 | 1574 (13·4%) |
19·9% | 15,921 | 17,963 (52·4%) |
6216 | 10,005,561 | 3726 | 154,949 | 25,417 | 139,084 |
| 50% PrEP + 90% ART | 43,212 | 81,427 (63·6%) |
7244 | 3569 (31·1%) |
15·8% | 13,981 | 19,903 (57·8%) |
10,526 | 10,029,653 | 8025 | 179,456 | 47,243 | 188,129 |
| 80% PrEP + 90% ART | 29,445 | 95,194 (74·6%) |
5974 | 4838 (43·9%) |
13·6% | 12,524 | 21,360 (62·0%) |
14,248 | 10,045,670 | 11,741 | 195,781 | 63,332 | 243,993 |
PrEP pre-exposure prophylaxis, ART antiretroviral thera\py, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year.
Costs including PrEP, first- and second-line drugs, HIV testing, genotype resistance testing, associated opportunistic infections, diagnosis and counselling.
Costs and quality-adjusted life years (QALYs) are net present values (3% annual discount rate) over 30 years.
Incremental costs and QALYs are relative to the status quo.
The negative number refers to increased drug-resistant infections compare to base case.
Sensitive analysis
We conducted sensitivity analyses to evaluate the potential impact of various model parameters on cost-effectiveness, including PrEP effectiveness on preventing infections with wild-type strains (10–100%), relative efficacy in preventing infection with resistant strains (0–100%), PrEP coverage (0–80%), the fraction of discontinuing PrEP per year (1–50%) and annual PrEP costs ($147·9–$3444·8), etc (Figure 5, Appendix Figures S5–10).
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Impacts of PrEP on the new infections and drug resistant infections
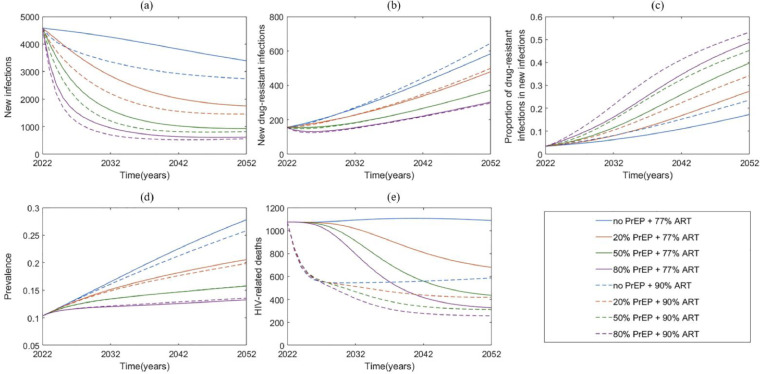
PrEP may greatly decrease new HIV infections and new drug-resistant infections. In the base case (no PrEP + 77% ART in 2027), we estimated that the cumulative numbers of new HIV infections and new drug-resistant infections from 2022 to 2052 would be 124,639 (95% CI: 70,875–178,403) and 10,812 (5932–15,693), respectively (Table 1, Figure 3a, b). 20% PrEP + 77% ART would decrease these two numbers over the next 30 years by 42,719 (25,052–60,386, 34·3% [33·0–35·7%]) and 1753 (1259–2247, 16·5% [12·9–20·1%]), respectively, and yield 51,405 (27,396–75,415) incremental QALYs, compared with the base case. 50% PrEP + 77% ART would decrease these two numbers by 72,912 (41,484–104,339, 58·5% [57·0–59·9%]) and 3521 (2314–4727, 32·9% [28·3–37·5%]), respectively, and yield 95,315 (50,232–140,398) incremental QALYs. 80% PrEP + 77% ART would decrease these two numbers by 89,645 (50,586–128,704, 71·8% [70·4–71·8%]) and 4709 (2938–6479, 43·9% [39·5–48·2%]), respectively, and yield 126,816 (66,831–186,801) incremental QALYs. Expanding ART coverage to 90% alone (no PrEP + 90% ART), 23,705 (13,660–33,750, 19·0% [16·2–21·8%]) new infections would be averted, and yield 125,211 (73,448–176,974) incremental QALYs (Table 1), but the new drug-resistant infections would increase by 582 (248–915, 5·6% [2·6–8·6%]).
Figure 3.
Projection of HIV epidemic trend among Chinese MSM from 2022 to 2052. (a) new infections; (b) new drug-resistant infections; (c) proportion of drug-resistant infections in new infections; (d) HIV/AIDS prevalence; (e) HIV-related death among diagnosed and treated. PrEP pre-exposure prophylaxis; ART antiretroviral therapy.
PrEP combined with expanded ART would further decrease the numbers of new HIV infections and new drug-resistant infections (Figure 3a, b, Table 1). 20% PrEP + 90% ART would reduce these two numbers by 57,533 (32,787–82,278, 44·9% [43·0–46·7%]) and 1574 (1163–1985, 13·4% [8·7–18·1%]) over the next 30 years, respectively, and yield 154,949 (89,662–220,237) incremental QALYs compared with the base case. 50% PrEP + 90% ART would reduce these two numbers by 81,427 (45,853–117,000, 63·6% [ 62·0-65·1%]) and 3569 (2291–4846, 31·1% [26·3–35·8%]), respectively, and yield 179,456 (102,570–256,342) incremental QALYs. 80% PrEP + 90% ART would reduce these two numbers by 95,194 (53,494–136,894, 74·6% [73·1–76·0%]) and 4838 (2944–6733, 43·9% [39·5–48·2%]), respectively, and yield 195,781 (111,080–280,483) incremental QALYs.
Impacts of PrEP on the prevalence and HIV-related deaths
PrEP would decrease the prevalence and disease-induced deaths. In the base case, HIV prevalence would increase to 27·8% [17·3–38·4%] in 2052 and the number of HIV-related deaths from 2022 to 2052 would be 33,884 (19,788–47,980) (Figure 3e, Table 1). 20% (50% or 80%) PrEP + 77% ART would reduce the prevalence to 20·6% [12·9–28·3%] (15·8% [10·3–21·3%] or 13·3% [8·9–17·6%]), and avert 5911 (2928–8893), 10,812 (5416–16,208), 13,913 (7087–20,739) deaths, respectively. 90% ART with no PrEP, 20%, 50%, 80% of PrEP would decrease the prevalence to 25·8% [16·4–35·3%], 19·9% [12·9–26·9%], 15·8% [10·6–21·0%] and 13·6% [9·4–17·8%], and avert 15,589 (9076–22,102), 17,963 (10,320–25,607), 19,903 (11,385–28,422), and 21,360 (12,242–30,478) deaths, respectively.
Economic outcomes
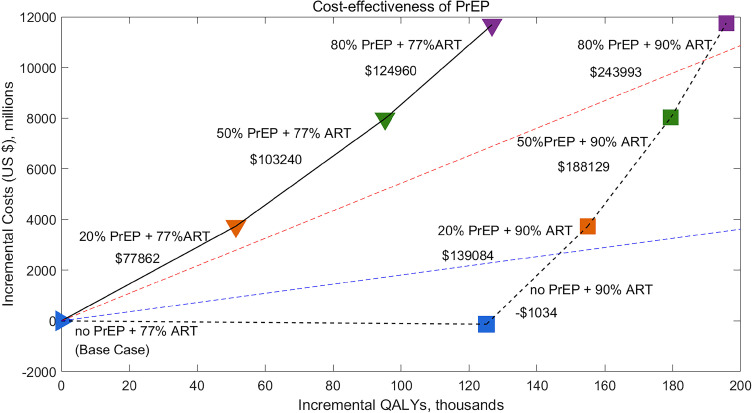
We found that no PrEP + 90%ART would be cost-saving over the next 30 years compare to the base case, and 20% or 50% PrEP + 90% ART would be cost-effective according to WHO standards.52,54 20% PrEP + 90% ART would cost an additional $3726 ($3327– $4125) million over the next 30 years, and has an ICER of $25,417 ($12,390–$38,445) per QALY gained compare to the base case. 50% PrEP + 90% ART would cost an additional $8025 ($7380–$8671) million, with an ICER of $47,243 ($23,756–$70,729) per QALY gained compare to the base case. However, only expanding PrEP while not expanding ART is not cost-effective (Figure 4, Table 1). 20% PrEP + 77% ART, 50% PrEP + 77% ART, and 80% PrEP + 77% ART, would cost $77,862 ($34,016–$121,708), $89,536 ($40,430–$138,642), and $98,338 ($45,824–$150,852) per QALY gained compare to the base case, respectively, which are not cost-effective. Moreover, 80% PrEP + 90% ART is also not cost-effective, with an ICER of $63,332 ($32,374–$94,290) per QALY gained.
Figure 4.
Incremental costs and QALYs of no PrEP, 20%, 50% or 80% PrEP with or without expanded ART (90% ART), compared with the base case (no PrEP + 77% ART). The black solid lines show the incremental cost-effectiveness ratio (ICER) relative to the next best strategy when expanded ART is not implemented. The dashed black lines show the ICER relative to the next best strategy when ART is expanded. Incremental costs and QALYs are calculated over a 30-year time horizon (2022–2052) and are discounted to the present at 3% annually. The dashed blue and red lines represent the GDP per capita in Jiangsu Province, China ($18,100 in 2020)49 and three times the GDP per capita, respectively. Interventions above the red dashed line are defined as not cost-effective, between red dashed line and blue dash line as cost-effective, below the blue dash line as highly cost-effective. Negative value denotes cost-saving. PrEP, pre-exposure prophylaxis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; GDP, gross domestic product.
Sensitive analysis
Our findings are qualitatively robust to parameter uncertainty. In one-way sensitivity analysis, we found that the annual costs and coverage of PrEP, and coverage of ART had the largest impact on the cost-effectiveness (Figure 5). If the annual cost of PrEP was 27% lower than the base case ($1200), then 20% PrEP +90% ART coverage would be highly cost-effective. If the annual cost of PrEP continued to decrease and became 64% lower than the base case ($588), then 50% PrEP + 90% ART would be highly cost-effective (Figure S9). The sensitivity analyses results suggest that PrEP should be initiated after ART coverage has increased to a high level. Results of one-way sensitivity analyses of other interventions are shown in Appendix Figures S5–S10.
Discussion
In this study, we have developed a comprehensive HIV transmission dynamic model to investigate the cost-effectiveness of daily oral PrEP involving acquired and transmitted ART-mediated drug resistance in China. Our results demonstrated that PrEP can prevent substantial new infections (23,705–95,194), new drug resistant infections (1574–4838), and disease-induced deaths (5911–21,360). Expanding ART coverage to 90% without PrEP is cost-saving, and combination of 20% (or 50%) PrEP and 90% ART are cost-effective with an ICER of $25,417 (or $47,243) per QALY gained, even considering the acquisition and transmission of ART-mediated drug resistance. If the annual budget was under $78 million, the optimal strategy is to expand ART coverage without PrEP. Based on the budget ($17 million annually, personal communication) in Jiangsu Province, it would be better to invest in diagnosing and treating more infected individuals. If the annual budget was improved to greater than $202 million, expanding PrEP coverage to above than 20% is advisable.
Our results showed that ART implementation alone would prevent 19% of HIV infections but increase drug resistance prevalence by 5·4% over a 30-year period, ART plus PrEP would futher decrease the incidence. These results were consistent with the study by Abbas et al.21 But they found that expanded ART plus PrEP would increase resistance prevalence, which may be attributed to the different initial DR prevalence between Africa and China. Despite the increasing proportion of new TDR infections, the incidence of TDR infections decreased. This apparent paradox has been shown by Supervie et al.20 that resistance appears to be increasing, but actually is decreasing. When PrEP interventions are implemented, we recommend to employ the number of new drug-resistance infections, rather than the proportion of new drug-resistant infections to monitor drug resistance in the entire population.40
The cost-effectiveness of PrEP depends on a variety of factors, including HIV prevalence, PrEP costs, the thresholds of cost-effectiveness and whether combined with ART. Our results demonstrated that daily oral PrEP targeting 20–80% MSM without expanded ART, with a $77,862–$98,338 per QALY gained, is not cost-effective. These results were consistent with the study in Zhang et al.27 that 20–80% PrEP among high-risk MSM is not cost-effective, with an ICER of $46,813–$52,008, and the cost reduction in PrEP by about 50% would achieve cost effectiveness. But it was different from the results in Hu et al.25 and Li et al.26 that PrEP is cost-effective among Chinese MSM, without considering the drug resistance. Taking ADR and TDR into consideration, our model evaluated the undermined effectiveness of PrEP on drug resistant infections. Impact of relative effectivenss of PrEP against drug-resisitant infections on ICERs was showed in the sensitive analysis (Figures 5 and S5–S10 in Appendix). And we not only evaluated the number of total HIV incidence prevented by PrEP but also the drug-resistant incidence averted by PrEP. Susceptible infected with drug resistant strains would increase the extra expenses including the costly second-line drugs and HIV genotype test, and the QALYs of drug resistant infections was lower. Our findingswere also different from those of Drabo et al.22 and Shen et al.23 that PrEP is cost-effective in the presence of drug resistance for MSM in San Francisco and Los Angeles. Two reasons may explain this discrepancy. First, HIV prevalence and drug-resistant prevalence were different between China and US. For example, the initial HIV prevalence in our study (10·2%) was lower than that among MSM in San Francisco (16%) and Los Angeles (24%), and the initial prevalence of ADR in our study was lower than 2%, but it was 25% in San Francisco, and the prevalence of multidrug resistance was 4·79% in Los Angeles.22,23 Second, the cost-effectiveness thresholds of 3 times GDP per capita ($10,500 in 2020) in China is much lower than that in US ($63,543 in 2020).54 Whereas combination 20% (or 50%) PrEP and 90% ART would be cost-effective with an ICER of $25,417 (or $47,243) per QALY gained. PrEP combined with expanded ART would yield substantial incremental QALYs only with a few increase in costs compared with PrEP alone. For example, 50% PrEP + 90% ART only add $47 million over the next 30 years or $1·56 million annually while yield 83,617 incremental QALYs relative to 50% PrEP+77% ART.
Our findings demonstrated that the cost of PrEP and the coverage of PrEP have important impact on the ICER. If the cost of PrEP reduced by about 64% (annual cost $588), 50% PrEP + 90% ART would be highly cost-effective (Figure 5). Given that patents for PrEP (Truvada) produced by Gilead Company will expire in 2024 in China,55 Chinese government has started negotiations with the pharmaceutical industry, and there were two types of generic TDF/FTC (Glyke and Taihe) have been approved in China in 2020,56,57 so a lower price can be expected. High costs of PrEP have been identified as common barriers to PrEP use58,59 and insurance systems excluding PrEP lead to further barriers to expand PrEP coverage.60 A meta-analysis showed the acceptability was 14·0–36·8% when payment was required, and if PrEP was provided for free, the acceptability would increase to 46·1–61·0%.61 The Chinese government may consider to negotiate with Gilead Company for bulk purchasing, or widely use of generic-drug to obtain PrEP at a lower price, and incorporate the PrEP into the medical insurance system to support the implementation of PrEP.
Our study has several limitations. First, we assumed that routine HIV testing for PrEP user was implemented every 3 months without considering that people using PrEP might move straightly from susceptible to diagnosed. We performed sensitivity analysis (Figure 5) by increasing the diagnose rate to account for this and found that it had little impact on the ICER. In contrast, low adherence to routine screening might compromise the effectiveness of PrEP. Our sensitive analysis mitigates this by accounting for the variations in effectiveness of PrEP on the ICERs. Second, we did not have a standard price of Truvada in China. We collected the price of PrEP from published papers and the website25,27,42,62,63 and used the average annual cost ($1638·9) in this analysis. The annual costs of PrEP are varied from $147·962 to $3444·825,27 as shown in the sensitive analysis and it may change the results of the cost-effectiveness of PrEP. The explanations of the results should be cautious. Third, we assumed an 8% annual rate of PrEP attrition, based on a cohort study in San Francisco,64 for the absence of real-world data among Chinese MSM. But our results should be qualitatively robust by changing it from 1% to 50% per year in sensitivity analyses. Fourth, we assumed that drug-resistant infections switched to second-line drugs in time, and all would be tested for drug resistance. This may increase the costs of the second-line drugs, but it has little impact on the result due to low acquired drug-resistance rate. Fifth, our analysis only evaluated the cost-effectiveness of daily oral PrEP, without directly considering on-demand oral PrEP and long-acting injectable PrEP,65, 66, 67, 68, 69 which may have lower costs and better adherence. We varied the costs and effectiveness of daily oral PrEP in the sensitive analysis to account for these potential effects and the rigorous cost-effectiveness analysis for on-demand and injectable PrEP would be left for further investigation. Finally, the cost-effectiveness of PrEP among Jiangsu cannot be simply generalized to China as a whole due to the great difference of HIV incidence and GDP. Setting-specific analysis was needed based on the local epidemic and economic level.
Conclusion
Expanding PrEP to 20% (or 50%) MSM combined with expanded ART in China would prevent a great number of total cases and drug resistant infections but it requires significant investment of money. These strategies would be cost-effective among MSM even considering the ADR and TDR. However, expanded ART alone may be the optimal policy under the current budgets. Incorporating PrEP into the insurance system and reducing its price to a low level would be beneficial to stimulate PrEP use among MSM.
Additional file
The supplementary appendix associated with this article, which describes model details and parameters estimation.
Ethics approval and consent to participate
The work was approved by the ethical committee of Nanjing Medical University (“F”, "CH”, “Nanjing Med U”, “FWA00001501”, “NANJING”, 11/21/2004), and an IRB (Institutional Review Board) approval was given prior to this study. We have read and have abided by the statement of ethical standards for manuscripts.
Contributors
M.S., Z.P. and X.J. conceived and designed the study. G.F., L.S., C.W., T.Q., Y.Y., extracted the HIV surveillance data from Jiangsu CDC. X.J. analyzed the data, carried out the analysis and performed numerical simulations. X.J. wrote the first draft of the manuscript. M.S. and Z.P. made the key revision. All the authors contributed to the writing of the paper and agreed on manuscript results and conclusions.
Data sharing statement
The data used in this study are referenced in the article and included in the supplementary information files.
Declaration of interests
All authors declare that they have no competing interests.
Acknowledgments
This work is supported by National Natural Science Foundation of China (grant number 82073673 (ZP), 12171387 (MS), 11801435 (MS)); China Postdoctoral Science Foundation (2018M631134 (MS), 2020T130095ZX (MS)); Natural Science Basic Research Program of Shaanxi Province (2019JQ-187 (MS)); Young Talent Support Program of Shaanxi University Association for Science and Technology (20210307 (MS)); the National S&T Major Project Foundation of China (2018ZX10715002-004 (ZP), 2018ZX10713001-001 (ZP)) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD (ZP)). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank Xi'an Jiaotong University for hosting X.J. while the research was conducted during her visiting.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100462.
Contributor Information
Mingwang Shen, Email: mingwangshen521@xjtu.edu.cn.
Gengfeng Fu, Email: fugf@jscdc.cn.
Zhihang Peng, Email: zhihangpeng@njmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Murphy E.L., Collier A.C., Kalish L.A., et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bavinton B.R., Pinto A.N., Phanuphak N., et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5:e438–e447. doi: 10.1016/S2352-3018(18)30132-2. [DOI] [PubMed] [Google Scholar]
- 3.Wittkop L., Günthard H.F., de Wolf F., et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis. 2011;11:363–371. doi: 10.1016/S1473-3099(11)70032-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R.K., Gregson J., Parkin N., et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18:346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO; 2019. HIV Drug Resistance Report.https://www.who.int/publications/i/item/WHO-CDS-HIV-19.21 Jul 1, 2019. Accessed 15 December 2020. [Google Scholar]
- 6.Clutter D.S., Jordan M.R., Bertagnolio S., Shafer R.W. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyrer C., Baral S.D., van Griensven F., et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant R.M., Lama J.R., Anderson P.L., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonner V.A., Dalglish S.L., Kennedy C.E., et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant R.M., Anderson P.L., McMahan V., et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack S., Dunn D.T., Desai M., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grohskopf L.A., Chillag K.L., Gvetadze R., et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64:79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 13.Molina J.M., Capitant C., Spire B., et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO; 2020. HIV Drug Resistance Surveillance in Countries Scaling Up Pre-exposure Prophylaxis.https://www.who.int/publications/i/item/9789240009813 Oct 30. Accessed 16 March 2021. [Google Scholar]
- 15.Holmes D. FDA paves the way for pre-exposure HIV prophylaxis. Lancet. 2012;380:325. doi: 10.1016/s0140-6736(12)61235-5. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO; 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection.https://www.who.int/publications/i/item/9789241549684 Jun 1, 2016. Accessed 16 July 2021. [PubMed] [Google Scholar]
- 17.Xu J., Tang W., Zhang F., Shang H. PrEP in China: choices are ahead. Lancet HIV. 2020;7:e155–e157. doi: 10.1016/S2352-3018(19)30293-0. [DOI] [PubMed] [Google Scholar]
- 18.Jin X., Wang H., Li H., et al. Real-time monitoring and just-in-time intervention for adherence to pre-exposure prophylaxis among men who have sex with men in China: a multicentre RCT study protocol. BMC Public Health. 2020;20:1160. doi: 10.1186/s12889-020-08709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A., Wang Q., Ye J., Liu L. Pre-exposure prophylaxis: Exploring suitable HIV prevention strategies for China. Chin J Epidemiol. 2021;42:357–363. doi: 10.3760/cma.j.cn112338-20200423-00628. [DOI] [PubMed] [Google Scholar]
- 20.Supervie V., García-Lerma J.G., Heneine W., Blower S. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci U S A. 2010;107:12381–12386. doi: 10.1073/pnas.1006061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas U.L., Glaubius R., Mubayi A., Hood G., Mellors J.W. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis. 2013;208:224–234. doi: 10.1093/infdis/jit150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drabo E.F., Hay J.W., Vardavas R., Wagner Z.R., Sood N. A Cost-effectiveness analysis of preexposure prophylaxis for the prevention of HIV among Los Angeles County men who have sex with men. Clin Infect Dis. 2016;63:1495. doi: 10.1093/cid/ciw578. -04. [DOI] [PubMed] [Google Scholar]
- 23.Shen M., Xiao Y., Rong L., Meyers L.A., Bellan S.E. The cost-effectiveness of oral HIV pre-exposure prophylaxis and early antiretroviral therapy in the presence of drug resistance among men who have sex with men in San Francisco. BMC Med. 2018;16:58. doi: 10.1186/s12916-018-1047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong N.S., Kwan T.H., Tsang O.T.Y., et al. Pre-exposure prophylaxis (PrEP) for MSM in low HIV incidence places: should high risk individuals be targeted? Sci Rep. 2018;8:11641. doi: 10.1038/s41598-018-30101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Q.H., Meyers K., Xu J.J., et al. Efficacy and cost-effectiveness of early antiretroviral therapy and partners' pre-exposure prophylaxis among men who have sex with men in Shenyang, China: a prospective cohort and costing study. BMC Infect Dis. 2019;19:663. doi: 10.1186/s12879-019-4275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Peng L., Gilmour S., et al. A mathematical model of biomedical interventions for HIV prevention among men who have sex with men in China. BMC Infect Dis. 2018;18:600. doi: 10.1186/s12879-018-3516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Peng P., Wu Y., et al. Modelling the epidemiological impact and cost-effectiveness of PrEP for HIV Transmission in MSM in China. AIDS Behav. 2019;23:523–533. doi: 10.1007/s10461-018-2205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L., Tang W., Hu H., et al. The impact of COVID-19 pandemic on HIV care continuum in Jiangsu, China. BMC Infect Dis. 2021;21:768. doi: 10.1186/s12879-021-06490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding J., Liu X., Hu H. The development report of 30 years AIDS prevention and control in Jiangsu Province. Jiangsu Prev Med. 2022;33:1–2. +7. [Google Scholar]
- 30.Zhou Y., Lu J., Zhang Z., Fu G.F. Risk factors associated with HIV drug resistance among ART virological failure patients taking first-line antiviral treatment from Jiangsu Province. Chin J Dis Control Prev. 2017;21:1191–1194. +1199. [Google Scholar]
- 31.Guo H., Xu X., Hu H., et al. Low prevalence of the transmitted HIV-1 drug resistance among newly diagnosed HIV-1 individuals in Jiangsu Province, China during 2009-2011. BMC Public Health. 2015;15:120. doi: 10.1186/s12889-015-1489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo L., Liu K., Liu H., et al. Trend of HIV-1 drug resistance in China: a systematic review and meta-analysis of data accumulated over 17 years (2001-2017) EClinicalMedicine. 2020;18 doi: 10.1016/j.eclinm.2019.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Lu J., Wang J., et al. Prevalence of HIV antiretroviral drug resistance and its impacts on HIV-1 virological failures in jiangsu, china: a cross-sectional study. Biomed Res Int. 2016;2016 doi: 10.1155/2016/1752437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B., Han X., Xu J., et al. Increase of RT-related transmitted drug resistance in non-CRF01_AE among HIV type 1-infected men who have sex with men in the 7 cities of China. J Acquir Immune Defic Syndr. 2015;68:250–255. doi: 10.1097/QAI.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 35.Jiangsu Bureau of Statistics . Jiangsu Bureau of Statistics; 2021. Jiangsu Provincial Population and Employment Statistics Yearbook.http://tj.jiangsu.gov.cn/col/col4009/index.html 2009-2019. Accessed 5 March. [Google Scholar]
- 36.Hu M., Xu C., Wang J. Spatiotemporal analysis of men who have sex with men in mainland China: social app capture-recapture method. JMIR Mhealth Uhealth. 2020;8:e14800. doi: 10.2196/14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing L.W., Cui Y.H., Yu H.M. Use of the multiplier method to estimate the size of populations most at risk of human immunodeficiency virus infection in China: a systematic review. Public Health. 2020;185:254–260. doi: 10.1016/j.puhe.2020.05.067. [DOI] [PubMed] [Google Scholar]
- 38.Shen M, Xiao Y, Xing H, Ruan Y. The impact of attrition on the transmission of HIV drug resistance: a mathematical modelling study. In submission.
- 39.Cohen M.S., McCauley M., Gamble T.R. HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS. 2012;7:99–105. doi: 10.1097/COH.0b013e32834f5cf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen M., Xiao Y., Rong L., Meyers L.A., Bellan S.E. Early antiretroviral therapy and potent second-line drugs could decrease HIV incidence of drug resistance. Proc Biol Sci. 2017;284 doi: 10.1098/rspb.2017.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X., Hou J., Song A., et al. Efficacy and safety of oral TDF-based pre-exposure prophylaxis for men who have sex with men: a systematic review and meta-analysis. Front Pharmacol. 2018;9:799. doi: 10.3389/fphar.2018.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Data Y. Drug bid-winning information inquiry. Truvada. 2021 https://db.yaozh.com/yaopinzhongbiao?comprehensivesearchcontent=%E8%88%92%E5%8F%91%E6%B3%B0& Accessed 3 June. [Google Scholar]
- 43.Peng G.P., Li W.H., Da Q. Demand forecast for the free antiretroviral drugs of AIDS in Hubei Province. J of Pub Health and Prev Med. 2010;21:35–37. [Google Scholar]
- 44.Ma L.P. Chinese Center for Disease Control and Prevention.; 2016. Cost, Effect and Effectiveness of Different Antiviral Therapy Strategies in AIDS Mono-positive Families. [Google Scholar]
- 45.Sun H.M. Nantong University; 2015. Epidemiological Analysis and Health Economical Evaluation of Scale-up of HIV Testing and Antiretroviral Therapy (ART) Among Men Who have Sex with Men (MSM) [Google Scholar]
- 46.Nichols B.E., Boucher C.A.B., van der Valk M., Rijnders B.J.A., van de Vijver D. Cost- effectiveness analysis of pre-exposure prophylaxis for HIV-1 prevention in the Netherlands: a mathematical modelling study. Lancet Infect Dis. 2016;16:1423–1429. doi: 10.1016/S1473-3099(16)30311-5. [DOI] [PubMed] [Google Scholar]
- 47.Zang X., Tang H., Min J.E., et al. Cost-effectiveness of the 'One4All' HIV linkage intervention in Guangxi Zhuang Autonomous Region, China. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichols B.E., Sigaloff K.C., Kityo C., et al. Increasing the use of second-line therapy is a cost-effective approach to prevent the spread of drug-resistant HIV: a mathematical modelling study. J Int AIDS Soc. 2014;17:19164. doi: 10.7448/IAS.17.1.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juusola J.L., Brandeau M.L., Owens D.K., Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156:541–550. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders G.D., Bayoumi A.M., Sundaram V., et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 51.National Bureal of Statistics . National Bureal of Statistic; 28, 2021. Statistical Communique of the People's Republic of China on National Economic and Social Development 2020.http://www.stats.gov.cn/tjsj/zxfb/202102/t20210227_1814154.html Accessed 12 July 2021. [Google Scholar]
- 52.World Health Organization . World Health Organization; 2001. Macroeconomics and Health: Investing in Health for Economic Development.http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf Report of the Commission on Macroeconomics and Health. Dec 20. Accessed 28 June 2020. [Google Scholar]
- 53.Jiangsu Provincial Bureau of Statistics . Jiangsu Provincial Bureau of Statistics; 2021. Jiangsu's Economic Aggregate Jumped to 10 Trillion Yuan.http://tj.jiangsu.gov.cn/art/2021/1/28/art_4027_9658080.html Jan 28. Accessed 9 July 2021. [Google Scholar]
- 54.The World Bank . The World Bank; 2021. GDP per capita (current US$) (Map)https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2020&most_recent_year_desc=true&start=2020&view=map Accessed 15 July. [Google Scholar]
- 55.MedsPaL Database. Patent card for China 2004 (CN201210094391). Jan 13, 2004. ttps://www.medspal.org/?keywords=Turvada&page=2 . Accessed 21 July 2021.
- 56.Chia Tai Tianqing Pharmaceutical Group Co. LTD . Chia Tai Tianqing Pharmaceutical Group Co LTD; 2022. The first Generic TDF/FTC has Approved.https://www.cttq.com/news/578867.htm Jun 11. Accessed 31 March 2022. [Google Scholar]
- 57.Anhui Baker Biopharmaceutical Co . Anhui Baker Biopharmaceutical Co; 2020. LTD. 2.8 Billion Big Varieties, Second Generic Emtricitabine Tenofovir Tablets in China was Belong to Anhui Beck.http://www.bcpharm.com/display.php?id=432 Nov 17. Accessed 31 March 2022. [Google Scholar]
- 58.Goedel W.C., Halkitis P.N., Greene R.E., Duncan D.T. Correlates of awareness of and willingness to use pre-exposure prophylaxis (PrEP) in gay, bisexual, and other men who have sex with men who use geosocial-networking smartphone applications in New York City. AIDS Behav. 2016;20:1435–1442. doi: 10.1007/s10461-016-1353-6. [DOI] [PubMed] [Google Scholar]
- 59.Mullins T.L.K., Zimet G., Lally M., Xu J., Thornton S., Kahn J.A. HIV care providers' intentions to prescribe and actual prescription of pre-exposure prophylaxis to at-risk adolescents and adults. AIDS Patient Care STDs. 2017;31:504–516. doi: 10.1089/apc.2017.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayer K.H., Agwu A., Malebranche D. Barriers to the wider use of pre-exposure prophylaxis in the United States: a narrative review. Adv Ther. 2020;37:1778–1811. doi: 10.1007/s12325-020-01295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng P., Su S., Fairley C.K., et al. A global estimate of the acceptability of pre-exposure prophylaxis for HIV among men who have sex with men: a systematic review and meta-analysis. AIDS Behav. 2018;22:1063–1074. doi: 10.1007/s10461-017-1675-z. [DOI] [PubMed] [Google Scholar]
- 62.Fan C., Jin Y.Y., Jia Z.W. Cost-effectiveness analysis of pre-exposure prophylaxis for men who have sex with men in China. J AIDS STD. 2018;24:692–696. [Google Scholar]
- 63.Zhong Y. Chongqing Medical University; 2018. Economic Evaluation of PrEP Prevention Strategy in MSM Population. [Google Scholar]
- 64.Liu A., Cohen S., Follansbee S., et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J., Xu J.J., Wang H.Y., et al. Preference for daily versus on-demand pre-exposure prophylaxis for HIV and correlates among men who have sex with men: the China Real-world Oral PrEP Demonstration study. J Int AIDS Soc. 2021;24:e25754. doi: 10.1002/jia2.25667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina J.M., Charreau I., Spire B., et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4:e402–e410. doi: 10.1016/S2352-3018(17)30089-9. [DOI] [PubMed] [Google Scholar]
- 67.Durand-Zaleski I., Mutuon P., Charreau I., et al. Costs and benefits of on-demand HIV preexposure prophylaxis in MSM. AIDS. 2018;32:95–102. doi: 10.1097/QAD.0000000000001658. [DOI] [PubMed] [Google Scholar]
- 68.van Vliet M.M., Hendrickson C., Nichols B.E., Boucher C.A., Peters R.P., van de Vijver D.A. Epidemiological impact and cost-effectiveness of providing long-acting pre-exposure prophylaxis to injectable contraceptive users for HIV prevention in South Africa: a modelling study. J Int AIDS Soc. 2019;22:e25427. doi: 10.1002/jia2.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith J.A., Garnett G.P., Hallett T.B. The potential impact of long-acting cabotegravir for HIV prevention in South Africa: a mathematical modeling study. J Infect Dis. 2021;224:1179–1186. doi: 10.1093/infdis/jiaa296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.