Abstract
Research into chloroquine resistance reversal in Plasmodium falciparum has revealed a widespread range of functionally and structurally diverse chemosensitizers. However, nearly all of these chemosensitizers reverse resistance optimally only at concentrations that are toxic to humans. Verapamil, desipramine, and trifluoperazine were shown to potentiate chloroquine accumulation in a chloroquine-resistant (CQr) strain of P. falciparum, while progesterone, ivermectin, and cyclosporin A were not shown to potentiate chloroquine accumulation. The simultaneous use of two or even three of these chemosensitizers at concentrations within their therapeutic ranges in humans displayed an additive effect in potentiating chloroquine accumulation in the CQr strain. The levels of resistance reversal achieved with these multiple combinations were comparable to those achieved with high concentrations of the single agents used to enhance the activity of chloroquine. No chemosensitizer, whether used singly or in combination, potentiated any change in chloroquine accumulation or a shift in the 50% inhibitory concentration for the chloroquine-sensitive strain. The use of combinations of chemosensitizers at concentrations not toxic to humans could effectively reverse chloroquine resistance without the marked toxicity from the use of a single agent at high concentrations. This cocktail of chemosensitizers may serve as a viable treatment to restore the efficacy of chloroquine in patients with malaria.
The spread of chloroquine (CQ) resistance in Plasmodium falciparum throughout most areas where malaria is endemic has necessitated alternate treatments for malaria. More recently, antimalarials such as mefloquine and halofantrine were developed, but indications are that these are becoming ineffective as resistance to them spreads (20).
There have been attempts to restore CQ's efficacy in vitro and in vivo by using it in combination with resistance reversers like promethazine and chlorpheniramine (16, 18). However, these compounds, which stimulate the uptake of CQ by resistant strains and drastically reduce the 50% inhibitory concentration (IC50), operate optimally as resistance reversers in vitro only at concentrations that are highly toxic in vivo. Work with multidrug-resistant (MDR) cancer cells has shown that it is possible to reverse anticancer agent resistance by using combinations of chemosensitizers at concentrations not toxic to humans (10). The levels of reversal obtained with these combinations were comparable to those obtained with the single agents used at their optimal concentrations.
In P. falciparum, two calcium channel blockers, verapamil (VPL) and fantofarone, have been shown to act synergistically in reversing CQ resistance (1). We selected several structurally and functionally diverse compounds to test CQ resistance (CQr) reversal in P. falciparum. VPL and desipramine (DES) are known resistance reversers in P. falciparum (2, 14). However, progesterone (PROG), ivermectin (IVM), trifluoperazine (TRF), and cyclosporine (CsA) have not been implicated in CQ resistance reversal, although they do reverse multidrug resistance in cancer cells (7, 11, 17). A combination of the chemosensitizers used at low concentrations was shown to work as effectively in vitro in reversing CQ resistance as the single compounds used at their optimal concentrations with CQ. This may yet prove to be an effective way of overcoming the CQ resistance without the toxicity associated with these chemosensitizers in vivo.
MATERIALS AND METHODS
Chemicals.
Chloroquine diphosphate, verapamil hydrochloride, desipramine hydrochloride, trifluoperazine dihydrochloride, PROG, CsA, and IVM were purchased from Sigma Chemical Co., St. Louis, Mo.
In vitro P. falciparum culture.
Two strains were selected for experimental work: D10, a CQ-sensitive (CQs) strain (donation from A. Cowman, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia), and RSA11, a CQr strain (Janet Freese, Medical Research Council, Durban, South Africa).
The IC50s of CQ for D10 and RSA11 are 11.63 and 339.2 nM, respectively (5, 6). The parasites were cultured by a method modified from that of Trager and Jenson (19). The parasites were maintained in type O-positive human red blood cells, 10% type A-positive human serum, and RPMI 1640 culture medium (Biowhittaker). The culture medium was supplemented with 1% sodium bicarbonate and gentamicin (40 mg/ml). The cultures were kept in continuous culture under a gas mixture of 4% CO2, 3% O2, and 93% N2. Cultures were synchronized in the ring stage with 5 volumes of 5% d-sorbitol (12).
Drug dilutions and solvent controls.
CQ, VPL, DES, and TRF were dissolved in distilled water, while PROG, IVM, and CsA were dissolved in ethanol. Appropriate controls were established for the solvents used and the combinations tested, but none showed any toxicity in either the CQ accumulation or the IC50 determination experiments.
Tritiated CQ accumulation.
Synchronized parasitized erythrocytes in the trophozoite growth stage (1% hematocrit, 5% parasitemia) were exposed to 1 nM [3H]CQ (18.8 Ci/mmol; Amersham) in a 1.5-ml microcentrifuge tube. Appropriate controls were established for the solvent. The tubes were then incubated at 37°C in a water bath for 1 h. For the combination studies, the parasites were first incubated for 15 min at 37°C in the presence of a fixed concentration of the chemosensitizers before the radioactive CQ was added. After incubation, the parasitized erythrocytes were centrifuged (Microfuge E; Beckman) and washed twice with ice-cold phosphate-buffered saline. The microcentrifuge tube tip containing the parasitized erythrocytes was then cut off and placed in a scintillation vial with 5 ml of scintillation fluid (Quicksafe A; Zinsser Analytic) and shaken overnight. The radioactivity within the vials was counted in a Packard Tri-Carb 4640 liquid scintillation spectrophotometer.
Parasite lactate dehydrogenase assay.
The IC50s for the parasites in the presence and absence of chemosensitizers was measured by a method modified from that Makler et al. (13). The parasites were maintained at 1% hematocrit and 2% parasitemia for 48 h along with the particular drug to be tested. For the combination studies, the parasites were incubated with serially diluted CQ in the presence of a fixed concentration of one or more of the chemosensitizers. The Malstat (Flow Inc.) reagent was used as an indicator of parasite viability.
RESULTS
Intrinsic antimalarial activities of chemosensitizers.
The IC50s of CQ and the six putative chemosensitizers tested with both strains are summarized in Table 1. There were no statistically significant differences between the results for the CQr and CQs strains used except for those for CQ, CsA, and IVM. Most of the IC50s fell within the micromolar range, suggesting a weak intrinsic antimalarial activity. These values are almost 1,000-fold higher than that of CQ for the CQs strain. However, the IC50 of CsA was within the nanomolar range, and CsA appeared to be more active against the CQs strain. In addition, it was more effective than CQ against the CQr strain.
TABLE 1.
IC50s showing the intrinsic antimalarial activities of CQ and the putative chemosensitizers performed with the CQs and CQr strains of P. falciparum
| Compound | IC50 (μM)a
|
|
|---|---|---|
| D10 | RSA11 | |
| CQ | 0.027 ± 0.003 | 0.326 ± 0.013 |
| VPL | 12.72 ± 0.877 | 12.68 ± 1.272 |
| DES | 13.90 ± 0.007 | 11.74 ± 4.244 |
| TRF | 5.660 ± 1.045 | 5.901 ± 1.794 |
| PROG | 24.68 ± 4.612 | 23.77 ± 3.567 |
| CsA | 0.158 ± 0.019 | 0.275 ± 0.026 |
| IVM | 18.85 ± 4.730 | 8.898 ± 0.998 |
Values represent the means ± standard deviations for three independent experiments, each of which was performed in duplicate. The values obtained for each strain are not significantly different (P ≥ 0.05) between strains except for those for CQ, CsA, and IVM.
Tritiated CQ accumulation in presence of chemosensitizers.
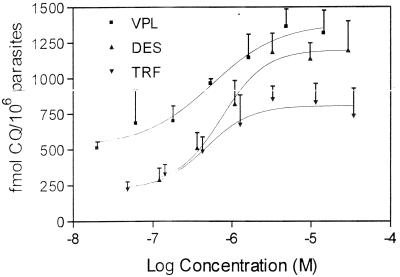
CQ accumulation in parasitized erythrocytes was evaluated in the presence of the putative chemosensitizers over a large range of concentrations. In the CQs strain D10, there was no significant increase in the level of accumulation of CQ in the presence of any of the chemosensitizers (data not shown). In the CQr strain, there was also no significant increase in the level of CQ accumulation with PROG, CsA, and IVM. However, there was a dose-dependent increase in uptake in the CQr strain with VPL, TRF, and DES (Fig. 1). The maximum levels of accumulation of CQ in the presence of these drugs were obtained at 5, 3, and 3 μM for VPL, DES, and TRF, respectively. The solvent control did not affect the level of CQ accumulation (data not shown).
FIG. 1.
Dose-response curves showing the increase in the level of CQ accumulation with the single chemosensitizers in the CQr strain. Values are the means ± standard deviations for three independent experiments, each of which was performed in duplicate.
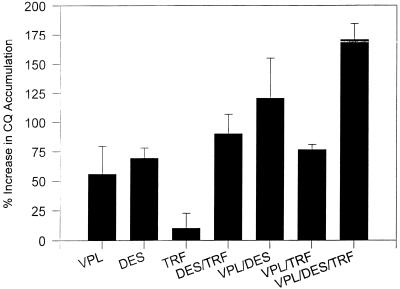
Tritiated CQ accumulation with simultaneous combinations of chemosensitizers in CQr strain.
From the data presented above it was decided that for the combination experiments chemosensitizer concentrations that would be regarded as nontoxic for humans would be used (15). The concentrations selected for VPL, DES, and TRF were 250, 175, and 50 nM, respectively. In the experiments whose results are presented in Fig. 2, CQ was incubated either singly or with combinations of either two or three chemosensitizers. In the CQr strain, an additive accumulation effect was observed with the combinations tested. There was no significant increase in the level of CQ accumulation in the CQs strain with any of the chemosensitizer combinations used (data not shown).
FIG. 2.
Effect of multiple combinations on the level of CQ accumulation in the CQr strain of P. falciparum. The chemosensitizers' concentrations are within the therapeutic level in humans. The concentrations of VPL, DES, and TRF are 250, 175, and 50 nM, respectively. The results are for two independent experiments, each of which was performed in duplicate.
Resistance reversal with simultaneous combinations of chemosensitizers in a CQr strain.
The ability of combinations of low concentrations of chemosensitizers to act additively to stimulate CQ uptake in the CQr strain suggested that these combinations might also have an additive effect in lowering the CQ IC50.
The IC50s of CQ used with a variety of combinations of chemosensitizers are shown in Table 2. The concentrations of the chemosensitizers used were the same as those used in the experiments whose results are presented in Fig. 2. The sensitivity of the CQr strain observed with each chemosensitizer used alone was increased, in some cases dramatically, with all of the combinations used. In two of the multiple combinations, VPL-TRF and VPL-DES-TRF, the shift in CQ sensitivity was so marked that it was comparable to or less than the sensitivity of the CQs strain to CQ.
TABLE 2.
IC50s of CQ alone and in combination with the chemosensitizers for CQs and CQr strains
| Drug combinationa | IC50 (nM)b
|
|
|---|---|---|
| D10 (CQS) | RSA11 (CQR) | |
| CQ | 26.30 ± 2.963 | 337.4 ± 8.202 |
| CQ + DES | 24.56 ± 4.830 | 109.7 ± 0.141 |
| CQ + TRF | 25.82 ± 6.781 | 295.6 ± 33.94 |
| CQ + VPL | 22.79 ± 2.222 | 167.6 ± 62.73 |
| CQ + DES-TRF | —c | 69.84 ± 42.50 |
| CQ + DES-VPL | — | 36.08 ± 23.58 |
| CQ + TRF-VPL | — | 26.56 ± 21.49 |
| CQ + DES-TRF-VPL | — | 6.958 ± 4.005 |
The concentrations of VPL, DES, and TRF were identical to those used in the radioactive chloroquine accumulation experiment whose results are shown in Fig. 2.
Values are the means ± standard deviations for two independent experiments, each of which was performed in duplicate.
—, These combinations were not tested for resistance reversal, as their individual components did not show any effect on the CQs strain.
There was no shift in the sensitivity of the CQs strain with any of the single chemosensitizers used.
DISCUSSION
The data in Table 1 show that the putative chemosensitizers have very low levels of antimalarial activity when they are used alone. Also, there were no statistically significant differences in their effects between the CQr and CQs strains used. The exception was CsA, which possessed antimalarial activity within the nanomolar range and for which there was a significant difference in activity between the two strains. It has been shown in vitro and in vivo that CsA exhibits significant antiprotozoal activity (for a review, see reference 4). CsA also appears to be more effective against the CQs strain than against the CQr strain. The reason for this difference is not known.
The CQ accumulation studies confirm that these chemosensitizers have no effect on the action of CQ against the CQs strain. Figure 1 illustrates the dose-response effects of VPL, TRF, and DES on increasing the level of CQ accumulation in the CQr strain. The optimal concentration for this CQ accumulation is in the toxic range for these compounds. None of the IVM, PROG, or CsA concentrations tested resulted in an increase in the level of accumulation of CQ, nor were they able to reverse resistance in the CQr P. falciparum strain (data not shown). Both IVM and CsA are highly effective at increasing anticancer drug uptake in MDR cancer cell lines (17). These results may indicate that the mechanism of resistance reversal in P. falciparum operates via a mechanism different from that in MDR cancer cells, even though there is clearly an overlap in some of the agents able to reverse resistance.
There is a maximum level of CQ accumulation that can be achieved with the single chemosensitizers (Fig. 1). When the different chemosensitizers were combined at their optimal concentrations, there was no additive effect on the level of CQ accumulation above the maximal level achieved with each of the single components (data not shown). This implies that there is a saturation point above which there is no further effect of the chemosensitizers on CQ accumulation. However, when combinations of chemosensitizers were mixed at concentrations which alone yielded submaximal levels of accumulation, an additive effect was observed (Fig. 2). The amount of CQ that accumulated with the chosen combinations did not reach the level of CQ that accumulated with the single drugs at their optimal concentrations (data not shown).
Despite this, the reversal effect observed with some combinations, in particular, the triple combination, resulted in a lowering of the IC50 for the CQr strain to that for the CQs strain. It is unclear why the levels of CQ accumulation in Fig. 2 do not correlate with the levels of reversal seen in Table 2. One would expect that higher levels of accumulation would result in a more pronounced resistance reversal; however, this was not observed. The enhanced reversal observed with the multiple combinations could be a result of some combined interaction between the compounds that is observed only by the 48-hour Malstat assay but not by the 1-h CQ accumulation assay.
It has previously been reported that combinations of chemosensitizers at nontoxic levels can be effectively used to reverse resistance in cancer cells (9, 10). It has also been reported that in combination, of VPL and fantofarone, both of which are calcium channel blockers, act synergistically in reversing CQ resistance in P. falciparum (1). In addition, it was recently demonstrated that certain plant compounds act synergistically in enhancing CQ activity in a CQr strain (8). It is clear from the work presented here that chemosensitizers from different classes of drugs can act synergistically to reverse CQ resistance. Since a large number of structurally and functionally different compounds are able to reverse CQ resistance in vitro, it may be possible to formulate a cocktail of drugs for use in vivo, with each compound used at concentrations sufficient to minimize the toxicity while maintaining the efficacy of treatment. It was recently shown that CQ resistance could be reversed in humans with a single antihistamine, chlorpheniramine or promethazine, and these would be potential candidates for use in a cocktail (16, 18). Clearly, however, both bioavailability and protein binding will need to be considered when candidate drugs for use in an in vivo cocktail are chosen. DES, which resulted in excellent in vitro resistance reversal, is not able to reverse resistance in humans owing to its high level of plasma protein binding (3). It is being investigated whether combinations of chemosensitizers can be used with CQ to reverse resistance in vivo. If it can be shown that these combinations are more effective in vivo than the single antihistamines currently being investigated and that no marked toxicity is associated with them, this multiple combination therapy should perhaps be considered as an alternative to the use of CQ in areas where CQ resistance is endemic but where no other alternatives are available.
ACKNOWLEDGMENTS
We thank the University of Cape Town Research Committee and the Medical Research Council of South Africa for the financial assistance received for this study.
REFERENCES
- 1.Adovelande J, Delèze J, Shrével J. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem Pharmacol. 1998;55:433–440. doi: 10.1016/s0006-2952(97)00482-6. [DOI] [PubMed] [Google Scholar]
- 2.Bitonti A J, Sjoerdsma A, McCann P P, Kyle D E, Oduola A M, Rossan R N, Milhous W K, Davidson D E J. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1998;242:1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- 3.Boulter M K, Bray P G, Howells R E, Ward S E. The potential of desipramine to reverse chloroquine resistance of Plasmodium falciparum is reduced by its binding to plasma protein. Trans R Soc Trop Med Hyg. 1993;87:303. doi: 10.1016/0035-9203(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 4.Chappell L H, Wastling J M. Cyclosporin A: antiparasite drug, modulator of the host-parasite relationship and immunosuppressant. Parasitology. 1992;105:S25–S40. doi: 10.1017/s0031182000075338. [DOI] [PubMed] [Google Scholar]
- 5.Foote S J, Kyle D E, Martin R K, Oduola A M, Forsyth K, Kemp D J, Cowman A F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990;345:255–259. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- 6.Freese J A, Markus M B, Golenser J. In vitro sensitivity of southern African reference isolates of Plasmodium falciparum to chloroquine and pyrimethamine. Bull W H O. 1991;69:707–712. [PMC free article] [PubMed] [Google Scholar]
- 7.Georges E, Sharom F J, Ling V. Multidrug resistance and chemosensitization: therapeutic implications for cancer chemotherapy. Adv Pharmacol. 1990;21:185–213. doi: 10.1016/s1054-3589(08)60343-9. [DOI] [PubMed] [Google Scholar]
- 8.Haruki K, Bray P G, Ono M, Ward S A. Potent enhancement of the sensitivity of Plasmodium falciparum to chloroquine by the bisbenzylquinoline alkaloid cephantharin. Antimicrob Agents Chemother. 2000;44:2706–2708. doi: 10.1128/aac.44.10.2706-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X F, Martin T J, Bell D R, Luise M, Zalcberg J R. Combined use of cyclosporin A and verapamil in modulating multidrug resistance in human leukemia cell lines. Cancer Res. 1990;50:2953–2957. [PubMed] [Google Scholar]
- 10.Hwang M, Ahn C H, Pine P S, Yin J J, Hryeyna C A, Light T, Aszalos A. Effect of combination of suboptimal concentrations of P-glycoprotein blockers on the proliferation of MDR1 gene expressing cells. Int J Cancer. 1996;65:389–397. doi: 10.1002/(SICI)1097-0215(19960126)65:3<389::AID-IJC19>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Juliano R L, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochem Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 12.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418–420. [PubMed] [Google Scholar]
- 13.Makler M T, Ries J M, Williams J A, Bancroft J E, Piper R C, Gibbons B L, Hinrichs D J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48(6):739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 14.Martin S K, Oduola A M, Milhous W K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 15.Moffat A C, editor. Clarke's isolation and identification of drugs in pharmaceuticals, body fluids and post-mortem materials. 2nd ed. London, Great Britain: The Pharmaceutical Press; 1986. [Google Scholar]
- 16.Oduola A M J, Sowunmi A, Milhous W K, Brewer T G, Kyle D E, Gerena L, Rossan R N, Salako L A, Schuster B G. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am J Trop Med Hyg. 1998;58:625–629. doi: 10.4269/ajtmh.1998.58.625. [DOI] [PubMed] [Google Scholar]
- 17.Pouliot J-F, L'Heureux F, Liu Z, Prichard K, Georges E. Reversal of P-glycoprotein-associated multidrug resistance by ivermectin. Biochem Pharmacol. 1997;53:17–25. doi: 10.1016/s0006-2952(96)00656-9. [DOI] [PubMed] [Google Scholar]
- 18.Sowunmi A, Oduola A M, Ogundahunsi O A, Falade C O, Gbotosho G O, Salaka L A. Enhanced efficacy of chloroquine-chlorpheniramine combination in acute uncomplicated falciparum malaria in children. Trans R Soc Trop Med Hyg. 1997;91:63–67. doi: 10.1016/s0035-9203(97)90399-0. [DOI] [PubMed] [Google Scholar]
- 19.Trager W, Jenson J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 20.Wilson C M, Volkman S K, Thaithong S, Martin R K, Kyle D S, Milhous W K, Wirth D F. Amplification of pfmdrl associated with mefloquine and halofantrine resistance in Plasmodium falciparum in Thailand. Mol Biochem Parasitol. 1993;57:151–160. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]