Abstract
Diabetes is a condition that affects a large percentage of the population and it is the leading cause of a wide range of costly complications. Diabetes is linked to a multi-fold increase in mortality and when compared to non-diabetics, the intensity and prevalence of COVID-19 ailment among diabetic individuals are more. Since its discovery in Wuhan, COVID-19 has grown rapidly and shown a wide range of severity. Temperature, lymphopenia, non-productive cough, dyspnoea, and tiredness are recognized as the characteristic of individuals infected with COVID-19 disease. In COVID-19 patients, diabetes and other related comorbidities are substantial predictors of disease and mortality. According to a recent study, SARS-CoV-2 (the virus responsible for covid-19 disease) may also lead to direct pancreatic harm, which could aggravate hyperglycemia and potentially cause the establishment of diabetes in formerly non-diabetic individuals. This bidirectional association of COVID-19 and diabetes load the burden on health care professionals throughout the world. It is recommended that gliptin medications be taken moderately, blood glucose levels must be kept under control, ACE inhibitors should be used in moderation, decrease the number of avoidable hospitalizations, nutritional considerations, and some other prevention measures, such as immunization, are highly recommended. SARS-CoV-2 may cause pleiotropic changes in glucose homeostasis, which could exacerbate the pathophysiology of pre-existing diabetes or result in new disease processes.
Keywords: COVID-19; Diabetes mellitus; SARS-CoV-2; Glucose homeostasis, Angiotensin-converting enzyme inhibitors
Graphical Abstract
1. Introduction
The severity of diabetes as a medical problem has risen considerably in the past two decades, making it a worldwide concern [1]. Diabetes afflicted 30 million people in 1985 and 285 million people in 2010. Globally, 700 million people are anticipated to be affected by diabetes by 2045. In 2019, 463 million people are affected by the disease [2], [3]. End-stage renal failure, blindness, and lower-limb abnormalities are all linked to diabetes. There is a higher chance of getting life-threatening illnesses in those who are affected by these ailments [4], [5].
The first pneumonia incidents of unknown origin were discovered in Wuhan in early December 2019, in Hubei Province, China. The SARS-associated coronavirus causes an acute respiratory tract disorder known as a severe acute respiratory syndrome (SARS). Coronavirus-2 (SARS-CoV-2) is a virus that belongs to the Coronaviridae family and was responsible for the epidemics of SARS and Middle East respiratory syndrome (MERS) in 2002 and 2008, which resulted in the deaths of hundreds of people [6]. On the dashboard of ‘WHO’ as of January 12, 2022, 314,319,655 worldwide verified incidences of COVID-19 have been documented, with 5523,538 fatalities. Coronaviridae is a class of encapsulated viruses having a positive RNA genome that is single-stranded [7], [8]. Structure and transmembrane crown-like glycoprotein spikes distinguish this virus from others [9]. The virus utilizes ACE2 (Angiotensin-Converting Enzyme 2) receptors to enter the cell membranes of the host [10]. When the virus invades the cell of the new host, seizes its genetic machinery and exploits it to produce viral proteins and genetic material. Once replication is completed, the virus escapes the cell by exocytosis, and the stress caused by the virus causes the cell to die [11]. An inflammatory response is triggered when proinflammatory cytokines are activated by the death or necrosis of cells infected with COVID-19. As a consequence of the increased numbers of cytokines in the body, the organism eventually experiences a "cytokine storm." [12]. Multi-organ collapse and hyper-inflammation are caused by the cytokine storm [13]. Diabetes screening to discover undiagnosed individuals is crucial with the COVID-19 pandemic [14]. In China, a study (meta-analysis) of COVID-19 patients was performed, indicating a 9.7% prevalence of DM, which is equivalent to China's overall diabetes mellitus incidences [15]. Among the 23,804 participants in the COVID-19 research in the United Kingdom, one-third of the patients had type 2 diabetes (T2D), whereas fewer than 2% had diabetes (Type 1). Diabetes patients have a 2.03–3.5 times greater risk of death in the hospital as compared to non-diabetic persons [16]. Covid-19 diabetes patients, compared to non-DM persons, had a greater risk of heart disease, according to a retrospective Chinese study [17]. Covid-19 diabetic patients who had glycated hemoglobin of more than 10% were much more susceptible to COVID-19 than those who had HbA 1c < 6.5%, according to population-based cohort research from England [18].
The virus was discovered to be a new encased RNA β-coronavirus [19]. This virus quickly spread from Wuhan to nearby places in China and it is still spreading around the world [20]. As per the World Health Organization (WHO), illness produced by SARS-CoV-2 is now officially characterized as coronavirus disease (COVID-19). Temperature, lymphopenia, non-productive cough, dyspnoea, and tiredness are recognized as the characteristic of individuals infected with COVID-19 disease. The symptoms of MERS and COVID-19 illness are strikingly similar [21], [22], [23], [24]. The most prevalent means of transmission are aerosols or direct exposure, and infection occurs through the respiratory system [25], [26]. The variables determining the degree of disease and death remain unclear owing to the uniqueness of sickness. However, it is assumed that those with pre-existing health issues, those who are older, and those who are taken to the hospital late all have a part in the severity of the problem. People having reoccurring health issues like high blood pressure and uncontrolled blood sugar level are considered high-risk factors for contracting the new coronavirus. In addition, it is projected that these individuals would have greater long-term problems and a higher death rate as a result of this terrible illness. COVID-19 has indirect impacts on persons who have underlying health problems. For example, while COVID-19 continues to overburden many health services throughout the world, a large proportion of patients (non-COVID-19) are unable to get the medical treatment they need owing to their pre-existing diseases. Moreover, many individuals have been impacted by the decrease in physical activity as a result of the global lockdowns enforced by most governments, which is particularly crucial for diabetic management. Diabetic patients have an increased chance of sickness, hospitalization, and even death as a result of these repercussions [27], [28]. In the coming years, the economic growth and tourist industry are expected to suffer greatly. Millions of people have lost their jobs, industries have suffered setbacks, and nearly every aspect of life has been impacted [29].
2. Structure of SARS-CoV-2
This virus belongs to the family Coronaviridae and the genus Beta-coronavirus. Its RNA is single-stranded and positive-sense [30]. The size of the genome of this virus is approximately 29.9 kb, which was recently sequenced and has 78% biological (sequence) homology with SARS-CoV [30]. The SARS-CoV-2 genomic RNA, ORF1a and ORF1b includes 66% of the total genome and is translated into two different proteins namely pp1a and pp1b. A papain-like protease (PLpro), commonly referred to as nsp3 (non-structural protein 3), and a 3 C-like protease (3CLpro), also known as nsp5, are both encoded in the viral genome. Both these proteases cut the polypeptides pp1a and pp1b and generated sixteen non-structural proteins (nsp) [32], [33]. Nsp12 is located in the midsection of the RNA-dependent RNA polymerase and is essential for coronavirus replication and transcription. The nsp12 and template-primer RNA pairing was greatly boosted by nsp7 and nsp8 [29], [34]. Residual 1/3rd of the genome is made up of overlapping ORFs that encode four important structural proteins: S, N, M, and E (spike glycoprotein, nucleocapsid protein, membrane protein, envelope protein), as well as other supplementary proteins [29], [33], [34], [35]. The S1 subunit is comprised of two subdomain units namely SD1 and SD2, one receptor-binding domain commonly referred to as RBD, and a signal peptide commonly termed as SP. The Membrane-fusion subunit (S2) contains the heptad repeat 1, heptad repeat 2, transmembrane and fusion peptides also termed HR1, HR2, TM, and FP respectively [36]. Structural proteins E, along with M and N proteins are thought to aid in the formation of virus-like particles ( Fig. 1) [37].
Fig. 1.
Schematic illustration of the structure of SARS-CoV-2 and the many proteins found inside it, including the spike protein, hemagglutinin esterase (HE) protein, nucleocapsid protein, and membrane protein.
3. Incubation interval and mechanism of propagation
The interval between infection and the development of illness is described as the incubation period. The median incubation duration was 4 days in a group comprising 1099 Chinese patients with lab-confirmed symptomatic COVID-19. Another research with 181 confirmed cases found that the median incubation time was around 5 days and by the 12th day, 97.5% of infected patients had developed symptoms [37], [38]. Depending on the incubation time of SARS-CoV and MERS-CoV, along with scientific data, the US CDC (Centers for Disease Control and Prevention) predicts COVID-19 symptoms to appear 2–14 days after infection. As a consequence, the international norms for monitoring and regulating the mobility of healthy persons have been set at fourteen days.
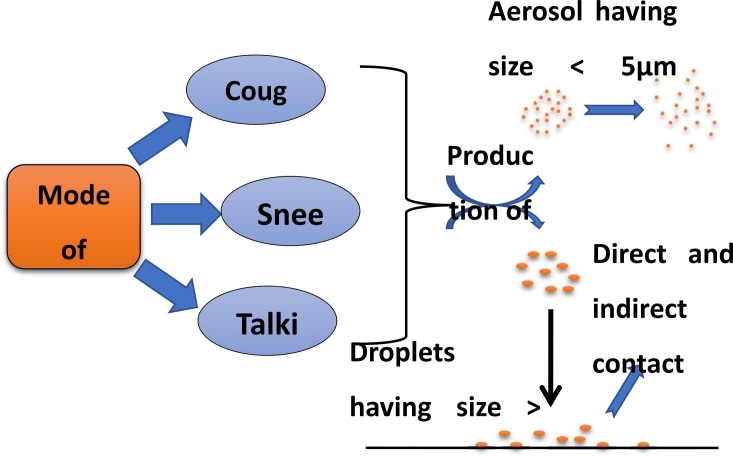
Most of the early patients of COVID-19 had previously been exposed to a Chinese seafood and animal market in their area, indicating that zoonotic exposure was the primary mechanism of transmission [39]. It has been discovered via viral genome sequencing that SARS-CoV-2 and the bat coronavirus may have shared an ancestral lineage, even though bats aren't sold at this seafood market [40]. Following that, cases were reported in the medical community as well as in people who had no previous contact with animals or who had visited Wuhan, showing the transmission of the disease from person to person [39]. The virus is most often disseminated by direct touch, aerosol, and tiny particles. The virus may transmit to those around when pulmonary droplets from a COVID-19 patient or droplets from a sneeze or cough are swallowed or inhaled. After touching a virus-infested surface or item, people may get infected if they place their hands over their lips, noses, or eyes [41]. Moreover, it has been demonstrated in the laboratory that if a pathogen is breathed or swallowed, it can survive for at least 3 h in aerosols and can be transferred in confined conditions [41], [42]. As a consequence, during aerosol-generating therapies such as endotracheal intubation, bronchoscopy, cardiac resuscitation, and so on, the airborne transfer is a concern ( Fig. 2) [40], [43].
Fig. 2.
The process by which SARS-CoV-2 spreads and is transmitted is as follows: production of aerosols and droplets by different modes such as sneezing and talking causes the virus to be suspended in the air or settle down depending on particle size, resulting in the development of sickness.
4. Diagnosis
COVID-19 cannot be diagnosed without a microbiologic examination. As there is a limited capacity for screening for COVID-19 in reported incidents, specific criteria for priority patients may be specified by local health agencies. However numerous testing methods have already been created, real-time fluorescence (RT-PCR) is the common model technique for coronavirus detection that identifies viruses in mucus, naso-oropharyngeal samples, and respiratory tract sputum [44].
5. What effect does diabetes mellitus have on COVID-19 and vice versa?
There are several plausible pathophysiological explanations for the connection between diabetes and the occurrence of corona virus-19. As the main line of defense against this infection, a weakened body's defensive system is seen in patients with uncontrolled diabetes [45]. Additionally, as demonstrated in COVID-19 sufferers, diabetes is a pro-inflammatory syndrome characterized by an improper and exaggerated cytokine response, where DM patients had significantly greater amounts of interleukin-6 (IL-6), ferritin, and C-reactive protein in their bloodstream compared non-DM individuals [46]. This demonstrates that individuals having unmanaged blood glucose levels are higher vulnerable to cytokine production outbursts, which consequently leads to rapid exacerbation of this devastating disease due to the development of ARDS and shock. Furthermore, preceding research found that COVID-19 subjects with diabetes mellitus had greater D-dimer values than those who did not have diabetes mellitus, probably indicating hemostatic system over-activation [46]. Hyper-activation of the coagulation cascade in COVID-19 in the context of a preexisting pro-thrombotic hypercoagulable state exacerbated by the simple presence of DM may result in severe thromboembolic outcomes and eventual mortality [47], [48].
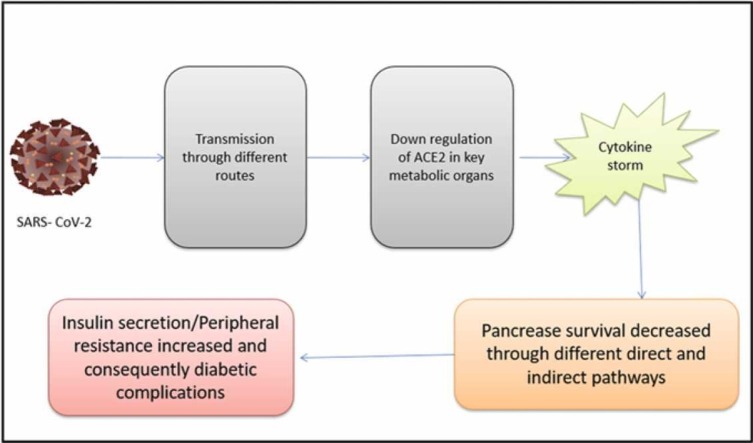
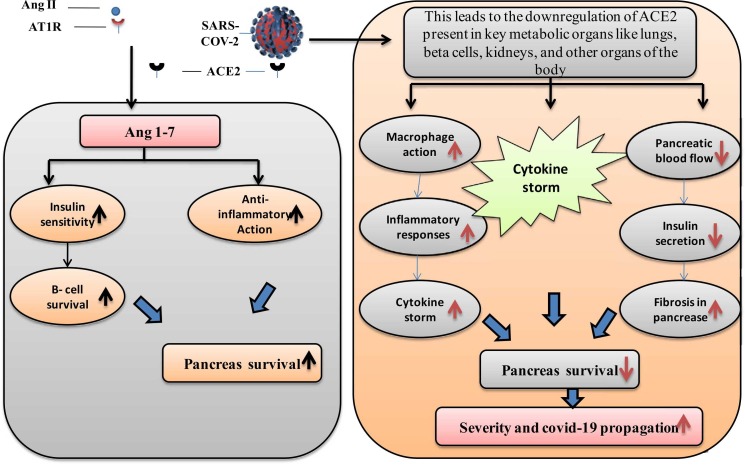
Diabetes mellitus is associated with decreased expression of angiotensin-converting enzyme 2 (ACE2), an enzyme found in the epithelial cells of alveoli, small intestine, vascular endothelium, and various other human organs. Under normal physiological conditions, ACE2 degrades angiotensin-II and, to a lesser degree, angiotensin-I into smaller peptides known as angiotensin (1−7) and angiotensin (1−9) respectively. Both ACE2 and Ang 1–7 have a beneficial effect in reversing inflammation and oxidative stress. Additionally, ACE2 has been found to protect against transmission of avian influenza A H5N1 [49]. As a consequence, the increased prevalence of ARDS and substantial lung damage associated with this catastrophic condition may be explained by decreased ACE2 expression in DM [50]. ACE inhibitors (ACEi) and angiotensin-receptor blockers have opposing effects that must be considered. ACEi/ARBs are often used as antihypertensive and reno-protective medicines in people with diabetes. Upregulation of ACE2 is connected to the use of ACEi/ARBs [51]. Ironically, for gaining entry into the recipient pneumocytes, coronavirus targets ACE2; hence enzyme overexpression would assist the corona virus's invasion and subsequent proliferation. As a result, ACE2 is downregulated and fails to defend the lungs after the pathogen utilizes the enzyme to allow access to the host organism. Hemoglobin's capacity to carry oxygenated blood may be impaired by the non-structural proteins of SARS-CoV-2, which target hemoglobin's β1-chain, according to recent research. Coronavirus may have a stronger tendency for attaching glycated Hb to non-glycated Hb, although this is only a hypothesis [52].
Diabetes patients with COVID-19 had a less survival rate and a greater mortality rate than non-diabetic patients, according to various studies reported. Dey et al. reported in their first study on 53 old males, who already suffered from diabetes and hypertension. By following a low-glycemic diet and other lifestyle changes, the diabetic patient was successfully controlling his condition. On the 10th day after getting an infection of COVID-19, serious complications were noticed including diabetic ketoacidosis, hyperglycemic blood level, imbalanced sodium, urea, and potassium level. COVID-19 was detected in the second investigation, which found that a 78-year-old man had been hospitalized previously with the same symptoms. On the ninth day of illness, he got HHS with bilateral pneumonia. He was already taking medicines for diabetes and hypertension. Complications became more drastic for both diabetic patients with the progression over time [53]. Additionally, Maddaloni et al. investigated the risk by researching 79 diabetes patients admitted to hospital with covid-19 illness and 158 control diabetic patients without corona virus-19. COVID-19 diabetic patients were shown to have a greater chance of COPD and chronic kidney disease (CKD) [54]. In a meta-analysis of 128 types of research, Shrestha et al. found that 44.93% of the participants had diabetes and hyperglycemia as their primary concerns. The total mortality rate for the diabetic and hyperglycemic patients was found to be approximately triple i.e., 26.62% corresponding to non-diabetic patient (9.26%). The fatality rate was observed at 24.96% in COVID-19 associated diabetic patients. Moreover, the numbers of adverse events were also more in DM patient than in non-diabetic subjects [55]. Fawares et al. described a case study of a 46-year-old diabetic male patient with beginning physiological parameters that were virtually normal, but who developed DKA and AKI as a result of a growing imbalance in physiological parameters [56]. Leon-Abarca JA et al. looked at the medical records of adult Mexican patients aged 20–90 who reported COVID-19-like symptoms in the previous week. The presence of DM was identified in 12.97% of the total number of records. According to the findings, diabetes patients were more likely to contract COVID-19 and develop pneumonia [57]. Liu Z et al. carried out a retrospective observational study for a large sample group (1880 patients). In contrast to earlier research with high sample sizes, the findings revealed contradictory observations that diabetes had no meaningful impact on COVID-19 patients’ prognosis but had a detrimental impact on their clinical course [58]. A retrospective cohort study was performed on 258 COVID-19 hospitalized patients (diabetic patient 63) by Zhang Y et al. Diabetic patients had substantially higher leukocyte and neutrophil counts, as well as greater fasting blood sugar, creatinine levels, and other important biological markers when hospitalized in contrast to individuals without diabetes. COVID-19 individuals were shown to have a high prevalence of diabetes (24%) [59]. Kumar A et al. analyzed 33 studies in their meta-analysis (16,003 patients). Diabetes was shown to be prevalent in 9.8% of covid-19 patients. Diabetes has been linked to a twofold rise in mortality and COVID-19 intensity corresponding to the euglycemic patient [60]. Li B et al. conducted a meta-analysis of six Chinese research (no. of patients 1527) and observed the prevalence of diabetes was found to be 9·7% [61]. COVID-19 individuals were studied in a retrospective cohort study from New York City by Petrilli et al. Altered blood glucose levels and overweight were more prevalent in hospitalized patients than in non-hospitalized patients ( Table 1) [62]. COVID-19 diabetics have a poor prognosis, but newer research has linked it to the emergence of new-onset diabetes as well.
Table 1.
Complications in pre-existing diabetic persons, as well as retrospective data from certain research, suggest that COVID-19 is responsible for the genesis of new diabetes.
| Complication in the pre-existing diabetic patient | ||||||||||||||
| Sr. No. | Patient age (yrs) | Gender | Symptoms (COVID-19 infection) | Disease history | Medication history | Management of co-morbid disease | Serious complications observed | Ref. | ||||||
| 1 | 53 | Male | Tiredness, myalgia, ageusia, hyposmia, and one incident of vomiting | Diabetes and hypertension | Nil | Diet, exercise, and lifestyle modification | DKA, plasma blood glucose 1543 mg/dL, Glycated hemoglobin 13.0%, blood urea 32.10–136.9 mg/dL, Na: 139–164, mEq/L, K: 4.1–5.3 mEq/L | [52] | ||||||
| 2 | 78 | Male | Mild fever and dry cough | Diabetes mellitus, hypertension, and recurrent ischemic stroke | Statins, ARBs (losartan), and oral hypoglycemic agents | NA | On 9th day of admission: Hyperosmolar hyperglycemic state (HHS), pulse 124b/min, BP 180/100, blood glucose 626 mg/dL, blood urea 64 mg/dL, serum Na: 167 mEq/L, serum K 4.2 mEq/L, serum osmolality 378 mOsm/kg | [53] | ||||||
| 3 | 46 | Male | Weakness, myalgia, hyposmia, vomiting, polydipsia, polyuria | Diabetes and hypertension | Nil | Diet, exercise, and lifestyle modification | Diabetic Ketoacidosis (DKA) and Acute Kidney Injury (AKI), thrombocytopenia, pneumonia, CRP 4.03–19.8 mg/ml, Na: 139–164mEq/L, serum K: 4.1–5.3 mEq/L | [55] | ||||||
| New onset of diabetes after COVID-19 | ||||||||||||||
| Sr. no. | Type of study | Mean Age (y) | Study population | Setting | Diabetic prevalence (%) | Ref. | ||||||||
| 1 | Retrospective | 64 | 258 | West Court of Union Hospital in Wuhan, China | 24 | [59] | ||||||||
| 2 | – | 5279 | The single academic medical center, New York City | 22.6 | [62] | |||||||||
| 3 | 61 | 453 | Wuhan Union Hospital | 11.7 | [63] | |||||||||
| 4 | 47 | 80 | Anhui Provincial Hospital | 27.5 | [64] | |||||||||
| 5 | 56 | 191 | Jinyintan Hospital and Wuhan Pulmonary Hospital | 19 | [65] | |||||||||
| 6 | 62 and 53 | 7337 | The multi-centered study, Hubei Province, China | 13 | [66] | |||||||||
| 7 | 66.6 and 68.5 | 59 | Vanvitelli University and San Sebastiano Caserta Hospital | 44 | [67] | |||||||||
DKA, Diabetic Ketoacidosis; HHS, Hyperosmolar hyperglycemic state; BP, Blood Pressure; AKI, Acute Kidney Injury; ARBs, Angiotensin receptor blocker; Na, Sodium; K, Potassium; mg/dL, milligram per deciliter; mEq/L, Mill equivalents per liter.
6. Exacerbation of inflammatory storm in diabetes
People with diabetes often have some degree of chronic inflammation, which may lead to cytokine storms and the death-causing consequences of COVID-19 [13], [68]. Guo et al. presented the first study of biochemical characteristics of diabetic COVID-19 persons, indicating that diabetic individuals have a significantly fewer amount of lymphocytes than non-diabetic people, while the number of neutrophils is considerably greater. In further retrospective investigations assessing the clinical parameters of COVID-19 diabetic and non-diabetic patients, elevated NLR, procalcitonin (PCT), and high-sensitivity C response protein (CRP) were also found [46]. Certain inflammation-related indicators were also enhanced in diabetes individuals, with interleukin (IL)− 6 being the most elevated among the different markers [69], [70]. Elevated NLR and CRP among COVID-19 individuals along with elevated inflammatory biomarkers were shown to be independent risk factors for disastrous outcomes [70], [71]. Furthermore, preliminary research indicates that tocilizumab which targets IL-6 could enhance COVID-19 therapy. Activation of the monocyte-macrophage cascade is shown by a significant increase in blood protein (ferritin), which is a key component of the cytokine storm. As a result, these findings suggest that patients with diabetes are more likely to trigger an inflammatory storm, resulting in fast COVID-19 precipitation ( Fig. 3) [72], [73], [74].
Fig. 3.
The effects of COVID-19 on pancreatic β-cells have been studied. SARS-COV-2 causes the downregulation of ACE-2, which is located over β-cells, increasing cytokine storm and fibrosis of the pancreas, as well as a reduction in pancreatic blood flow and insulin secretion, all of which contribute to decreased pancreas survival and severity of disease progression in the patient. ACE2, Angiotensin-converting enzyme 2; Ang 1–7, Angiotensin 1–7; AT1R, Angiotensin II receptor type 1; SARS-COV-2, severe acute respiratory syndrome corona virus-2.
7. Relationship between COVID-19 and diabetes mellitus
Chronic diabetes and uncontrolled blood sugar levels are substantial risk factors in people who have been infected by several viruses [75], [76], [77]. COVID-19 infection and mortality were more common in older individuals with chronic diseases like diabetes, according to various reports published worldwide. In COVID-19 people, there is much less data on glucose homeostasis and the establishment of acute diabetic complications such as ketoacidosis. COVID-19 diabetics may result in increased stress levels and the production of hormones (glucocorticoids and catecholamines) which elevate the blood sugar content and improper glucose fluctuation [78]. Retrospective research from Wuhan, on the other hand, revealed that almost 10% of COVID-19 diabetics had a minimum single hypoglycemic episode [64]. The activation of pro-inflammatory monocyte and the activation of platelets have been related to reducing blood glucose levels, all of which have been linked to a greater cardiovascular risk in diabetic patients [79]. Despite this, little is known about the mechanisms through which these individuals’ inflammatory and immunological responses function, if hyper or hypoglycemia affects virus pathogenicity, or whether the virus has an effect on insulin release or blood glucose control. Diabetes mellitus is a chronic inflammatory disease that causes lots of new metabolic and vascular issues that might make it difficult to fight infections. Additional production of AGEs, interleukins, and tissue necrosis factor (pro-inflammatory cytokines), as well as oxidative stress that drives tissue inflammation, are all promoted by hyperglycemia and insulin resistance. This inflammatory process might be the underlying cause of greater susceptibility to infections, as well as worse infection results in diabetic individuals [80], [81].
There have been several links established between immune system deficits and hyperglycemia, but the clinical relevance of various in vitro modifications is still up in the air [82]. Diabetes that is poorly managed has been connected to a reduced lymphocyte proliferation in response to a variety of sensory stimuli and additionally modified phagocyte responses [83]. Atypical impeded hypersensitivity response and complement proteins stimulation-related abnormalities were documented among diabetics [82], [84]. In vitro experiments have demonstrated that high sugar levels promote influenza virus infection and replication in pulmonary epithelial cells, indicating that high glycemic content in the blood may enhance viral replication in vivo as well [85] . Experiments on animals have indicated that structural lung changes such as increased blood vessel permeability and collapsed pulmonary epithelium are associated with diabetes [86]. Diabetic patients, on the other hand, have a considerable decrease in pulmonary function tests which is linked to elevated glycemic content in plasma [87].
8. Treatment for COVID-19 diabetes patients
In diabetics with COVID-19 infection, regular monitoring of blood glucose levels is important, both during hospitalization and quarantine [88]. Maximum mild infections should be handled as normal, except for inhibitors of the sodium-glucose cotransporter-2 which may lead to higher rates of dehydration and diabetic ketoacidosis and necessitate cautious renal function surveillance. Metformin has been associated with lactic acidosis, hence it is recommended that patients with moderate or severe illness discontinue taking it while receiving hospital treatment. Oral hypoglycemic drug (Sulfonylurea) dosage modifications should be made following patient sugar levels, limited food intake, and the danger of hypoglycemia. In addition, discontinuation is highly advocated in hospitalized patients [89]. Since the usage of dipeptidyl peptidase-4 inhibitors has been associated with a higher risk of upper respiratory tract infections (RTIs), they do not reduce the risk of pneumonia and there is little evidence in favor or opposition regarding their usage [90]. Considering the notion that insulin usage in COVID-19 diabetics is linked to poor consequences, insulin treatment appears to remain a preferred option for hospitalized sick people. All oral anti-diabetic medications should be stopped in hospitalized patients. Depending on the specific therapy strategy, insulin dosages may need to be changed. For quick hyperglycemia treatment, fast-acting insulin is employed in individuals who receive basal insulin; blood glucose variation following insulin treatment necessitates vigilant and continuous monitoring [89]. In a retrospective, multicenter trial in China, when compared to improperly managed patients, effective glucose level control (glycemic index 3.9–10.0 mmol/L) has been linked to a lower risk of death [66]. In COVID-19 diabetic patients admitted to the ICU, the most pressing challenges are maintaining proper glycemic control and managing the high insulin requirements. Intravenous steroids, vitamin C, or other drug prescriptions may all lead to increased glucose fluctuation. Depending on the treatment strategy, the physician should choose the most appropriate customized insulin regimen [91]. Hydroxychloroquine has been reported to lower viral load and glycated hemoglobin (HbA1c) in several countries as COVID-19 infection prevention [92]. Hydroxychloroquine, on the other hand, may induce hypoglycemia. Furthermore, hydroxychloroquine in conjunction with metformin has the potential to be hazardous [93]. Despite the positive effects of hydroxychloroquine on glycemic management; long-term treatment in diabetic individuals has been linked to CVS issues and vision problems. Using hydroxychloroquine in individuals with diabetes and COVID-19 may cause severe ventricular arrhythmias, particularly when taken with medications that lengthen the QT interval of ECG [94].
9. Immunization in diabetes patients against COVID-19
Type 2 diabetics have a greater risk of morbidity and death from COVID-19 disease. As a consequence, coronavirus disease 2019 causes higher hospital readmission rates and severity of illness in patients with T2D [95]. Furthermore, a patient’s prognosis with COVID-19 is worsened by poor glycemic management, which increases the likelihood of ICU treatment due to mechanical resuscitation, trauma, and multi-organ failure [95]. The importance of timely and proper immunization in the primary prevention of disease cannot be underscored. Pneumococcal pneumonia immunization is recommended regularly and other viral diseases are suggested for people having diabetes [96]. Even though past research has shown that people with uncontrolled diabetes had reduced antibody responses to several viral vaccinations [97], [98]. Because of recent advancements in vaccine manufacture, patients with diabetes who get vaccinations may now establish a normal immune response. The effectiveness and safety of pneumococcal vaccination have been found to range from 56% to 81% in numerous case-control studies [99], [100]. In people with diabetes, the side effects of immunization are typically minor. 1568 diabetic patients were vaccinated against pneumonia and influenza at an Indian diabetes clinic. Only joint and muscular discomfort, fever, local rash, and enlarged glands were reported as adverse effects. There were no serious allergic responses recorded. Only 17 out of 2057 DM patients who had pneumococcal vaccines reported slight injection site pain or redness [99]. Several COVID-19 vaccinations have been produced, each with varied effectiveness and safety. Vaccines like BNT162b2 (developed by Pfizer-BioNTechUSA, Germany), mRNA-1273 (developed by Moderna, USA), AZD1222 (ChAdOx1) (developed by Oxford-AstraZeneca Jenner Institute), Sputnik V (developed by Gamaleya Research Institute of Epidemiology and Microbiology Russia), NVX-CoV2373 (developed by Novavax, Inc. USA), CoronaVac (developed by Inovac Biotech China), JNJ-78436735 or Ad26. COV2. S (developed by Johnson & Johnson (Janssen Biotech, Inc., USA)), Covaxin (invented by Bharat Biotech India), Covishield (invented by Serum Institute, Pune, India) [101], [102], [103], [104], [105], [106], [107]. Efficacy of these vaccines was found to be reported at 50.65–95%. Overweigh, CVD, pulmonary illness, and diabetes were among the comorbidities evaluated in the clinical studies [108].
10. Diabetes treatment for non-infected individuals
Since diabetes management has been shown to have a detrimental impact on prognosis and increase the chance of infection hence during the COVID-19 epidemic, tight glucose management is essential [109]. Prevention plans that work often involve things like social isolation and thorough cleaning [73]. However, such limits on travel and quarantine should not compromise the availability of effective healthcare services. As a consequence, during the era of this devastating disease, access to healthcare practitioners should be assured [109]. As a result, telehealth services might be a cost-effective way to interact with patients or perhaps identify possible diabetic complications in the early stage, such as indicators of blood glucose dysregulation or infection. Patients should also receive adequate medicine and a glucose assay kit for usage at home. In certain circumstances, access to social care specialists may be required, given the importance of stress management for mental and physical health stability [109], [110]. There is insufficient evidence to recommend discontinuing hypertension, diabetes, or dyslipidemia drugs; frequent use of anti-diabetic medicines and insulin is recommended [73]. Despite various theories indicating that long-term use of ACE inhibitors and ARBs may enhance the likelihood and severity of infection due to COVID-19 disease, the European Council on Hypertension (ESC Council) strongly advises that patients continue to use their routine hypertension drugs since there is no evidence that they are harmful. The clinical outcomes of ACEi/ARBs in patients with diabetes and hypertension who have COVID-19 were similar to those in the control group, according to a recent study. A healthy lifestyle is required to maintain great glycemic control that includes a balanced diet, frequent cardiovascular activity, and low-weight resistance training [111], [112].
11. Conclusion and future directions
Diabetes mellitus is a leading cause of a cascade of costly complications, when it strikes young people, it may drive them out of work. Furthermore, COVID-19 illness is a severe respiratory disorder and its virus spread throughout the globe, infecting and killing millions of individuals. Symptoms of COVID-19 include throat infection, temperature, non-productive cough, lethargy, and diarrhea in all patients. Uncontrolled glucose level is linked to a rise in the intensity of COVID-19 sickness. Pathophysiology, on the other hand, remains unclear. Recognizing the link between COVID-19 and diabetes might lead to therapeutic clinical interventions, however, evidence is scarce on the subject. According to the data, diabetes must always be regarded as a major cause of the severity of COVID-19 symptoms and the ideal function is to lower the exposure to corona sources. Healthcare systems must design programs to reduce diabetes patients’ exposure to the risk of disease [113], [114], [115], [64]. Prompt quarantine, diagnosis, and treatment may all aid in the control of the disease and improve the result. In COVID-19 patients, unmanaged glycemic content and other degenerative diseases are major determinants of illness and death. Diabetes and COVID-19 are two diseases that have a global impact. Approximate 14.5% of COVID-19 patients had diabetes, placing them at greater risk for the severity and lethality of COVID-19. Prevention is the best function, followed by the use of evidence-based therapies. Gliptin medicines should be used in moderation, blood glucose levels must be kept under control, ACE inhibitors should be used in moderation, decrease the number of avoidable hospitalizations, and nutritional considerations and vaccination are some of the recommendations for considering the preventive measure. Therefore, after contracting COVID-19, patients should be monitored for blood glucose levels, alert for ARDS, and given nutritional counseling and referrals as soon as feasible. Diabetes has been demonstrated in tests to impact viral entry into cells and the inflammatory response. As a result, it's possible that SARS-CoV-2 may cause pleiotropic changes in glucose homeostasis, which could exacerbate the pathophysiology of pre-existing diabetes or result in new disease processes. Future research is critically required to learn more about possible genetic predispositions in different populations, the main pathophysiological processes of the correlation between COVID-19 and diabetes, and its therapeutic care.
Funding
Fundings for the publication of this paper are provided by University of Oradea, Oradea, Romania, by an Internal project.
CRediT authorship contribution statement
Prateek Sharma, Tapan Behl, Neelam Sharma, Sukhbir Singh: Conceived the idea and wrote the article. Ajmer Singh Grewal, Ali Albarrati: Literature review. Mohammed Albratty, Abdulkarim M. Meraya: Figure work. Simona Bungau: Proof read.
Declaration of Interest
There is no conflict of interest in the submission of the manuscript.
Data availability
No data was used for the research described in the article.
References
- 1.Ramachandran A., Snehalatha C., Shetty A.S., Nanditha A. Trends in prevalence of diabetes in Asian countries. World J. Diabetes. 2012;3:110–117. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin. Pr. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Grewal A.S., Lather V., Charaya N., Sharma N., Singh S., Kairys V. Recent developments in medicinal chemistry of allosteric activators of human glucokinase for type 2 diabetes mellitus therapeutics. Curr. Pharm. Des. 2020;26:2510–2552. doi: 10.2174/1381612826666200414163148. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P., Singh S., Thakur V., Sharma N., Grewal A.S. Novel and emerging therapeutic drug targets for management of type 2 diabetes mellitus. Obes. Med. 2021;24 doi: 10.1016/j.obmed.2021.100329. [DOI] [Google Scholar]
- 5.Grewal A.S., Sekhon B.S., Lather V. Recent updates on glucokinase activators for the treatment of type 2 diabetes mellitus. Mini Rev. Med Chem. 2014;14:585–602. doi: 10.2174/1389557514666140722082713. [DOI] [PubMed] [Google Scholar]
- 6.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8:S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.C., Bai W.Z., Hashikawa T. Response to Commentary on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J. Med Virol. 2020;92:707–709. doi: 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palm N.W., Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat. Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafidh K., Abbas S., Khan A., Kazmi T., Nazir Z., Aldaham T. The clinical characteristics and outcomes of COVID-19 infections in patients with diabetes at a tertiary care center in the UAE. Dubai Diabetes Endocrinol. J. 2021;26:1–6. doi: 10.1159/000512232. [DOI] [Google Scholar]
- 15.Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X., Qiu K., Zhang J., Zeng T., Chen L., Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes ObesMetab. 2020;22:1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., Knighton P., Holman N., Khunti K., Sattar N., Wareham N.J., Young B., Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., Feng J., Yan S., Guan Y., Xu D., He G., Chen C., Xiong X., Liu L., Li H., Tao J., Peng Z., Wang W. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 18.N. Holman, P. Knighton, P. Kar, J. O'Keefe, M. Curley, A. Weaver, E. Barron, C. Bakhai, K. Khunti, N.J. Wareham, N. Sattar, B. Young, J. Valabhji, Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study, Lancet Diabetes Endocrinol 8:823–833. https://doi.org/10.1016/S2213–8587(20)30271–0. [DOI] [PMC free article] [PubMed]
- 19.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2019;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 22.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., Makhdoom H.Q., Zumla A.I., Memish Z.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J. Antimicrob. Agents. 2019;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S., Du B. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X., Fan Y., Wan Y.L., Shi H. A diabetic patient with 2019-nCoV (COVID-19) infection who recovered and was discharged from hospital. J. Thorac. Imaging. 2020;35:W94–W95. doi: 10.1097/RTI.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers L.C., Lavery L.A., Joseph W.S., Armstrong D.G. All feet on deck-the role of podiatry during the COVID-19 pandemic: preventing hospitalizations in an overburdened healthcare system, reducing amputation and death in people with diabetes. J. Am. Podiatr. Med Assoc. 2020 doi: 10.7547/20-051. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad T., Baig Haroon, M., Hui J. Coronavirus Disease 2019 (COVID-19) Pandemic and Economic Impact. Pak. J. Med Sci. 2020;36:S73–S78. doi: 10.12669/pjms.36.COVID19-S4.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng Q., Peng R., Yuan B., Zhao J., Wang M., Wang X., Wang Q., Sun Y., Fan Z., Qi J., Gao G.F., Shi Y. Structural and Biochemical Characterization of the nsp12-nsp7-nsp8 Core Polymerase Complex from SARS-CoV-2. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Disco. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 37.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou A., Song Q., Peng Y., Liao X., Huang P., Liu W., Xiang Z., Liu Q., Jiang M., Xiang X., Deng D., Chen P. Symptoms at disease onset predict prognosis in COVID-19 disease. Libyan J. Med. 2022;17:2010338. doi: 10.1080/19932820.2021.2010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalava K. First respiratory transmitted food borne outbreak? Int J. Hyg. Environ. Health. 2020;226 doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adhikari S.P., Meng S., Wu Y.J., Mao Y.P., Ye R.X., Wang Q.Z., Sun C., Sylvia S., Rozelle S., Raat H., Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect. Dis. Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO, Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 29 March 2020, World Health Organization. (2020) 〈https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-COVID-19-implications-for-ipc-precaution-recommendations〉 Accessed 15 January 2022.
- 44.WHO, Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. World Health Organization. (2020) 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidanceAccessed〉 15 January 2022.
- 45.Jafar N., Edriss H., Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med Sci. 2016;351:201–211. doi: 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., Zhao L., Fan H., Luo S., Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res Rev. 2020;31 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn E.J., Grant P.J. Type 2 diabetes: an atherothrombotic syndrome. Curr. Mol. Med. 2005;5:323–332. doi: 10.2174/1566524053766059. [DOI] [PubMed] [Google Scholar]
- 48.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin. Pr. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:1–7. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tikellis C., Thomas M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J. Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cure E., Cumhur M. Cure, Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes MetabSyndr. 2020;14:349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenzhong L., Hualan L. COVID-19: Attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. Camb.: Camb. Open Engag. 2021 doi: 10.33774/chemrxiv-2021-dtpv3-v10. [DOI] [Google Scholar]
- 53.Dey R.K., Hilmy A.I., Imad H.A., Yoosuf A.A., Latheef A.A. COVID-19 and emergencies in patients with diabetes: two case reports. J. Med Case Rep. 2021;15:57. doi: 10.1186/s13256-020-02659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maddaloni E., D’Onofrio L., Alessandri F., Mignogna C., Leto G., Coraggio L., Sterpetti S., Pascarella G., Mezzaroma I., Lichtner M., Pozzilli P., Agrò F.E., Rocco M., Pugliese F., Mastroianni C.M., Buzzetti R. Clinical features of patients with type 2 diabetes with and without COVID-19: A case control study (COVIDiab I) Diabetes Res Clin. Pr. 2020;169 doi: 10.1016/j.diabres.2020.108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shrestha D.B., Budhathoki P., Raut S., Adhikari S., Ghimire P., Thapaliya S., Rabaan A.A., Karki B.J. New-onset diabetes in COVID-19 and clinical outcomes: A systematic review and meta-analysis. World J. Virol. 2021;10:275–287. doi: 10.5501/wjv.v10.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fawares F.F., Haddad R., Fawares B. Diabetes and COVID-19: Case study. Int Med Case Rep. J. 2021;10:3. doi: 10.46998/IJCMCR.2021.10.000242. [DOI] [Google Scholar]
- 57.Leon-Abarca J.A., Portmann-Baracco A., Bryce-Alberti M., Ruiz-Sánchez C., Accinelli R.A., Soliz J., Gonzales G.F. Diabetes increases the risk of COVID-19 in an altitude dependent manner: An analysis of 1,280,806 Mexican patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Li J., Huang J., Guo L., Gao R., Luo K., Zeng G., Zhang T., Yi M., Huang Y., Chen J., Yang Y., Wu X. Association Between Diabetes and COVID-19: A Retrospective Observational Study With a Large Sample of 1,880 Cases in Leishenshan Hospital, Wuhan. Front Endocrinol. 2020;11:478. doi: 10.3389/fendo.2020.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Cui Y., Shen M., Zhang J., Liu B., Dai M., Chen L., Han D., Fan Y., Zeng Y., Li W., Lin F., Li S., Chen X., Pan P. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res Clin. Pr. 2020;165 doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou W., Ye S., Wang W., Li S., Hu Q. Clinical Features of COVID-19 Patients with Diabetes and Secondary Hyperglycemia. J. Diabetes Res. 2020;2020:3918723. doi: 10.1155/2020/3918723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., Li H., Zhang P., Song X., Chen X., Xiang M., Zhang C., Bai L., Xiang D., Chen M.M., Liu Y., Yan Y., Liu M., Mao W., Zou J., Liu L., Chen G., Luo P., Xiao B., Zhang C., Zhang Z., Lu Z., Wang J., Lu H., Xia X., Wang D., Liao X., Peng G., Ye P., Yang J., Yuan Y., Huang X., Guo J., Zhang B.H., Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sardu C., D'Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Paolisso G., Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maddaloni E., Buzzetti R. COVID-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab. Res Rev. 2020;31 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., Byrne C.D., Zheng K.I., Chen Y.P., Eslam M., George J., Zheng M.H. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335–337. doi: 10.1016/j.diabet.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin. Pr. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin. Drug Invest. 2020;40:511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoen K., Horvat N., Guerreiro N.F.C., de Castro I., de Giassi K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 2019;19:964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Sun G.Z., Yang G.R., Zhang X.L., Wang L., Xu X., Xu X.P., Chan J.C. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 77.Banik G.R., Alqahtani A.S., Booy R., Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol. Sin. 2016;31:81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W., Lu J., Gu W., Zhang Y., Liu J., Ning G. Care for diabetes with COVID-19: advice from China. J. Diabetes. 2020;12:417–419. doi: 10.1111/1753-0407.13036. [DOI] [PubMed] [Google Scholar]
- 79.Iqbal A., Prince L.R., Novodvorsky P., Bernjak A., Thomas M.R., Birch L., Lambert D., Kay L.J., Wright F.J., Macdonald I.A., Jacques R.M., Storey R.F., McCrimmon R.J., Francis S., Heller S.R., Sabroe I. Effect of Hypoglycemia on Inflammatory Responses and the Response to Low-Dose Endotoxemia in Humans. J. Clin. Endocrinol. Metab. 2019;104:1187–1199. doi: 10.1210/jc.2018-01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59:99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 81.Petrie J.R., Guzik T.J., Touyz R.M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol. Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 83.Moutschen M.P., Scheen A.J., Lefebvre P.J. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. DiabeteMetab. 1992;18:187–201. [PubMed] [Google Scholar]
- 84.Ilyas R., Wallis R., Soilleux E.J., Townsend P., Zehnder D., Tan B.K., Sim R.B., Lehnert H., Randeva H.S., Mitchell D.A. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216:126–131. doi: 10.1016/j.imbio.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohio H.P., Adamson A.L. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444:301–309. doi: 10.1016/j.virol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 86.Popov D., Simionescu M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur. Respir. J. 1997;10:1850–1858. doi: 10.1183/09031936.97.10081850. [DOI] [PubMed] [Google Scholar]
- 87.Lange P., Groth S., Kastrup J., Mortensen J., Appleyard M., Nyboe J., Jensen G., Schnohr P. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur. Respir. J. 1983;2:14–19. [PubMed] [Google Scholar]
- 88.Rhee E.J., Kim J.H., Moon S.J., Lee W.Y. Encountering COVID-19 as endocrinologists. Endocrinol. Metab. 2020;35:197–205. doi: 10.3803/EnM.2020.35.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin. Pr. 2020;162 doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamdy O., Gabbay R.A. Early Observation and Mitigation of Challenges in Diabetes Management of COVID-19 Patients in Critical Care Units. Diabetes Care. 2020;43:e81–e82. doi: 10.2337/dc20-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes MetabSyndr. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajeshkumar N.V., Yabuuchi S., Pai S.G., Maitra A., Hidalgo M., Dang C.V. Fatal toxicity of chloroquine or hydroxychloroquine with metformin in mice. Biorxiv. 2020 doi: 10.1101/2020.03.31.018556. [DOI] [Google Scholar]
- 94.Infante M., Ricordi C., Fabbri A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: Risks and benefits at the time of COVID-19 pandemic. J. Diabetes. 2020;12:659–667. doi: 10.1111/1753-0407.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sardu C., Gargiulo G., Esposito G., Paolisso G., Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by COVID-19. Cardiovasc Diabetol. 2020;19:76. doi: 10.1186/s12933-020-01047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.American Diabetes Association Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S37–S47. doi: 10.2337/dc20-S004. [DOI] [PubMed] [Google Scholar]
- 97.Smith S.A., Poland G.A. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care. 2000;23:95–108. doi: 10.2337/diacare.23.1.95. [DOI] [PubMed] [Google Scholar]
- 98.Li Volti S., Caruso-Nicoletti M., Biazzo F., Sciacca A., Mandarà G., Mancuso M., Mollica F. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Arch. Dis. Child. 1998;78:54–57. doi: 10.1136/adc.78.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kesavadev J., Misra A., Das A.K., Saboo B., Basu D., Thomas N., Joshi S.R., Unnikrishnan A.G., Shankar A., Krishnan G., Unnikrishnan R., Mohan V. Suggested use of vaccines in diabetes. Indian J. Endocrinol. Metab. 2012;16:886–893. doi: 10.4103/2230-8210.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shapiro E.D. Prevention of pneumococcal infection with vaccines: an evolving story. JAMA. 2012;307:847–849. doi: 10.1001/jama.2012.194. [DOI] [PubMed] [Google Scholar]
- 101.Polack F.P., Thomas S.J., Kitchin N., et al. Clinical trial group. safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Voysey M., Clemens S.A.C., Madhi S.A., et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pal R., Sachdeva N., Mukherjee S., Suri V., Zohmangaihi D., Ram S., Puri G.D., Bhalla A., Soni S.L., Pandey N., Bhansali A., Bhadada S.K. Impaired anti-SARS-CoV-2 antibody response in non-severe COVID-19 patients with diabetes mellitus: A preliminary report. Diabetes MetabSyndr. 2021;15:193–196. doi: 10.1016/j.dsx.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dispinseri S., Lampasona V., Secchi M., Cara A., Bazzigaluppi E., Negri D., Brigatti C., Pirillo M.F., Marzinotto I., Borghi M., Rovere-Querini P., Tresoldi C., Ciceri F., Scavini M., Scarlatti G., Piemonti L. Robust Neutralizing Antibodies to SARS-CoV-2 Develop and Persist in Subjects with Diabetes and COVID-19 Pneumonia. J. Clin. Endocrinol. Metab. 2021;106:1472–1481. doi: 10.1210/clinem/dgab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lampasona V., Secchi M., Scavini M., Bazzigaluppi E., Brigatti C., Marzinotto I., Davalli A., Caretto A., Laurenzi A., Martinenghi S., Molinari C., Vitali G., Di Filippo L., Mercalli A., Melzi R., Tresoldi C., Rovere-Querini P., Landoni G., Ciceri F., Bosi E., Piemonti L. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63:2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.WHO, Interim recommendations for use of the ChAdOx1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™). World Health Organization, 2021. 〈https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222–2021.1Accessed〉 15 January 2022.
- 109.Kosinski C., Zanchi A., Wojtusciszyn A. Diabetes and COVID-19 infection. Rev. Med. 2020;16:939–943. [PubMed] [Google Scholar]
- 110.Angelidi A.M., Belanger M.J., Mantzoros C.S. Commentary: COVID-19 and diabetes mellitus: What we know, how our patients should be treated now, and what should happen next. Metabolism. 2020;107 doi: 10.1016/j.metabol.2020.154245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Simone G. Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. Eur. Soc. Cardiol. 2020:13. [Google Scholar]
- 112.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., Liu C., Xiong M., Deng A., Zhang Y., Zheng L., Huang K. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 113.Cheng Y.J., Kanaya A.M., Araneta M.R.G., Saydah S.H., Kahn H.S., Gregg E.W., Fujimoto W.Y., Imperatore G. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim E.S., Chin B.S., Kang C.K., Kim N.J., Kang Y.M., Choi J.P., Oh D.H., Kim J.H., Koh B., Kim S.E., Yun N.R., Lee J.H., Kim J.Y., Kim Y., Bang J.H., Song K.H., Kim H.B., Chung K.H., Oh M.D. Korea National Committee for Clinical Management of COVID-19. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J. Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.