Abstract
Coronavirus disease 2019 is known to impact older people more severely and to cause persistent symptoms during the recovery phase, including cognitive and neurologic ones. We investigated the cognitive and neurologic features of 100 elderly patients with confirmed diagnosis of coronavirus disease 2019 evaluated in the postacute phase through a direct neuropsychological evaluation consisting on Mini Mental State Examination and 8 neuropsychological tests. Overall, a total of 33 participants were found to perform at a level considered to be pathologic; more specifically, 33%, 23%, and 20% failed on Trial Making, Digit Span Backwards, and Frontal Evaluation Battery tests, respectively.
Keywords: Long COVID, Elderly, Cognitive impairment
Key points
-
•
We asked whether coronavirus disease 2019 can have a long-term impact on cognitive function in the elderly.
-
•
In this cohort study of 100 elderly individuals assessed on average 3 months after acute coronavirus disease 2019, we found a high prevalence of failed neuropsychological tests.
-
•
We found that coronavirus disease 2019 is capable of eliciting persistent measurable neurocognitive alterations in the elderly, particularly in the areas of attention and working memory.
Introduction
Since December 2019, when the first cases of the coronavirus disease 2019 (COVID-19) were confirmed in the Chinese Hubei region, the pandemic of severe acute respiratory syndrome coronavirus 2 continues to plague populations and health systems around the world. A number of descriptions now cover the long-term symptoms of the disease, which include fatigue, shortness of breath, and pain.1 , 2 Neurologic involvement and psychological symptoms owing to or related to the disease are described to affect up to one-third of infected people.3, 4, 5 These symptoms include a wide spectrum of manifestations that are often loosely described as a general mental slowness often named foggy brain or COVID fog.6 Such symptoms can characterize both the acute phase and the convalescent period, during which patients report an ill-defined sense of not feeling their best or of not having fully recovered their previous well-being in the physical, occupational, or social domains.7 In some studies, cognitive problems were tied to a diagnosis of dementia5 and concern is particularly high for the aged populations.
Because the availability of clinical data remains poor, the aim of the present study was to investigate the neurologic and cognitive features of a sample of elderly patients with confirmed diagnosis of COVID-19 evaluated in the postacute phase through a direct neuropsychological evaluation.
Methods
Since April 21, 2020, a postacute outpatient service for individuals recovering from COVID-19 was established at our institution. All patients with a previous diagnosis of COVID-19 who met criteria for discontinuation of quarantine were considered eligible (no fever for 3 consecutive days, improvement in other symptoms, and 2 negative test results for severe acute respiratory syndrome coronavirus 2 taken 24 hours apart).
Once enrolled, each participant underwent a number of evaluations (described elsewere8), including a detailed history, neurologic objective examination, and specific anamnesis for general and neurologic symptomatology. For the purpose of this study, we enrolled individuals over the age of 65 years.
Cognitive Evaluation
After the anamnestic evaluation and neurologic objectivity, each patient underwent a neuropsychological evaluation that included Mini Mental State Examination9 and 8 more specific neuropsychological tests: the Rey Auditory Verbal Test was used to investigate immediate and deferred memory10; selective attention and visual–spatial exploration were assessed with Multiple Features Target Cancellation Test11 , 12; the Trial Making Test assessed selective, divided, and alternating, attention together with other features such as psychomotor speed, visuospatial research ability and working memory13 , 14; the Digit Span Forward and Backward evaluated the verbal short-term and working memory capacity15; and the Frontal Assessment Battery evaluates composite multidimensional domains and was used to screen for global executive dysfunction including behavioral, affective, motivational and cognitive components.16 , 17
Of each neuropsychological test were reported the raw scores, the scores adjusted for age and educational level and gender (where appropriate), and standardized scores on a 5-point ordinal scale (Equivalent Scores).18 A test Equivalent Score of 0 was considered pathologic, a score of 1 was classified as borderline, and scores of 2 to 5 were considered consistent with normal performance.
Other Evaluations
Psychiatric domains were evaluated with the Hamilton Anxiety19 , 20 and Depression21 , 22 scales and the Kessler Psychological Distress scales,23 , 24 and anxiety, depressive symptoms, and global psychological distress were evaluated with a cut-off of 7, 7, and 19 in each scale total score, respectively. The Pittsburg Sleep Quality Index25 was used to assess sleep quality and disturbances.
Case severity was assessed with the 7-category ordinal scale,26 which classifies participants based on the need for hospitalization and O2 administration into 1 to 2, nonhospitalized; 3, hospitalized, not requiring supplemental oxygen; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; 6, hospitalized, requiring invasive mechanical ventilation, extracorporeal membrane oxygenation, or both; and 7, death. Given the differences in the clinical manifestations and severity between sexes,27 results were also compared by sex.
Statistical Analysis
Descriptive analyses and comparisons were obtained through ANOVA and χ2 tests where appropriate. The P value was set to less than .05 for statistical significance. Given the descriptive basis of the analyses no correction of significance levels was used. All analyses were conducted using R version 4.1.3 (R Foundation, Vienna, Austria).
This study was approved by the Università Cattolica and Fondazione Policlinico Gemelli IRCCS Institutional Ethics Committee. Written informed consent was obtained from all participants.
Results
We present data from 100 individuals (mean age, 73.4 ± 6.1 years; 35% female) assessed at our institution from April 23, 2020, to November 30, 2020. The general characteristics of the study participants, stratified by sex, are described in Table 1 . Females presented a lower prevalence of diabetes mellitus and a higher prevalence of thyroid disorders. In contrast, males showed less persistence of post–COVID-19 symptoms and on average a smaller decrease in quality-of-life scores.
Table 1.
Sample characteristics
| Total |
Males |
Females |
p |
|
|---|---|---|---|---|
| n = 100 | n = 65 | n = 35 | ||
| General information | ||||
| Age (years) | 73.4 (6.1) | 73.4 (5.8) | 73.5 (6.7) | 0.957 |
| Females | 35 (35%) | |||
| BMI (kg/m2) | 26.1 (3.9) | 26.2 (3.7) | 25.9 (4.1) | 0.695 |
| Education (years) | 12.7 (8.7) | 13.0 (5.5) | 12.2 (12.7) | 0.678 |
| Not employed | 80 (80%) | 51 (78.5%) | 29 (82.9%) | 0.793 |
| Flu vaccination | 53 (53%) | 40 (61.5%) | 13 (37.1%) | 0.046 |
| Antipneumococcal vaccination | 21 (21%) | 17 (26.2%) | 4 (11.4%) | 0.16 |
| Regular physical activity | 60 (60%) | 37 (56.9%) | 23 (65.7%) | 0.784 |
| Smoking status | 0.115 | |||
| Nonsmoker | 37 (37%) | 20 (30.8%) | 17 (48.6%) | |
| Active smoker | 6 (6%) | 5 (7.7%) | 1 (2.9%) | |
| Former smoker | 52 (52%) | 38 (58.5%) | 14 (40%) | |
| Unknown | 5 (5%) | 2 (3.1%) | 3 (8.6%) | |
| Pre-COVID clinical features | ||||
| Cardiovascular conditions | 69 (69%) | 49 (75.4%) | 20 (57.1%) | 0.098 |
| Chronic heart disease | 19 (19%) | 16 (24.6%) | 3 (8.6%) | 0.092 |
| Atrial fibrillation | 12 (12%) | 7 (10.8%) | 5 (14.3%) | 0.847 |
| Heart failure | 8 (8%) | 6 (9.2%) | 2 (5.7%) | 0.817 |
| Stroke | 2 (2%) | 0 (0%) | 2 (5.7%) | 0.231 |
| Hypertension | 58 (58%) | 41 (63.1%) | 17 (48.6%) | 0.234 |
| Diabetes mellitus | 19 (19%) | 17 (26.2%) | 2 (5.7%) | 0.027 |
| Renal failure | 9 (9%) | 6 (9.2%) | 3 (8.6%) | 1 |
| Thyroid disease | 24 (24%) | 9 (13.8%) | 15 (42.9%) | 0.003 |
| COPD | 22 (22%) | 15 (23.1%) | 7 (20%) | 0.919 |
| Active cancer | 7 (7%) | 4 (6.2%) | 3 (8.6%) | 0.967 |
| Immune disease | 8 (8%) | 3 (4.6%) | 5 (14.3%) | 0.189 |
| COVID-19 events | ||||
| Seven category ordinal scale | 0.092 | |||
| 2. Not hospitalized | 12 (12%) | 5 (7.7%) | 7 (20%) | |
| 3. Hospitalized, not requiring O2 | 14 (14%) | 6 (9.2%) | 8 (22.9%) | |
| 4. Hospitalized, requiring O2 | 43 (43%) | 31 (47.7%) | 12 (34.3%) | |
| 5. Hospitalized, requiring HFNC/NIV | 16 (16%) | 12 (18.5%) | 4 (11.4%) | |
| 6. Hospitalized, requiring intubation/ECMO | 15 (15%) | 11 (16.9%) | 4 (11.4%) | |
| Drug treatments | ||||
| Treatment for COVID-19 pneumonia | 81 (81%) | 56 (86.2%) | 25 (71.4%) | 0.128 |
| Anti retrovirals | 80 (80%) | 56 (86.2%) | 24 (68.6%) | 0.067 |
| Hydroxychloroquine | 80 (80%) | 55 (84.6%) | 25 (71.4%) | 0.19 |
| Anti-IL6 | 40 (40%) | 29 (44.6%) | 11 (31.4%) | 0.29 |
| Azithromycin | 42 (42%) | 31 (47.7%) | 11 (31.4%) | 0.174 |
| Other antibiotics | 48 (48%) | 35 (53.8%) | 13 (37.1%) | 0.166 |
| Enoxaparin | 73 (73%) | 47 (72.3%) | 26 (74.3%) | 1 |
| Corticosteroids | 15 (15%) | 9 (13.8%) | 6 (17.1%) | 0.883 |
| Antiplatelet drugs | 19 (19%) | 16 (24.6%) | 3 (8.6%) | 0.092 |
| Length of stay (days) | 23.3 (16.1) | 25.4 (15.8) | 18.8 (16.0) | 0.072 |
| Post COVID-19 | ||||
| Days since first symptoms | 96.5 (45.3) | 93.2 (40.7) | 102.7 (52.8) | 0.319 |
| Days since hospital discharge | 62.1 (39.7) | 58.3 (34.4) | 70.2 (48.9) | 0.25 |
| N. persistent symptoms | 3.0 (2.5) | 2.6 (2.3) | 3.8 (2.9) | 0.02 |
| Persistent symptoms | 0.114 | |||
| No symptoms | 17 (17%) | 14 (21.5%) | 3 (8.6%) | |
| 1–2 symptoms | 33 (33%) | 23 (35.4%) | 10 (28.6%) | |
| ≥3 symptoms | 50 (50%) | 28 (43.1%) | 22 (62.9%) | |
| Decrease in QoL (EQ-VAS) | −10.1 (14.0) | −7.7 (13.1) | −14.7 (14.8) | 0.022 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; EQ-VAS, EuroQol visual analog scale; HFNC, high-flow nasal cannulae; NIV, noninvasive ventilation; QoL, quality of life.
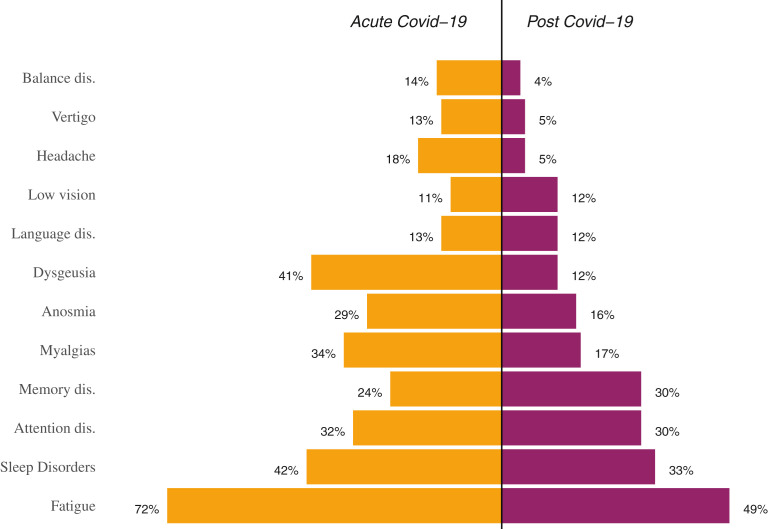
On average, the assessment was performed 96.5 days after the onset of COVID-19 symptoms. Fatigue was reported by half of the enrolled participants; apart from that, as shown in Fig. 1 , very high rates of persistent neurologic symptoms were reported in the domains of memory, attention, and sleep. The outcome of neuropsychological testing is described in Table 2 and Fig. 2 . On average, the adjusted Mini Mental State Exam score was 28.2 ± 1.7, as expected in a study sample consisting of fairly educated individuals with no history of cognitive impairment. No significant differences were observed within the severity groups. Importantly, 33%, 23%, and 20% of participants achieved either pathologic or borderline performances on the Trial Making, Digit Span Backwards, and Frontal Evaluation Battery tests, respectively. It is also notable that on the neuropsychological assessment a total of 33 participants were found to perform at a level considered to be pathologic.
Fig. 1.
Neurologic symptoms reported in the acute and recovery phase.
Table 2.
Neuropsychological tests
| Total |
Sex |
Severity – Seven Category Ordinal Scale |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not Hospitalized |
Hospitalized |
P Value | ||||||||
| Males |
Females |
P Value |
2. At Home |
3. No O2 |
4. O2 |
5. HFNC/NIV |
6. Intubation/ECMO |
|||
| n = 100 | n = 65 | n = 35 | n = 12 | n = 14 | n = 43 | n = 16 | n = 15 | |||
| MMSE | ||||||||||
| Corrected | 28.2 (1.7) | 28.4 (1.7) | 27.7 (1.8) | 0.068 | 28.2 (1.9) | 28.5 (1.5) | 27.9 (2.1) | 28.2 (1.3) | 28.7 (0.8) | 0.48 |
| Rey’s immediate recall | ||||||||||
| Corrected | 42.6 (7.8) | 41.8 (8.0) | 44.1 (7.3) | 0.171 | 43.1 (7.0) | 42.5 (8.7) | 41.7 (7.5) | 42.8 (8.8) | 44.7 (8.0) | 0.804 |
| Equivalent | 3.1 (1.1) | 3.0 (1.2) | 3.5 (0.9) | 0.044 | 3.4 (0.9) | 3.1 (1.1) | 3.0 (1.2) | 3.1 (1.1) | 3.5 (1.1) | 0.638 |
| Failed | 2 (2%) | 2 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 1 (6.7%) | ||
| Borderline | 9 (9%) | 8 (12.3%) | 1 (2.9%) | 0 (0%) | 2 (14.3%) | 5 (11.6%) | 2 (12.5%) | 0 (0%) | ||
| Rey’s delayed recall | ||||||||||
| Corrected | 8.7 (2.9) | 8.2 (2.9) | 9.5 (2.7) | 0.03 | 9.5 (2.7) | 9.0 (2.8) | 8.1 (2.9) | 8.4 (3.1) | 9.6 (2.8) | 0.327 |
| Equivalen | 3.0 (1.3) | 2.8 (1.3) | 3.3 (1.2) | 0.03 | 3.3 (1.2) | 3.0 (1.5) | 2.8 (1.3) | 2.7 (1.4) | 3.3 (1.2) | 0.58 |
| Failed | 7 (7%) | 6 (9.2%) | 1 (2.9%) | 0 (0%) | 1 (7.1%) | 4 (9.3%) | 1 (6.2%) | 1 (6.7%) | ||
| Borderline | 10 (10%) | 6 (9.2%) | 4 (11.4%) | 2 (16.7%) | 2 (14.3%) | 3 (7%) | 3 (18.8%) | 0 (0%) | ||
| MFTC | ||||||||||
| Time corrected | 50.8 (31.7) | 51.4 (25.5) | 49.7 (41.3) | 0.797 | 44.4 (26.7) | 58.4 (26.1) | 48.6 (36.4) | 46.9 (25.4) | 59.3 (32.5) | 0.522 |
| Time equivalent | 3.8 (0.8) | 3.8 (0.6) | 3.6 (1.0) | 0.133 | 3.6 (1.0) | 3.6 (0.9) | 3.8 (0.7) | 3.9 (0.3) | 3.7 (1.0) | 0.703 |
| Failed | 2 (2%) | 1 (1.5%) | 1 (2.9%) | 0 (0%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 1 (6.7%) | ||
| Borderline | 2 (2%) | 0 (0%) | 2 (5.7%) | 1 (8.3%) | 1 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| False alarms corrected | 0.7 (2.5) | 0.6 (2.9) | 0.7 (1.3) | 0.858 | 2.6 (6.4) | 0.8 (1.9) | 0.4 (1.0) | 0.2 (0.8) | 0.3 (0.8) | 0.651 |
| False alarms equivalent | 3.5 (1.1) | 3.7 (0.9) | 3.2 (1.4) | 0.042 | 2.8 (1.8) | 3.4 (1.2) | 3.6 (0.9) | 3.7 (1.0) | 3.7 (1.0) | 0.149 |
| Failed | 7 (7%) | 3 (4.6%) | 4 (11.4%) | 3 (25%) | 1 (7.1%) | 1 (2.3%) | 1 (6.2%) | 1 (6.7%) | ||
| Borderline | 2 (2%) | 1 (1.5%) | 1 (2.9%) | 0 (0%) | 0 (0%) | 2 (4.7%) | 0 (0%) | 0 (0%) | ||
| Frontal Assessment Battery | ||||||||||
| Corrected | 15.8 (1.9) | 15.9 (1.8) | 15.7 (2.1) | 0.717 | 16.1 (2.2) | 15.6 (2.0) | 15.9 (1.9) | 15.9 (1.1) | 15.6 (2.2) | 0.952 |
| Equivalent | 2.8 (1.4) | 2.9 (1.4) | 2.7 (1.5) | 0.556 | 2.8 (1.6) | 2.8 (1.4) | 2.8 (1.4) | 2.9 (1.1) | 2.6 (1.8) | 0.978 |
| Failed | 12 (12%) | 6 (9.2%) | 6 (17.1%) | 2 (16.7%) | 2 (14.3%) | 4 (9.3%) | 0 (0%) | 4 (26.7%) | ||
| Borderline | 8 (8%) | 7 (10.8%) | 1 (2.9%) | 1 (8.3%) | 0 (0%) | 5 (11.6%) | 2 (12.5%) | 0 (0%) | ||
| Digit Span Forward | ||||||||||
| Corrected | 6.0 (1.1) | 6.0 (1.1) | 5.9 (1.1) | 0.444 | 5.8 (0.9) | 5.8 (1.1) | 6.0 (1.1) | 6.2 (1.2) | 6.0 (1.0) | 0.918 |
| Equivalent | 3.2 (1.3) | 3.2 (1.2) | 3.1 (1.4) | 0.479 | 3.2 (1.3) | 2.9 (1.6) | 3.2 (1.3) | 3.4 (1.3) | 3.1 (1.1) | 0.917 |
| Failed | 8 (8%) | 4 (6.2%) | 4 (11.4%) | 1 (8.3%) | 2 (14.3%) | 4 (9.3%) | 1 (6.2%) | 0 (0%) | ||
| Borderline | 4 (4%) | 3 (4.6%) | 1 (2.9%) | 0 (0%) | 1 (7.1%) | 1 (2.3%) | 1 (6.2%) | 1 (6.7%) | ||
| Digit Span Backwards | ||||||||||
| Corrected | 4.1 (1.0) | 4.2 (1.0) | 3.9 (1.0) | 0.26 | 4.3 (0.8) | 4.0 (0.8) | 4.1 (1.0) | 3.8 (0.9) | 4.1 (1.1) | 0.701 |
| Equivalent | 2.7 (1.3) | 2.8 (1.3) | 2.5 (1.4) | 0.279 | 3.1 (1.2) | 2.6 (1.2) | 2.8 (1.4) | 2.6 (1.5) | 2.6 (1.4) | 0.852 |
| Failed | 8 (8%) | 4 (6.2%) | 4 (11.4%) | 0 (0%) | 0 (0%) | 5 (11.6%) | 2 (12.5%) | 1 (6.7%) | ||
| Borderline | 15 (15%) | 8 (12.3%) | 7 (20%) | 2 (16.7%) | 3 (21.4%) | 4 (9.3%) | 3 (18.8%) | 3 (20%) | ||
| Trail Making | ||||||||||
| Corrected | 118.2 (100.6) | 93.9 (86.8) | 163.5 (109.7) | <.001 | 185.2 (121.7) | 94.3 (91.1) | 112.2 (99.2) | 98.4 (89.8) | 125.5 (94.8) | 0.136 |
| Equivalent | 2.3 (1.5) | 2.7 (1.4) | 1.6 (1.5) | <.001 | 1.5 (1.7) | 2.9 (1.5) | 2.4 (1.6) | 2.4 (1.4) | 2.1 (1.4) | 0.231 |
| Failed | 21 (21%) | 8 (12.3%) | 13 (37.1%) | 6 (50%) | 2 (14.3%) | 8 (18.6%) | 2 (12.5%) | 3 (20%) | ||
| Borderline | 11 (11%) | 7 (10.8%) | 4 (11.4%) | 0 (0%) | 0 (0%) | 6 (14%) | 3 (18.8%) | 2 (13.3%) | ||
Abbreviations: ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannulae; MFTC, Multiple Features Target Cancellation test; MMSE, Mini Mental State Exam.
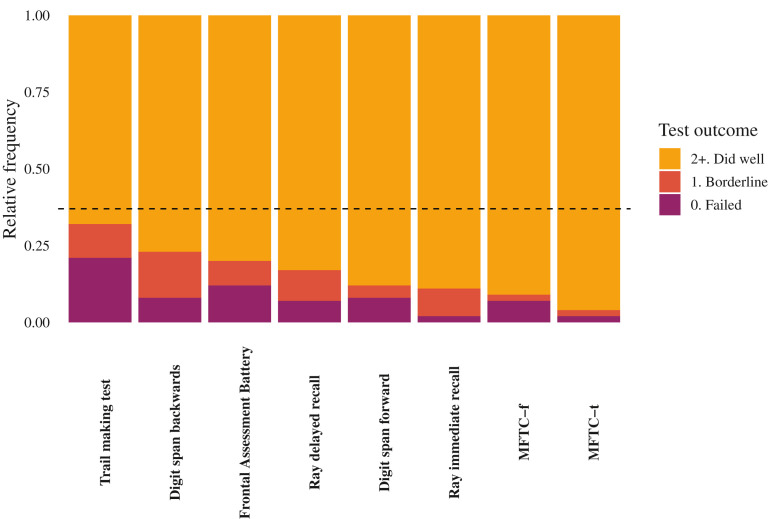
Fig. 2.
Neuropsychological tests. The figure shows, for each neuropsychological test, the proportion of patients with fair (light color), borderline (darker color) or failed (dark color) outcome. Equivalent scores (ES) were used to rate participants: those with a score of 2 or more, 1, or zero were classified as having normal, borderline or pathologic performance respectively. Horizontal dashed line indicates the overall prevalence of participants classified as having a pathologic neuropsychological test (ie, ≥1 ES of zero and ≥1 ES of 1) MFTC, Multiple Features Target Cancellation test.
Discussion
This single-center study investigated the cognitive status of a group of elderly people post–COVID-19 through a battery of neuropsychological tests. Interviewed on average 3 months after the onset of the first symptoms of COVID-19, a significant proportion of participants reported persistent sleep (33%), attention (30%), and memory (30%) symptoms. These findings are consistent with several previous studies5 , 28 and the well-established notion that COVID-19 leaves behind a burden of persistent symptoms pertaining to many organ systems.1
When directly tested with the neuropsychological battery, 33%, 23%, and 20% of participants failed the Trial Making, Digit Span Backwards, and Frontal Evaluation Battery tests, respectively, showing impairment in visuoperceptual skills, selective and divided attention, working memory, short-term verbal memory, and executive functions. These data expand the preliminary knowledge acquired in 2 previous studies with evidence of attention deficit,29 visuoperception, naming, and fluency.30
An important finding from the study is that approximately 1 in 3 participants presented at neuropsychological tests with at least 1 overtly pathologic score in conjunction with at least 1 borderline pathologic test. This finding, together with average Mini Mental State Exam scores above the cutoff of 23 could represent a rough estimate of post–COVID-19 mild cognitive impairment. Such a value does not differ from that obtained in other studies based on telephone interviews.31
This study had many methodological limitations owing to the design and circumstances under which it was conducted. It is a single-center study with no control group or longitudinal follow-up. In addition, after an initial phase in which people were contacted from the hospital’s patient lists, later people from the local area began to request to be followed at our center. Therefore, it is not possible to exclude that people with a greater burden of disease were included. Importantly no premorbid neuropsychological evaluation was available. Indeed, the sole use of neuropsychological tests could have inaccurately estimated the problem because an unknown proportion of participants could have presented pathologic performance on tests, regardless of COVID-19.
Summary
COVID-19 is capable of eliciting persistent neurocognitive alterations. These alterations are measurable with widely available test batteries and seem particularly relevant in the areas of executive functions in general and attention and working memory specifically. In the context of this ongoing pandemic, it is imperative to intensify and expand research in the field as these cognitive derangements may represent an early stage of mild cognitive impairment in the elderly.
Clinics care points
-
•
For many elderly people, Covid-19 represents an event with non-negligible cognitive sequelae.
-
•
Since in many cases these people have never been previously studied cognitively, the clinician is confronted with the question of whether what is being observed is a more or less temporary effect of Covid-19 or on the contrary represents the onset of a cognitive impairment of a different nature although possibly triggered or made more readily apparent by Covid-19.
-
•
In this sensitive population group, therefore, we recommend to proactively look for emerging cognitive deficits and to plan a reassessment of the cognitive picture at regular intervals.
Footnotes
The authors declare to have no conflicts of interest.
Funded by: ITALYLETR.
Supplementary data
References
- 1.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021 doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janiri D., Carfì A., Kotzalidis G.D., et al. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry. 2021 doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taquet M., Luciano S., Geddes J.R., et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham E.L., Clark J.R., Orban Z.S., et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann Clin Translational Neurol. 2021 doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenforde M.W., Kim S.S., Lindsell C.J., et al. Symptom duration and risk factors for delayed return to usual health among[1] M. W. Tenforde et al., “Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United. MMWR Morbidity Mortality Weekly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemelli Against COVID-19 Post-Acute Care Study Group Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613–1620. doi: 10.1007/s40520-020-01616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Carlesimo G.A., Sabbadini M., Fadda L., et al. Different components in word-list forgetting of pure amnesics, degenerative demented and healthy subjects. Cortex. 1995;31(4):735–745. doi: 10.1016/s0010-9452(13)80024-x. [DOI] [PubMed] [Google Scholar]
- 11.Gainotti G., Marra C., Villa G. A double dissociation between accuracy and time of execution on attentional tasks in Alzheimer’s disease and multi-infarct dementia. Brain. 2001;124(Pt 4):731–738. doi: 10.1093/brain/124.4.731. [DOI] [PubMed] [Google Scholar]
- 12.Standardizzazione e taratura italiana di test neuropsicologici. Spinnler H., Tognoni G., editors. Iialian J Neurol Sci. 1987;8(6):120. [PubMed] [Google Scholar]
- 13.Giovagnoli A.R., Del Pesce M., Mascheroni S., et al. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 14.Bowie C.R., Harvey P.D. Administration and interpretation of the trail making test. Nat Protoc. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 15.Monaco M., Costa A., Caltagirone C., et al. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci. 2013;34(5):749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- 16.Dubois B., Slachevsky A., Litvan I., et al. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 17.Appollonio I., Leone M., Isella V., et al. The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci. 2005;26(2):108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 18.Capitani E., Laiacona M. Composite neuropsychological batteries and demographic correction: standardization based on equivalent scores, with a review of published data. The Italian Group for the Neuropsychological Study of Ageing. J Clin Exp Neuropsychol. 1997;19(6):795–809. doi: 10.1080/01688639708403761. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Maier W., Buller R., Philipp M., et al. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman M., Martinez J.H., Young D., et al. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150(2):384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Andrews G., Slade T. Interpreting scores on the Kessler Psychological Distress scale (K10) Aust N Z J Public Health. 2001;25(6):494–497. doi: 10.1111/j.1467-842x.2001.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 24.Kessler R.C., Andrews G., Colpe L.J., et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 25.Buysse D.J., Reynolds C.F., Monk T.H., et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scully E.P., Haverfield J., Ursin R.L., et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H., Lu S., Chen J., et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129(January):98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amalakanti S., Arepalli K.V.R., Jillella J.P. Cognitive assessment in asymptomatic COVID-19 subjects. VirusDisease. 2021 doi: 10.1007/s13337-021-00663-w. Mci) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y.H., Chen Y., Wang Q.H., et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022 doi: 10.1001/JAMANEUROL.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.