Abstract
Background
We evaluate the overall effectiveness of the nationwide vaccination campaign using ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines in preventing Covid-19 in South Korea.
Methods
The National Surveillance System with the National Immunization Registry were linked to form a large-linked database for assessment. Age-adjusted incidence of SARS-CoV-2 infection, severe disease, and death by vaccination status are calculated. Weekly vaccine effectiveness was calculated based on incidence rate ratio (IRR) between fully-vaccinated and unvaccinated persons, as: IRR = incidence rate of vaccinated / incidence rate of unvaccinated. We estimate the cumulative SARS-CoV-2 outcome overtime comparing the observed case with predicted cases without vaccination.
Results
Age-adjusted incidence in unvaccinated persons (5.69 per 100,000 person-day) was 2.7 times the rate in fully vaccinated (2.13 per 100,000 person-day) persons, resulting effectiveness against SARS-CoV-2 infection of 63%. Vaccine effectiveness against severe disease and death were 93% and 95%, respectively. Between March and October 2021, estimated Covid-19 related outcomes averted by vaccinations were: 46,508 infections, 3,424 severe diseases, and 718 deaths.
Conclusions
We found significant protection for national Covid-19 vaccination campaign against Covid-19 severe disease, and death in target populations, but there was an unexpected decreased protection against SARS-CoV-2 infection, highlighting the importance of continued surveillance and assessment.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Vaccine, Effectiveness
1. Introduction
Since the emergence of severe acute respiratory disease syndrome coronavirus-2 (SARS-CoV-2), coronavirus disease 2019 (Covid-19) has caused 248 million cases with over 5 million deaths globally, as of November 2021 [1]. After widespread use of viral vector-based and mRNA-based Covid-19 vaccines, the incidence, hospitalization, and death due to SARS-CoV-2 decreased noticeably in many countries and regions; however, little is known about the vaccine effectiveness in Asian countries [2].
Introduction of Covid-19 vaccines began in February 2021 in South Korea, with two viral vector-based vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S) and two mRNA-based vaccines (BNT162b2 and mRNA-1273) adopted by the Korean national vaccination campaign. Working in conjunction with the national immunization technical advisory group, Korean government instituted a targeted vaccination program delivered through vaccination centers and primary care [3]. Vaccinations were initially offered to high risk persons in long-term care facilities and healthcare professionals, which were expanded to all persons aged 12 years and above, by October 2021 [4].
Over the course of the pandemic, all reverse transcription polymerase chain reaction (RT-PCR) confirmed Covid-19 cases were reported to the government, while all Covid-19 vaccinations were registered to the national immunization registry [4], [5]. By linking these two large databases, we aimed to evaluate the overall effectiveness of the national vaccination campaign among persons ≥ 18 years of age, in preventing SARS-CoV-2 infection, severe disease case, and Covid-19 related death in South Korea.
2. Methods
2.1. Study population and design
In this study, we report the number of SARS-CoV-2 infections, Covid-19 severe disease cases, and Covid-19-related deaths; and estimate the cases averted via the direct effect of nationwide Covid-19 vaccination program, between March 13, 2021 (surveillance week 11) and October 2, 2021 (surveillance week 40), in all adults aged 18 years and older in Korea. The study assesses the incidence rates of SARS-CoV-2 infections and severe disease cases, and subsequent case fatality rates among all individuals who were vaccinated with at least one dose, with at least 14 days of follow-up.
2.2. PCR testing and vaccination program
In Korea, SARS-CoV-2 RT-PCR testing had been widely available since the beginning of pandemic, which is mandated for close contacts of infected person, people with febrile or respiratory symptoms, or people coming from abroad [4]. All RT-PCR-confirmed SARS-CoV-2 infections, severe diseases, and deaths are reported to the government on daily basis. In the case who wishes to be tested regardless of above criteria, non-PCR based testing can be done, which then are reflexed to RT-PCR testing, hence are notified to the government. From January 2020 to February 2021, a total of 89,902 RT-PCR-confirmed SARS-CoV-2 cases were reported in Korea, out of 51 million residents [4].
The nationwide Covid-19 vaccination program was launched on February 26, 2021. To date, four vaccines have been authorized by the Korean Ministry of Food and Drug Administration: ChAdOx1 nCoV-19 (AstraZeneca), BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Janssen/Johnson & Johnson). Except for Ad26.COV2.S, all vaccines require a two-dose vaccination schedule at different time intervals.
Given the supply constrains with the vaccines in the earlier phase, the government had the complete control over the vaccine distribution in different target groups. Vaccines were administered in vaccination centers or outpatient clinics with no out-of-pocket costs. Initially in the February, the ChAdOx1 nCoV-19 vaccination was offered to the residents and workers at long-term care facilities (LTCFs), and the BNT162b2 to the healthcare professionals (HCPs) at Covid-19 designated hospitals (Supplementary Table 1 ). By June and July, Ad26.COV2.S and mRNA-1273 vaccines were introduced to different target population. Between the surveillance weeks 11 and 40, the vaccination coverage rate has increased in a stepwise manner, largely due to intermittent supply constraints of the vaccines (Supplementary Fig. 1).
Table 1.
Number of SARS-CoV-2 Cases among Persons Aged ≥18 Years, by Selected Characteristics and Vaccination Status, South Korea, February - September, 2021
| Characteristics | Vaccination Status, no. (%) |
p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Fully Vaccinated* | Partially Vaccinated | Unvaccinated | ||||||
| Total no. of cases infected | 172,049 | 13,479 | 21,414 | 137,156 | |||||
| Vaccine | |||||||||
| ChAdOx1 nCoV-19 | 16,099 | (9.4) | 5,711 | (42.4) | 10,388 | (48.5) | - | - | <0.001 |
| BNT162b2 | 14,250 | (8.3) | 3,989 | (29.6) | 10,261 | (47.9) | - | - | |
| mRNA-1273 | 813 | (0.5) | 48 | (0.4) | 765 | (3.6) | - | - | |
| Ad26.COV2.S | 2,941 | (1.7) | 2,941 | (21.8) | - | - | - | - | |
| ChAdOx1 (prime) / BNT162b2 (booster) | 790 | (0.5) | 790 | (5.9) | - | - | - | - | |
| Median age, years (IQR) | 44 | (30-56) | 57 | (40-65) | 44 | (29-52) | 37 | (27-48) | <0.001 |
| Age group, yrs | |||||||||
| 18-29 | 42,301 | (24.6) | 1,074 | (8.0) | 2,205 | (10.3) | 39,022 | (28.5) | <0.001 |
| 30-39 | 30,227 | (17.6) | 2,751 | (20.4) | 1,910 | (8.9) | 25,566 | (18.6) | |
| 40-49 | 33,852 | (19.7) | 1,433 | (10.6) | 3,085 | (14.4) | 29,334 | (21.4) | |
| 50-59 | 32,694 | (19.0) | 1,313 | (9.7) | 5,704 | (26.6) | 25,677 | (18.7) | |
| 60-69 | 21,362 | (12.4) | 3,335 | (24.7) | 6,675 | (31.2) | 11,352 | (8.3) | |
| 70-79 | 7,876 | (4.6) | 2,148 | (15.9) | 1,603 | (7.5) | 4,125 | (3.0) | |
| ≥80 | 3,737 | (2.2) | 1,425 | (10.6) | 232 | (1.1) | 2,080 | (1.5) | |
| Sex | |||||||||
| Female | 82,817 | (48.1) | 6,074 | (45.1) | 10,666 | (49.8) | 66,077 | (48.2) | <0.001 |
| Male | 89,232 | (51.9) | 7,405 | (54.9) | 10,748 | (50.2) | 71,079 | (51.8) | |
| Severe Disease** | 3,849 | (2.2) | 197 | (1.5) | 409 | (1.9) | 3,243 | (2.4) | <0.001 |
| Death*** | 625 | (0.4) | 54 | (0.4) | 60 | (0.3) | 511 | (0.4) | 0.086 |
*Persons were considered fully vaccinated ≥14 days after receipt of the second dose in a 2-dose series (ChAdOx1 nCoV-19, BNT162b2, or mRNA-1273 vaccines) or after 1 dose of the single-dose Ad26.COV2.S vaccine; partially vaccinated ≥14 days after receipt of the first dose and <14 days after the second dose in a 2-dose series; and unvaccinated <14 days receipt of the first dose of a 2-dose series or 1 dose of the single-dose vaccine.
**Severe Disease: COVID-19 patients treated with high flow oxygen therapy, mechanical ventilator, ECMO (Extracorporeal Membrane Oxygenation), CRRT (Continuous Renal Replacement Therapy) within 28 days of laboratory confirmation.
***Death: COVID-19 patients who died within 28 days of laboratory confirmation.
2.3. Data source
We linked the Covid-19 National Surveillance System with the National Immunization Registry to form a large-linked database (LLDB), comprising 172,049 PCR-confirmed Covid-19 cases and 53,253,662 person’s immunization registry data (Supplementary Fig. 2). Of those, 9,139,041 children, adolescents, and foreigners, 41,951 overseas vaccinated people, 459 persons with missing data, and 89,107 people with prior infection were excluded. Among 43,983,105 eligible individuals, we had 8,558,970 unvaccinated people (19.5%), 8,600,605 ChAdOx1 nCoV-19 fully vaccinated people (19.6%), 9,284,531 BNT162b2 fully vaccinated people (21.1%), 994,567 mRNA-1273 fully vaccinated people (2.3%), 1,243,614 Ad26.COV2.S fully vaccinated people (2.8%), and 1,572,671 persons who were primed with ChAdOx1 nCoV-19 and boosted with BNT162b2 (3.6%).
2.4. Case definition
A case with Covid-19 severe disease is defined as a Covid-19 patient treated with high flow oxygen therapy, mechanical ventilator, ECMO (extracorporeal membrane oxygenation), CRRT (continuous renal replacement therapy) within 28 days of RT-PCR confirmation. The Covid-19-related death is defined as Covid-19 patients who died within 28 days of RT-PCR confirmation.
Vaccination status was defined at the time of their SARS-CoV-2 laboratory confirmation (Supplementary Figure 3): A) unvaccinated, measured from zero doses to 0–13 days after the first dose of Covid-19 vaccines; B) partially vaccinated, measured from 14 days after the first dose through 13 days after the second dose; C) fully vaccinated, measured 14 days after the second dose (ChAdOx1 nCoV-19, BNT162b2, or mRNA-1273 vaccines) or after one dose of the single-dose Ad26.COV2.S vaccine.
2.5. Analysis
We first compare the type of vaccine, age group, sex, health outcome (severe disease, death) for fully vaccinated, partially vaccinated, and unvaccinated persons. Age-adjusted incidence of SARS-CoV-2 infection, severe disease, and death by vaccination status are calculated. The rates were stratified by vaccination status and were calculated as ratio between the number of events and total observation period per 100,000 person-time in days. Weekly vaccine effectiveness was calculated based on incidence rate ratio (IRR) between fully-vaccinated and unvaccinated persons, as: IRR = incidence rate of vaccinated / incidence rate of unvaccinated.
We then estimate the cumulative SARS-CoV-2 outcome overtime comparing the crude number of cases with predicted cases without vaccination. To estimate the weekly number of averted cases, we used the following formula: cases averted = weekly number of observed persons*vaccine coverage rate*(expected number of cases in unvaccinated - observed number of cases in vaccinated); using direct standardization to adjust for difference in population structure between vaccinated and unvaccinated persons. Daily, age-adjusted incidence rates were calculated for vaccinated (fully + partially) persons and unvaccinated persons for three health outcomes. The person-days for vaccinated individuals for each day was estimated by multiplying the proportion of people who were at least partly vaccinated by population. The person-days for unvaccinated individuals for each day was determined by subtracting the number of person-days contributed by vaccinated persons from the total census population.
This study was conducted as a legally mandated public health investigation under the authority of the Korean Infectious Diseases Control and Prevention Act (No. 12,444 and No. 13392) and was not a research that was subject to institutional review board approval; therefore, written informed consent was not required.
3. Results
The number of vaccinations has increased from 20,878 (0.3% of target population) in week 11 to 35,424,135 doses by week 40, 2021. During the same period, 172,049 cases of SARS-CoV-2 infections among residents aged ≥ 18 years were reported nationally, including 13,479 (7.3%) in fully vaccinated persons, 21,414 (12.4%) in partially vaccinated persons, and 137,156 (79.7%) in unvaccinated persons (Table 1). The largest percentage of cases were among adults aged 60–69 years in fully vaccinated persons (24.7%) and partially vaccinated persons (31.2%); while among unvaccinated persons, 18–29 years comprised the largest percentage (28.5%). Among fully vaccinated persons, median age was higher (57 years) compared to partially vaccinated (44 years) and unvaccinated (37 years) persons (P < 0.001). Among fully vaccinated persons, 42.4% had received ChAdOx1 nCoV-1 vaccine, 29.6% had received BNT162b2 vaccine, and 21.8% received Ad26.COV2.S vaccine (Table 1). Lower percentage of fully vaccinated persons had severe disease (1.5%) compared to partially vaccinated persons (1.9%) and unvaccinated persons (2.4%) (P < 0.001). Supplementary Table 2 shows baseline characteristics of study population.
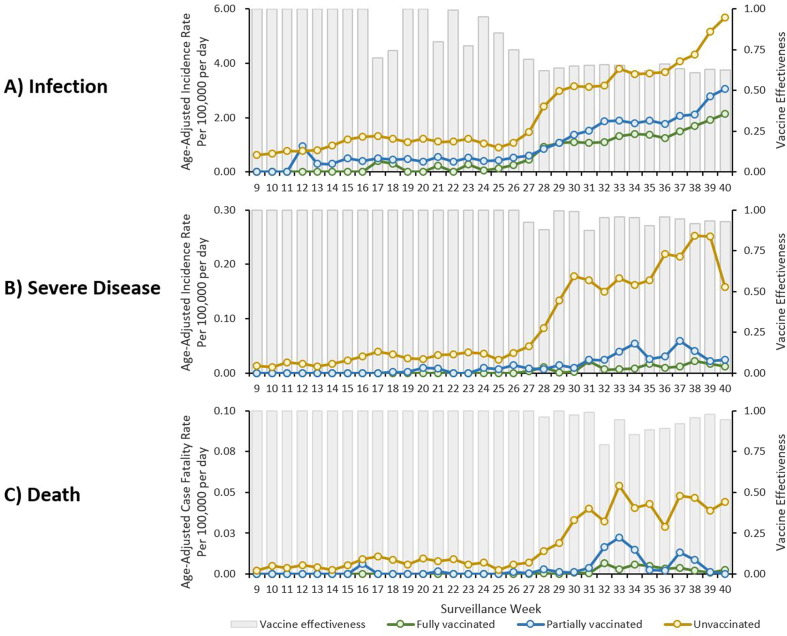
Among all Korea residents, the age-adjusted incidence increased among unvaccinated, partially vaccinated, and fully vaccinated persons, with the highest rates among unvaccinated persons after week 28 (Fig. 1 A). By week 40, in unvaccinated persons, the age-adjusted incidence (5.69 per 100,000 person-day) was 1.9 times the rate in partially vaccinated (3.05 per 100,000 person-day) and 2.7 times the rate in fully vaccinated (2.13 per 100,000 person-day) persons, resulting vaccine effectiveness against SARS-CoV-2 infection of 63%. The age-adjusted incidence for severe disease and case fatality rate for partially and fully vaccinated persons remained relatively low compared to the rate for unvaccinated persons (Fig. 1B and 1C). By week 40, the age-adjusted incidence for severe disease in unvaccinated persons (0.16 per 100,000 person-day) was 8–16 times that in partially vaccinated and fully vaccinated persons (0.02 per 100,000 person-day and 0.01 per 100,000 person-day, respectively), resulting vaccine effectiveness of 93%. The age-adjusted case fatality rate in unvaccinated persons (0.04 per 100,000 person-day) was higher than the rate in partially vaccinated persons (none) and fully vaccinated persons (0.002 per 100,000 person-day), which result in vaccine effectiveness of 95%.
Fig. 1.
Weekly Trend in Age-Adjusted Incidence of SARS-CoV-2 Infection, Severe Disease, and Death, by Vaccination Status, South Korea, February - September 2021.
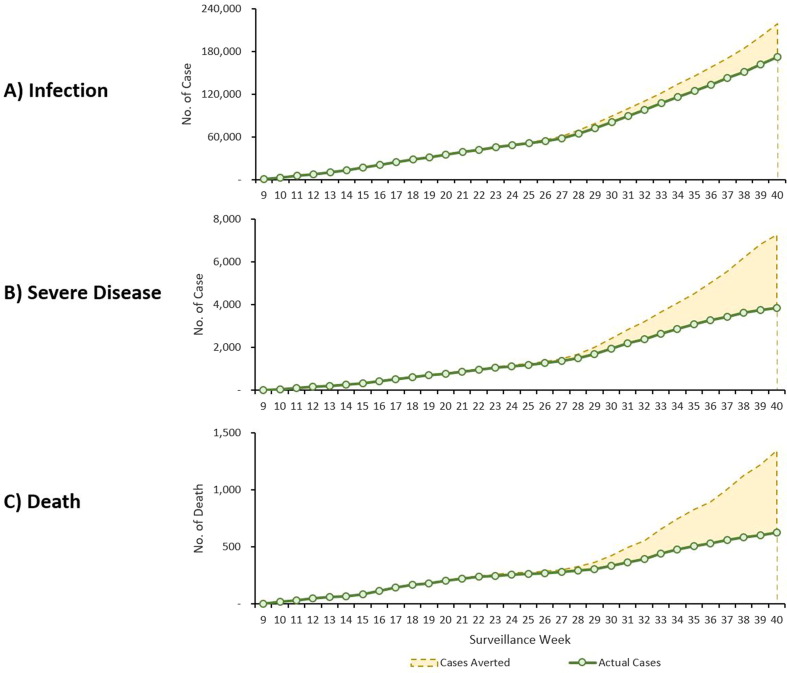
Fig. 2 shows a nationwide weekly time series of the actual number of cumulative Covid-19 A) infection, B) severe disease, and C) death and predicted number of cumulative numbers that would have occurred in the absence of vaccinations. Between the surveillance weeks 11 and 40, estimated number of Covid-19 related outcomes averted by vaccinations were 46,508 infections, 3,424 severe diseases, and 718 deaths.
Fig. 2.
Cumulative SARS-CoV-2 Outcome Overtime Comparing Observed Cases with Nationwide Vaccination and Predicted Cases without Vaccination by Outcome, South Korea, February - September 2021.
4. Discussion
In this national cohort study, we estimated the effectiveness of Covid-19 vaccine against SARS-CoV-2 infection in almost 170,000 people aged 18 years and greater in South Korea. Among the target group, the vaccine effectiveness against severe disease and death from SARS-CoV-2 infection of 93%, and 95%, respectively. In Israel, BNT162b2 was highly effective in preventing symptomatic (97.0%) and asymptomatic (91.5%) SARS-CoV-2 infections and COVID-19-related hospitalizations (97.5%), severe disease (97.5%), and (96.7%) death [6]. These figures may differ by vaccines and target population. From the U.S. Veterans Health Administration, vaccine effectiveness against death for age 65 years was 73.0% for Ad26.COV2.S, 81.5% for mRNA-1273, and 84.3% for BNT162b2; vaccine effectiveness against death for age ≥ 65 years was 52.2% for Ad26.COV2.S, 75.5% for mRNA-1273, and 70.1% for BNT162b2 [7]. However, our results are consistent with overall vaccine estimates against SARS-CoV-2-related severe disease or death previously reported by other countries. A retrospective cohort study using a large integrated health database in the United States showed a 93% (95% CI 84–96) effectiveness of BNT162b2 against hospital admissions [8]. A cohort study from Scotland showed vaccine effectiveness against death from SARS-CoV-2 was 90% (95% CI, 83 to 94) for BNT162b2 and 91% (95% CI, 86 to 94) for ChAdOx1 nCoV-19 [9]. In Qatar, a matched test-negative case-control study showed an effectiveness against severe, critical or fatal Covid-19 of 93.4% (95% CI, 85.4–97.0%) for BNT162b2 and 96.1% (95% CI, 71.6–99.5%) for mRNA-1273 vaccines [10].
Our findings suggest a high vaccine effectiveness for preventing serious disease and death from target population, however, as in studies from other countries, the vaccines may have reduced effectiveness in preventing SARS-CoV-2 infections. We report an unanticipated finding of decreased vaccine effectiveness against SARS-CoV-2 infection since surveillance week 24–26 (or June of 2021). The findings were corroborated by other observational studies in the U.K., reporting reduced effectiveness of Covid-19 vaccine against delta variant, compared with other variants. A test-negative case–control showed that the effectiveness of BNT162b2 was 93.7% (95% CI, 91.6 to 95.3) among persons with the alpha variant and 88.0% (95% CI, 85.3 to 90.1) among those with the delta variant [11]. Another U.K. study showed the reduced effectiveness of 10–13% for BNT162b2 and 16% for ChAdOx1 in preventing delta-related infections compared to alpha variant [12]. Since June 2021, the delta variant has emerged as the predominant strain in Korea, which may have affected the effectiveness in preventing SARS-CoV-2 infection, as shown in our study [4], [13]. The reduced effectiveness over time may be affected by waning immunity, or the secondary vaccine failure. In all age groups in Israel, immunity against delta variant waned in a few months after receipt of the second dose of vaccine [14]. A matched test-negative case-control study from Qatar showed that the variant-specific effectiveness waned in the same for BNT162b2, however, the protection against hospitalization and death persisted for 6 months [15]. A study from Israel reported a significantly lower incidence of booster-dose recipients compared with non-booster dose recipients, where delta variant was the predominant virus [16]. Since there has been emergence of delta variant affecting vaccine effectiveness against SARS-CoV-2 infection, these results may lead to public need for the booster doses in Korea [17].
Despite the reduced effectiveness against SARS-CoV-2 infection over time, our results suggest residual protection against severe disease and death in target population if they had been fully vaccinated. Our study demonstrates that the national vaccination campaign was instrumental to reducing the Covid-19 related severe disease and death. As postulated by previous modelling study, high vaccination coverage in conjunction with continued nonpharmaceutical intervention may be crucial in mitigating from Covid-19 [18]. As the new variants may emerge among unvaccinated individuals, our findings underscore the public health need to accelerate vaccination coverage and close the vaccination coverage gaps.
Our study has number of limitations. First, this is an observation study, in which neither vaccination or Covid-19 testing were made at random. It is plausible that the vaccination status may have affected chances of getting tested. Second, the difference in relevant characteristics between vaccinated and unvaccinated population were not controlled beyond sex and age group. Given the limited clinical and demographic data obtained through nationally-collected surveillance system, it was challenging to emulate the target trial to estimate vaccine effectiveness.
Despite these limitations, our study included accurate immunization registry of 53 million people and more than 170,000 RT-PCR confirmed Covid-19 case, which allowed us to present a real-world data from a large scale. Prior to this study, there were limited opportunities to directly evaluate the public vaccination program against SARS-CoV-2 in a national scale. We have presented a weekly estimation of incidence rates over time to avoid overestimation of vaccine effectiveness. The data shows dose–response to the vaccination in terms of number of doses and window period on effectiveness against SARS-CoV-2 infection, severe disease, and death. Given the presence of validated, real-time, internet-based immunization registry, the completeness is high because all the supply and reimbursement process was controlled by the government. Lastly, the use of complete national database may overcome the selection bias that can be introduced by non-random missing data.
In summary, we found significant protection for national Covid-19 vaccination campaign against SARS-CoV-2 severe disease, and death in target populations, but there was an unexpected finding of decreased protection against SARS-CoV-2 infection. This highlights the importance of continued surveillance of the effectiveness of Covid-19 vaccines. With a potential for waning immunity and vaccine escape by new variant, prevention of severe disease and death through increased vaccine uptake and booster doses in vulnerable population, along with other preventive measures, will be important in mitigating Covid-19 pandemic.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank the COVID-19 Vaccination Task Force and Division of National Immunization, Korea Disease Control and Prevention Agency for their dedication to provide public health services in mitigating COVID-19 pandemic. Additionally, we are grateful to the staff at the local health departments for their dedication.
Disclaimers
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Korea Disease Control and Prevention Agency or the institutions with which the authors are affiliated.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: November 11, 2021).
- 2.Fiolet T., Kherabi Y., MacDonald C.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021 doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi M.J., Choi W.S., Seong H., Choi J.Y., Kim J.H., Kim Y.J., et al. Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea. J Korean Med Sci. 2021;36 doi: 10.3346/jkms.2021.36.e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus (COVID-19), Republic of Korea: Korea Diseaes Control and Prevention, 2021. Available online: http://ncov.mohw.go.kr/en/ (last cited: November 11, 2021).
- 5.Kennedy D.S., Vu V.K., Ritchie H., Bartlein R., Rothschild O., Bausch D.G., et al. COVID-19: Identifying countries with indicators of success in responding to the outbreak. Gates open research. 2020;4:62. doi: 10.12688/gatesopenres.13140.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet (London, England). 2021;397:1819-29. [DOI] [PMC free article] [PubMed]
- 7.Cohn B.A., Cirillo P.M., Murphy C.C., Krigbaum N.Y., Wallace A.W. Science; New York, NY: 2021. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. eabm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (London, England) 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh A., Robertson C., Taylor B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. New England J Medicine. 2021 doi: 10.1056/NEJMc2113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021 doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. The New England journal of medicine. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.D., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021 doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.K. How to deal with the Delta variant this fall. Osong public health and research perspectives. 2021;12:201–202. doi: 10.24171/j.phrp.2021.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning Immunity after the BNT162b2 Vaccine in Israel. New England J medicine. 2021 doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. The New England journal of medicine. 2021 doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. New England J Medicine. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobucci G. Covid-19: How is the UK's vaccine booster programme faring? BMJ (Clinical research ed) 2021;375 doi: 10.1136/bmj.n2702. [DOI] [PubMed] [Google Scholar]
- 18.Min K.D., Tak S. Dynamics of the COVID-19 epidemic in the post-vaccination period in Korea: a rapid assessment. Epidemiology Health. 2021;43 doi: 10.4178/epih.e2021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.