As the COVID-19 pandemic progressed, shortages in personal protective equipment (PPE) and inadequately protected clinicians were increasingly reported in the media [1,2]. Clinicians became concerned about airborne transmission of the virus, especially during aerosol generating procedures (AGPs). It is critical that we learn as much as possible from this pandemic so as to be better prepared for inevitable future pandemics. We conducted a global cross-sectional survey from representative multinational sample to quantify available PPE, determine novel PPE-related practices, and assess the infrastructure available during the early stages of the COVID-19 pandemic.
The Children's Hospital of Philadelphia Institutional Review Board (IRB) determined this study did not meet the regulatory definition of human subject research. There was little literature supporting clinical care practices [3]. An international panel of ten anesthesiologists and intensivists (five from the United States of America (USA), three from India, one each from Canada and Italy) from large nationally recognized hospitals designed, tested, piloted and disseminated the survey. This cross-sectional survey used a structured branched-logic questionnaire. The initial survey drafts underwent several revisions, with inputs from all authors. The survey was pretested with four randomly selected physicians (two each from the USA and India) independent of survey creation.
The final survey included 23 questions (Included as online supplement). Questions examined 1) the availability of PPE during AGPs, 2) infrastructure and equipment related to AGP management, and 3) use of novel PPE practices. The survey population comprised anesthesiologists and intensivists knowledgeable about their institutional PPE-related practices. This was a non-probability-based global sample representing the institutions available to participate in the study. Institutions were recruited through list server using email and the WhatsApp Messenger application. Using RedCap [4], e-mail invitations were sent to potential respondents between May 23, 2020 and June 13, 2020 with a maximum of six automated reminders sent to non-respondents. One respondent completed the survey at each institute. Responses were collected between May 23, 2020 and June 25, 2020.
The institutions were categorized by country: USA; other high-income; low/low-middle/upper-middle income countries (collectively termed LMIC) based on World Bank definitions (gross national income per capita cut off of $12,695) [5]. High income countries were separated into the USA and other high-income countries because of media reports citing significantly more PPE shortages in the USA. The infrastructure-related questions explored the presence of negative-pressure rooms, availability and use of video laryngoscopy, barrier techniques, and use of viral filters for anesthesia circuits. Questionnaire-based screening and laboratory testing for COVID-19, and the reuse and disinfection of PPE across institutions were assessed. Standard descriptive statistics are reported as n (%). Analyses were performed using SAS (version 9.4, SAS Institute).
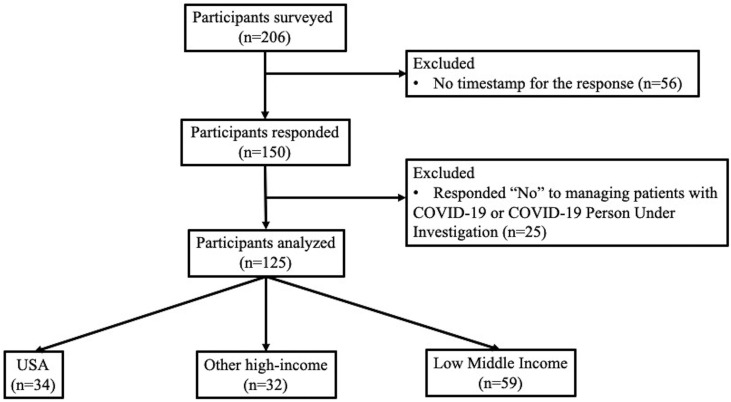
The survey was sent to 206 institutions across 37 countries. The response rate was 72.8% (n = 150). Out of 150 who responded, 125 answered ‘Yes’ to a question on whether their hospital cared for patients with COVID-19 (Fig. 1 ). There were 66 institutions in 15 high-income and 59 institutions in 22 LMICs. Fifty-two percent had mixed (adult and pediatric) and 38% had pediatric practices. Most of the institutions were tertiary or national referral hospitals (72%) (Supplemental Table 1).
Fig. 1.
Flow diagram of respondents in the survey.
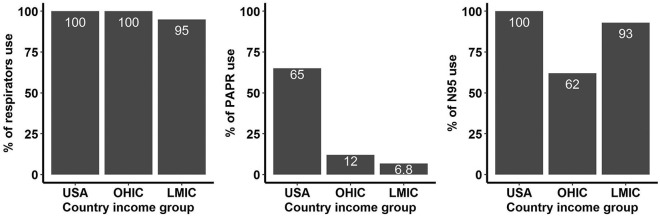
The distribution of PPE availability for each item according to income group is shown in the Table 1 . Among the individual PPE components, respirators were universally used by institutions in the US and institutions in other high-income nations and 95% of institutions in LMIC. Powered Air Purifying Respirator (PAPR) was used in 65% of US institutions, 12% in other high-income and 6.8% in LMICs (Supplemental Figure). Isolation gowns were used in 97% of the USA institutions, 75% of other high-income and 66% of LMICs. Shoe covers were used in 94% of USA, 85% LMIC and 69% other high-income countries.
Table 1.
The distribution of personal protective equipment availability by income group.
| Characteristic (N = 125) | USA, N = 34 (27%) | Other high income, N = 32 (26%) | Low-middle income, N = 59 (47%) |
|---|---|---|---|
| Respirators | 34 (100%) | 32 (100%) | 56 (95%) |
|
22 (65%) | 4 (12%) | 4 (6.8%) |
|
34 (100%) | 20 (62%) | 55 (93%) |
|
0 (0%) | 2 (6.2%) | 1 (1.7%) |
|
3 (8.8%) | 4 (12%) | 8 (14%) |
|
1 (2.9%) | 14 (44%) | 10 (17%) |
|
1 (2.9%) | 13 (41%) | 4 (6.8%) |
| Eye protection | 30 (88%) | 24 (75%) | 48 (81%) |
| Face Shield | 34 (100%) | 29 (91%) | 51 (86%) |
| Gloves | 34 (100%) | 31 (97%) | 56 (95%) |
| Isolation Gown | 33 (97%) | 24 (75%) | 39 (66%) |
| Hair Cover | 34 (100%) | 30 (94%) | 50 (85%) |
| Surgical Mask | 30 (88%) | 22 (69%) | 40 (68%) |
| Scrubs | 30 (88%) | 27 (84%) | 43 (73%) |
| Shoe Cover | 32 (94%) | 22 (69%) | 50 (85%) |
FFP = Filtering Face Piece; PAPR = Powered Air Purifying Respirators; USA = United States of America.
Respirator reuse were reported at 94% institutions within the USA and LMIC (Supplemental Table 2). Respirator disinfection occurred at 72% in institutions within the USA, 22% in other high-income and 41% LMIC. The response rates for infrastructure like negative pressure rooms, video laryngoscope, barriers to reduce aerosolization, viral filter practices for anesthesia circuits and COVID-19 testing are provided in the Supplemental table 3.
The international survey of COVID-19 practices respondents from the USA and other high income countries reported using respirators on all their cases. Majority of respondents from LMICs also used respirators (95%). N95 seemed to be most popular respirator while PAPR was more popular in the USA. Institutions in the USA and LMIC had higher N95 availability than institutions in other high-income countries. Respondents from US-based institutions also reported reuse, re-sterilization, and use of novel PPE, such as barriers, more often than respondents from other high-income countries. This finding is consistent with media reports regarding PPE shortages. Reusing and re-sterilizing PPE was not rigorously evaluated at the time of initial pandemic waves. A recent study on N95 decontamination and reusability concluded that institutions should not reuse respirators unless they must [6]. Although logical, this reuse practice was evident during the pandemic. If repurposing of respirators is to be done, which may be inevitable in many parts of the world, an appropriate ultraviolet system is essential and respirators meeting specific design criteria may be reasonable for repurposing using ultraviolet disinfection [7]. Additionally, institutions should maintain adequate emergency stockpiles in anticipation of future pandemics.
Our study has limitations. There may be selection bias because institutes may not have been representative of an entire country. There is a possibility of responder bias. Respondents may have answered questions recalling their most recent experience and not their institution's global experience. The findings were based on the absolute availability of PPE (yes/no) and not the amount of PPE available. The other limitation is that the supplies varied on a daily basis in almost every hospital during the first wave. Since the hospitals often switched between different PPE during the first wave, the availability of any single technology may not represent the level of protection available to staff.
To conclude, during the time of this survey, all respondents from high income countries and majority of LMIC had some form of respirator. Reuse of N95 was reported by most respondents in the USA and LMIC and by two-thirds of respondents from other high income countries. The disinfecting process followed a similar trend.
The following are the supplementary data related to this article.
Supplementary Fig. S1.
This is a histogram showing the distribution of respirator use on the far left, percentage of powered air purifying respirator (PAPR) in the middle, percentage of N95 use on the far right compared between income levels on the x-axis. (OHIC = Other high-income countries; LMIC = low-middle income countries).
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinane.2022.110881.
Financial disclosures
Rajeev Iyer has research funding from the Masimo Foundation, Irvine, CA, United States.
John Fiadjoe has research funding from the Anesthesia Patient Safety Foundation, United States.
No external funds were used for this project.
All other authors have no financial disclosures to report.
None reported by any of the remaining authors.
Contributions
Name: Rajeev Iyer MD.
Contribution: This author conceptualized the idea and helped with the survey questionnaire, data collection, data analysis, and manuscript preparation.
Name: Clyde Matava MBChB.
Contribution: This author assisted with the survey questionnaire, data analysis, and manuscript preparation.
Name: Vittori Alessandro MD.
Contribution: This author assisted with the survey questionnaire, data analysis, and manuscript preparation.
Name: Elizabeth T. Drum MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: Priti G Dalal MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: Faye Evans MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: Heather Griffis PhD.
Contribution: This author helped with data analysis and data interpretation and critically reviewed the manuscript.
Name: Hongyan Liu PhD.
Contribution: This author helped with the data analysis, data interpretation, and manuscript preparation.
Name: Kamal Kajal MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: Goverdhan D Puri MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: Elsa Varghese MD.
Contribution: This author helped with the survey questionnaire and data interpretation, and critically reviewed the manuscript.
Name: John Fiadjoe MD.
Contribution: This author assisted with the survey questionnaire, data analysis, and manuscript preparation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The funding obtained by Rajeev Iyer and John Fiadjoe are for other projects. These are financial disclosures with NO conflict of interest for this manuscript.
References
- 1.Dev N., Meena R.C., Gupta D.K., Gupta N., Sankar J. Risk factors and frequency of COVID-19 among healthcare workers at a tertiary care Centre in India: a case-control study. Trans R Soc Trop Med Hyg. Mar 24, 2021 doi: 10.1093/trstmh/trab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma L., Zou S., Liu Y., Lai J., Yang J. The application of Hazard vulnerability analysis in the prevention and control of COVID-19 in medical institutions. Iran J Public Health. Feb 2021;50(2):271–279. doi: 10.18502/ijph.v50i2.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng L., Qiu H., Wan L., et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. Jun 2020;132(6):1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groups WBCaL 2022. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed January 24.
- 6.Peters A., Palomo R., Ney H., et al. The COVID-19 pandemic and N95 masks: reusability and decontamination methods. Antimicrob Resist Infect Control. May 29, 2021;10(1):83. doi: 10.1186/s13756-021-00921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ontiveros C.C., Shoults D.C., MacIsaac S., et al. Specificity of UV-C LED disinfection efficacy for three N95 respirators. Sci Rep. Jul 28, 2021;11(1):15350. doi: 10.1038/s41598-021-94810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3