Abstract
We established a straightforward murine model of oropharyngeal candidiasis. Mice were immunosuppressed with cortisone acetate, anesthetized, and then inoculated by placing cotton wool balls saturated with Candida albicans sublingually for 2 h. A prolonged, reproducible infection was induced. This model may be useful for antifungal screening or pathogenesis studies.
Oropharyngeal candidiasis (OPC) is the most common fungal infection in patients with AIDS (3, 7, 9). Animal models of OPC are useful for evaluating the efficacy of antifungal agents and investigating the pathogenesis of this infection (8). In this study, we established and characterized a new model of OPC in mice.
Specific pathogen-free male ddY mice (5 weeks old; Japan SLC, Inc., Shizuoka, Japan) were used. All animal experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee of Sankyo Co., Ltd. Mice were immunosuppressed with 4 mg of cortisone acetate (Sigma Chemical Co., St. Louis, Mo.) administered subcutaneously on the day before and 1 and 3 days after inoculation. The mice were also given tetracycline hydrochloride (Achromycin V; Lederle Japan, Ltd., Tokyo, Japan) in their drinking water (0.5 mg/ml), starting the day before infection. All experiments used at least five mice per treatment group.
Candida albicans SANK51486 from our laboratory was used for this study. This strain is susceptible to fluconazole (FLC) with an MIC of 0.25 μg/ml, as determined by the NCCLS M27A broth microdilution method (5). Before inoculation, the mice were anesthetized by intraperitoneal injection with 26 μg of dimorpholamine (Theraptique; Eisai Co., Ltd., Tokyo, Japan), 207 μg of xylazine (Bayer Yakuhin, Ltd., Osaka, Japan), and 1.04 mg of sodium pentobarbital (Nembutal; Dainabot Co., Ltd., Tokyo, Japan). Next, 3-mm-diameter cotton wool balls (Hakujuji Inc., Tokyo, Japan) were saturated with 100 μl of a suspension containing 108 blastospores per ml and then placed sublingually in the oral cavity for 2 h.
To determine the number of viable organisms in the tissues, each mouse was sacrificed, and the mandible with attached tissue and the esophagus were excised. After removing the bone and teeth, the tissue was homogenized and serial dilutions were plated onto Sabouraud dextrose agar plates containing 10 μg of chloramphenicol per ml for colony counting.
For histopathological analysis, the excised tissue was fixed with formalin and embedded in paraffin, after which thin sections were prepared and stained with periodic acid Schiff (PAS). To study the effects of FLC on the course of OPC, the drug (Diflucan; Pfizer Pharmaceuticals Inc., Tokyo, Japan) was dissolved in 0.5% sodium carboxymethyl cellulose (Wako Pure Chemical Industries, Osaka, Japan) and administered orally once daily, starting on day 3 postinoculation. The control mice received 0.2 ml of the vehicle.
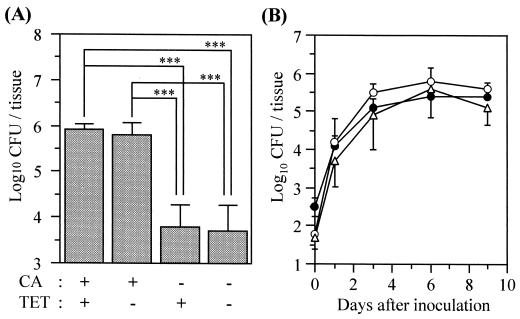
Initially, we investigated the effects of cortisone acetate and tetracycline on the organism burden in the oral tissues on day 6. Groups of mice were treated as follows: cortisone acetate plus tetracycline; cortisone acetate alone; tetracycline alone; and no treatment (Fig. 1A). The number of organisms in the oral tissues of mice receiving cortisone acetate was significantly greater than in mice that did not receive this drug, regardless of whether they received tetracycline. These results indicate that cortisone acetate is necessary for inducing and maintaining a high level of infection. Although tetracycline had no effect on the number of fungi in the oral tissues, we used it to prevent bacterial superinfection.
FIG. 1.
Development of OPC in mice. (A) Effect of cortisone acetate and tetracycline on the numbers of viable C. albicans cells in the oral tissue on day 6. Data are the mean plus standard deviation for five mice per group. ∗∗∗, P < 0.001 by the Tukey test. CA, cortisone acetate; TET, tetracycline. (B) Time course of OPC in mice treated with cortisone acetate and tetracycline. Data are the mean ± standard deviation for five mice per group. Open circles, tongue; closed circles, nonlingual oral tissue; open triangles, esophagus.
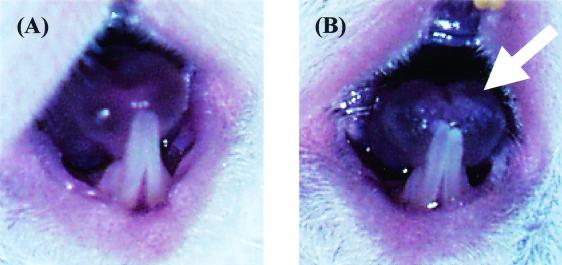
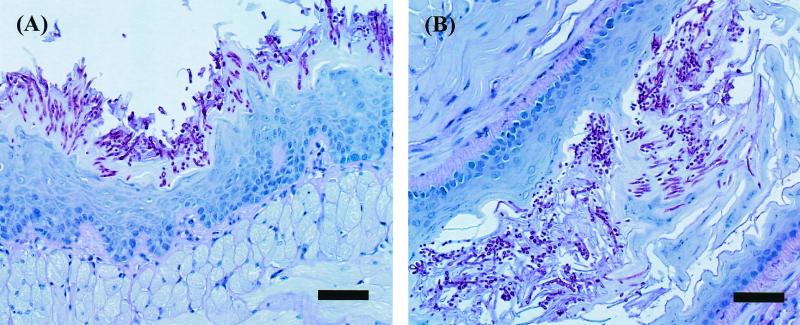
Next, we investigated the time course of infection in the tongue, the nonlingual oral tissue, and the esophagus. In these tissues, the number of viable cells progressively increased over time and then remained at 5 to 6 log10 CFU/tissue from day 3 to at least day 9 (Fig. 1B). In this experiment, white patches, which are a common clinical feature in human OPC, were observed to cover virtually the entire dorsum of the tongue beginning on day 2 or 3 and remaining throughout the experiment (Fig. 2). PAS staining of the tongue and esophagus demonstrated that the C. albicans cells had invaded the superficial epithelial layer of the mucosa and that both mycelial and blastoconidial forms of the organism were present (Fig. 3). These histological findings are similar to those for humans with OPC.
FIG. 2.
White patches on the tongue of a mouse inoculated with C. albicans. The tongues of an uninfected (A) and infected (B) mouse were photographed on day 3. The arrow indicates the white patches on the tip of the tongue.
FIG. 3.
Histopathological analysis of the tongue (A) and the esophagus (B) tissue of mice infected with C. albicans for 4 days (PAS stain; bar = 50 μm).
We also investigated the virulence of another C. albicans isolate, SC5314 (2), in this model of OPC and observed that it induced a level of infection similar to that induced by strain SANK51486 (data not shown). Therefore, our findings are reproducible with two different strains of C. albicans.
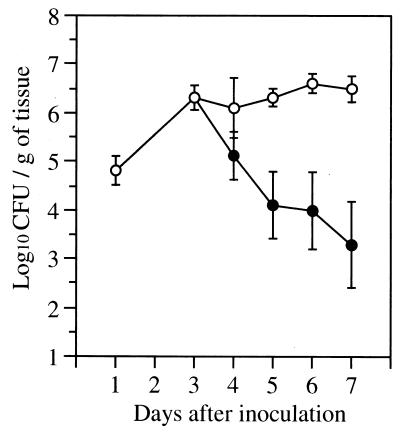
To determine if this model could be used to assess the efficacy of antifungal agents for the treatment of OPC, we evaluated the efficacy of FLC. When FLC was administered at 10 mg/kg of body weight/dose starting on day 3, the number of CFU in the oral tissues progressively decreased from day 4 to day 7. In contrast, the oral fungal burden of the control mice remained constant over this time (Fig. 4). Also, 2 days of therapy with FLC at doses of 2 and 10 mg/kg/dose reduced the oral fungal burden on day 5 by 1.3 log10 and 2.7 log10, respectively, compared to that of control mice (P < 0.001 for each comparison, Dunnett's test). The oral fungal burden of mice receiving FLC at 0.4 mg/kg/dose was similar to that of control mice (data not shown). These results demonstrate the dose-dependent efficacy of FLC in this model.
FIG. 4.
The number of viable C. albicans cells in the oral tissue of mice that were treated with daily FLC at 10 mg/kg/dose starting on day 3. Results are the mean ± standard deviation for six mice per time point. Open circles, control mice; closed circles, mice treated with FLC.
To date, a number of animal models have been developed for studying OPC (1, 4, 6, 8, 10). Some of these models, such as the one presented here, are best suited for evaluating the therapeutic efficacy of selected antifungal agents (1, 4, 6, 10). However, these antifungal agents must have favorable pharmacokinetics in the animal model that is being used. Simple animal models of OPC that are used for antifungal screening may also be appropriate for screening C. albicans mutants for loss of virulence. Those mutants with diminished virulence can be evaluated further in more sophisticated (and expensive) animal models, such as the CD4+-depleted mouse or the sialadenectomized rat.
In summary, we have established a new technique for inducing sustained OPC in mice. The inoculation procedure using a cotton wool ball is rapid and simple. The infection induced by this method is reproducible, and the animal-to-animal variability in the tissue fungal burden is low. These characteristics suggest that this model may be useful for basic antifungal screening.
Acknowledgments
S.G.F. was supported by Public Health Service grant R01 DE13974.
REFERENCES
- 1.Flattery A M, Abruzzo G K, Gill C J, Smith J G, Bartizal K. New model of oropharyngeal and gastrointestinal colonization by Candida albicans in CD4+ T-cell-deficient mice for evaluation of antifungal agents. Antimicrob Agents Chemother. 1996;40:1604–1609. doi: 10.1128/aac.40.7.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillum A M, Tsay E Y H, Kirsch D R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 3.Klein R S, Carol A H, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 4.Martinez A, Regadera J, Jimenez E, Santos I, Gargallo-Viola D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob Agents Chemother. 2001;45:1008–1013. doi: 10.1128/AAC.45.4.1008-1013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 6.Petraitis V, Petraitiene R, Groll A H, Sein T, Schaufele R L, Lyman C A, Francesconi A, Bacher J, Piscitelli S C, Walsh T J. Dosage-dependent antifungal efficacy of V-echinocandin ( LY303366) against experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. Antimicrob Agents Chemother. 2001;45:471–479. doi: 10.1128/AAC.45.2.471-479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samaranayake L P, Holmstrup P. Oral candidiasis and human immunodeficiency virus infection. J Oral Pathol Med. 1989;18:554–564. doi: 10.1111/j.1600-0714.1989.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 8.Samaranayake Y H, Samaranayake L P. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangeorzan J A, Bradley S F, He X, Zarins L T, Ridenour G L, Tiballi R N, Kauffman C A. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh T J, Gonzales C E, Piscitelli S, Bacher J D, Peter J, Terres R, Shetti D, Katsov V, Kligys K, Lyan C A. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J Clin Microbiol. 2000;38:2369–2373. doi: 10.1128/jcm.38.6.2369-2373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]