Abstract
We found that an extract of Arctostaphylos uva-ursi markedly reduced the MICs of β-lactam antibiotics, such as oxacillin and cefmetazole, against methicillin-resistant Staphylococcus aureus. We isolated the effective compound and identified it as corilagin. Corilagin reduced the MICs of various β-lactams by 100- to 2,000-fold but not the MICs of other antimicrobial agents tested. The effect of corilagin and oxacillin was synergistic. Corilagin showed a bactericidal action when added to the growth medium in combination with oxacillin.
Drug resistance in pathogenic bacteria is a serious problem in the treatment of patients infected with such bacteria. It is currently very difficult to discover new antibiotics or to develop new antimicrobial drugs. Thus, it is urgently necessary to find new drugs or to devise new methods that are effective for the treatment of infectious diseases caused by drug-resistant bacteria. Several mechanisms of bacterial drug resistance are known (16). We have been trying to find inhibitors of drug resistance systems in bacteria. Such inhibitors, if not toxic to humans, must be very valuable for the treatment of patients infected with drug-resistant bacteria.
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of hospital-acquired (nosocomial) infections in many countries. MRSA infections are very difficult to cure because MRSA is resistant not only to β-lactams, such as methicillin, but also to most other antimicrobial agents. For most MRSA strains, vancomycin and its analog teicoplanin are the only effective antimicrobial agents. However, the emergence of MRSA strains with intermediate-resistance to vancomycin has been reported (8). Thus, it is extremely important to find new drugs or alternative therapies that are effective against MRSA.
Although the mechanisms of β-lactam resistance in S. aureus are not fully understood yet, the mecA gene, which codes for a low-affinity penicillin-binding protein PBP2′, is the most important gene responsible for the resistance (22). Several other genes, such as fem, fmt, llm, and sigB, are also involved (2, 9, 14, 23).
It has been reported previously that catechins from green tea (Camellia sinensis) (21), compound P from green tea (25), epicatechin gallate from green tea (18), tellimagrandin I and rugosin B from rose red (Rosa canina) (19), totarol from totara tree (15), and baicalin from Scutellaria amoena (12) markedly reduced the MIC of β-lactams. Also, there are several chemical compounds, such as a triazine dye (Cibacron blue F3GA), Triton X-100, Polidocanol, polyoxytungstates, and a tripeptide composed of carbobenzoxy diphenylalanine-proline-phenylalanine alcohol (LY301621), that have been reported to reduce the MIC of β-lactams when used in combination with β-lactams against MRSA (3, 5, 7, 10, 20).
Recently, we found that an extract of Arctostaphylos uva-ursi markedly reduced the MIC of β-lactams in MRSA. We tried to isolate the effective compound. We report here the isolation and identification of the effective compound and its some properties.
MRSA strains OM481, OM505, OM584, and OM623 were clinical isolates from Okayama University hospital. The strains OM505 and OM584 are β-lactamase positive and PBP2′ inducible, and OM 481 and OM623 are β-lactamase negative and PBP2′ constitutive. Methicillin-sensitive S. aureus (MSSA) strain 209P was used as a control strain. MRSA cells were grown, and the MICs were measured as described previously (19). Viable cell numbers were measured as described previously (19). The fractional inhibitory concentration (FIC) index was calculated as reported earlier (6). The effects of the drugs were interpreted to be indicative of synergy or indifference when the index was <0.5 or >0.5, respectively.
Extract from A. uva-ursi leaves was concentrated by using a rotary evaporator. The concentrated extract was subjected to column chromatography over Toyopearl HW-40C (Toso Co.) and eluted with H2O and aqueous methanol (20, 60, and 100%) in a stepwise manner, and with 70% acetone at the final step. The eluate with 60% methanol that showed the highest effect on the reduction of the MIC of oxacillin against MRSA was further subjected to chromatography over MCI GEL CHP20P (Mitsubishi Chemical Co.) by using 5% methanol as a solvent. Active fractions were collected and purified by preparative high-pressure liquid chromatography on YMC-pack ODS-AQ324 (1 by 30 cm) using 30% methanol with 0.001% trifluoroacetic acid as an eluant. The structure of the isolated compound was determined by 1H-NMR spectral analysis.
The extract of A. uva-ursi leaves (Uva-ursi Fluid Extract; Shiseido Co.), antimicrobial agents, and chemicals used in this study were purchased from commercial sources.
We have been trying to find compounds that make MRSA susceptible to various antimicrobial agents currently used. During the course of our studies, we found that extract of A. uva-ursi markedly reduced the MICs of β-lactam antibiotics against MRSA. This implies that some effective compound(s) is contained in the A. uva-ursi extract.
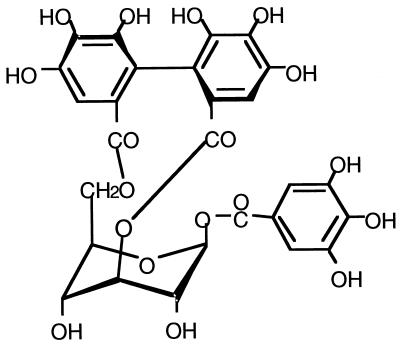
We isolated the effective compound from the A. uva-ursi extract after several steps of column chromatography. We identified the compound as corilagin (17), a polyphenol (Fig. 1). Corilagin itself showed weak anti-MRSA activity (data not shown). The MIC of corilagin against the two MRSA strains used in this study was 128 μg/ml.
FIG. 1.
Structure of corilagin.
The addition of much lower concentrations (16 μg/ml) than the MIC (128 μg/ml) of corilagin markedly decreased the MIC of oxacillin and other β-lactams against MRSA strains tested (Table 1). Such a dramatic effect was not observed with MSSA strain 209P (Table 1). Corilagin did not give such a remarkable effect on the MICs of other types of antibacterial agents tested (Table 1). However, some reduction in the MICs caused by corilagin was observed in strain OM481 and OM584 with streptomycin and tetracycline but not in OM505 and OM623 (Table 1).
TABLE 1.
MICs of various antibacterial agents in MSSA and MRSA in the absence or presence of corilagin
| Antibiotica | Corilaginb | MIC (μg/ml) for strain:
|
||||
|---|---|---|---|---|---|---|
| 209P | OM481 | OM505 | OM584 | OM623 | ||
| OXA | − | 0.12 | 512 | 256 | 256 | 256 |
| + | 0.06 | 1 | 1 | 0.25 | 1 | |
| CMZ | − | 0.06 | 128 | 32 | 64 | 128 |
| + | 0.06 | 0.5 | 0.06 | 0.5 | 0.06 | |
| IPM | − | 0.03 | 64 | 8 | 64 | 64 |
| + | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | |
| PCG | − | 0.12 | 4 | 4 | 4 | 2 |
| + | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | |
| ERY | − | 0.25 | >1,024 | >1,024 | >1,024 | >1,024 |
| + | 0.12 | >1,024 | >1,024 | >1,024 | >1,024 | |
| FOF | − | 0.5 | >1,024 | 512 | >1,024 | >1,024 |
| + | 0.5 | >1,024 | 128 | >1,024 | >1,024 | |
| OFX | − | 0.25 | 2 | 1 | 8 | 8 |
| + | 0.25 | 2 | 1 | 4 | 8 | |
| STR | − | 8 | 4 | 8 | 8 | 16 |
| + | 4 | 1 | 8 | <1 | 16 | |
| TET | − | 0.25 | 4 | 1 | 128 | 128 |
| + | 0.12 | 0.06 | 0.5 | 32 | 128 | |
| VAN | − | 0.06 | 0.06 | 0.06 | 0.06 | 0.12 |
| + | 0.03 | 0.06 | 0.12 | 0.06 | 0.12 | |
OXA, oxacillin; CMZ, cefmetazole; IPM, imipenem; PCG, benzylpenicillin; ERY, erythromycin; FOF, fosfomysin; OFX, ofloxacin; STR, streptomycin; TET, tetracycline; VAN, vancomycin.
MICs were determined in the absence (−) or presence (+) of 16 μg of corilagin/ml.
Since both oxacillin and corilagin possess antibacterial activity against MRSA, although not strong, it is necessary to assess whether the anti-MRSA effect observed in the presence of the two drugs is an additional one or a synergistic one. Therefore, we determined the MICs of oxacillin against MRSA strains in the absence or presence of 16 μg of corilagin per ml and calculated the FIC index. The index was 0.13 in all four MRSA strains. Therefore, we concluded that the effect observed was a synergistic one. Previously, we reported that the FIC index for oxacillin plus tellimagrandin I was 0.39 (19). This means that corilagin is much more effective than tellimagrandin I in the synergistic reduction of the MIC for oxacillin against MRSA. On the other hand, the index for oxacillin with corilagin was 0.75 in MSSA.
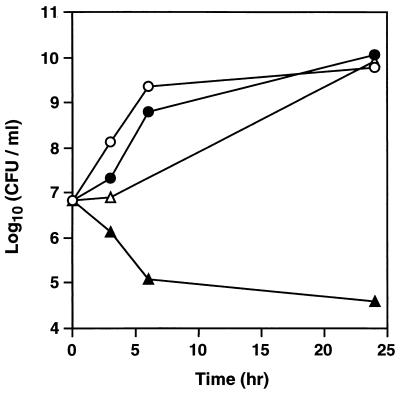
Thereafter, we investigated the effect of oxacillin plus corilagin on MRSA viability to determine whether the effect was bactericidal or bacteriostatic. Viable cell numbers of MRSA OM584 increased in the absence of oxacillin or corilagin (Fig. 2). Slightly lower growth was observed in the presence of oxacillin (5 μg/ml), and much lower growth was observed in the presence of corilagin (16 μg/ml) (Fig. 2). On the other hand, simultaneous addition of oxacillin and corilagin greatly reduced viable cell numbers. Thus, we concluded that the effect of oxacillin plus corilagin was bactericidal.
FIG. 2.
Effect of corilagin and oxacillin on viable cell number of MRSA. S. aureus OM584 cells grown in Mueller-Hinton broth were diluted with fresh medium and incubated at 32°C under aerobic conditions in the absence (○) or presence of oxacillin (5 μg/ml) (●), corilagin (16 μg/ml) (▵), or oxacillin (5 μg/ml) plus corilagin (16 μg/ml) (▴). Samples were taken at the indicated time points, and viable cell numbers were counted.
Corilagin dramatically reduced the MICs of β-lactams in MRSA strains but not in the MSSA strain 209P (Table 1). The reduced MIC values of β-lactams against MRSA determined in the presence of corilagin were at the same level as those obtained with the MSSA strain (Table 1). This implies that corilagin inhibits PBP2′ present in MRSA cells but not other PBPs present in MSSA cells. It is important to make the mechanism of action of corilagin clear. There are two possibilities regarding the mechanism of action of corilagin: (i) inhibition of PBP2′ activity and (ii) inhibition of production of PBP2′. Our preliminary results support the notion that the major action of corilagin is to inhibit the activity of the PBP2′. Shimamura and coworkers found recently that epigallocatechin gallate, which reduced the MIC of oxacillin against MRSA, did not inhibit production of PBP2′ in MRSA (T. Shimamura et al., unpublished results). They suggested that damages in the cell wall and in the cell membrane caused by epigallocatechin gallate and an increase in the permeability would be responsible for the potent synergy.
Corilagin markedly reduced the MICs of β-lactams in both β-lactamase-positive MRSA (OM505 and OM584) and β-lactamase-negative MRSA (OM481 and OM623). These results imply that corilagin also inhibits the action or production of β-lactamase. It has been reported that Compound P (epicatechin gallate according to J. M. T. Hamilton-Miller [unpublished results]) from green tea inhibited β-lactamase action by preventing the secretion of β-lactamase from cells (25). It has been also suggested that baicalin inhibits β-lactamase (12).
Previously, we reported that epicatechin gallate (18), tellimagrandin I, and rugosin B (19) markedly reduced the MICs of β-lactams against MRSA. Here, we isolated corilagin as a more effective compound than these three. These are all polyphenols. The structure of corilagin is similar to that of tellimagrandin I, and they are both classified as hydrolyzable tannin, especially ellagitannin. Corilagin could be hydrolyzed to glucose, ellagic acid, and gallic acid. None of these (i.e., glucose, ellagic acid, or gallic acid), however, reduced the MICs of β-lactams against MRSA (data not shown).
Corilagin has been reported to show several activities, such as antifungal (11), antiviral (13, 24), or antimicrobial (1) activity, and antihypertensive effect in rats (4). In addition of these reported activities, we found a novel activity of corilagin, potentiation of activity of β-lactams against MRSA.
Leaves of A. uva-ursi and its extract are described in the Japanese Pharmacopoeia as medicine. The extract is known to contain antimicrobial agents and is orally administered for the treatment of some infectious diseases. The traditional oral administration of the extract suggests that the toxicity of the extract, if present, is very low for humans.
Acknowledgments
We thank Y. Hirai for providing us with the MRSA strains used in this study, Manuel F. Varela for critically reading the manuscript, and T. Shimamura for sending us a preprint of his very interesting study on epigallocatechin gallate.
REFERENCES
- 1.Adesina S K, Idowu O, Ogundaini A O, Oladimeji H, Olugbade T A, Onawunmi G O, Pais M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Aacalypha hispida. Phytother Res. 2000;14:371–374. doi: 10.1002/1099-1573(200008)14:5<371::aid-ptr625>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Berger-Bachi B, Barberis-Maino L, Strassle A, Kayser F H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 3.Bruns W, Keppeler H, Baucks R. Suppression of intrinsic resistance to penicillins in Staphylococcus aureus by Polidocanol, a dodecyl polyethyleneoxid ether. Antimicrob Agents Chemother. 1985;27:632–639. doi: 10.1128/aac.27.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J T, Lin T C, Hsu F L. Antihypertensive effect of corilagin in the rat. Can J Physiol Pharmacol. 1995;73:1425–1429. doi: 10.1139/y95-198. [DOI] [PubMed] [Google Scholar]
- 5.Eid C N, Halligan N G, Nicas T I, Mullen D L, Butler T F, Loncharich R J, Paschal J W, Schofield C J, Westwood N J, Cheng L. Tripeptide LY301621 and its diastereomers as methicillin potentiators against methicillin-resistant Staphylococcus aureus. J Antibiot. 1997;50:283–285. [PubMed] [Google Scholar]
- 6.Eliopoulos G M, Moellering R C. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. [Google Scholar]
- 7.Fukuda N, Yamase T, Tajima Y. Inhibitory effect of polyoxytungstates on the production of penicillin-binding proteins and β-lactamase against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 1999;22:463–470. doi: 10.1248/bpb.22.463. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Hanaki H, Ito T, Yabuta K, Ogura T, Tenover F C. Methicillin-resistant Stapylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–146. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Komatsuzawa H, Sugai M, Ohta K, Fujiwara T, Nakashima S, Suzuki J, Lee C Y, Suginaka H. Cloning and characterization of fmt gene which affects the methicillin resistance level and autolysis in the presence of Triton X-100 in methicillin-resistant Staphyloccoccus aureus. Antimicrob Agents Chemother. 1997;41:2355–2361. doi: 10.1128/aac.41.11.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. The effect of Triton X-100 on the in-vitro susceptibility of methicillin-resistant Staphylococcus aureus to oxacillin. J Antimicrob Chemother. 1994;34:886–897. doi: 10.1093/jac/34.6.885. [DOI] [PubMed] [Google Scholar]
- 11.Latte K P, Kolodziej H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z Naturforsch. 2000;55:467–472. doi: 10.1515/znc-2000-5-625. [DOI] [PubMed] [Google Scholar]
- 12.Liu I X, Durham D G, Richards R M E. Baicalin synergy with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other β-lactam-resistant strain of S. aureus. J Pharm Pharmacol. 2000;52:361–366. doi: 10.1211/0022357001773922. [DOI] [PubMed] [Google Scholar]
- 13.Liu K C, Lin M T, Lee S S, Chiou J F, Ren S, Lien E J. Antiviral tannins from two Phyllanthus species. Planta Med. 1999;65:43–46. doi: 10.1055/s-1999-13960. [DOI] [PubMed] [Google Scholar]
- 14.Maki H, Yamaguchi T, Murakami K. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J Bacteriol. 1994;176:4993–5000. doi: 10.1128/jb.176.16.4993-5000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolson K, Evans G, O'Toole P W. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpens. FEMS Microbiol Lett. 1999;179:233–239. doi: 10.1111/j.1574-6968.1999.tb08733.x. [DOI] [PubMed] [Google Scholar]
- 16.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 17.Okuda T, Yoshida T, Mori K. Brevifolin, corilagin, and other phenols from Geranium thunbergii. Phytochemistry. 1975;14:1877–1878. [Google Scholar]
- 18.Shiota S, Shimizu M, Mizushima T, Ito H, Hatano T, Yoshida T, Tsuchiya T. Marked reduction in the MIC of β-lactams in methicillin resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis) Biol Pharm Bull. 1999;22:1388–1390. doi: 10.1248/bpb.22.1388. [DOI] [PubMed] [Google Scholar]
- 19.Shiota S, Shimizu M, Mizushima T, Ito H, Hatano T, Yoshida T, Tsuchiya T. Restoration of effectiveness of β-lactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from rose red. FEMS Microb Lett. 2000;185:135–138. doi: 10.1111/j.1574-6968.2000.tb09051.x. [DOI] [PubMed] [Google Scholar]
- 20.Shirai S, Sugai M, Komatsuzawa H, Ohta K, Yamakido M, Suginaka H. A triazine dye, Cibacron blue F3GA, decreases oxacillin resistance levels in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1278–1280. doi: 10.1128/aac.42.5.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi O, Cai Z, Toda M, Hara Y, Shimamura T. Appearance of antibacterial activity of oxacillin against methicillin-resistant Staphylococcus aureus (MRSA) in the presence of catechin. Kansenshogaku Zasshi. 1995;69:1126–1134. doi: 10.11150/kansenshogakuzasshi1970.69.1126. [DOI] [PubMed] [Google Scholar]
- 22.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989;171:2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H X, Wan M, Dong H, But P P, Foo L Y. Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol Pharm Bull. 2000;23:1072–1076. doi: 10.1248/bpb.23.1072. [DOI] [PubMed] [Google Scholar]
- 25.Yam Y S, Hamilton-Miller J M T, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2′ synthesis, and β-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]