Abstract
The susceptibility of Candida albicans to several fatty acids and their 1-monoglycerides was tested with a short inactivation time, and ultrathin sections were studied by transmission electron microscopy (TEM) after treatment with capric acid. The results show that capric acid, a 10-carbon saturated fatty acid, causes the fastest and most effective killing of all three strains of C. albicans tested, leaving the cytoplasm disorganized and shrunken because of a disrupted or disintegrated plasma membrane. Lauric acid, a 12-carbon saturated fatty acid, was the most active at lower concentrations and after a longer incubation time.
Candida albicans is normally present in small numbers in the oral cavity, lower gastrointestinal tract, and female genital tract. Most C. albicans infections are caused by endogenous flora exept in cases of direct mucosal contact with lesions, for example, through sexual intercourse. With a breakdown of host defenses, the organism can produce diseases ranging from superficial skin or mucous membrane infections, e.g., oral lesions called thrush and vaginal candidiasis, to systemic involvement of multiple organs. Infections are often a complication of broad-spectrum antibacterial therapy. Candida infections of visceral organs have a particularly strong association with immunologic compromise or other violations of normal defense mechanisms (10).
Medium-chain free fatty acids and their corresponding 1-monoglycerides have been found to have a broad spectrum of microbicidal activity against enveloped viruses and various bacteria in vitro (5, 7, 11, 12, 14), including pathogens like herpes simplex virus (8, 12), Neisseria gonorrhoeae (2), Chlamydia trachomatis (1), group A streptococci, group B streptococci (GBS), and Staphylococcus aureus (3). The mechanism by which these lipids kill bacteria is not known, but electron microscope studies indicate that they disrupt cell membranes (1, 3, 12). The lipids are commonly found in natural products, for example, in milk, and are therefore likely to be nontoxic to mucosas, at least at low concentrations. In milk and at mucosas these compounds are considered to be potent inhibitors of many human pathogens or parasites (4, 5). This work was done in order to find if some fatty acids or their 1-monoglycerides might inactivate C. albicans and therefore be useful for treatment of infections of skin and mucosas caused by this pathogen. Apparently, no such studies have previously been done, except for a work by Kabara et al. (6), who studied the MICs of medium-chain fatty acids against C. albicans.
Fatty acids and 1-monoglycerides (the purest grade) were purchased from Sigma Chemical Co., St. Louis, Mo. Stock solutions were made in ethanol: 0.5 M for monomyristin and 1 M for all the other fatty acids and monoglycerides.
One C. albicans strain (strain I) was obtained from the American Type Culture Collection (ATCC 28516). Strains II and III are recent clinical isolates. Both were isolated from cervical swabs and identified by a germ tube test. Cultures were prepared from frozen stocks. For each experiment, the yeasts were streaked on Sabouraud agar medium plates and incubated at 37°C in an atmosphere of 5% CO2 for 24 h. The yeast suspensions used in experiments with 10-min incubation times were prepared by removing the colonies from the culture plate with a loop and suspending them in heart infusion broth by vortexing. If needed, broth was added to bring the suspension to a standard density of 108 to 109 CFU per ml. Assay of antiyeast activity was performed by diluting stock solutions of lipids in heart infusion broth to the desired concentration by vortexing them at the highest speed for 1 min at 37°C. The solutions showed a little turbidity, which varied among the lipids but was less for lipids with short or unsaturated fatty acid chains. Two hundred microliters of a lipid solution was thoroughly mixed with 200 μl of yeast suspension in a 5-ml round-bottom tube (12 by 75 mm; Falcon). The tubes were incubated at 37°C in a water bath and shaken every 2 min. Yeast cells mixed with broth alone and with 1% ethanol in broth were used as controls. Samples (100 μl) were removed and diluted 10-fold in sterile physiological saline, and the number of viable yeast cells was determined by streaking 10 μl of 10−2 to 10−6 dilutions and 100 μl of 10−1 dilution on Sabouraud agar plates with a pipette tip. Each streaking was done in duplicate. The yeast colonies were counted after incubation for 24 h at 37°C in a CO2 incubator. The titers (log10 CFU) of lipid-yeast mixtures were analyzed by the Tukey-Kramer method of multiple comparisons among pairs of means and were deemed significantly different from the control, i.e., broth without ethanol and lipid, when the probability was <0.01.
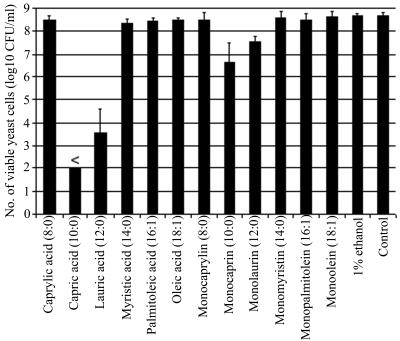
The inactivation time of 10 min or less was selected as a criterion for fast and effective antiyeast activity of a lipid. A comparison of the activities is shown in Fig. 1. The bars represent the mean titer of colony-forming yeast (log10 CFU/ml) of all three strains of C. albicans after incubation with a 10 mM concentration of fatty acids and monoglycerides for 10 min at 37°C. Each strain was tested at least twice. Capric acid (10:0 [number of carbon atoms/number of double bonds]) caused the greatest reduction of the infectivity titers (≥6.75 log10 CFU) compared to the control: no colonies were detectable in 100 μl of the 10−1 dilution, which was the lowest dilution tested. When the mean titers of all three C. albicans strains treated with lipids were compared to the control, lauric acid (12:0) and monocaprin (10:0), in addition to capric acid, showed a significant difference from the control (P < 0.01). To further compare the antiyeast activities against C. albicans, the lipids showing the highest activities, capric acid and lauric acid, were tested at lower concentrations at 37°C for 10 min. Table 1 shows that capric acid did not have a significant activity when diluted to a concentration of 5 mM. Lauric acid, which caused a significant reduction in infectivity titers at a 10 mM concentration, still showed significant activity when diluted to 5 mM (P < 0.01) but not at the 2.5 mM concentration. The titers of samples treated with 1% ethanol, which is the dilution used in 10 mM lipid solutions, were not significantly different from the titer of the control. These results are partly in agreement with those of Kabara et al. (6), who found capric, lauric, and palmitoleic acids to be inhibitory to C. albicans after 18 h of incubation. Discrepancies in the two studies may be due to differences in the experimental conditions, particularly the much shorter incubation time used in our study.
FIG. 1.
Number of C. albicans cells after treatment with 10 mM fatty acids and monoglycerides for 10 min at 37°C. At the top of the column for capric acid, < indicates that no colonies were detectable in 100 μl of the 10−1 dilution, which was the lowest dilution tested. The error bars indicate the standard deviation of the mean for all three strains, each tested at least two times.
TABLE 1.
Inactivation of C. albicans by medium-chain fatty acids after 10-min incubation at 37°C
| Fatty acid | Concn (mM)b | No. (log10 CFU/ml) of viable yeast cellsa
|
||
|---|---|---|---|---|
| Ic | II | III | ||
| Capric acid (10:0)f | 10 | ≤2.00 ± NAde | ≤2.00 ± NA | ≤2.00 ± NA |
| 5 | 8.07 ± 0.42g | 8.20 ± 0.27 | 8.03 ± 0.38 | |
| 2.5 | 8.73 ± 0.06 | 8.57 ± 0.06 | 8.47 ± 0.15 | |
| Lauric acid (12:0) | 10 | 2.47 ± 0.31h | 4.00 ± 0.60h | 4.27 ± 0.65h |
| 5 | 6.47 ± 0.06h | 6.50 ± 0.36h | 6.87 ± 0.50h | |
| 2.5 | 8.67 ± 0.06 | 8.37 ± 0.06 | 8.40 ± 0.10 | |
| Control | 8.75 ± 0.11 | 8.68 ± 0.10 | 8.62 ± 0.13 | |
| 1% ethanol | 8.70 ± 0.00 | 8.70 ± 0.10 | 8.60 ± 0.10 | |
Mean (± standard deviation) of at least three determinations.
Final concentration of fatty acids.
Strains are defined in the text.
≤ Indicates that no colonies were detectable in 100 μl of the 10−1 dilution, which was the lowest dilution tested.
NA, not available.
Number of carbon atoms/number of double bonds.
The same results were obtained for lipids diluted in broth containing either 1 or 0.5% ethanol.
Significantly different from control (P < 0.01).
To ascertain whether the reduction in titer is due to cell killing, the viability of the yeast cells was determined by staining C. albicans strain I with trypan blue. Control cells not incubated with capric acid did not stain with trypan blue, indicating live cells with intact cell membranes. In contrast, cells treated with 10 mM capric acid for 30 min stained blue, indicating dead cells. The dead cells showed the same density and distribution as the untreated control cells, which demonstrates that the loss of titer is due to killing of the cells and not to clumping or prevention of yeast cell growth on the agar.
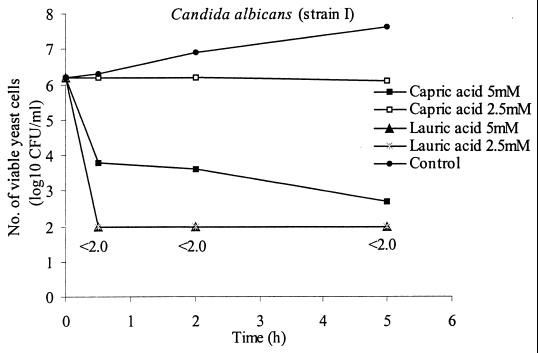
The killing activities of lipids against strain I of C. albicans were tested after longer incubation times. Cultures were prepared from frozen stock and suspended in heart infusion broth. Unlike in the 10-min experiments, the suspensions were diluted 1,000-fold in broth and grown for 4 h in a 50-ml Erlenmeyer flask before being tested; the cells were in their early growth phase and at a final density of 6.2 log10 CFU/ml. Samples (0.5 ml) were withdrawn from the Erlenmeyer flask and thoroughly mixed with 0.5 ml of 10, 5, or 2.5 mM capric and lauric acids, respectively, in 14-ml round-bottom tubes (17 by 100 mm; Falcon). Cells mixed with broth were used as controls. Samples (100 μl) were taken at time zero, and the mixtures were then incubated at 37°C in a water bath and rotated at 320 rpm. One hundred-microliter samples were then removed after 30 min and 2 and 5 h. The samples were serially diluted 10-fold in physiological saline, and the number of viable cells was determined as described previously for the 10-min incubation assay. The results are shown in Fig. 2. Lauric acid was the most active, showing greater-than-16,000-fold (4.2 log10 CFU) reduction of titer after 30 min at 5 and 2.5 mM concentrations. At 5 mM concentration, capric acid was the second most active lipid and showed about 3,200-fold reduction after 5 h of incubation. Most of the killing took place during the first 30 min. Capric acid at a concentration of 2.5 mM and lauric acid at 1.25 mM (data not shown) showed no significant killing activity but inhibited proliferation after 5 h of incubation.
FIG. 2.
Killing activities of 5 and 2.5 mM concentrations of capric acid and lauric acid against C. albicans (strain I) incubated at 37°C for 30 min and 2 and 5 h. The values are the means from three experiments, with standard deviations ranging from 0 to 0.38; <2.0 indicates that no colony-forming yeast cells were detectable in 100 μl of the 10−1 dilution. Candida cells mixed with broth without lipid were used as a control. Addition of 1% ethanol to the control had no effect on cell growth.
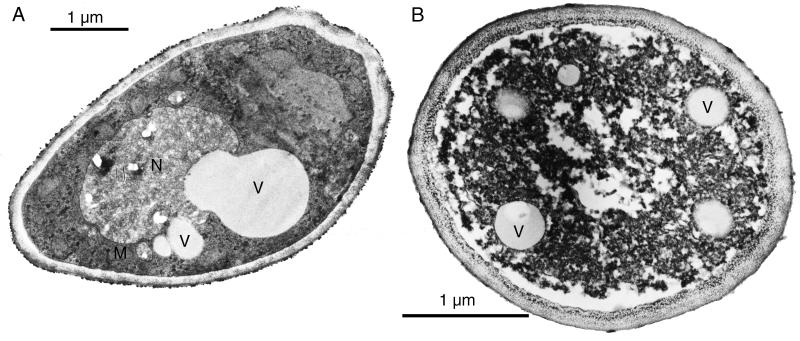
The effects on C. albicans after treatment with 10 mM capric acid for 30 min were further studied by TEM and are visualized in Fig. 3. A 200-μl suspension of C. albicans (strain I) was mixed with an equal volume of 20 mM capric acid in heart infusion broth, and the mixture was incubated at 37°C. Samples were removed after 30 min and immediately diluted fivefold in sterile physiological saline. C. albicans incubated for 30 min with an equal volume of heart infusion broth without capric acid was diluted fivefold and used as a control. The diluted samples were fixed with 5% glutaraldehyde for 2 h at room temperature. The fixed cells were then dehydrated in a graded series of ethanol, stained with uranyl acetate, and embedded in Spurr (Pelco International, Redding, Calif.). Sections were made with an ultramicrotome (Reichert-Jung), stained with lead citrate (Reynolds), and examined in a Philips 300 transmission electron microscope at 80 kV.
FIG. 3.
Effects of capric acid on the ultrastructure of C. albicans as demonstrated by TEM of ultrathin sections of yeast cells. (A) Cell from the control sample with intact cytoplasm, nucleus (N), vacuoles (V), and mitochondria (M). (B) Cell from samples treated with 10 mM capric acid for 30 min, demonstrating disorganization of the cytoplasm. No visible changes are seen in the shape or size of the cell wall.
No visible changes were seen in the shape or size of the cell wall of the yeast after treatment with capric acid (Fig. 3). The results show disorganization of the cytoplasm, which is probably due to changes in hydrostatic turgor pressure within the cell. Previous studies (3) have shown that after treatment with monocaprin, which is the most active lipid against GBS, the plasma membranes of GBS are no longer visible, indicating their disintegration by the lipid. Also, no changes were detectable in the structure of the bacterial cell wall. There is a distinct difference between the activity profiles of lipids for C. albicans and GBS, since in contrast to C. albicans, monocaprin is both fast and effective in killing GBS. This can possibly be explained by differences of peptidoglycan polymers in the GBS cell wall on one hand and microfibrillar polymers of β-glucans and chitin of the C. albicans cell wall on the other.
Twofold dilutions of capric acid, lauric acid, and monocaprin from 0.15 to 10 mM concentrations were tested for cytotoxicity in monolayers of A-549 cells, a hypotriploid human epithelial cell line (ATCC CCL-185). The cells were grown in 96-well microtiter plates (Nunclon) in RPMI 1640 medium (GIBCO) containing 12% (vol/vol) heat-inactivated fetal calf serum, 2 mM l-glutamine, 45 mM sodium bicarbonate, and 0.05 mg of gentamicin per ml until the cells had formed a confluent monolayer. The lipids were diluted in broth. One hundred microliters of each lipid dilution was added to four wells, and cell viability was determined in two wells after 10 and 30 min, respectively, at 37°C by trypan blue exclusion.
The lowest concentrations of monocaprin causing complete lysis of the cell layers were 1.25 mM in 10 min and 0.6 mM in 30 min. The lowest concentration of lauric acid causing complete lysis of the cells in 10 and 30 min was 2.5 mM. Capric acid showed the same effect at 5 mM concentration in 10 min and 2.5 mM in 30 min. Even if lipids are toxic in cell cultures, studies have shown that they are not toxic to skin and mucosa at much higher concentrations. Thus, hydrogels containing monocaprin at a concentration of 5 mg/ml (20 mM) have been shown not to cause irritation of the vaginal mucosas of mice and rabbits (13) or the skin of hairless mice (9).
In summary, the results show that both capric and lauric acids are active in killing C. albicans and may therefore be useful for treatment of infections caused by that pathogen or others that infect the skin and mucosa, possibly in conjunction with antibiotic therapy over a longer period of time.
Acknowledgments
This work was supported by a grant from the Research Fund of the University of Iceland and the Icelandic Research Council.
We thank Sigfús M. Karlsson and Erla Sigvaldadóttir for their help in this work.
REFERENCES
- 1.Bergsson G, Arnfinnsson J, Karlsson S M, Steingrímsson Ó, Thormar H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1998;42:2290–2294. doi: 10.1128/aac.42.9.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsson G, Steingrímsson Ó, Thormar H. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob Agents Chemother. 1999;43:2790–2792. doi: 10.1128/aac.43.11.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsson, G., J. Arnfinnsson, Ó. Steingrímsson, and H. Thormar. Killing of gram-positive cocci by fatty acids and monoglycerides. APMIS, in press. [DOI] [PubMed]
- 4.Isaacs C E, Kim K S, Thormar H. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann N Y Acad Sci. 1994;724:457–464. doi: 10.1111/j.1749-6632.1994.tb38947.x. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs C E, Litov R E, Thormar H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 6.Kabara J J, Swieczkowski D M, Conley A J, Truant J P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabara J J. Fatty acids and derivatives as antimicrobial agents. In: Kabara J J, editor. The pharmacological effect of lipids. St. Louis, Mo: American Oil Chemists Society; 1978. pp. 1–14. [Google Scholar]
- 8.Kristmundsdóttir T, Árnadóttir S, Bergsson G, Thormar H. Development and evaluation of microbicidal hydrogels containing monoglyceride as the active ingredient. J Pharm Sci. 1999;88:1011–1015. doi: 10.1021/js9900396. [DOI] [PubMed] [Google Scholar]
- 9.Neyts J, Kristmundsdóttir T, De Clercq E, Thormar H. Hydrogels containing monocaprin prevent intravaginal and intracutaneous infections with HSV-2 in mice: impact on the search for vaginal microbicides. J Med Virol. 2000;61:107–110. doi: 10.1002/(sici)1096-9071(200005)61:1<107::aid-jmv17>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Ryan K J. John C. Sherris (ed.), Medical microbiology, an introduction to infectious diseases. 2nd ed. New York, N.Y: Elsevier Science Publishing Co., Inc.; 1990. Candida and other opportunistic fungi; pp. 651–657. [Google Scholar]
- 11.Shibasaki I, Kato N. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria. In: Kabara J J, editor. The pharmacological effects of lipids. St. Louis, Mo: American Oil Chemists Society; 1978. pp. 15–24. [Google Scholar]
- 12.Thormar H, Isaacs C E, Brown H R, Barshatzky M R, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thormar H, Bergsson G, Gunnarsson E, Georgsson G, Witvrouw M, Steingrímsson Ó, De Clercq E, Kristmundsdóttir T. Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex Transm Infect. 1999;75:181–185. doi: 10.1136/sti.75.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh J K, Arsenakis M, Coelen R J, May J T. Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis. 1979;140:322–328. doi: 10.1093/infdis/140.3.322. [DOI] [PubMed] [Google Scholar]