Abstract
Background
The present study aimed to evaluate the safety and prophylactic efficacy of add-on Comprehensive Ayurveda and mindfulness-based Yoga (CAY) regimen to standard care among HealthCare Workers (HCWs) against COVID-19.
Materials and methods
This prospective single-blind (outcome assessor-blinded) RCT was conducted in tertiary care hospital in Delhi during July 2020–April 2021. HCWs of both sexes were randomized to add-on CAY intervention or control group. The primary outcomes were the incidence of confirmed COVID-19 positive cases and influenza-like illness events (ILI). Secondary outcomes were anxiety (GAD-7), depression (PHQ-9), and quality of life (SF-36) at the end of 12 weeks.
Results
Three hundred fifty-six participants (181 in intervention and 175 in the control group) were randomized. With the modified intention to treat approach, we analyzed 309 participants. The mean age for the intervention and control group was 39.3 ± 10.1 and 36.6 ± 10 years, respectively. Incidence of COVID-19 event was higher in control group compared to CAY group (16 of 164 [9.8%] vs. 11 of 145 [7.6%]; P = 0.50). The incidence of ILI events was also higher in the control group as compared to the CAY group (14 of 164 [8.5%] vs 9 of 145 [6.2%]). The health change domain of the SF-36 questionnaire showed statistically significant improvement in the CAY group as compared to the control group (P < 0.01).
Conclusion
Incidence of COVID-19 and ILI events was lower in the CAY group compared with the contr ol group, though the difference is not statistically significant.
Keywords: Ayurveda, Yoga, COVID-19, Anxiety, RCT, Complementary therapy
1. Introduction
Coronavirus disease 2019 (COVID-19) has quickly spread worldwide since its origin in Wuhan, China. According to the World Health Organisation (WHO) data, there were 404,910,528 confirmed cases globally by the end of February 11, 2022, resulting in 5,783,776 deaths [1]. COVID-19 is caused by the infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in pulmonary, cardiovascular, neuropsychiatric and dermatologic complications such as dyspnoea, pneumonia, arrythmia, brain fog, and skin rash [2].
There have been global vaccination campaigns with a total of 10,095,615,243 vaccine doses administered by February 6, 2022 to control the spread of COVID-19 [1]. However, there are several challenges related to vaccine production scale, pricing, and affordability for broader outreach to remote areas [3]. Therefore, preventive measures are still emphasized and relied upon, notably since newer strains of SARS-CoV-2 have been reported in different regions of the world. Across the world, healthcare workers were provided with vaccination with a priority, owing to their frequent potential exposures. However, there is uncertainty about whether a vaccine will eliminate the threat due to COVID-19 and its new variants [4].
Several studies have examined pharmacological prophylaxis of COVID-19 and demonstrated the inefficacy of hydroxychloroquine, ivermectin, lopinavir, ritonavir, and ribavirin [5]. Hence, there is great interest worldwide in exploring effective medications to prevent the SARS-CoV-2 transmission.
Recent studies have shown an emerging trend of using Complementary and Alternative Medicine (CAM) as a prophylactic method of containing the SARS-CoV-2 [6,7]. Many pre-clinical and clinical studies have demonstrated the efficacy of CAM in the prevention and management of COVID-19 in India and around the world [[8], [9], [10]]. A meta-analysis of herbal formulations examining their effectiveness against COVID-19 revealed their beneficial effect when combined with modern medicine [11,12].
Ayurveda and yoga are indigenous healthcare systems that have been practiced for thousands of years [13]. There are several Ayurveda herbs with antiviral properties reported in in-silico and randomized controlled trials (RCT), such as Tinospora cordifolia [14], Withania somnifera [15], and Glycyrrhiza glabra [16]. The Ministry of AYUSH, Govt. of India has published preventive measure guidelines for self-care advocating the use of Ayurveda herbal medicines along with advice to adopt yoga-based lifestyle modules as a prophylactic measure against COVID-19 [6]. Several computational studies have shown that specific Ayurveda herbs like guduchi [17] (T. cordifolia) and kalmegh [18] (Andrographis paniculata) exhibit antiviral activity against SARS-CoV-2 proteins. Specifically, in-silico studies have reported active phytoconstituents of T. cordifolia and A. paniculata to be effective against different structural proteins of SARS-CoV-2, e.g., 3CLpro, PLpro, Mpro, RdRp, and spike protein. These proteins are responsible for replication, transcription, and host cell recognition of the virus [19]. T. cordifolia [20] and A. paniculata [21] are reported to be safe in animal and human studies. Clinical and pre-clinical studies using T. cordifolia have shown improvement in depression and anxiety-like behaviour [22]. Yoga and mindfulness have proven to be effective in reducing stress, anxiety, depression and improving quality of life in HCWs [[23], [24]]. Hence, the current study was designed to test the hypothesis whether administering an add-on CAY regimen to standard care among HCWs in a tertiary care hospital during COVID-19 prevent SARS-CoV-2 infection, alleviate psychological stress and improve quality of life.
2. Methods
2.1. Study design
This prospective single-blind, two-arm, parallel-group, randomized controlled trial (CTRI no. - CTRI/2020/06/026151) was conducted by the Centre for Integrative Medicine & Research (CIMR), All India Institute of Medical Sciences (AIIMS), New Delhi, India in collaboration with the Department of Medicine, AIIMS, New Delhi, India and All India Institute of Ayurveda, New Delhi, India. The enrolment began in July 2020 and ended in January 2021, with the last follow-up completed in April 2021. The Institutional Ethics Committee (IEC), AIIMS, New Delhi, India approved the study protocol, (IEC No. 460/22.05.2020, RP-03/2020, OP-12/04.09.2020). The GAD-7, PHQ-9, and SF-36 questionnaires were evaluated by a clinical psychologist at CIMR who was blinded to the allocation of groups.
2.2. Participants
HCWs of both sexes, aged 18–60 years working at AIIMS, New Delhi, India with a risk of COVID-19 exposure were screened telephonically for eligibility. The study included participants with controlled co-morbid conditions receiving ongoing standard medical care. Those with symptoms compatible with COVID-19, active or prior COVID-19 history, and pregnant or lactating women were excluded from the study. Patients with clinically significant uncontrolled endocrine, hepatic, renal, cardiovascular, gastrointestinal, respiratory, haematological, or neurological illnesses were excluded. Participants with additional psychological, familial, sociological, or geographical circumstances that would find compliance with the protocol challenging were also excluded. Participants who were willing to provide written informed consent and follow the trial procedures were enrolled.
2.3. Randomization
Randomization was carried out in blocks of 2, 4, and 6 by an independent statistician who was not involved in the outcome assessment using sequences generated by www.sealedenevelope.com (an online randomization program). Stratification was done based on gender. The eligible participants were randomly allocated to a 1:1 ratio to either the add-on CAY regimen group or the control group (under standard care, i.e., following COVID-19 prophylactic guidelines issued by the Ministry of Health and Family Welfare and Ministry of AYUSH, Govt. of India) for 12 weeks. Allocation concealment was done with a serially numbered opaque sealed envelope. The envelopes were maintained by a person who was not a part of the study.
2.4. Sample size
Primary outcome measure of the current RCT was to assess the prophylactic effect of the intervention on the event rate of COVID-19 in HCWs. A study conducted by Yeung et al. [25] reported the number of probable cases of SARS in HCWs as 21% globally. Another study protocol by Sylvain et al. [26] assumed that prophylactic or pre-emptive therapy would result in a 50% risk reduction of infection from SARS-CoV-2.
Considering the above studies, we assumed the COVID-19 incidence rate to be 21% in standard care group and at least 50% risk reduction in primary outcome measure, a sample size of 406 (203 in each group) was calculated with a two-sided α of 0.05, 80% power, and 1:1 allocation ratio. A sample size of 452 (226 in each group) was finalized with a dropout rate of 10%.
2.5. Details of intervention
Participants in the intervention group received an add-on CAY regimen for 12 weeks. The Ayurveda intervention included samshamni vati (two 250 mg tablets) and kalmegh vati (two 250 mg tablets) given twice daily after food. A specifically designed yoga module of 30 min duration consisted of preparatory loosening and breathing practices, asana (physical posture), pranayama (breathing techniques), and deep relaxation technique were administered virtually by the institutionally qualified yoga therapists. The yoga module was primarily developed by the yoga physicians at the study site after a thorough review of traditional and contemporary literature to cater the needs of HCWs to combat COVID-19 related risk factors. Subsequently, the module was sent to fifteen yoga experts across the nation for content validation. C. H. Lawshe's method was used for content validation by the statistician. The yoga practices with a cut-off value of ≥0.49 were retained, and the rest were removed. We incorporated the suggestions from the yoga experts, and after a thorough discussion, the yoga module was finalized (Table 1 ). A total of sixteen online yoga sessions were conducted, i.e., five sessions during the first week of recruitment and one session each week from the second to the twelfth week. In addition, participants were provided with a video recording of the complete yoga module and a booklet containing detailed instructions with visual demonstration of yoga practices to encourage daily yoga practices at home. All randomized intervention group participants were given a logbook to record their compliance and adherence to the intervention, viz., details about Ayurveda medicines intake and yoga practice. In the intervention group, both drugs were administered from the date of randomization until the clinical event (i.e., COVID-19 confirmed with RT-PCR) or study completion, whichever occurred earlier. Participants were instructed to take medicines and practice yoga at the same time each day. Participants of both the groups were counselled and advised to follow the guidelines issued by the Ministry of Health and Family Welfare, Govt. of India, and to continue with the regimen (if any) prescribed by the consulting physician for the prevention of COVID-19.
Table 1.
Validated yoga module followed by the intervention group.
| S. No. | Practice | No. of Rounds/Timing |
|---|---|---|
| 1. | Breath awareness | Few cycles |
| 2. | Breathing practices: | 3 rounds each (5 min) |
| · Hands in and out breathing | ||
| · Hand stretch breathing | ||
| · Ankle stretch breathing | ||
| 3. | Preparatory practices: | 3 rounds each (5 min) |
| · Neck rotation | ||
| · Shoulder rotation | ||
| · Lumbar stretch | ||
| 4. | Asana | 1 round each (7 min) |
| · Tadasana | ||
| · Trikonasana | ||
| · Vakrasana | ||
| · Setubandhasana | ||
| · Bhujangasana | ||
| 5. | Pranayama | 5 min |
| · Nadishuddhi pranayama | ||
| · Kapalbhati pranayama | ||
| · Bhramari pranayama | ||
| 6. | Relaxation · Deep relaxation technique (modified yoga nidra) |
8 min |
2.6. Study assessment
Weekly telephonic review was scheduled for 12 weeks which included an inquiry about the incidence of COVID-19 compatible symptoms or confirmed COVID-19, and ILI symptoms, e.g., fever, cough, cold. In addition, adherence to medication and yoga, other additional physical exercises/medicine/home remedies, and side effects from the medications were also recorded over the telephone.
2.7. Outcome assessment
2.7.1. Primary outcome
The primary outcome was the incidence of COVID-19 (confirmed by RT-PCR) and the number of ILI events like fever, cough, cold, and sore throat among the enrolled participants during the 12 weeks by self-report.
2.7.2. Secondary outcome
-
1.
Generalized Anxiety disorder (GAD-7): GAD-7 was used to assess anxiety symptoms of individuals in the past 2 weeks at baseline and after 12 weeks. It is a 7-item scale, and the response lies on a four-point Likert scale ranging from 0 (not at all) to 3 (nearly every day). The overall score ranges from 0 to 21, with a higher score indicating more symptom severity. The scale has good reliability, criterion, construct, factorial and procedural validity [[27], [28], [29]].
-
2.
Patient health questionnaire (PHQ-9): PHQ-9 was used to assess depressive symptoms of participants in past 2 weeks at baseline and after 12 weeks. It is a 9-item scale and has a four-point Likert scale ranging from 0 to 3. The total score ranges from 0 to 27, with a higher score indicating more severe symptoms. The scale has good internal consistency, test-retest reliability, construct and criterion validity [30,31].
-
3.
Health-related quality of life using short-form survey (SF-36): The SF-36 was used to evaluate health-related quality of life and has 36-items. The scale has eight subscales (physical function, role limitations-physical, bodily pain, general health, vitality, social function, role limitations-emotional and mental health). The score ranges from 0 (worst condition) to 100 (best condition) for each sub-scale, with a higher score indicating better health. The SF-36 is shown to have high reliability and validity [31,32].
2.8. Statistical analysis
Continuous variables were reported as mean ± SD, and categorical variables as frequency with percentage. For continuous outcomes within a group, pre-post comparisons were made by paired t-test, and for between-group comparisons, two sample t-test was used to compare outcomes at baseline and follow up. Chi-square or Fisher's exact test was used to compare categorical variables between two study groups. Log-rank test was used to compare the COVID-19 incidence between the study groups, and the cox proportional hazard model was also used to compare the age-adjusted incidence. All comparisons were two-sided. P < 0.05 was set as the cut-off of statistical significance. G-power was used to calculate the sample size and post-hoc power calculation. Stata version 14.1 was used for statistical analysis.
2.9. Premature discontinuation of the trial
We planned to recruit 452 participants within six months of the study initiation; however, the Covaxin phase III trial by Bharat Biotech began at the study site in November 2020, which led to a reduction in the recruitment rate of the HCWs in the trial. Consequently, enrollment ceased due to recruitment failure, with continued follow-up for those already enrolled. Accordingly, enrolment was suspended in January 2021, and outcome data of the enrolled participants was collected till April 2021.
3. Results & discussion
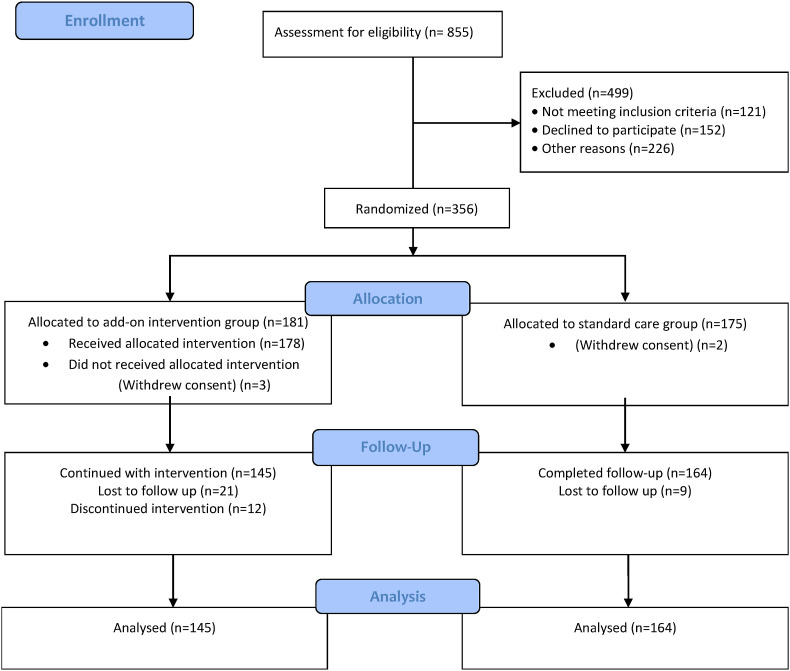
The present study is a randomized, controlled, single-blind (outcome assessor-blinded) trial evaluating the effect of add-on CAY as pre-exposure prophylaxis against COVID-19 in HCWs of a tertiary care hospital in India. A total of 855 HCWs were screened from July 2020 to January 2021, and 357 were enrolled and randomized to either the intervention or control group. Post randomization, 182 participants were in the intervention group, and 175 participants were in the control group. After considering loss to follow-ups and discontinuation from the intervention, 145 participants in the intervention group and 164 in the control group were analyzed for primary outcome. Participant flow and follow-ups have been described using a CONSORT diagram (Fig. 1 ). With a modified intention to treat approach, we analyzed 309 participants. Table 2 summarizes the socio-demographic data of the participants. The mean age of the study participants in the intervention group was 39.4 ± 10.1 years, whereas, in the control group, it was 36.6 ± 10 years. The HCWs enrolled were predominantly males (54.7%). Out of the participants who reported pre-existing co-morbid conditions, 2.7% reported diabetes, while 10.6% reported hypertension.
Fig. 1.
CONSORT diagram depicting the flow of participants and follow-ups.
Table 2.
Socio-demographic characterizes of the participants in both the groups.
| Characteristics | Control (n = 164) | Intervention (n = 145) | P value |
|---|---|---|---|
| Age (in yrs) mean ± S.D. | 36.6 ± 10 | 39.3 ± 10.1 | 0.01* |
| Gender: Male n (%) | 93 (56.7) | 76 (52.4) | 0.44 |
| Comorbidities | |||
| - Hypertension | 16 | 12 | 0.62 |
| - Other cardiovascular disorders | 01 | 02 | 0.50 |
| - Type 2 Diabetes Mellitus | 04 | 08 | 0.19 |
| - Bronchial Asthma | 0 | 01 | 0.29 |
| Renal disease | 01 | 0 | 0.33 |
| Cancer | 01 | 0 | 0.33 |
3.1. Primary outcomes
COVID-19 positivity rate was 7.6% (11 out of 145 participants) and 9.8% (16 out of 164 participants) in the intervention group and control group respectively (HR = 0.99; 95% C.I. 0.79–1.24, P = 0.96). After adjusting age at baseline, the model gives, HR = 0.99; 95% C.I. 0.78–1.24, P = 0.94. A post-hoc power calculation was also performed using G-power [33]. For the primary outcome, the achieved power was 20.7% at the two-sided alpha of 5%. At the end of the 12 weeks, there were more positive participants in the control group (n = 20) compared with the intervention group (n = 12), but the difference was not statistically significant (P = 0.50) (Table 3 ). The participants who developed symptoms were advised for home quarantine and followed the standard COVID-19 treatment guidelines as per the consulting physician of the Department of Medicine, AIIMS, New Delhi, India.
Table 3.
Comparison of primary outcomes across two study groups.
| Characteristics | Control n (%) | Intervention n (%) |
|---|---|---|
| Covid-19 positive cases | 16 (9.8) | 11 (7.6) |
| Influenza like Illness symptoms | 14 (8.5) | 09 (6.2) |
Out of the 11 COVID-19 positive participants in the intervention group, one had a history of bronchial asthma (controlled) for eight years, and one had been suffering from hypertension for three years. Among the control group participants, three were known hypertensive (controlled and on medication), and one had hypothyroidism.
Lower incidence of ILI symptoms was observed in the intervention group (n = 9) compared to the control group (n = 14). Out of the nine participants who had ILI symptoms, two participants had hypertension, two suffered from diabetes mellitus, and one had bronchial asthma. Among the control group participants, one had hypertension, and the other had cancer. All these participants had controlled illness and were on medication. However, no statistically significant difference was found between the control group (8.5%) and the intervention group (6.2%) concerning the incidence of ILI symptoms (P = 0.43) (Table 3). The reasons for no significant effect observed may be related to inadequate power due to unexpected termination. Similar challenges were also observed in other pre-exposure prophylaxis trials, where the trials were prematurely ended due to incomplete recruitment or futility after interim analysis [34,35]. However, it is noteworthy that CAY administered patients had fewer cases of COVID-19 and ILIs than those who received standard care alone.
The results of our study differ from those reported in recent RCTs assessing the prophylactic effect of herbal/polyherbal formulations against COVID-19. In contrast to our study duration of 12 weeks, these studies had assessed the effect of the intervention for one month only, i.e., 30 days [36,37]. Studies have evaluated the effect of chyavanprash (an Ayurveda polyherbal formulation) vs standard care in HCWs and reported no COVID-19 cases in both the groups [36]. Another double-blind placebo-controlled RCT assessed the use of Neem (Azadirachta indica) capsules for 30 days and found a significant difference in the number of COVID-19 positive cases in the two groups [37]. Our study was the first attempt to demonstrate the prophylactic effect of the CAY intervention for a longer duration, i.e., twelve weeks. Treatment duration is an essential factor in assessing the prophylactic effect of the intervention [38]. Especially in the case of HCWs, as they are at sustained high risk of contracting COVID-19 [39]. With a longer duration, the incidence of COVID-19 positive and ILI events did not show a statistically significant difference (though the number of positive cases was higher in the control group).
3.2. Secondary outcomes
Among the secondary outcomes, scores of PHQ-9, and GAD-7 at baseline in both the groups indicated that the participants were under subthreshold depression (score 0–4 indicate minimal/no depression) and anxiety (score 0–4 indicate minimal/no anxiety). HCWs with minimal anxiety and depression are more likely to be motivated to learn coping skills and adapt to the newer interventions [40]. After 12 weeks, scores of GAD-7, PHQ-9, and emotional well-being (assessed in SF-36) showed statistically significant difference (within-group) in both intervention (P < 0.01) and control (P < 0.01) group and the percentage improvement is higher in the intervention group (Table 4 ). Previous studies have reported the efficacy of yoga in reducing depression and anxiety [41]. Significant improvement in the GAD-7 and PHQ-9 in the control group may be due to the younger age group of the participants and increased awareness about physical and mental health in this pandemic. The 'health change' domain of the SF-36 showed a statistically significant improvement in the intervention group compared to the control group. This domain captures perceived health improvement within the previous year. The result showed a ‘self-rated’ improvement from the baseline scores after 12 weeks of intervention.
Table 4.
Score comparison of PHQ-9, GAD-7 and domains of SF-36 across the two study groups.
| Outcome | Timeline | Control | Intervention |
P-value (b/w group) |
P-value (within group) Baseline vs 12 week |
|
|---|---|---|---|---|---|---|
| P1 | P2 control | P3 intervention | ||||
| Patient Health Questionnaire −9 | Baseline | 2.5 ± 2.5 | 3 ± 2.5 | 0.15 | <0.01* | <0.01* |
| 12-Week | 1.8 ± 2 | 1.8 ± 2 | 0.97 | |||
| Generalized Anxiety Disorder Questionnaire - 7 | Baseline | 3.4 ± 2.8 | 3.8 ± 3 | 0.32 | <0.01* | <0.01* |
| 12-Week | 2 ± 2.3 | 2.2 ± 2.4 | 0.54 | |||
| Physical functioning | Baseline | 92.3 ± 10.4 | 93.4 ± 9.2 | 0.34 | 0.15 | 0.24 |
| 12-Week | 94.1 ± 9.7 | 94.7 ± 11.1 | 0.65 | |||
| Role limitations due to physical health | Baseline | 95.5 ± 12.8 | 96.8 ± 12.3 | 0.37 | 0.99 | 0.55 |
| 12-Week | 96.2 ± 11.3 | 97.3 ± 10.1 | 0.42 | |||
| Role limitations due to emotional problems | Baseline | 96.7 ± 11.9 | 97 ± 11 | 0.79 | 0.61 | 0.52 |
| 12-Week | 97.3 ± 11.5 | 98.2 ± 9.7 | 0.53 | |||
| Energy/fatigue | Baseline | 68.1 ± 15.2 | 67.8 ± 16.8 | 0.86 | <0.01* | <0.01* |
| 12-Week | 74.4 ± 15.3 | 74.3 ± 15.5 | 0.99 | |||
| Emotional well-being | Baseline | 76.6 ± 12.1 | 75.8 ± 13.3 | 0.91 | <0.01* | <0.01* |
| 12-Week | 80.9 ± 10.5 | 82.9 ± 11.9 | 0.16 | |||
| Social functioning | Baseline | 92 ± 11.2 | 91.1 ± 13.3 | 0.51 | <0.01* | 0.06 |
| 12-Week | 96 ± 8.7 | 94.8 ± 9.1 | 0.30 | |||
| Pain | Baseline | 88 ± 18.4 | 86.7 ± 19.9 | 0.56 | 0.03* | 0.83 |
| 12-Week | 91.3 ± 16.3 | 87.7 ± 18.4 | 0.10 | |||
| General health | Baseline | 74.8 ± 16.6 | 72.7 ± 17.5 | 0.29 | 0.14 | 0.17 |
| 12-Week | 77.6 ± 16.5 | 76.8 ± 17.3 | 0.71 | |||
| Health change | Baseline | 50 ± 15.4 | 50.7 ± 16.4 | 0.69 | 0.70 | <0.01* |
| 12-Week | 51.2 ± 14.6 | 59.4 ± 18 | <0.01 | |||
P1 = calculated P value compared to intervention and control group; P2 = calculated P value within the control group; P3 = calculated P value within the intervention group.
3.3. Adherence to the medication and adverse events
Most of the participants (60%) adhered to the protocol, attending at least 33% of the online yoga sessions and consuming at least 50% of the prescribed Ayurveda herbal drugs. The intervention group participants reported a few adverse events such as skin rashes, abdominal discomfort, acid reflux, and mild hypoglycemia episodes after taking kalmegh tablets. The symptoms were of mild to moderate grade and subsided after discontinuation of the medication within 2–3 days. All the side effects were minor. However, no participant in the study experienced serious adverse events on the Common Toxicity Criteria for Adverse Events (CTCAE) scale, hospitalizations, or death.
3.4. Need for hospitalization
Only one participant in the intervention gorup with COVID-19 required hospitalization. Rest of the COVID-19 positive participants were asymptomatic or had mild to moderate symptoms and had complete recovery.
4. Limitations
Our study has several limitations. First, the study was an outcome assessor-blinded trial with standard control without any placebo for the intervention. Second, the dropout rate of intervention group was 19%. Since the study centre was a tertiary care hospital, the high workload of HCWs associated with COVID-19 cases might have led to the discontinuation of the structured yoga protocol/Ayurveda treatment for 12 weeks. Third, any prophylaxis trial is directly related to disease frequency. A decline in the number of active cases after the first wave of the pandemic during the study and the initiation of the vaccination drive at AIIMS, New Delhi, India in November 2020 led to the unexpected termination of the trial [42]. This left the trial underpowered. Moreover, the study hospital was a tertiary care facility located in New Delhi; thus, it might not adequately represent COVID-19 prevalence and exposure risk in other regions of India.
5. Conclusion
Compared to the intervention group, more participants in the control group had COVID-19 during the study duration, though the difference is statistically insignificant. CAY intervention showed significant improvement in GAD-7, PHQ -9, and several domains of the SF-36 questionnaire. However, further RCTs are required to validate the effect of comprehensive Ayurveda and yoga interventions against SARS-CoV-2 infection among HCWs.
Author disclosure statement
No competing interests exist.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
Vandna Sharma: Conceptualization, Writing - original draft, Methodology, Writing - review & editing; Bharat Krushna Khuntia: Conceptualization, Writing - original draft, Methodology, Writing - review & editing; Manish Soneja: Writing - review & editing, Supervision; Vitthal G Huddar: Resources, Writing - review & editing; S Ramakrishnan: Writing - review & editing, Resources; Payal Sharma: Methodology, Writing - review & editing, Validation; Shubhangi Rathore: Investigation, Writing - review & editing; Varun Valliappan: Resources, Writing - review & editing; Mohit Wadhawan: Resources, Writing - review & editing; Varun Chhabra: Resources, Writing - review & editing; Aman Agarwal: Formal analysis, Writing - review & editing; Mansingh Jat: Resources; Arvind Kumar: Resources; Tanuja Manoj Nesari: Resources, Supervision Gautam Sharma: Conceptualization, Writing, review and editing, Supervision, Project administration.
Declaration of competing interest
None.
Acknowledgement
The authors would like to thank the nursing officers, yoga instructors, and all non-technical staff of the Center for Integrative Medicine and Research (CIMR), AIIMS, New Delhi, India for their contribution to the conduct of the trial. The authors like to acknowledge the All India Institute of Ayurveda, New Delhi, for their support throughout the study. We also acknowledge the Ministry of AYUSH, Govt. of India, for their support through the Center of Excellence grant for Yoga and Ayurveda to the CIMR, AIIMS, New Delhi, India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctcp.2022.101601.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.W.C. (COVID-19) Dashboard, WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data, (n.d.).
- 2.Desai A.D., Lavelle M., Boursiquot B.C., Wan E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022;322:C1–C11. doi: 10.1152/AJPCELL.00375.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Heal. Policy Www.Thelancet.Com. 2021;397 doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellet J., Pepper M.S. A covid-19 vaccine: big strides come with big challenges. Vaccines. 2021;9:1–14. doi: 10.3390/vaccines9010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez V.G., Salas A.A., Ballestín S.S. Antiviral therapeutic approaches for sars-cov-2 infection: a systematic review. Pharmaceuticals. 2021;14 doi: 10.3390/ph14080736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushell W., Castle R., Williams M.A., Brouwer K.C., Tanzi R.E., Chopra D., Mills P.J. Meditation and yoga practices as potential adjunctive treatment of SARS-CoV-2 infection and COVID-19: A Brief Overview of Key Subjects. 2020. 26, 547-556. [DOI] [PubMed]
- 7.Nandan A., Tiwari S., Sharma V. Exploring alternative medicine options for the prevention or treatment of coronavirus disease 2019 (COVID-19) - a systematic scoping review. medRxiv. 2020:1–40. [Google Scholar]
- 8.Charan J., Bhardwaj P., Dutta S., Kaur R. Use of complementary and alternative medicine (CAM) and home remedies by COVID-19 patients : a telephonic survey. Indian J. Clin. Biochem. 2021;36:108–111. doi: 10.1007/s12291-020-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira D., Prieto-Garcia J.M., Boylan F., Estrada O., Fonseca-Bazzo Y.M., Jamal C.M., Magalhães P.O., Pereira E.O., Tomczyk M., Heinrich M. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020;11:1–44. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of Ayurveda and yoga for COVID-19 prophylaxis. J. Alternative Compl. Med. 2020;26:360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- 11.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 2020;9:1–20. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan A.Y., Gu S., Alemi S.F. Research Group for Evidence-based Chinese Medicine, Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J. Integr. Med. 2020;18:385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipton C.M. 2021. Susruta of India , an Unrecognized Contributor to the History of Exercise Physiology; pp. 1553–1556. [DOI] [PubMed] [Google Scholar]
- 14.Jena S., Munusami P., Mm B., Chanda K. Computationally approached inhibition potential of Tinospora cordifolia towards COVID-19 targets. VirusDisease. 2021;32:65–77. doi: 10.1007/s13337-021-00666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashyap V.K., Dhasmana A., Yallapu M.M., Chauhan S.C. Withania somnifera as a potential future drug molecule for. COVID-19. 2020;2:7–10. doi: 10.4155/fdd-2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava V., Yadav A., Sarkar P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater. Today Proc. 2020 doi: 10.1016/j.matpr.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krupanidhi S., Peele K.A., Venkateswarulu T.C., Ayyagari V.S., Bobby N., Babu D.J., Narayana A.V., Aishwarya G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2 : an in silico study inhibitory activity on SARS-CoV-2 : an in silico study. J. Biomol. Struct. Dyn. 2020:1–5. doi: 10.1080/07391102.2020.1787226. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukardiman M. Ervina, Pratama M.R.F., Poerwono H., Siswodihardjo S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Ness. J. Adv. Pharm. Technol. Res. 2020;11:157–162. doi: 10.4103/japtr.JAPTR_84_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan N.A., Pandian C.J., Jeyakanthan J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J. Biomol. Struct. Dyn. 2021;39:4415–4426. doi: 10.1080/07391102.2020.1777901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alajmi M.F., Mothana R.A., Al-rehaily A.J., Khaled J.M. 2018. Antimycobacterial Activity and Safety Profile Assessment of Alpinia Galanga and Tinospora Cordifolia; p. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mag P., Singh D.P., Awasthi H., Luqman S., Singh S., Mani D. Tinospora Cordifolia and Solanum Nigrum Against Paracetamol Induced Hepatotoxicity; 2015. Hepatoprotective Effect of A Polyherbal Extract Containing Andrographis Paniculata; pp. 375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra R., Manchanda S., Gupta M., Kaur T., Saini V. Tinospora cordifolia ameliorates anxiety-like behavior and improves cognitive functions in acute sleep deprived rats. Nat. Publ. Gr. 2016:1–15. doi: 10.1038/srep25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cocchiara R.A., Peruzzo M., Mannocci A., Ottolenghi L., Villari P., Polimeni A., Guerra F., La Torre G. 2019. The Use of Yoga to Manage Stress and Burnout in Healthcare Workers : A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Torre G., Raffone A., Peruzzo M., Calabrese L., Cocchiara R.A., D'Egidio V., Leggieri P.F., Dorelli B., Zaffina S., Mannocci A., Brocca F., Cianfanelli S., Dalmasso G., Giannandrea A., Tasco S. Yoga and mindfulness as a tool for influencing affectivity, anxiety, mental health, and stress among healthcare workers: results of a single-arm clinical trial. J. Clin. Med. 2020;9:1–13. doi: 10.3390/jcm9041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan-Yeung M. Severe acute respiratory syndrome (SARS) and healthcare workers. Int. J. Occup. Environ. Health. 2004;10:421–427. doi: 10.1179/oeh.2004.10.4.421. [DOI] [PubMed] [Google Scholar]
- 26.Lother S.A., Abassi M., Agostinis A., Bangdiwala A.S., Cheng M.P., Drobot G., Engen N., Hullsiek K.H., Kelly L.E., Lee T.C., Lofgren S.M., MacKenzie L.J., Marten N., McDonald E.G., Okafor E.C., Pastick K.A., Pullen M.F., Rajasingham R., Schwartz I., Skipper C.P., Turgeon A.F., Zarychanski R., Boulware D.R. Post-exposure prophylaxis or pre-emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): study protocol for a pragmatic randomized-controlled trial. Can. J. Anesth. 2020;67:1201–1211. doi: 10.1007/s12630-020-01684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer R.L., Kroenke K., Williams J.B.W., Lo B. A Brief Measure for Assessing Generalized Anxiety Disorder. Arch. Intern. Med. 2022;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 29.De Man J., Absetz P., Sathish T., Desloge A., Haregu T., Oldenburg B., Johnson L.C.M., Thankappan K.R., Williams E.D. Are the PHQ-9 and GAD-7 suitable for use in India? A Psychometric Analysis. Front. Psychol. 2021;12:1–14. doi: 10.3389/fpsyg.2021.676398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.K. Kroenke, R.L. Spitzer, J.B.W. Williams, The PHQ-9, 46202 (n.d.) 606–613. [DOI] [PMC free article] [PubMed]
- 31.Brazier E., Harper R., Jones N.M.B., Cathain A.O., Thomas K.J., Usherwood T., Westlake L. General practice Validating the SF-36 health survey questionnaire : new outcome. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha R., Van Den Heuvel W.J.A., Arokiasamy P. Validity and reliability of MOS short form health survey (SF-36) for use in India. 2013. 38, 22-26. [DOI] [PMC free article] [PubMed]
- 33.Erdfelder E., Faul F., Buchner A. GPOWER: a general power analysis program. Behav. Res. Methods Instrum. Comput. 1996;28:1–11. doi: 10.3758/BF03203630. [DOI] [Google Scholar]
- 34.Rajasingham R., Bangdiwala A.S., Nicol M.R., Skipper C.P., Pastick K.A., Axelrod M.L., Pullen M.F., Nascene A.A., Williams D.A., Engen N.W., Okafor E.C., Rini B.I., Mayer I.A., Mcdonald E.G., Lee T.C., Li P., Mackenzie L.J., Balko J.M., Dunlop S.J., Hullsiek K.H., Boulware D.R., Lofgren S.M., Abassi M., Balster A., Collins L.B., Drobot G., Krakower D.S., Lother S.A., Mackay D.S., Meyer-Mueller C., Selinsky S., Solvason D., Zarychanski R., Zash R. Hydroxychloroquine as pre-exposure prophylaxis for coronavirus disease 2019 (COVID-19) in healthcare workers: a randomized trial. Clin. Infect. Dis. 2021;72:E835–E843. doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/nejmoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta N.S.A., Madan A., Yadav B., Mundada P., Singhal R., Pandey Y., Agarwal R., Tripathi A., Rana R., Sharma B.S., Rao B.C.S., Bharti Chyawanprash for the prevention of COVID-19 infection among healthcare workers: A Randomized Controlled Trial. BMJ. 2021:1–21. [Google Scholar]
- 37.Nesari T.M., Bhardwaj A., ShriKrishna R., Ruknuddin G., Ghildiyal S., Das A., Pandey A.K., Chaudhary N., Soman G., Barde M. Neem (azadirachta indica A. Juss) capsules for prophylaxis of COVID-19 infection: a pilot, double-blind, randomized controlled trial. Alternative Ther. Health Med. 2021 http://www.ncbi.nlm.nih.gov/pubmed/33891569 [PubMed] [Google Scholar]
- 38.Colditz G.A., Taylor P.R. Prevention trials: their place in how we understand the value of prevention strategies. Annu. Rev. Publ. Health. 2010;31:105–120. doi: 10.1146/annurev.publhealth.121208.131051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poletti P., Tirani M., Cereda D., Guzzetta G., Trentini F., Marziano V., Toso C., Piatti A., Piccarreta R., Melegaro A., Andreassi A., Gramegna M., Ajelli M., Merler S. Seroprevalence of and risk factors associated with SARS-CoV-2 infection in health care workers during the early COVID-19 pandemic in Italy. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang L., Ma S., Chen M., Yang J., Wang Y., Li R., Yao L., Bai H., Cai Z., Xiang Yang B., Hu S., Zhang K., Wang G., Ma C., Liu Z. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav. Immun. 2020;87:11–17. doi: 10.1016/j.bbi.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Manincor M., Bensoussan A., Smith C.A., Barr K., Schweickle M., Donoghoe L.-L., Bourchier S., Fahey P. Individualized yoga for reducing depression and anxiety, and improving well-being: a randomized controlled trial. Depress. Anxiety. 2016;33:816–828. doi: 10.1002/da.22502. [DOI] [PubMed] [Google Scholar]
- 42.Kumar V.M., Pandi-Perumal S.R., Trakht I., Thyagarajan S.P. Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. Npj Vaccines. 2021;6 doi: 10.1038/s41541-021-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.