Abstract
Randomised controlled trials (RCTs) usually provide the best evidence for treatments and management. Historically, older people have often been excluded from clinical medication trials due to age, multimorbidity and disabilities. The situation is improving, but still the external validity of many trials may be questioned. Individuals participating in trials are generally less complex than many patients seen in geriatric clinics.
Recruitment and retention of older participants are particular challenges in clinical trials. Multiple channels are needed for successful recruitment, and especially individuals experiencing frailty, multimorbidity and disabilities require support to participate. Cognitive decline is common, and often proxies are needed to sign informed consent forms. Older people may fall ill or become tired during the trial, and therefore, special support and empathic study personnel are necessary for the successful retention of participants.
Besides the risk of participants dropping out, several other pitfalls may result in underestimating or overestimating the intervention effects. In nonpharmacological trials, imperfect blinding is often unavoidable. Interventions must be designed intensively and be long enough to reveal differences between the intervention and control groups, as control participants must still receive the best normal care available. Outcome measures should be relevant to older people, sensitive to change and targeted to the specific population in the trial. Missing values in measurements are common and should be accounted for when designing the trial.
Despite the obstacles, RCTs in geriatrics must be promoted. Reliable evidence is needed for the successful treatment, management and care of older people.
Keywords: randomised controlled trial, clinical trial, older people
Key Points
Older people have historically been excluded from drug trials. This hampers external validity of trial findings.
Recruitment and drop-outs of older participants are challenges in clinical trials. Means to prepare for better recruitment and retention are presented in this article.
Some pitfalls may overestimate or underestimate the trial effects such as imperfect blinding, mild intervention, contamination or unsensitive outcome measures.
Introduction
Clinical trials, especially randomised controlled trials (RCTs), provide the best evidence to guide decision making in healthcare. RCTs can effectively eliminate confounding factors (known and unknown) and minimise bias [1].
The first published RCT in medicine was conducted in 1948 to test the effect of streptomycin on pulmonary tuberculosis [2]. By the late 20th century, RCTs were being widely used to prove the efficacy of new treatments, such as new medications.
In geriatrics, RCTs have long been used to test the effectiveness of geriatric care models. Whereas these were established from an early date in the UK, they were mainly tested in a series of RCTs in the USA [3]. Though comprehensive management models were tested in rigorous trials, older patients (75+) were generally excluded from medication trials until recent years.
Exclusion of older people from trials
Despite the fact that older people use the largest share of all medications, they have been clearly underrepresented in clinical trials [4]. This problem has concerned treatments for cancer, cardiovascular disease and many other illnesses [5, 6], albeit international regulatory agencies have provided guidance for conducting clinical drug trials among older adults [7]. Although the inclusion of them in drug trials has lately been increasing [8], trial participants often differ from the patients seen in geriatric clinics: the participants may be fitter and may not suffer from major comorbidities, dementia or functional decline [9]. One recent example was COVID-19 vaccine trials, in which only 1.7% of the study populations were 75+ years, whereas they were the first age group that had to be vaccinated [10].
The exclusion of trial subjects based on age, multimorbidity or functional decline compromises the external validity of trials. This limitation must be accounted for when applying findings to geriatric practice. For example, older people can respond differently than younger patients to therapy [5]. They are more prone to adverse effects and drug–drug interactions. To better assess the risks and benefits to the patients actually using medications, those patients must be represented in trials.
In addition to attitudes or the toxicity of drugs, several obstacles prevent older people from being included more generally in trials [5] (Table 1). The reasons may be individual, practical or administrative. Older people (or their relatives) may hesitate to participate, they may need more time than younger people to be assessed and the logistics and costs of transporting them to the trial site may be challenging for study personnel. Obtaining informed consent may require proxy participation. Older people with multimorbidity issues can present difficulties in defining indication of the drug for administrative bodies and may increase the financial strain on companies testing new drugs [5]. Heterogeneous study participants and dropping out of the study may also threaten internal validity [1].
Table 1.
Challenges and solutions in including older people in clinical trials
| Challenges/problems | Limiting factors | Consequence | Successful strategies to overcome the problem |
|---|---|---|---|
| Exclusion of older people from clinical trials, especially drug trials | Attitudes towards older people limit their participation. Restrictive exclusion criteria (e.g. age, comorbidity, polypharmacy, functional limitations) due to cautions related to potential adverse effects and drug–drug interactions in older people. Older people take more time to assess. Older people need support for transportation and an accessible study site. Higher drop-out rate for older people. Cognitive decline may limit understanding and ability to sign informed consent form. |
The trial participants are limited to younger people, those with one disease/disorder who are healthier and have higher education levels than the background population. Poor external validity of findings, results cannot be generalised to geriatric populations. People with cognitive decline do not participate |
Geriatricians should speak in public for older people. Older people should be involved in the planning phase of the trial. Minimise exclusion criteria: consider a pilot for older people. Pharmacokinetics and dynamics of the study drug should be investigated in older people; have flexible dosing of a new drug resources should be allocated to older participants. Consider oversampling of the oldest participants. Those experiencing cognitive decline should be encouraged to have a proxy with them during the appointment. The proxy may translate essential information to the participant and sign the informed consent form. |
| Recruitment | Older people do not receive or consider invitations due to: problems in literacy, visual or auditory impairments Older people hesitate to participate, belittling the significance of their participation, considering problems related to comorbidities, disabilities and practical problems with participation. Older people’s relatives consider older people’s participation too risky and/or too difficult. |
Too few older people are recruited and/or recruitment takes time. | Plan several recruitment strategies, pilot them and allow time: surveys, announcements in local newspapers, talks in retirement clubs, allow older people to recruit their peers, invitations sent via primary care practices or other healthcare facilities. Foster a good personal relationship with local healthcare professionals to ensure their commitment. Use plain language and large letters in invitation announcements. Monitor recruitment and adapt it accordingly. Develop personal relationships between recruiters and older people/participants. Encourage older people and their relatives to recognise the importance of participation and show them how the trial will ease and support older people’s participation. |
| Retention | Older people become tired during follow up. Control participants are disappointed in being assigned to the control group and tend to drop out. Older people fall ill, are hospitalised and die of various causes. |
The drop-out rates are high among older people and may dilute the findings. | Create a strategy to take care of participants at risk of dropping out. Ensure a pleasant trial experience for all. Consider an attention intervention for the control group. Have consistent study nurses and researcher throughout the trial and nurture their relationship with participants. Be flexible with assessment schedules. Consider alternative study visits: home visits, telephone contacts, proxy responses. Use participants’ peer support and group dynamics to support their commitment. Use supportive telephone contacts and postal cards to retain people in the trial. |
Efficacy or effectiveness?
It is well known that RCTs are often explanatory trials testing efficacy with more-or-less pre-selected participants and done under a carefully controlled research context [11]. For example, the Hypertension in the Very Elderly Trial investigated the effect of intensified antihypertensive treatment on stroke incidence in hypertensive patients over the age of 80 years. Less than 2% of the identified patients were eligible for the trial [9]. The Systolic Blood Pressure Intervention Trial (SPRINT) trial included 34% of identified hypertension patients, but it excluded such patient groups as those with diabetes or dementia, and nursing home residents [12].
Explanatory trials aim to ensure good internal validity for reliable results, which means that the observations accurately reflect what they are intended to measure [1]. If the randomisation procedure is successful, confounding factors are distributed evenly throughout the groups, and the effect of the treatment (independent variable) may be evaluated in terms of its outcome (dependent variable) [1]. RCTs can eliminate selection bias (or ‘healthy user bias’), which is common in observational studies. For example, hormone replacement therapy seemingly had protective effects on cardiovascular outcomes in observational studies until RCTs were conducted that demonstrated the complex pattern of risks and benefits, especially in older women [13].
The problem with a well-designed RCT having good internal validity is its potential for poor real-life applicability if highly selected participants do not represent normal geriatric patients. In addition to RCTs, observational studies are valuable for assessing treatment effectiveness, remembering though that adherence also often differs between patients in trials and in real life. However, one population-based cohort study suggested that particularly for cardiovascular drugs, the RCT results and real-life applicability do not necessarily differ [14].
Pragmatic RCTs test effectiveness in practice with relatively unselected participants and under flexible conditions; in this way, pragmatic RCTs may inform decisions about practice. Pragmatic trials in geriatrics have both pros and cons, which will be further discussed below.
Recruitment and retention
It is a common problem that ‘patients disappear when the trial starts’, even if the target disease or syndrome is common in the general population. Recruitment is always challenging and may need a large survey to screen the target population for individuals with the target syndrome. It is worth using multiple channels to reach potential trial participants, as has been done for studies on the geriatric syndromes of loneliness [15] and frailty [16]. Newspaper/web announcements, approaching people in retirement clubs or at primary care facilities may be helpful.
There are also challenges related to recruiting frail, incompetent people for trials, e.g. in terms of obtaining informed consent from patients with dementia. However, routine measures exist for obtaining informed consent from the closest proxies, who can act as advocates for the would-be participant.
Another issue is how to retain participants during the trial and its follow-up. Older people often become tired or sick and may easily discontinue the trial. Participants in the control group may be disappointed with their assigned role as controls. Good, empathic study nurses performing assessments are worth their weight in gold. Furthermore, if financially feasible the control participants could receive the same intervention after the trial follow-up is over. For those falling ill, it is important that they are supported. In a trial on loneliness, we sent ‘Get well soon’ cards to participants who were hospitalised during the study [17]. Providing peer support is probably the best way to retain older people during the intervention (Table 1).
Randomisation
Randomisation of participants should be performed in an appropriate way not prone to manipulation. Researchers or assessors may, whether consciously or unconsciously, steer participants into either intervention or control groups. This potential pitfall should be inhibited by establishing a separate centre performing randomisation and assigning participants by computer-generated random numbers (Table 2).
Table 2.
Factors threatening the validity and generalisability of the trial findings
| Problem | How problem is realised? | Consequence | Successful strategies to overcome the problem |
|---|---|---|---|
| Restrictive exclusion criteria | Trial recruits highly selected, relatively healthy participants with single or low number of diseases, without major disabilities or other risks. | Trial may produce overly positive—although internally valid—results that are not generalisable to real-world/geriatric patients. | Mimimising exclusion criteria. |
| Problems with randomisation | Using randomisation techniques prone to manipulation (sealed envelopes, drawing pieces of paper from a hat, block randomisation with the same size blocks). Heterogeneity of participants leads to an imbalance in how the characteristics of the participants are distributed in the intervention and control groups. |
Researcher may consciously or unconsciously choose participants that he/she wants in the intervention group, resulting in an imbalance in the intervention and control groups. Intervention and control groups differ from each other in important ways. The findings are skewed by differing characteristics. |
Computer-generated random numbers, separate randomisation centre. In block randomisation the blocks should have different sizes not known to the researchers. Randomisation can be performed using stratification according to participants’ important characteristics. The analyses can be adjusted for baseline measures. |
| Imperfect blinding | Nonpharmacological trials are often non-blinded. Nonpharmacological trials may be single-blinded (participants in the intervention know they are in active treatment but researchers/assessors are blinded of allocation). |
Researchers/assessors may consciously or unconsciously favour participants in the intervention group, leading to overly positive findings. The participants may experience the overly positive effects of an intervention knowing that they are in active treatment. | Drug trials should be double-blind. Nonpharmacological trials should at least be single-blinded, and the participants should be blinded about the main outcome measures. |
| Hawthorn effect | People tend to improve when they receive attention. | Intervention group improves, but it is difficult to interpret whether it is due to intervention or purely to the attention they receive. | Control group may be offered an ‘attention’ intervention. Study assessors should be equally empathetic to intervention and control participants. |
| Intervention too mild | It is unethical to leave control group without care, and the normal healthcare and services are very good. The intervention must be administered on top of other healthcare services. The intervention itself makes sense and seems effective, but the control group receiving normal healthcare is also receiving very good care. |
The difference between new treatment and normal care is difficult to show. | Consider planning how to make the intervention as strong as possible. Consider involving older people in the planning phase. Consider how to motivate intervention participants and enhance their self-efficacy with the new treatment. |
| Competent, enthusiastic interventionist(s) treat(s) highly motivated volunteer participants | The problem materialises when the trial findings are implemented and distributed to healthcare providers. | Trial may produce overly positive results. Findings cannot be generalised to real-world healthcare. Trial shows good efficacy, but the findings are difficult to implement: in real life, professionals are busy, not so enthusiastic about new treatments. |
Incorporate a qualitative study together with the trial. Explore and describe the essential elements important for effectiveness. This will help with the implementation phase. Consider multiple study sites and multiple methods to also recruit participants from lower social classes who may face difficulties in participating. Improve adherence in real life to fully capture trial benefits. |
| Contamination of the control group | The control group receives an intervention or similar treatment as the intervention group. The professionals taking care of the intervention group receive training at their healthcare unit and they move from the intervention unit to the control unit, administering their know-how there. |
Common in healthcare. When professionals hear about a new treatment, they are eager to try new interventions in their own way. This dilutes the trial effects. |
Plan the intervention by considering how any possible contamination might be incorporated into the trial. Consider keeping the background healthcare professionals blind about the intervention. |
| Missing values in measurements or missing data | Older people may have difficulties answering all questions, they become tired during long assessment sessions or refuse to take part in some assessments (e.g. cognitive tests) | Difficult to use incomplete data, and missing scales may dilute the results. | Consider a pilot phase to estimate assessment times and the amount of missing data. Prioritise scales and measures and start with the most important ones. Use alternative methods to gather data (postal surveys, home visits, telephone contacts, proxy responses). A limited number of missing values may be imputed, and researchers should be aware of problems related to imputation methods. Describe adequately the characteristics of those with missing values. |
| Drop-outs | Older people become tired during the follow-up. They fall ill, are hospitalised or pass away. Controls tend to drop out more easily than intervention participants due to being disappointed with their group allocation. |
Drop-outs dilute the findings and decreases the power of the trial. Impossible to implement ‘intention-to-treat’ analyses. The number continuing on to the last follow-up differ between groups and may overestimate the effect of the intervention if ‘per-protocol’ analysis is used. |
Ensure a pleasant trial experience for all. See Table 1 → retention. Calculate power of the trial by considering drop-out rates. Appropriate statistical analyses using two measurement points (e.g. GEE-models) may help overcome this problem. Consider allowing the intervention for the control group after the trial follow-up is over. |
Heterogeneity of the patient population may pose a risk to the outcome of randomisation especially in small studies and those with heterogenous older participants. This can be avoided by controlling baseline characteristics in the final analyses or by stratified randomisation [1]. Participants with or without certain characteristics or at various study sites may be randomised in separate groups, as was done in the SPRINT trial [12].
Blinding
Information bias occurs in observational studies when, for example, cancer participants recall their risk factors differently than control participants or when the data collection method is systematically different between study groups. RCTs aim to reduce information bias by blinding both participants and observers.
Double blinding is applicable in drug trials, but it is much more challenging in nonpharmacological interventions. Single blinding (where the observers are blinded to group allocation but the participants are aware of their group) is feasible but poses some challenges. Participants are often eager to share their intervention experiences with study nurses and do not always remember to keep silent about their allocation [18]. Blinding is important because it reduces the Hawthorne effect—one type of placebo effect: people tend to improve if they receive attention [19]. Due to the Hawthorne effect, pre-post designs without a control group often yield positive results. Recent non-pharmacological RCTs have attempted to reduce the Hawthorne effect by giving extra attention also to control participants. For example, in the SPRINTT project, which investigated the effects of intensive physical activity and individualised nutrition counselling on preventing mobility disability, the control group also received health lectures [16].
Problems related to intervention
Several problems with RCTs may bias the results, either by diluting the findings or making them overly positive. Moreover, statistically significant differences between the intervention and control groups are not necessarily clinically meaningful. The pretrial power calculation should be based on a clinically important difference between groups.
One challenge in demonstrating current effectiveness of the intervention is that normal practice is already fairly good and the intervention must be built on top of other treatments. With RCTs, the control group also receives good treatment, probably even better than in ‘real life’. Thus, the intervention must be strong and different enough to show its effects. This problem became evident in the cognitive training trial [20]. The intervention participants received 2 hours of cognitive training per week in a day centre; the control participants received social stimulation in the same day centre. The two groups did not differ in terms of their cognitive outcomes [20], possibly because the stimulating activities of the control group also improved cognition [21]. The strength of an intervention can be enhanced by various participatory methods. Older people should be involved when planning interventions. Empowering older people is also a good motivator for retaining participants during the trial.
Contamination is involved when control participants, for various reasons, receive similar treatments as the intervention group. This naturally dilutes the differences between the groups. Contamination is a major problem especially in pragmatic trials, when the intervention is targeted at people in close contact with each other, for example in wards or nursing homes. In the cognitive training trial, some daycare nurses implemented cognitive training to control participants at the same time that the RCT intervention was being performed [20]. Performing cluster randomisation (wards/units instead of individuals) is one way to overcome this problem (Table 2).
‘Intention-to-treat’ analysis, i.e. analysing the data of all participants in the original groups, is an important principle required by high-quality journals. ‘Per-protocol’ analysis, involving only subjects complying with the protocol, may give overly positive results. However, it can also give information of ‘true’ potential of treatment and thus stimulate ways to improve adherence.
RCTs may yield an overly rosy picture of effectiveness when the intervention is carried out by enthusiastic, competent professionals. In addition, the participants are often more motivated, educated and knowledgeable than the background population. Consequently, their adherence rate is often better than among general patients. Research has demonstrated that when the findings are then applied to a broader context or real-life situation, the effectiveness is diluted. This finding was evident in an implementation trial done as part of the COTiD programme. The original trial showed the efficacy of the occupational therapist’s home visits on the daily functioning of patients with dementia, but the implementation study had no effects [22, 23]. It is a common phenomenon that when the findings are implemented in practice, the real-life effectiveness is not the same as in the original trial. Effectiveness can be impaired by a more heterogenous population and/or lower adherence rates than in the efficacy trial. On the other hand, the treatment effect in real life also includes a potential placebo effect, which is not identified in a careful medication RCT, which only gives the net efficacy.
Outcome measures
The outcomes chosen for a trial should be relevant for older people. They usually include quality of life (QOL), functioning or the ability to live independently. Furthermore, the outcomes should assess the type of population involved in the trial. For example, people in nursing homes with major disabilities should be assessed with basic activities of daily living (ADL) scales, whereas older people living independently should be assessed with scales measuring their instrumental activities. Cognitive and QOL scales should also be adjusted according to the population. Otherwise, trial procedures can be frustrating for the participants and scales will suffer from floor or ceiling effects.
It is often practical to choose continuous scales that are sensitive to change. Power calculations show that fewer participants are needed for a trial where the primary outcome is continuous rather than dichotomous. Previous trials involving older people may help the investigator identify valid and sensitive scales (Table 2). The clinical differences between ‘hard’ and surrogate endpoints should nevertheless be identified. This has recently been discussed after the FDA gave accelerated approval for the use of aducanumab for the treatment of Alzheimer’s disease based primarily on surrogate data (amyloid lowering effects) [24].
Missing values in measurements
Participant attrition during the intervention dilute the differences between the intervention and control groups.
In nursing home trials or trials on people with dementia, 20–30% of participants may pass away or drop out for other reasons during the follow-up [20, 25]. This leads to a problem with missing measurements towards the end of follow-up. This may be overcome by using sophisticated statistical methods that take into account two measurement points and extrapolate them for the whole follow-up period or else by imputing missing values for participants who did not complete the trial. However, the method of imputation should be chosen carefully by considering the particular characteristics of the patient population. Patients with dementia tend to decline fast in functioning and cognition regardless of the intervention being used. Often the drop-out rate is higher in the control group. If the ‘last observation carried forward’ method is used for imputation, the control participants may exhibit a better outcome than they deserve (Table 2). Often in dementia trials effectiveness means a slower decline in functioning in intervention group than in the control group [18].
More practical problems and tips when performing RCTs
RCTs are much more complicated and expensive to plan than observational studies. Some researchers may be afraid of ethical problems related to RCTs: the control group does not receive a potentially beneficial treatment or the intervention group might receive a harmful treatment. However, researchers several decades ago observed that people participating in clinical trials have generally better outcomes than their counterparts in the general population, irrespective of group allocation [26].
Researcher should pay attention to definitions related to ‘PICO’ (patients, intervention, control, outcome). Trial participants and intervention procedures should be described in sufficient enough detail that the trial findings can be implemented in practice. Figure 1 provides a rough determination of older people’s target groups.
Figure 1.
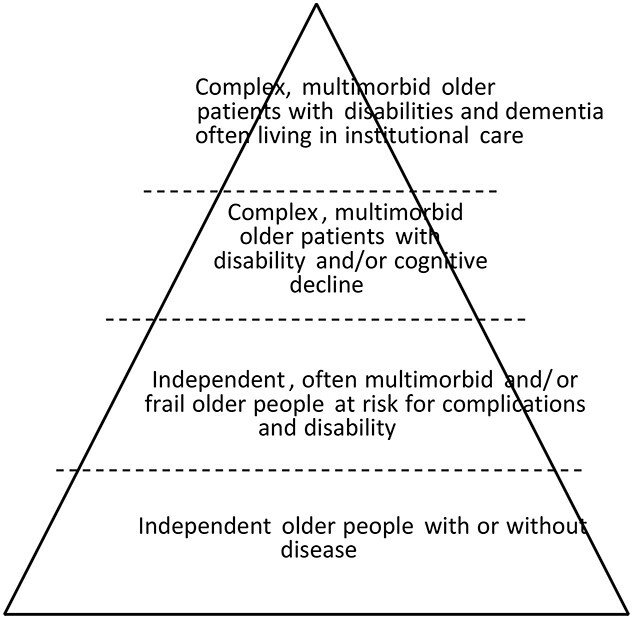
Older people in clinical trials are heterogeneous. The trial target group should be described in detail. This triangle may roughly help describe the target population.
An existing problem that causes potential bias towards positive findings has been the tendency of journals to accept positive results rather that null results [27]. Also, investigators and sponsors may be less enthusiastic with ineffectual trials. Preregistration of trials has been quite important in reducing the potential for such bias; it is important that researchers do not fruitlessly repeat negative trials.
It may take 5–10 years—or even longer—until the trial findings (intervention or management model) are properly implemented. It may not be feasible to use complicated intervention models in busy everyday practice. Consequently, an intervention may be modified—and perhaps diluted. It would be helpful if the investigators have explored all the essential and minimum elements to make the intervention effective using qualitative methods during the trial—e.g. observations, interviews with participants and interventionists. This was done in the Finnish Loneliness Trial [17], and its results have now been successfully implemented throughout Finland and in several other countries [28].
RCTs among older people should be promoted
RCTs involving both medication and management models for older people should be actively promoted. There are plenty of relevant study questions that should be explored in geriatrics, where the proper management of care is often complex and expensive. Cost-effectiveness studies of various management models for older people are still rare, but cost issues are important for politicians. It is imperative for geriatricians to keep these issues on the public agenda and demonstrate the benefits of research also from an economic standpoint.
There are other practical reasons why the participation of older people in trials is worth promoting. Older people are often altruistic, and by participating they desire to help future patients. Since the absolute risk for older patients is usually higher than for younger adults, fewer participants are needed to show treatment benefit. Therefore, the number of participants in trials involving older people can be counted in the hundreds as opposed to the thousands needed for trials involving younger adults.
RCT is not the answer to everything
A longitudinal, observational study of good quality may sometimes be a better choice than an RCT of poor quality. Especially RCTs involving life-style intervention with a long duration are difficult to perform in older people, since they move, fall ill, or die. In addition, if a common treatment, like antihypertensives or statins, has been integrated into healthcare treatment, it is unethical or impossible to leave the control group without such a treatment.
The current requirements for obtaining informed consent from each trial participant makes RCTs expensive and complicated. We cannot randomise practices, wards, or healthcare units without acquiring consent. It has been said that ‘you can do interventions for all your patients but not half your patients’. Investigating healthcare practices or management models would be much easier as a register-based trial than obtaining thousands of informed consents.
RCTs are often too rigid to give straightforward answers to straightforward questions. Also, systematic reviews and meta-analyses are no better than the trials included in them. For example, a meta-analysis on surgical face masks that includes 11 RCTs aiming to prevent the transmission of respiratory illnesses could not ascertain their benefit in preventing COVID-19 [29].
Conclusion
More pragmatic clinical trials are needed to improve evidence of effective treatments, management and care of older people. Particular barriers recognised in trials have included recruitment and the retention of older participants [30]. Furthermore, a number of other pitfalls are often encountered during the research process, which the investigator should be aware of when designing and conducting a clinical trial. They involve the risk of either overestimating or underestimating the effectiveness of the intervention, which may also threaten the validity and generalisability of the trial findings.
Age and Ageing journal 50th anniversary commentary series.
Declaration of Conflicts of Interest
TES participates in several randomised drug trials funded by pharmaceutical companies (Including Amgen, Merck, Novo Nordisk, Pfizer, Sankyo, Sanofi, Servier).
Declaration of Sources of Funding
Open access funded by Helsinki University Library.
References
- 1. Hartung DM, Touchette D. Overview of clinical research design. Am J Health Syst Pharm 2009; 66: 398–408. [DOI] [PubMed] [Google Scholar]
- 2. Treatment of pulmonary tuberculosis with streptomycin and para-aminosalicylic acid; a Medical Research Council investigation. Br Med J 1950; 2: 1073–85. [PMC free article] [PubMed] [Google Scholar]
- 3. Grimley EJ. Geriatric medicine: a brief history. BMJ 1997; 315: 1075–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crome P, Lally F, Cherubini Aet al. Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging 2011; 28: 667–77. [DOI] [PubMed] [Google Scholar]
- 5. Lang KJ, Lidder S. Under-representation of the elderly in cancer clinical trials. Br J Hosp Med (Lond) 2010; 71: 678–81. [DOI] [PubMed] [Google Scholar]
- 6. McMurdo M. Clinical research must include more older people. BMJ 2013; 346: f3899. [DOI] [PubMed] [Google Scholar]
- 7. Cherubini A, Del Signore S, Ouslander J, Semla T, Michel JP. Fighting against age discrimination in clinical trials. J Am Geriatr Soc 2010; 58: 1791–6. [DOI] [PubMed] [Google Scholar]
- 8. Caughey GE, Inacio MC, Bell JS, Vitry AI, Shakib S. Inclusion of older people reflective of real-world clinical practice in cardiovascular drug trials. J Am Heart Assoc 2020; 9: e016936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheppard JP, Lown M, Burt Jet al. Generalizability of blood pressure lowering trials to older patients: cross-sectional analysis. J Am Geriatr Soc 2020; 68: 2508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veronese N, Petrovic M, Benetos Aet al. Special interest group in systematic reviews and meta-analyses and the task force on pharmaceutical strategy of the European Geriatric Medicine Society (EuGMS). Underrepresentation of older adults in clinical trials on COVID-19 vaccines: a systematic review. Ageing Res Rev 2021; 71: 101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zwarenstein M, Treweek S, Gagnier JJet al. CONSORT group; pragmatic trials in healthcare (Practihc) group. "improving the reporting of pragmatic trials: an extension of the CONSORT statement". BMJ 2008; 337: a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manson JE, Chlebowski RT, Stefanick MLet al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013; 310: 1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tinetti ME, McAvay G, Trentalange M, Cohen AB, Allore HG. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: population based cohort study. BMJ 2015; 351: h4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitkala KH, Routasalo P, Kautiainen H, Tilvis RS. Effects of psychosocial group rehabilitation on health, use of health care services, and mortality of older persons suffering from loneliness: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2009; 64: 792–800. [DOI] [PubMed] [Google Scholar]
- 16. Landi F, Cesari M, Calvani Ret al. SPRINTT Consortium. The "Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies" (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res 2017; 29: 89–100. [DOI] [PubMed] [Google Scholar]
- 17. Savikko N, Routasalo P, Tilvis S, Pitkälä K. Psychosocial group rehabilitation for lonely older people: a description of intervention and participants’ feedback. Int J Older People Nurs 2010; 5: 16–24. [DOI] [PubMed] [Google Scholar]
- 18. Pitkälä KH, Pöysti MM, Laakkonen MLet al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med 2013; 173: 894–901. [DOI] [PubMed] [Google Scholar]
- 19. McCarney R, Warner J, Iliffe S, Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol 2007; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kallio EL, Öhman H, Hietanen Met al. Effects of cognitive training on cognition and quality of life of older persons with dementia. J Am Geriatr Soc 2018; 66: 664–70. [DOI] [PubMed] [Google Scholar]
- 21. Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012; CD005562. [DOI] [PubMed] [Google Scholar]
- 22. Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006; 333: 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Döpp CM, Graff MJ, Teerenstra S, Olde Rikkert MG, Nijhuis-van der Sanden MW, Vernooij-Dassen MJ. Effectiveness of a training package for implementing a community-based occupational therapy program in dementia: a cluster randomized controlled trial. Clin Rehabil 2015; 29: 974–86. [DOI] [PubMed] [Google Scholar]
- 24. Liu KY, Howard R. Can we learn lessons from the FDA's approval of aducanumab? Nat Rev Neurol 2021; 17: 715–22. [DOI] [PubMed] [Google Scholar]
- 25. Pitkälä KH, Juola AL, Kautiainen Het al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc 2014; 15: 892–8. [DOI] [PubMed] [Google Scholar]
- 26. Coronary Drug Project Research Group . Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med 1980; 303: 1038–41. [DOI] [PubMed] [Google Scholar]
- 27. Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009; 2009: MR000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansson A, Savikko N, Pitkala KH. Training professionals to implement a group model for alleviating loneliness among older people – 10-year follow-up study-up. Educ Gerontol 44: 119–27. [Google Scholar]
- 29. Nanda A, Hung I, Kwong Aet al. Efficacy of surgical masks or cloth masks in the prevention of viral transmission: systematic review, meta-analysis, and proposal for future trial. J Evid Based Med 2021; 14: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buttgereit T, Palmowski Aet al. Barriers and potential solutions in the recruitment and retention of older patients in clinical trials—lessons learned from six large multicentre randomized controlled trials. Age Ageing 2021; 50: 1988–96. [DOI] [PubMed] [Google Scholar]


