Abstract
4-Phenylbutyric acid (4PBA) is utilized as a drug to treat urea cycle disorders and is also being studied as a potential anticancer drug that acts via its histone deacetylase (HDAC) inhibitor activity. During a search to find small molecules that affect plant regeneration in Arabidopsis, we found that 4PBA treatment promotes this process by mimicking the effect of exogenous auxin. Specifically, plant tissue culture experiments revealed that a medium containing 4PBA enhances callus formation and subsequent shoot regeneration. Analyses with auxin-responsive or cytokinin-responsive marker lines demonstrated that 4PBA specifically enhances AUXIN RESPONSE FACTOR (ARF)-dependent auxin responses. Our western blot analyses showed that 4PBA treatment does not enhance histone acetylation in Arabidopsis, in contrast to butyric acid and trichostatin A, other chemicals often used as HDAC inhibitors, suggesting this mechanism of action does not explain the observed effect of 4PBA on regeneration. Finally, mass spectroscopic analysis and genetic approaches uncovered that 4PBA in Arabidopsis plants is converted to phenylacetic acid (PAA), a known natural auxin, in a manner independent of peroxisomal IBR3-related β-oxidation. This study demonstrates that 4PBA application promotes regeneration in explants via its auxin activity and has potential applications to not only plant tissue culture engineering but also research on the plant β-oxidation pathway.
Keywords: auxin, histone acetylation, plant tissue culture, regeneration
Introduction
Plant tissue culture is a technique for the conversion of plant cell developmental fate which often involves several physiological phases, including cell cycle reactivation, stem cell reformation, and de novo organogenesis (Sugiyama 2015). It is firmly established that the balance of two phytohormones, auxin and cytokinin, is critical for the cell fate conversion of explant tissue to shoots, roots, or somatic embryos in tissue culture (Skoog and Miller 1957; Steward et al. 1958). Wounding is also another critical signal that triggers the formation of an unorganized cell mass, known as callus, and subsequent tissue regeneration (Ikeuchi et al. 2017; Iwase et al. 2015b, 2011, 2017). Accumulating evidence suggests that cell fate conversion is accompanied by epigenetic alterations, such as changes in DNA methylation and histone modifications, that affect gene expression networks (Ikeuchi et al. 2015; Lee and Seo 2018). Indeed, both auxin treatment and wounding stress alter histone modifications, including histone acetylation, which positively regulates the expression of cellular reprogramming-related genes (Rymen et al. 2019; Yamamuro et al. 2016). Trichostatin A (TSA) and butyric acid (BUA), well-known inhibitors of histone deacetylases (HDACs) that increase the level of acetylated histones, can be added during tissue culture to enhance plant tissue regeneration (Bie et al. 2020; Lee et al. 2020; Li et al. 2014; Wójcikowska et al. 2018). TSA treatment additionally alters auxin responsiveness (Li et al. 2014). Therefore, two questions arise: whether phytohormones and histone modifications mutually affect phase changes in plant regeneration processes and whether other chemicals used as HDAC inhibitors, for instance, 4-phenylbutyric acid (4PBA) (Kim et al. 2020; Kusaczuk et al. 2016), enhance the efficiency of regeneration.
Auxin was the first recognized plant hormone and controls plant development, growth, and cell reprogramming (Enders and Strader 2015). Plants produce both polar transportable auxin indole-3-acetic acid (IAA) and non-polar-transportable phenylacetic acid (PAA) (Sugawara et al. 2015). Indole-3-butyric acid (IBA) is a natural IAA-related metabolite and known to be enzymatically converted to active IAA via β-oxidation in peroxisomes (Zolman et al. 2007). What has become clear in recent years is that auxin responses involve histone acetylation-mediated epigenetic regulation. In the best-characterized auxin response pathway, the gene network regulated by the ARF transcription factors is affected by auxin concentration-dependent degradation of the Aux/IAA repressor proteins, which negatively regulate the ARFs by forming ARF-Aux/IAA protein complexes. At low concentrations of auxin, a TOPLESS-Histone deacetylation (HDAC) complex binds to domain I of the Aux/IAA protein, which is thought to cause transcriptional repression by deacetylating histones on genomic regions targeted by ARFs (Nguyen et al. 2020). Under high auxin concentrations, the Aux/IAA proteins are ubiquitinated and subsequently degraded, leading to activation of ARF target genes. One of the latest models explaining ARF-mediated activation of transcription at higher auxin concentrations is that ARFs bind to histone acetyltransferases (HATs) via the SNF/SWI complex, resulting in histone acetylation and activating the expression of ARF target genes (Nguyen et al. 2020).
Control of epigenetic status by, for example, application of small molecules which alter epigenetic modifications in plant cells, can enhance or repress cell fate conversion during tissue culture. Therefore, enhancer/inhibitor screening is a fascinating strategy for understanding the molecular details associated with cell fate change during plant regeneration while simultaneously establishing efficient plant tissue culture methods. This research found that 4PBA can enhance callus formation and tissue regeneration by acting as an auxin, likely at least in part because it is converted to PAA within the plant. Our data further suggest that this conversion from 4PBA to PAA might proceed via peroxisome-independent β-oxidation. Also, despite its documented activity as an HDAC inhibitor in other organisms, we were unable to detect an effect of 4PBA on histone acetylation in plant tissue via western blot.
Materials and methods
Plant material, growth condition, and regeneration assay
All plants used in this research, including the wild type, DR5-GUS (Ulmasov et al. 1997), TCS-GUS (Müller and Sheen 2008), pex5-1 (CS3949), and ibr3-9 (SALK_033467) (Zolman et al. 2007), are in the Arabidopsis Columbia background. For normal growth, plants were grown on soil at 22°C with a photoperiod of 16 h of white light and 8 h of dark. To check callus formation and tissue regeneration, we used etiolated hypocotyl explants obtained from 7-day-old dark-grown plants cultured on germination medium supplemented with full-strength Murashige-Skoog (MS) salt (Wako), 1% sucrose (Wako), and 0.6% Gelzan CM (Sigma-Aldrich). Subsequently, we incubated the approximate 5–7 mm hypocotyl explants on MS medium supplemented with Gamborg’s B5 vitamin (Sigma-Aldrich), 2% glucose (Wako), 0.05% 2-(N-morpholino) ethanesulfonic acid (MES; Sigma-Aldrich), and 0.8% phytoagar. Based on this recipe, we prepared both a hormone-free medium and a callus-inducing medium, the latter of which contains 5.37 µM NAA (Sigma-Aldrich) and 4.44 µM BA (Sigma-Aldrich). For the root elongation assay and adventitious root assay, we used the germination medium. Chemicals tested in this study were dissolved in DMSO (Wako) and then added to media, giving a final concentration of NAA (5.4 µM), BA (4.4 µM), 4PBA (20 µM, 1 mM), BUA (20 µM, 1 mM), TSA (5 µM), IBA (5.3 µM), and IAA (1 µM). We added 0.1% DMSO as a mock treatment since the chemical stocks were prepared at 1,000X working concentration.
Microscopy
GUS staining was performed as previously described (Kertbundit et al. 1991), and stained samples were observed using a Leica M165 C stereomicroscope.
Protein extraction and western blot
Protein isolation and western blotting were performed in duplicate and as previously described (Rymen et al. 2019) with some modifications. 5-day-old whole seedlings cultured in half-strength MS liquid media containing 1% sucrose were treated by chemicals for 24 h, and then the whole tissue of approximately 25 mg fresh weight was frozen in liquid nitrogen and ground using an MB1200 Multi-beads Shocker (Yasui Kikai). The total amount of extracted proteins was quantified with the Qubit dsDNA High Sensitivity Assay Kit, and 1 µg of protein was used for SDS-PAGE. After transfer to a PVDF membrane, histone H3, H4 and their modifications were detected with anti-histone H3 (ab1791, Abcam), anti-histone H3K9Ac (ab10812, Abcam), anti-histone H3K14Ac (ab52946, Abcam), anti-histone H3K23Ac (07-355, Merck Millipore), anti-histone H3K27Ac (ab4729, Abcam), anti-histone H4 (ab10158, Abcam) and anti-histone H4Ac (06-866, Merck Millipore).
Hormone quantification
PAA and IAA quantification by liquid chromatography-tandem mass spectrometry (LC-MS/MS) were performed as previously described (Aoi et al. 2020).
Results
4PBA enhances callus formation and regeneration likely via an ARF-dependent auxin response pathway
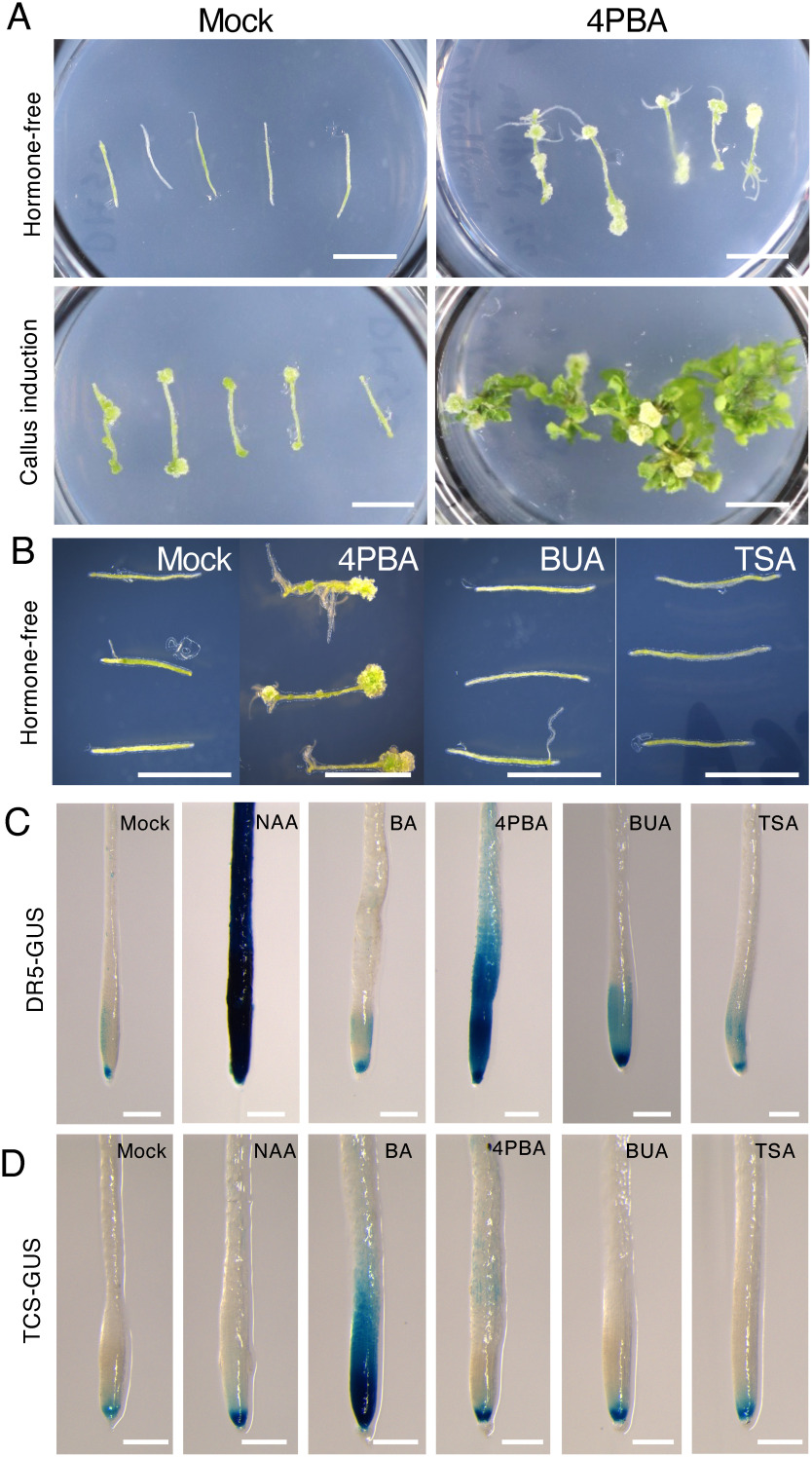
In our recent screen to identify small molecules which affect plant regeneration, we found that 4PBA, listed as a drug used to treat urea cycle disorders and an HDAC inhibitor in animal research (Kim et al. 2020; Palir et al. 2017; Peña-Quintana et al. 2017), has positive effects on both callus and adventitious root formation in hormone-free medium (Figure 1A). In callus induction medium (CIM), which contains both exogenous auxin and cytokinin, 4PBA at 20 µM addition greatly enhances callus formation and shoots regeneration from the hypocotyl segments compared to mock (DMSO)-treated explants (Figure 1A). However, explants cultured with 1 µM 4PBA was almost indistinguishable from mock-treated explants (Supplementary Figure S1), indicating that 4PBA is effective in the enhancement of callus formation and regeneration at relatively high concentration such as centimilli-molar levels. On the other hand, we confirmed that TSA and even BUA, other HDAC inhibitors often used in plant research, did not enhance callus formation on phytohormone-free medium (Figure 1B). Since ectopic callus formation and de novo shoot regeneration occur in Arabidopsis explants cultured on medium containing a higher concentration of auxin and/or cytokinin (Ikeuchi et al. 2019; Iwase et al. 2015a), we performed the promoter-reporter assays to examine if the 4PBA affects auxin and/or cytokinin responses. We also assayed BUA and TSA to check whether HDAC inhibitors commonly alter responses of the two phytohormones. The DR5-GUS marker lines, which is used to detect ARF-dependent auxin responses (Ulmasov et al. 1997), showed remarkable GUS activity in both NAA and 4PBA treated roots as expected, whereas BUA- or TSA-treated roots were indistinguishable from mock-treated DR5-GUS plants (Figure 1C). In the case of TCS-GUS, which has cis-elements for type-B ARR target sites and marks cytokinin responses (Müller and Sheen 2008), noticeable enhancement of the GUS activity was detected only in BA-treated samples (Figure 1D). These observations suggest that 4PBA enhances auxin responses but not cytokinin responses, at least those detectable using the TCS-GUS reporter. We could also conclude that the major HDAC inhibitors, BUA and TSA, do not behave like 4PBA; they do not enhance auxin and cytokinin responses, at least to the level detectable using the DR5-GUS and TCS-GUS reporters in roots.
Figure 1. 4-Phenylbutyric acid (4PBA) promotes regeneration and ARF-dependent auxin responses. (A) Addition of 4PBA to medium enhances callus formation and tissue regeneration. Callus formation and root regeneration in Arabidopsis hypocotyl explants (upper right panel) cultured on 4PBA-containing medium for 28 days without exogenous phytohormones, compared to mock (DMSO)-treated explants cultured on hormone-free medium (upper left panel). Shoots regeneration from callus of hypocotyl explants cultured on medium containing NAA, BA and 4PBA for 28 days (lower right panel) compared to mock-treated explants cultured in the presence of NAA, BA and DMSO (lower left panel). (B) Other HDAC inhibitors, butyric acid (BUA) and trichostatin A (TSA) fail to promote callus formation in the phytohormone-free condition. Callus formation in 4PBA-containing medium after 28 days of growth in tissue culture but not in hypocotyl explants cultured on BUA, TSA, and the mock containing medium lacking exogenous phytohormones. For (A and B), chemicals were used at the following concentrations: 4PBA (20 µM), NAA (5.4 µM), BA (4.4 µM), BUA (20 µM), TSA (20 µM). (C) 4PBA treatment activates auxin-responsive gene expression, as detected using the DR5-GUS reporter. An NAA-treated DR5-GUS plant root shows strong GUS activity. A 4PBA-treated root also displays enhanced DR5-GUS expression, whereas no obvious enhancement is seen in roots treated with the cytokinin BA, either of two HDAC inhibitors, BUA or TSA. (D) 4PBA treatment does not obviously activate the cytokinin-responsive TCS-GUS reporter. A BA-treated root shows strong TCS-GUS activity, but 4PBA and other chemicals do not produce a detectable enhancement of cytokinin responses. For (C and D) 5 day-old Arabidopsis seedlings were treated at the following concentrations for 24 h: 4PBA (20 µM), NAA (5.4 µM), BA (4.4 µM), BUA (20 µM), TSA (20 µM). Scale bar, 5 mm (A and B), 250 µm (C and D). The experiments were done at least twice, using 3 to 5 plants for the callus formation assay (A and B), and 10–15 seedlings for the GUS assay (C and D).
Although the usage of 4PBA in recent plant research has been mainly to attenuate ER stress via its chemical chaperon activity (Mira et al. 2017; Welch and Brown 1996), our literature survey unveiled that 4PBA had been counted as one of the chemicals that showed auxin activity in the very early period of plant hormone research. Thimann and Schneider tested several chemicals, including γ-phenyl-butyric acid (a synonym for 4PBA) in their Avena/Pisum tests where tissue elongation and curvature effects induced by auxin were examined (Thimann and Schneider 1939). The relative auxin activity of γ-phenyl-butyric acid, however, is quite weak compared to IAA, e.g., only 3% in the Pisum stem curvature test (Thimann and Schneider 1939).
4PBA does not enhance histone H3 and H4 acetylation at least as detectable by WB
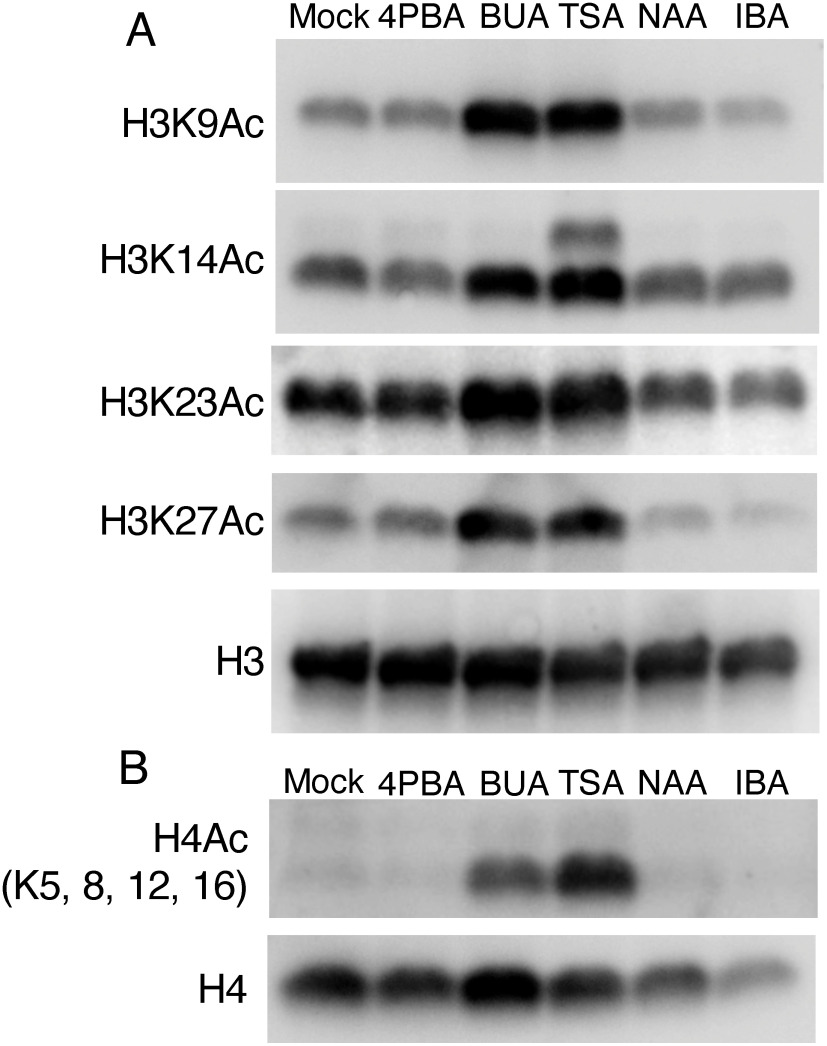
To investigate whether the enhancement of regeneration by 4PBA might be due to its HDAC inhibitor activity, in addition to its auxin activity, we performed western blot analyses to check histone acetylation levels in 4PBA-treated Arabidopsis seedlings. As previously reported in other research performed in Arabidopsis and Brassica napus (Lee et al. 2020; Li et al. 2014; Menge et al. 2017), both TSA-treated and BUA-treated Arabidopsis seedlings exhibited increases for all types of histone acetylation we examined, i.e., H3K9Ac, H3K14Ac, H3K23Ac, H3K27Ac and H4Acs (detected H4K5Ac, K8Ac, K12Ac and K16Ac), compared to mock-treated tissues (Figure 2). In contrast, we found that histone acetylation levels of 4PBA-treated Arabidopsis seedling are indistinguishable from mock-treated tissue. It is worth noting that the concentrations of inhibitors we tested (1 mM 4PBA, 1 mM BUA, and 5 µM TSA) have been used in animal studies to suppress HDAC activity. Plus, these concentrations of BUA and TSA also have been used in plant studies as HDAC inhibitors, indicating that 4PBA does not have the inhibitory effect on HDACs seen for TSA and BUA in this experimental condition, at least not to a level detectable by western blot. Since auxin treatment itself drives histone acetylation-related machinery to affect downstream gene expression (Yamamuro et al. 2016), we also tested the effects of two different auxins, NAA and IBA, on histone acetylation levels by western blot. At the concentration 5.4 µM NAA and 4.9 µM IBA in which callus formation and adventitious root regeneration occurs in Arabidopsis explants (Figure 1; Ikeuchi et al. 2019; Ludwig-Müller et al. 2005), both auxin treatments led to acetylation levels comparable to mock-treated seedlings (Figure 2), indicating that alteration of histone acetylation in these auxin conditions is not detectable, at least via western blot. Taken together with our observation that neither TSA nor BUA treatment enhances callus formation on phytohormone-free medium (Figure 1B), these data suggest that increasing histone acetylation by treatment with either of these chemicals alone in explants is likely not sufficient to promote callus formation. In addition, treatment of explants with auxin at concentrations that induce callus formation does not appear to substantially alter histone acetylation.
Figure 2. 4-Phenylbutyric acid (4PBA) treatment does not enhance histone H3 and H4 acetylation as detectable by western blot. (A) western blots using antibodies against the indicated acetylated histone H3 residue (K9Ac, K14Ac, K23Ac and K27Ac) were performed using protein extracted from chemical treated Arabidopsis seedlings. Treatment with either of two known HDAC inhibitors, butyric acid (BUA) and trichostatin A (TSA), increases the level of all test marks, whereas treatment with 4PBA or either of two types of auxins, naphthalene acetic acid (NAA) and indole-3-butyric acid (IBA), produces similar results to that observed in mock (DMSO) treated plants. (B) western blot using antibodies against the acetylated histone H4 (detects K5Ac, K8Ac, K12Ac, K16Ac) performed using protein extracted from chemical-treated Arabidopsis seedlings. Similar to (A), treatment with BUA or TSA increases the level of H4Ac, whereas treatment with 4PBA, NAA or IBA has no effect. 5 day-old Arabidopsis seedlings were treated at the following concentrations for 24 h: 4PBA (1 mM), BUA (1 mM), TSA (5 µM), NAA (5.4 µM), and IBA (5.3 µM). The experiments were done twice with approximately 25 mg fresh weight seedlings as plant material for each test.
Auxin effects of 4PBA are independent of peroxisomal β-oxidation
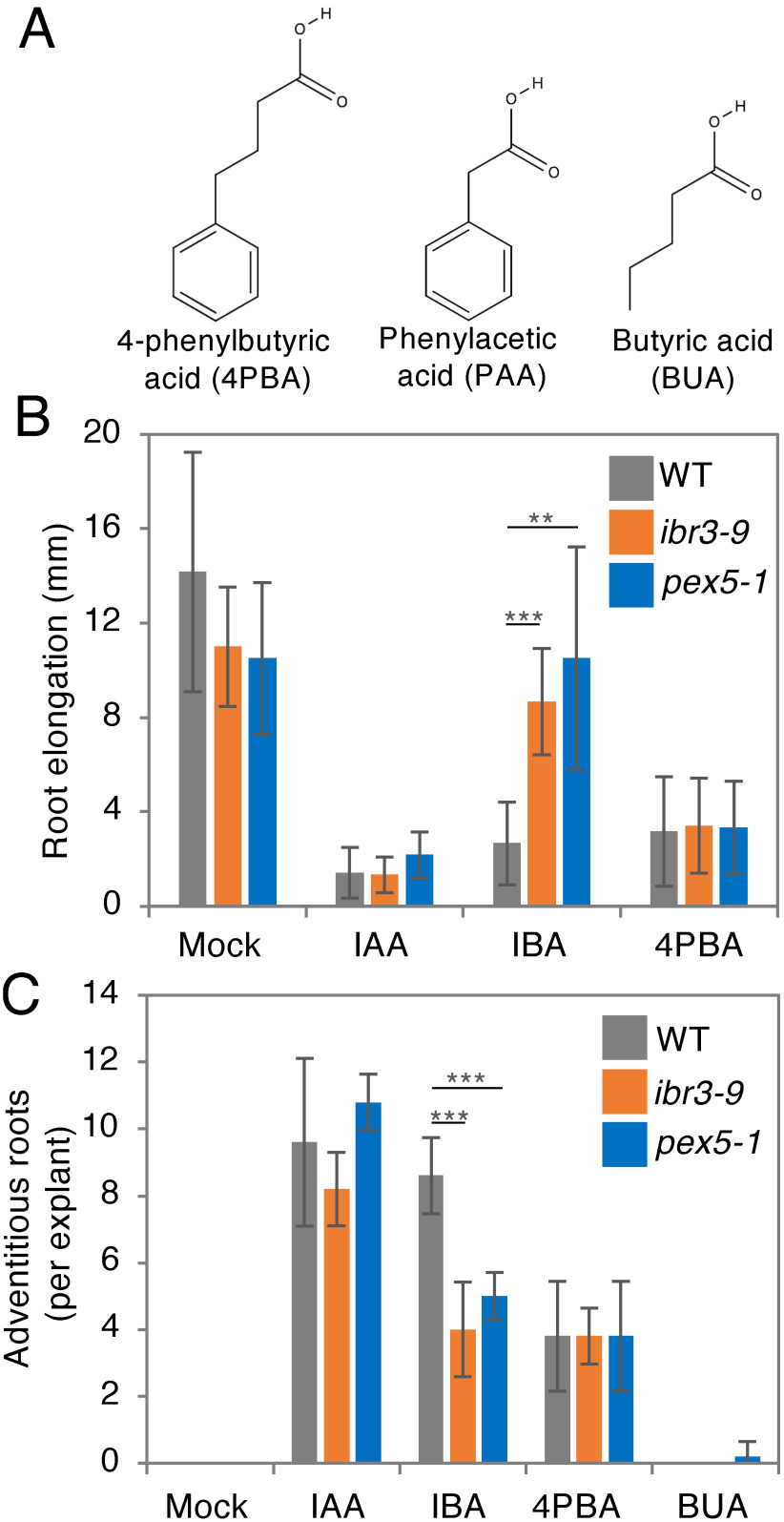
A natural IAA-related metabolite, IBA, and even a synthetic auxin, 2,4-DB, undergo β-oxidation, thus producing the active auxin species IAA and 2,4-D, respectively, in a reaction that is catalyzed by peroxisome-localized β-oxidation enzymes (Strader and Bartel 2009). Peroxisome biogenesis mutants, such as pex5-1, and mutants defective in β-oxidation enzymes, like ibr3-9, are resistant to exogenous IBA and 2,4-DB but sensitive to IAA and 2,4-D (Strader and Bartel, 2009; Woodward and Bartel 2005; Zolman et al. 2007), indicating that the conversion of IBA to IAA or of 2,4-DB to 2,4-D involves peroxisomal β-oxidation. 4PBA is structurally similar to PAA, a natural auxin (Sugawara et al. 2015; Thimann and Schneider 1939); the structural difference between the two compounds is that the side chain attached to the benzene ring has two fewer carbons in PAA (Figure 3A). Therefore, we hypothesized that 4PBA was β-oxidized to PAA by peroxisomal enzymes. As previously reported (Strader and Bartel 2009; Zolman et al. 2007), we observed that primary root elongation is attenuated in wild-type plants when treated with IAA or IBA, whereas both ibr3-9 and pex5-1 exhibited insensitivity to IBA (Figure 3B). Surprisingly, both mutants showed a short root phenotype identical to wild-type plants on 4PBA-containing medium, indicating that the auxin effect of 4PBA is independent of peroxisomal β-oxidation. We also confirmed this by examining adventitious root generation in auxin-treated hypocotyls, where we found that both ibr3-9 and pex5-1 form fewer adventitious roots compared to the wild type when treated with IBA, whereas no differences among the three genotypes were observed in the presence of 4PBA (Figure 3C). Note that treatment with the HDAC inhibitor BUA (Figure 3B) alone has no promotive effect on adventitious root formation in this condition, implying that adventitious root generation promoted by 4PBA is independent of its HDAC inhibitory effect and, additionally, that the benzene ring structure is essential for its effect as auxin (Figure 3C).
Figure 3. 4-Phenylbutyric acid (4PBA) works as an auxin in a peroxisomal β-oxidation-independent manner. (A) Molecular structures of 4PBA, phenylacetic acid (PAA) and butyric acid (BUA). (B) Root elongation inhibition by indole-3-butyric acid (IBA) treatment is abolished in both ibr3-9 and pex5-1 mutants where peroxisomal β-oxidation of IBA to indole-3-acetic acid (IAA) is disrupted, as previously reported (Strader and Bartel 2009; Zolman et al. 2007). 4PBA-treated plants show clear root elongation inhibition similar to those treated with IAA, with no difference in phenotypes between wild-type plants and the two β-oxidation mutants observed in this condition. (C) Enhancement of adventitious roots formation by IBA is significantly compromised in both ibr3-9 and pex5-1 mutants. 4PBA treatment enhances adventitious root formation compared to mock-treated hypocotyl explants or those treated with the HDAC inhibitor butyric acid (BUA), and wild-type plants and the two β-oxidation mutants respond similarly to 4PBA in this assay. Chemicals were used at the following concentrations for treatments: IAA (1 µM), IBA (8 µM), 4PBA (20 µM), and BUA (20 µM). Data are mean±SD. Statistical significance was determined by t-test, n=6 for (B) and 5 for (C); *** p<0.001, ** p<0.01.
4PBA is converted to PAA presumably in a manner independent of peroxisomal β-oxidation
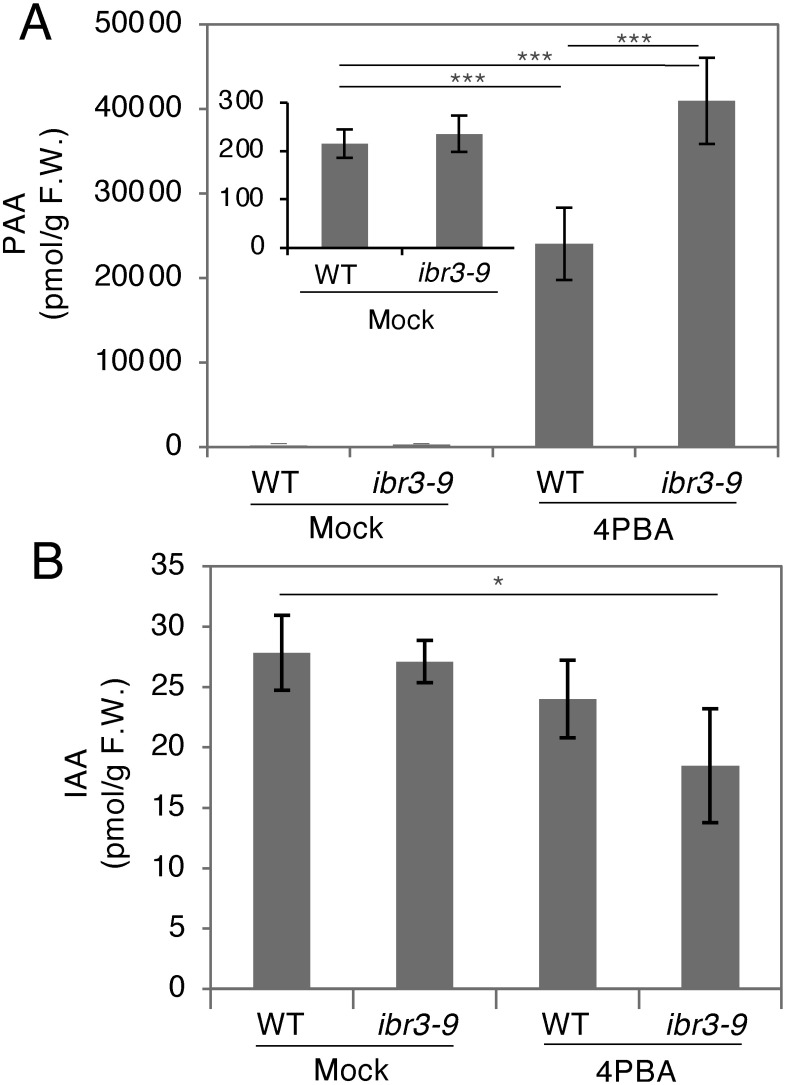
To investigate if 4PBA affects the endogenous auxin concentration in plants, we directly quantified PAA and IAA in 4PBA-treated seedlings and compared levels with mock-treated seedlings (Figure 4). To our surprise, the level of endogenous PAA was about 100 times higher in the 4PBA-treated wild-type seedlings compared with mock-treated plants (Figure 4A). In contrast, the endogenous level of IAA in the 4PBA-treated wild-type seedlings was comparable to that of mock-treated wild-type seedlings (Figure 4B). We also checked auxin concentrations in the ibr3-9 mutant and found that both PAA and IAA levels in the mutant are indistinguishable from the wild type in the mock-treated seedlings, whereas the level of PAA is significantly higher in mutant seedlings treated with 4PBA compared to the wild type (Figure 4A). We additionally tested the PAA concentration in liquid culture medium lacking seedlings but supplemented with 4PBA and found that PAA was not detected, suggesting that 4PBA was converted to PAA in plants. These results, together with the data from the auxin response tests performed with the pex5-1 and ibr3-9 mutants (Figure 3), suggest that in Arabidopsis seedlings, 4PBA is converted into PAA in a manner independent of peroxisomal β-oxidation.
Figure 4. Phenylacetic acid (PAA) accumulates to higher levels in the 4-phenylbutyric acid (4PBA) treated-Arabidopsis seedlings. (A) PAA concentrations in mock (DMSO) or 4PBA-treated seedlings. PAA accumulates to a level approximately 100 times higher in 4PBA-treated plants than in mock-treated plants. This hyper-accumulation is further increased in the ibr3-9 mutant, where the level of PAA is approximately 200 times higher than in mock-treated plants. The smaller bar graph seen inside the main graph shows PAA levels in mock-treated wild-type or ibr3-9 plants. (B) IAA concentrations in mock (DMSO)- or 4PBA-treated seedlings. Wild type, mock-treated ibr3-9 mutant, and 4PBA-treated wild-type plants all have similar levels of IAA. 4PBA-treated ibr3-9 seedlings contain significantly less IAA than mock-treated wild-type plants. 5 day-old Arabidopsis seedlings were treated for 24 h with 4PBA (20 µM). Data are mean±SD. Statistical significance was determined by t-test, n=4 for (A) and (B); * p<0.01, *** p<0.001.
Discussion
In this study, we confirmed that 4PBA, which was classified as an auxin based on the Avena/Pisum tests published by Thimann and Schneider (1939), affects plant tissues in a manner similar to other auxins. We found that the 4PBA promotes activation of ARF-dependent auxin-responses and promotes callus formation and tissue regeneration when applied during tissue culture (Figure 1). Furthermore, our data showed that the 4PBA is converted into PAA, one of the natural auxins, likely mainly via a pathway independent from peroxisomal β-oxidation.
Since it has been demonstrated, in animal studies, that 4PBA is an HDAC inhibitor and increases histone acetylation to a level detectable via western blotting (Kim et al. 2020; Kusaczuk et al. 2016), we expected it to have a similar effect in plants. However, it turns out that an enhancement of histone H3 and H4 acetylation is not detectable in 4PBA-treated Arabidopsis seedlings, at least when evaluated by western blotting (Figure 2), suggesting that 4PBA does not have a strong action as an HDAC inhibitor in plants. As we observed previously that histone modification-related inhibitors do not always produce alterations detectable via western blot analyses (Rymen et al. 2019), we cannot rule out the possibility that 4PBA works as an HDAC inhibitor in plants. To clarify this point, it will be necessary to perform genome-wide ChIP-sequencing experiments following 4PBA treatment to examine histone acetylation at individual loci.
It is also interesting to note that potent HDAC inhibition itself does not cause auxin or cytokinin responses detectable using a DR5 or TCS reporter, respectively. In addition, auxin treatment sufficient to enhance callus formation and tissue regeneration does not noticeably increase histone acetylation. This stands in contrast to treatment with the HDAC inhibitor BUA or TSA, which substantially increases the level of histone H3/H4 acetylation but alone is not sufficient to promote callus formation or induce auxin/cytokinin responses. Moreover, the NAA, IBA and 4PBA treatments do not increase global histone acetylation (Figures 1, 2). These results imply that auxin-mediated histone acetylation does not occur universally across the genome.
It is known that the 4PBA is converted to PAA in human cells when the chemical is taken as a drug to treat urea cycle disorder (Peña-Quintana et al. 2017). A recent study using fibroblast cell lines demonstrated that a mutation in a pivotal mitochondrial β-oxidation enzyme, such as in Medium-chain acyl-coenzyme A dehydrogenase (MCAD), blocks the conversion of 4PBA to PAA (Palir et al. 2017), suggesting that mitochondrial β-oxidation is critical for this process to occur in human cells. Since the existence of the mitochondrial β-oxidation pathway has been proposed in plants (Masterson and Wood 2009), we hypothesize that the peroxisome-independent production of PAA observed in this study (Figures 3, 4) might be due to mitochondrial β-oxidation. Although it is still unclear whether 4PBA itself has auxin effects, a point which will be important to investigate in future work, it is likely that the observed positive effect of this compound on DR5-GUS expression and callus formation (Figure 1), as well as its negative effect on root elongation (Figure 3), is caused at least in part by its degradation product, PAA. Supporting this hypothesis, it was reported previously that PAA enhances callus formation and suppresses primary root elongation (Leuba and LeTourneau 1990; Sugawara et al. 2015).
Taken together, our data point to a potential use for PBA in plant tissue culture engineering and in research focused on understanding plant β-oxidation pathways. One of the next important questions to address is what the benefits of using 4PBA in plant tissue culture are compared to other auxins. An arisen important question is whether the regeneration-inducing effect of 4PBA can be explained simply by the auxin action of 4PBA-derived PAA. We are so far not sure if the 4PBA itself has an auxin effect. Since we have noticed that increased NAA levels alone are sufficient to enhance shoot regeneration in Arabidopsis hypocotyl segments cultured on BA-containing medium (Ikeuchi et al. 2019; Iwase et al. 2015a), so we believe it is not because of a specific function of PAA but rather due to a common effect of auxins. Moreover, a synthesis pathway for this molecule in plants is unknown, based on our knowledge. Therefore, it will be interesting to explore whether plants produce 4PBA as a precursor for PAA. Additionally, exploring if the chemical chaperon activity of 4PBA contributes to its promotive effect on regeneration is another exciting challenge for future studies.
Acknowledgments
We thank Chika Ikeda, Mariko Mouri and Noriko Doi for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan to A.I. (20H04893 and 20K06694), H.K. (20K21419) and K.S. (20H05911 and 20H03284), from PRESTO, Japan Science and Technology Agency to A.I. (JPMJPR20D2) and from the Japan Society for the Promotion of Science to D.S.F. (19F19781)
Abbreviations
- ARF
AUXIN RESPONSE FACTOR
- Aux/IAA
AUXIN/INDOLE-3-ACETIC ACID
- BA
benzyl adenine
- BUA
butyric acid
- ChIP
chromatin immunoprecipitation
- GUS
β-glucuronidase
- IBA
indole-3-butyric acid
- IBR
IBA-response
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NAA
naphthaleneacetic acid
- PEX
PEROXIN
- SNF/SWI
sucrose non-fermentable/switch
- TCS
two component sensor
- TSA
trichostatin A
- 2,4-D
2,4-dichlorophenoxyacetic acid
- 2,4-DB
2,4-dichlorophenoxybutyric acid
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: IBR3 (At3g06810) and PEX5 (At5g56290).
Supplementary Data
References
- Aoi Y, Tanaka K, Cook SD, Hayashi KI, Kasahara H (2020) GH3 auxin-amido synthetases alter the ratio of indole-3-acetic acid and phenylacetic acid in Arabidopsis. Plant Cell Physiol 61: 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie XM, Dong L, Li XH, Wang H, Gao XQ, Li XG (2020) Trichostatin A and sodium butyrate promotes plant regeneration in common wheat. Plant Signal Behav 15: 1820681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders TA, Strader LC (2015) Auxin activity: Past, present, and future. Am J Bot 102: 180–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K (2019) Molecular mechanisms of plant regeneration. Annu Rev Plant Biol 70: 377–406 [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, Heyman J, Watanabe S, Seo M, De Veylder L, et al. (2017) Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol 175: 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Sugimoto K (2015) Control of plant cell differentiation by histone modification and DNA methylation. Curr Opin Plant Biol 28: 60–67 [DOI] [PubMed] [Google Scholar]
- Iwase A, Harashima H, Ikeuchi M, Rymen B, Ohnuma M, Komaki S, Morohashi K, Kurata T, Nakata M, Ohme-Takagi M, et al. (2017) WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 29: 54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Ikeuchi M, Sugimoto K (2015a) Molecular mechanisms on callus formation: Accelerators and brakes. Bot Soc Jpn Rev (Shokubutsu Kagaku no Saizensen) 6: 2–22 (in Japanese) [Google Scholar]
- Iwase A, Mita K, Nonaka S, Ikeuchi M, Koizuka C, Ohnuma M, Ezura H, Imamura J, Sugimoto K (2015b) WIND1-based acquisition of regeneration competency in Arabidopsis and rapeseed. J Plant Res 128: 389–397 [DOI] [PubMed] [Google Scholar]
- Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, et al. (2011) The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in arabidopsis. Curr Biol 21: 508–514 [DOI] [PubMed] [Google Scholar]
- Kertbundit S, De Greve H, Deboeck F, Van Montagu M, Hernalsteens JP (1991) In vivo random beta-glucuronidase gene fusions in Arabidopsis thaliana. Proc Natl Acad Sci USA 88: 5212–5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee YS, Jeong S, Kim D, Chon S, Pak YK, Kim S, Ha J, Kang I, Choe W (2020) A small molecule, 4-phenylbutyric acid, suppresses HCV replication via epigenetically induced hepatic hepcidin. Int J Mol Sci 21: 5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaczuk M, Krętowski R, Bartoszewicz M, Cechowska-Pasko M (2016) Phenylbutyrate—a pan-HDAC inhibitor—suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol 37: 931–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Seo PJ (2018) Dynamic epigenetic changes during plant regeneration. Trends Plant Sci 23: 235–247 [DOI] [PubMed] [Google Scholar]
- Lee MH, Lee J, Choi SH, Jie EY, Jeong JC, Kim CY, Kim SW (2020) The effect of sodium butyrate on adventitious shoot formation varies among the plant species and the explant types. Int J Mol Sci 21: 8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuba V, LeTourneau D (1990) Auxin activity of phenylacetic acid in tissue culture. J Plant Growth Regul 9: 71–76 [Google Scholar]
- Li H, Soriano M, Cordewener J, Muiño JM, Riksen T, Fukuoka H, Angenent GC, Boutilier K (2014) The histone deacetylase inhibitor trichostatin A promotes totipotency in the male gametophyte. Plant Cell 26: 195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Vertocnik A, Town CD (2005) Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 56: 2095–2105 [DOI] [PubMed] [Google Scholar]
- Masterson C, Wood C (2009) Influence of mitochondrial β-oxidation on early pea seedling development. New Phytol 181: 832–842 [DOI] [PubMed] [Google Scholar]
- Menge A, Ageeva A, Georgii E, Bernhardt J, Wu K, Durner J, Lindermayr C (2017) Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant Physiol 173: 1434–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira MM, Huang S, Kapoor K, Hammond C, Hill RD, Stasolla C (2017) Expression of Arabidopsis class 1 phytoglobin (AtPgb1) delays death and degradation of the root apical meristem during severe PEG-induced water deficit. J Exp Bot 68: 5653–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Tran GB, Nguyen NH (2020) Homeostasis of histone acetylation is critical for auxin signaling and root morphogenesis. Plant Mol Biol 103: 1–7 [DOI] [PubMed] [Google Scholar]
- Palir N, Ruiter JPN, Wanders RJA, Houtkooper RH (2017) Identification of enzymes involved in oxidation of phenylbutyrate. J Lipid Res 58: 955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Quintana L, Llarena M, Reyes-Suárez D, Aldámiz-Echevarria L (2017) Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: Patient perspectives. Patient Prefer Adherence 11: 1489–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymen B, Kawamura A, Lambolez A, Inagaki S, Takebayashi A, Iwase A, Sakamoto Y, Sako K, Favero DS, Ikeuchi M, et al. (2019) Histone acetylation orchestrates wound-induced transcriptional activation and cellular reprogramming in Arabidopsis. Commun Biol 2: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Steward FC, Mapes MO, Mears K; Organization in Cultures Grown from Freely Suspended Cell (1958) Growth and organized development of cultured cells. II. Organization in cultures growtn from freely suspended cells. Am J Bot 45: 705–708 [Google Scholar]
- Strader LC, Bartel B (2009) The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor lndole-3-butyric acid. Plant Cell 21: 1992–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, Mashiguchi K, Tanaka K, Hishiyama S, Sakai T, Hanada K, Kinoshita-Tsujimura K, Yu H, Dai X, Takebayashi Y, et al. (2015) Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol 56: 1641–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M (2015) Historical review of research on plant cell dedifferentiation. J Plant Res 128: 349–359 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Schneider CL (1939) The relative activities of different auxins. Am J Bot 26: 328–333 [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Creation of a highly active synthetic AuxRE. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Brown CR (1996) Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1: 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcikowska B, Botor M, Morończyk J, Wójcik AM, Nodzyński T, Karcz J, Gaj MD (2018) Trichostatin A triggers an embryogenic transition in Arabidopsis explants via an auxin-related pathway. Front Plant Sci 9: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol Biol Cell 16: 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro C, Zhu JK, Yang Z (2016) Epigenetic modifications and plant hormone action. Mol Plant 9: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B (2007) IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol Biol 64: 59–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.