Abstract
Background:
In patients with secondary upper limb lymphedema, positive correlations have been observed between the dermal back flow (DBF) type and visualization of lymph nodes around the clavicle, between the former and the lymph flow pathway type, and between the latter and the visualization of lymph nodes around the clavicle when using single photon-emission computed tomography/computed tomography/lymphoscintigraphy (SPECT-CT LSG).
Methods and Results:
We analyzed the associations between the visualization of inguinal lymph nodes, the lymph flow pathway type, and the DBF type using SPECT-CT LSG in 81 patients with unilateral secondary lower limb lymphedema by statistical analysis using Fisher's exact test. We revealed that the lymph flow pathways in the lower limb can be classified into nine types because the type in the lower leg is not always equal to the type in the thigh. Associations were observed between the visualization of inguinal lymph nodes and types of DBF (p < 0.01), between the types of lymph flow pathway in the thighs and visualization of the inguinal lymph nodes (p = 0.02), and between the lymph flow pathway types in the thighs and lower legs (p < 0.01).
Conclusion:
Detriment to the superficial lymph flow pathways in the lower limb appears to usually start from the proximal side, and deep pathways are considered to become dominant from a compensatory perspective as lymphedema severity increases.
Keywords: SPECT-CT lymphoscintigraphy, lower limb lymphedema, lymph flow pathways, dermal back flow, inguinal lymph nodes
Introduction
In many developed countries, malignant neoplasm treatment is a major cause of secondary lower limb lymphedema. Approximately one in six patients who undergo surgical treatment for solid malignancies develops lymphedema.1 Particularly for gynecological cancer, 10%–49% of patients who undergo pelvic lymph node dissection and postoperative radiation develop secondary lymphedema.2–4
Lymphovenous anastomosis, a major surgical treatment for lymphedema, is used to access the remnant functional lymph flow pathways.5,6 Therefore, lymph flow pathway investigation in the limbs of patients with lymphedema may contribute to cancer survivors' quality of life.
Although some cadaver studies revealed dermal or subcutaneous lymph flow pathways, few studies have examined vital lymph flow pathways under the deep fascia.7–9 Secondary lower limb lymphedema clinical severity is relevant to the dermal back flow (DBF) type in conventional lymphoscintigraphy.10 DBF in lymphoscintigraphy images indicates reverse lymph flow from the collecting ducts to the dermis owing to a disorder of the superficial collecting ducts. This lesion type is usually exacerbated from the proximal side in the limbs, such that the severity increases as the DBF appears more distally.10,11 Fewer patients with secondary upper limb lymphedema had clavicular lymph nodes observed on lymphoscintigraphy as the severity progressed.11
We use single photon-emission computed tomography/computed tomography/lymphoscintigraphy (SPECT-CT LSG) to evaluate the association between the lymph flow pathways and visualize lymph nodes around the clavicle in patients with secondary upper limb lymphedema. A previous study highlighted the association between DBF type, vital lymph flow pathway type, and clavicular lymph node visualization, suggesting that the superficial lymph flow pathways, the channels in the subcutaneous layer, were the main stream to the clavicular lymph nodes.12
Similar mechanisms in the lymph flow pathways and lymph nodes in the inguinal region and relationship with DBF type are suspected among patients with secondary lower limb lymphedema.12,13 We aimed to verify this hypothesis and confirm the lymph flow pathways under the deep fascia.
Materials and Methods
Ethics statement
The Yokohama City University Hospital Ethics Committee approved the use of SPECT-CT LSG images and other patient data (Study Nos. B110707025 and B151105012). The study conformed to the Declaration of Helsinki, 2013 revision. The patients provided written informed consent for use of their clinical data.
Patient selection
In total, 112 patients were diagnosed with secondary lower limb lymphedema based on history and clinical findings. All these patients (5 men, 107 women) underwent SPECT-CT between August 2012 and June 2016. The number of affected limbs was 143 because 31 patients had both lower limbs affected. No patients were treated surgically before receiving an imaging diagnosis with SPECT-CT. When examinations were repeated during the study period, only the initial result was included. The patients' mean age was 55.7 years (±12.4; range 25–82 years).
SPECT-CT LSG and data evaluation
All patients underwent SPECT-CT LSG according to our institute's standard protocol. The radioisotope was 200 mBq/mL 99mTc-human serum albumin. The medium was injected into the subcutaneous tissue of the interdigital spaces between the first and second toes and between the fourth and fifth toes of both feet. A total of 160 MBq of medium was applied to each patient; 40 MBq in each interdigital space. Two hours after radioisotope injection, images of each patient's lower body from the toe to the diaphragm were taken using a SPECT-CT system equipped with a dual-headed gamma camera; Symbia T16 (Siemens Healthineers, Erlangen, Germany). Pseudo-colors were added to the obtained SPECT-CT images by relative evaluation of the accumulated radioisotope. Areas with high accumulation were marked with warm colors; areas with low accumulation were marked with cold colors (Fig. 1). The lower limbs were classified into five types according to the DBF location.10 They were also categorized in two groups based on inguinal lymph node visualization (positive or negative), as in previous literature.14 Meanwhile, the lymph flow pathways were classified into two groups based on the SPECT-CT LSG images as previously reported: superficial pathways included the subcutaneous layer and deep pathways the muscle or periosteal layer.12 Then, the lymph flow pattern was categorized based on the number of lymph flow pathways and the radioisotope signal intensity according to the pseudocolors in the cine images as superficial dominant (dominant superficial pathways), deep dominant (dominant deep pathways), and complex (both pathways functioned almost equally) types. A plastic surgeon and a fourth-year medical student with no experience evaluating SPECT-CT LSG images first assessed the images independently; a radiologist checked the evaluation of the results and general findings of the images. Final evaluations were made after both assessments had been enrolled; split decisions were corrected by discussion between the two evaluators. Eighty-one unilaterally affected limbs of the 143 limbs were studied.
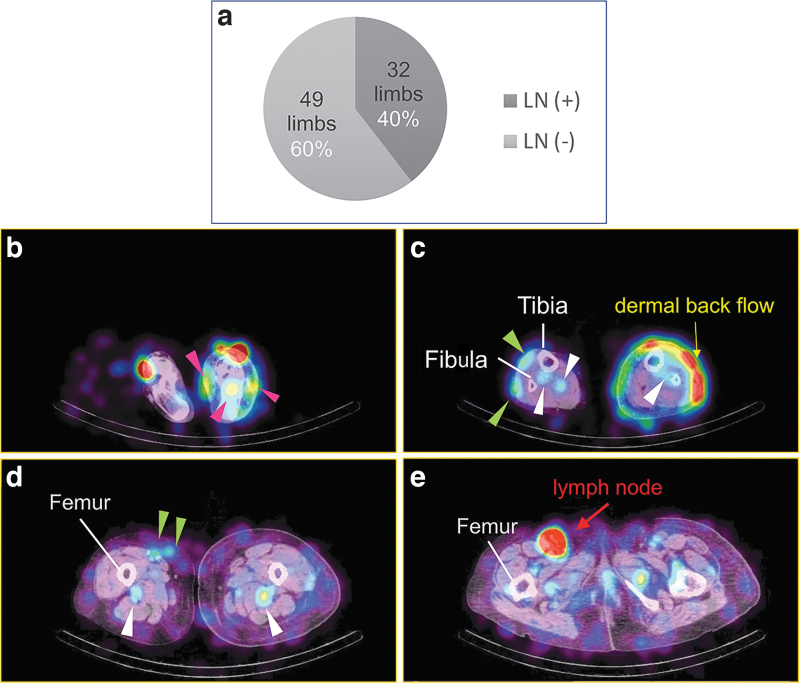
FIG. 1.
Classification of visualization of inguinal lymph nodes and the lymph flow type of lower limbs on SPECT-CT LSG images. (a) Ratio of the limbs of ipsilateral inguinal lymph nodes visualized on the SPECT-CT images. Inguinal lymph nodes were observed on the affected side in 40% of unilateral lower limb lymphedema cases. (b) A SPECT-CT LSG image of a secondary lower limb lymphedema patient: foot level stasis and spread in a subcutaneous layer of lymph is observed in the affected side (pink arrowheads). Additionally, subcutaneous tissue of the affected side is thicker than on the opposite side, right. (c) A SPECT-CT LSG image of a secondary lower limb lymphedema patient: distal lower leg level superficial lymph flow in the subcutaneous layer (green arrowheads) and in deep layers such as in the muscles or around the bones (white arrowheads) in the right (unaffected) side was observed. DBF in the left (affected) side was observed in a wide area whose signal is strong in front of the fibula. Meanwhile, lymph flow in the deep layer is observed (white arrowheads) in the affected side as well as on the right side. (d) A SPECT-CT LSG image of a secondary lower limb lymphedema patient: middle level of the thigh. The level of tracer intensity of the superficial pathways (green arrowheads) is almost equal to that of the deep pathway (white arrowheads) in the unaffected side. In contrast, in the affected side, the intensity of the tracer in subcutaneous tissue is weak while that of the deep flow behind the femur is conspicuous (white arrowheads). (e) A SPECT-CT LSG image of a secondary lower limb lymphedema patient: level of the inguinal lymph node high-level intensity of the tracer is observed in the superficial inguinal lymph node area in the right side, whereas no signal is found in the same area in the left. Besides, in this level of slice, only a weak signal of tracer is noticed in subcutaneous layer in both sides, while relatively strong signal areas are found in the muscle layer: deep pathways in both sides. DBF, dermal back flow; SPECT-CT LSG, single photon-emission computed tomography/computed tomography/lymphoscintigraphy.
Data analyses
The primary data were organized with FileMaker Pro 11 Advanced (Claris International, Inc., Santa Clara, CA), then compiled into secondary data with Microsoft Excel for Mac 2011 (Microsoft Japan Co. Ltd., Tokyo, Japan). For statistical analyses, Fisher's exact test was performed using R for Windows (x64 3.1.3). As a null hypothesis, each factor was thought to be independent of the others. p < 0.05 was considered statistically significant.
Results
Patient background
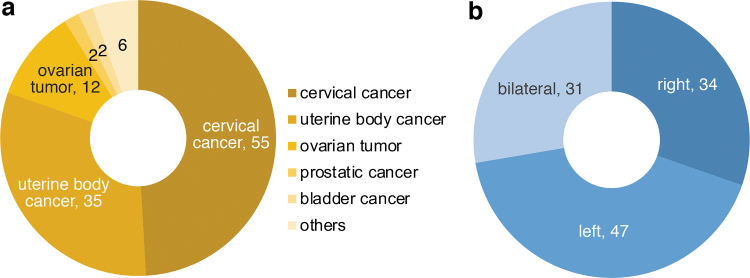
More than half the primary diseases affecting the patients in our study were uterine body and cervix cancers (Fig. 2). Among the 81 unilaterally affected lower limbs, 34 cases involved the right side (Fig. 2).
FIG. 2.
Background of the patients. (a) The primary disease of the patients. About 80% of the primary diseases were gynecological cancers. (b) Laterality of the affected lower limbs. About 30% of the patients were affected on both sides. The left side was affected slightly more than the right side in the unilateral cases.
Inguinal lymph node visualization
Inguinal lymph nodes were observed in 32 cases [LN (+) group], while the other 49 cases had no detectable lymph nodes (Supplementary Table S1 and Fig. 1). In contrast, 80 of 81 unaffected sides revealed lymph nodes in the inguinal region, and only 1 case had no lymph nodes (Supplementary Table S2).
Classification based on DBF type
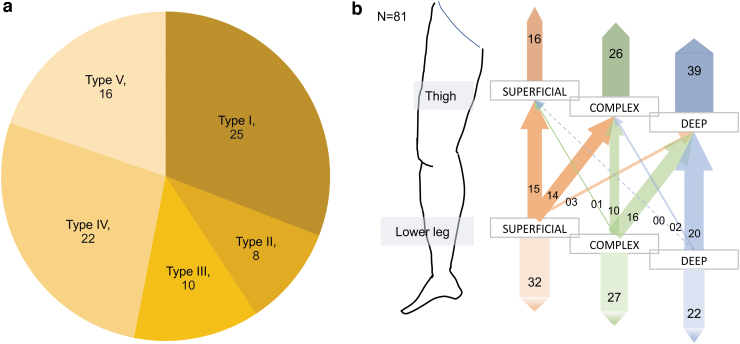
All patients were categorized into five groups based on the DBF pattern. Relatively mild cases (types I–III) accounted for about half the affected limbs (Fig. 3a).
FIG. 3.
Results of the basic analysis of the affected limbs. (a) Classification of DBF type of the affected limbs. The numbers in each sector indicate the numbers of the affected limbs. Almost half of the limbs are included in severe cases. (b) Classification of the affected lower limbs based on the lymph flow types in the thigh and the lower leg. In the limbs with superficial dominance in the lower legs, almost half of the limbs are superficial dominant in the thigh. On the contrary, over 90% of cases deep dominant in the lower leg are also deep dominant in the thigh.
Lymph flow pathway type classification
Split decisions for classifying the lymph flow pathway type occurred in over half the lower limb lymphedema cases; however, few cases of upper limb lymphedema had inconsistent assessments between the two evaluators. One important reason was that the lymph flow pathway type in the lower leg often differed from that in the thigh; therefore, the lymph flow pathway type could not be categorized as one single flow in a limb as in upper limb lymphedema patients. Therefore, we divided the lower limb into two parts, the thigh and the lower leg, and evaluated the lymph flow type of each part separately (Supplementary Movie S1). Therefore, most cases of deep dominance in the thigh were classified as deep dominance in the lower legs (Fig. 3b and Supplementary Fig. S1).
Based on these fundamental findings, some associations were noted among inguinal lymph node visualization, DBF type, and lymph flow type. The details are presented below.
Association between inguinal lymph node visualization and DBF type
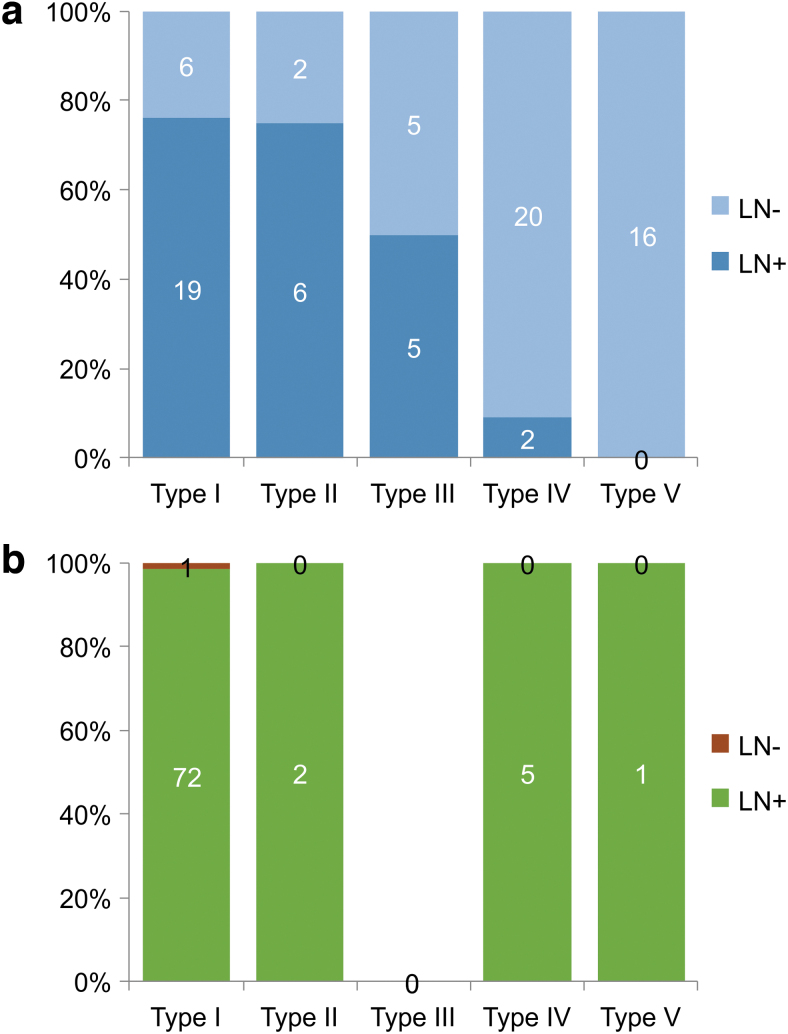
First, relevance was observed between inguinal lymph node visualization and DBF type in the affected limbs (p = 1.255E-09; Fisher's exact test, Supplementary Table S1 and Fig. 4a). In the LN (+) group, 19 of 32 limbs were classified as type I, which was the largest group, and 6 limbs were classified as type II. On the unaffected side, 72 limbs were type I DBF classification, whereas 5 limbs were type IV (Supplementary Table S2 and Fig. 4b). In contrast, in the LN (−) group, 20 limbs were type IV, followed by 16 type V limbs (Supplementary Table S1 and Fig. 4a).
FIG. 4.
The ratio of the lower limbs in each type of DBF based on the visualization of the lymph nodes in the inguinal region. (a) The affected side. The lymph nodes in the inguinal region came to be obscure, as the DBF appears in the distal side of the lower limb (p = 5.61E-09). LN (+): lymph nodes were visualized in the SPECT-CT LSG images, LN (−): lymph nodes were absent in the SPECT-CT LSG images. (b) The unaffected side. The lymph nodes in the inguinal region were observed in almost all the cases in the unaffected lower limbs, except for one case in type I. LN (+): lymph nodes were visualized in the SPECT-CT LSG images, LN (−): lymph nodes were absent in the SPECT-CT LSG images.
Association between inguinal lymph node visualization and lymph flow type
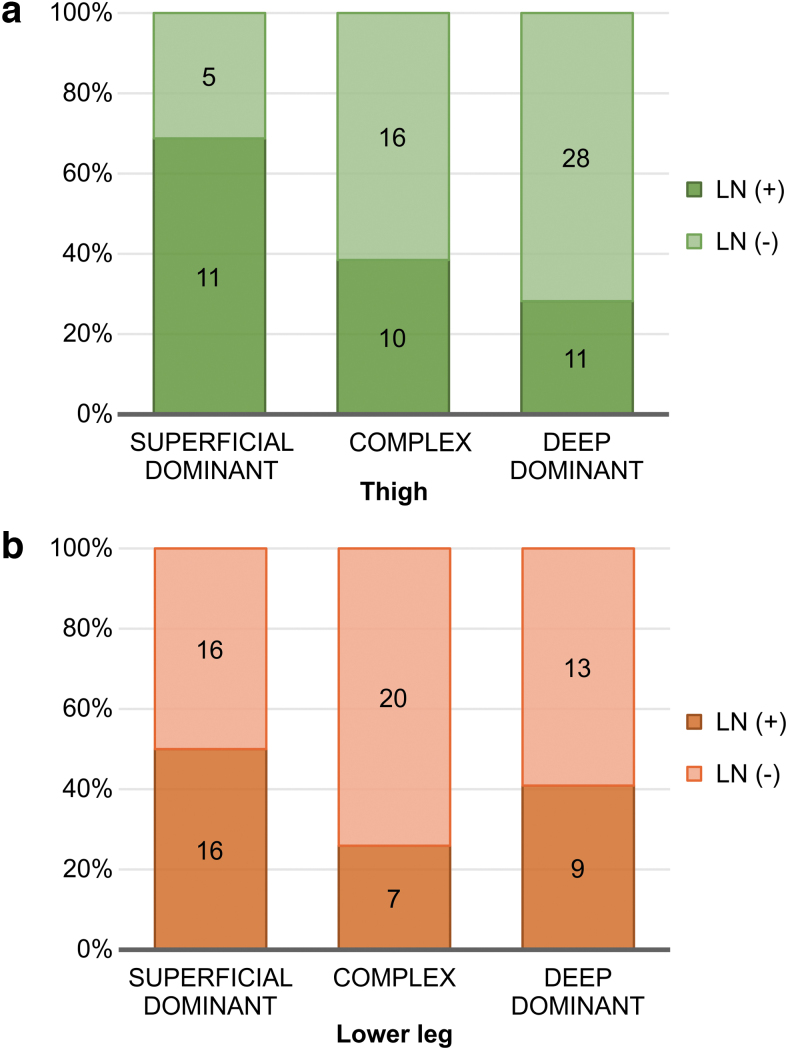
Next, a relevant association was observed between the lymph flow pathway type in the thigh and inguinal lymph node visualization. There was no significant difference in the lymph flow pathway type in the thigh in the LN (+) group; superficial dominant, deep dominant, and complex limbs had a frequency of 11, 11, and 10, respectively (Fig. 5a). In contrast, there was a tendency for deep dominance in the thigh of the LN (−) group, with superficial dominant, deep dominant, and complex limbs representing 5, 28, and 16 times, respectively (Supplementary Table S3, p = 2.07E-02 Fisher's exact test, Fig. 5b). Meanwhile, no association was identified between the lymph flow pathway type in the lower leg and inguinal lymph node visualization (Supplementary Table S3, p = 1.772E-01; Fisher's exact test, Fig. 5).
FIG. 5.
Association between the visualization of the inguinal lymph nodes and the lymph flow pathways in the affected limbs. (a) The association in the thigh. Statistical analysis revealed that the two factors are not significantly independent (p = 2.07E-02; Fisher's exact test). (b) The association in the lower leg visualization of the inguinal lymph nodes and the lymph flow pathways in the affected limbs are independent based on statistical analysis (p = 1.772E-01; Fisher's exact test).
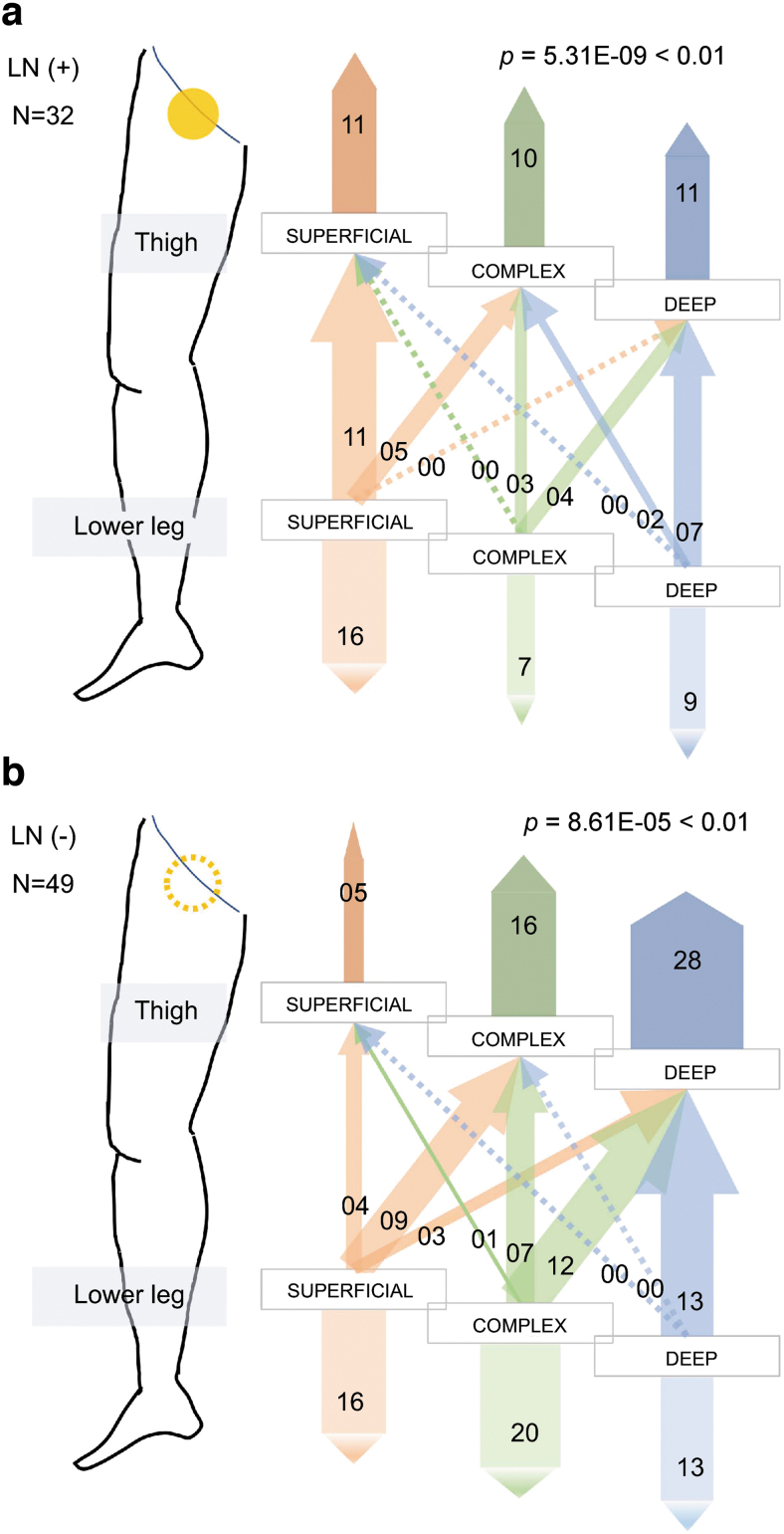
In the LN (+) group, the lymph flow pathway type in the thigh was implicated to be statistically associated with that in the lower leg (Supplementary Table S4 and Fig. 6a, p = 5.31E-09; Fisher's exact test, Supplementary Fig. S2). Furthermore, in the LN (−) group, the lymph flow pathway type in the thigh was statistically associated with that in the lower leg (Supplementary Table S5 and Fig. 6b, p = 8.61E-05; Fisher's exact test, Supplementary Fig. S2).
FIG. 6.
Association between the lymph flow pathways in the thigh and in the lower leg in the affected limbs. (a) The association in the LN (+) cases. The lymph flow pathways in the thigh and in the lower leg are not statistically independent (p = 5.31E-09; Fisher's exact test). (b) The association in the LN (−) cases. Statistical analysis revealed that the lymph flow pathways in the thigh and in the lower leg are not significantly independent (p = 8.61E-05; Fisher's exact test). Filled yellow circle indicates that lymph nodes were positive and dotted yellow circle indicates absence of lymph nodes.
Association between DBF type and lymph flow pathway type
To investigate the relationship between DBF type and lymph flow pathway type in the thigh and lower leg, more than 167 limbs should have been involved based on a statistical test: degree of freedom = 8, α = 0.05, power = 0.8, effect size = 0.3. Therefore, we investigated the inclination between DBF type and lymph flow pathway type. The results suggested that as the severity increased, the lymph flow became deep dominant both in the thighs and lower legs.
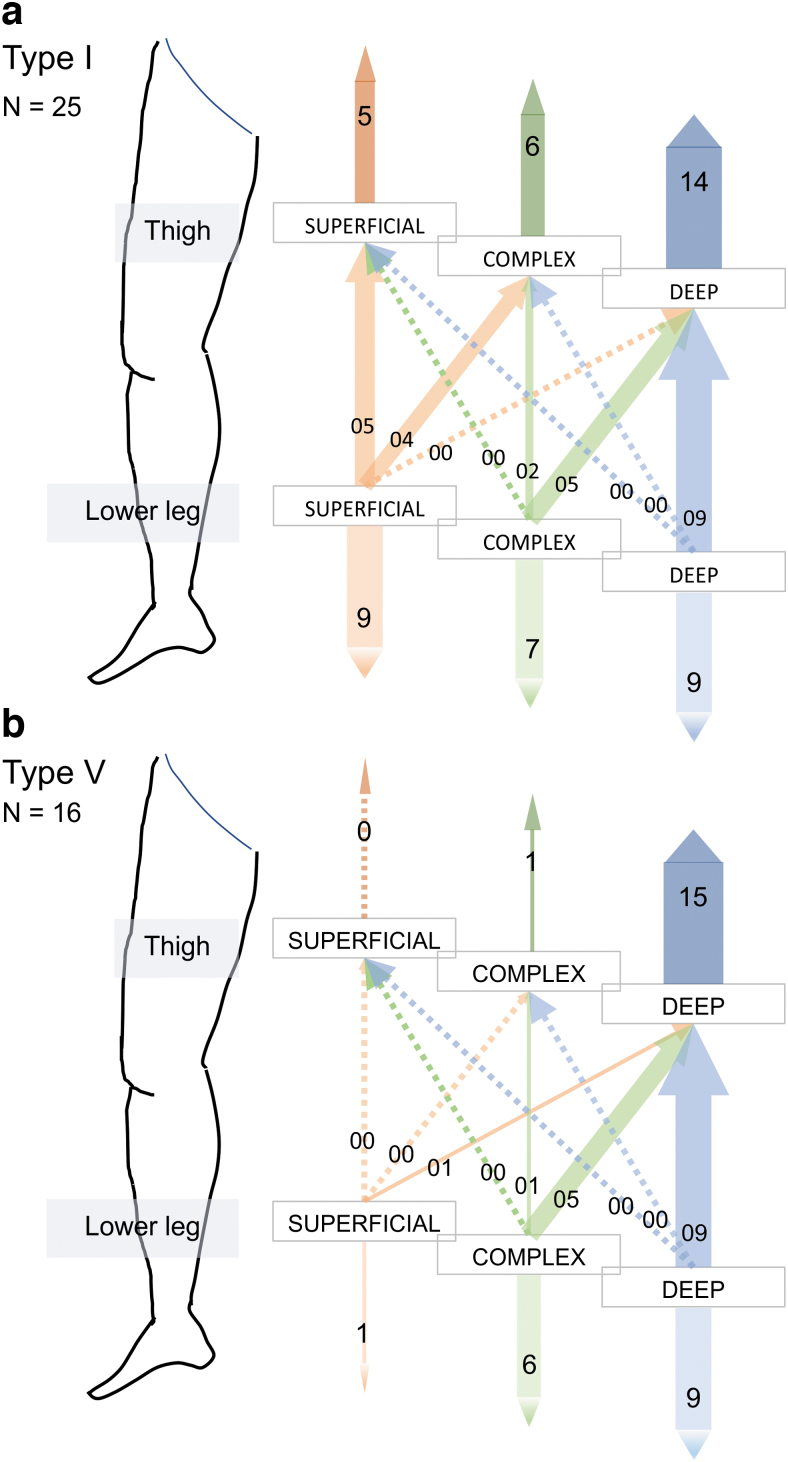
First, type I DBF, which is the clinical stage and imaging diagnosis of the mildest severity, and type V DBF, which is the most severe type clinically, were compared. As the severity increased, the deep pathways seemed to become dominant both in the thigh and lower legs (Fig. 7 and Supplementary Fig. S3). In contrast, type I–III cases, considered to be mild to moderate clinically, included superficial dominant cases both in the thigh and lower legs (Supplementary Fig. S4). However, with type I DBF classification, most cases were categorized into deep dominant in the thigh and in the lower leg like type I DBF in patients with secondary upper limb lymphedema.12 Furthermore, these seven patients were women, five of whom were affected in the right limb and two in the left. On the unaffected side, inguinal lymph nodes were observed on imaging in all cases, whereas the lymph flow types of many of the limbs were categorized as deep dominant both in the thigh and lower leg (Supplementary Table S6 and Supplementary Fig. S5).
FIG. 7.
Association between the lymph flow pathways in the thigh and in the lower leg in the affected limbs of type I and type V DBF classification patients. (a) Type I lower limbs. Most of the affected lower legs classified into superficial dominant or complex in the thigh were also classified as superficial dominant or complex in the lower leg. However, no limb was classified into deep dominant in the thigh and superficial dominant in the lower leg, although nine limbs were classified into deep dominant in the thigh and deep dominant in the lower leg. (b) Type V lower limbs. Almost all of the limbs were considered to be deep dominant in the thigh. Among the 15 limbs out of 16, 9 limbs were classified into deep dominant in the lower leg.
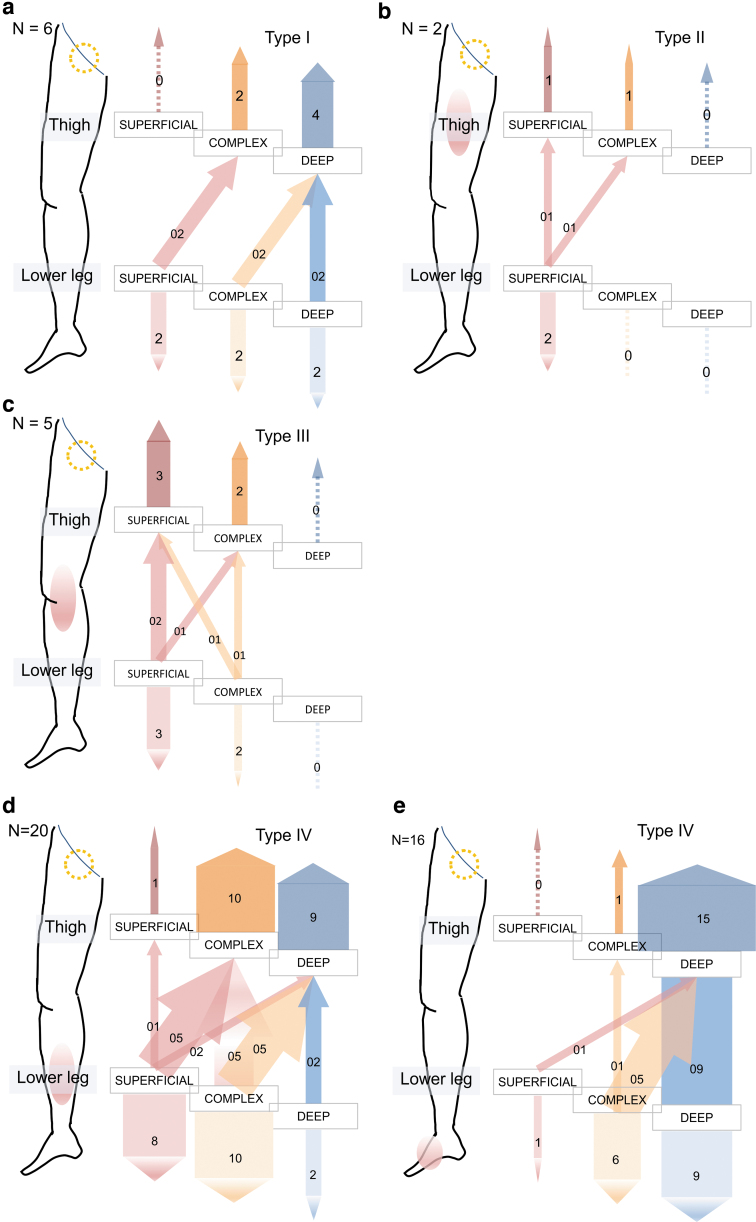
Next, the affected limbs in the LN (−) group were examined closely because the number of inguinal lymph nodes appeared to decrease as the DBF type became severe. There was no affected limb of the superficial dominant type in the thigh of type I DBF in the LN (−) group and no deep dominant case in the thighs of type II or III DBF (Fig. 8a–c). In contrast, only a few type IV and V cases were observed with superficial dominance in the thigh (Fig. 8d, e). Therefore, as the severity increased, the lymph flow became deep dominant both in the thighs and lower legs.
FIG. 8.
Association between the type of DBF and the type of lymph flow pathways in the cases whose inguinal lymph nodes were negative in the affected side. (a) Graph of type I limbs. No limbs were considered to be superficial dominant in the thigh, whereas only six limbs were classified in this type. (b) Graph of type II limbs. There was no limb classified into deep dominant in the thigh, whereas only two cases were classified into this type. (c) Graph of type III limbs. There was no limb classified into deep dominant in the thigh, as type II. (d) Graph of type IV. Only 1 limb out of 20 was classified into superficial dominant in the thigh. In viewpoint of lower leg, only 8 limbs out of 20 were considered to be superficial dominant. (e) Graph of type V. In contrast to type II and type III, no limb was regarded as superficial dominant in the thigh while only 1 limb out of 16 was classified into superficial dominant in the lower leg. Dotted yellow circles indicate that the lymph nodes were negative.
Comparing the LN (+) group with the LN (−) group, the number of limbs was greater in the LN (−) group than in the LN (+) group, in which lymph flow pathways in the thigh and lower legs were classified as deep dominant. However, in terms of the lymph flow pathway types, an association was observed between the thigh and the lower leg regardless of inguinal lymph node visualization [LN (+): p = 5.607E-5, LN (−): p = 8.607E-5, Fisher's exact test].
Discussion
First, contrary to expectations, many cases had a different lymph flow pathway type in the thigh compared with that in the lower leg. This is a notable difference between the lymph flow pathways in the upper and lower limbs; however, lymph flow pathways under the deep fascia were observed in both the lower and upper limbs. Therefore, we categorized lymph flow pathways in the lower limbs into nine types and those in the upper limbs into only three types.
Second, inguinal lymph node visualization was associated with clinical symptoms of secondary lower limb lymphedema because lymph nodes were detected in 80 of 81 unaffected sides. This suggests that inguinal lymph nodes keep lymph drainage active, which avoids the clinical symptom of edema. In contrast, in the affected side, the LN (+) group was the major group in DBF types I and II, while the LN (−) group was the major group in the IV and V DBF types. These findings suggest that inguinal lymph nodes respond not only to secondary lower limb lymphedema severity but also to the DBF lymph flow system. This contrasts with the popliteal lymph nodes described in a previous report about secondary lower limb lymphedema.15,16
Apart from these, there were few cases of type IV and V DBF cases in the clinically unaffected side; in two cases, the inguinal lymph nodes were absent on SPECT-CT LSG images. Because the clinical diagnosis of unilateral secondary lower limb lymphedema is based on patients' subjective symptoms, bilateral lower limb lymphedema could be diagnosed in some patients based on their own awareness.
Third, an association was suspected between inguinal lymph node visualization and lymph flow pathway type in the thigh, with the former considered to be independent of the lymph flow pathway type in the lower leg. These findings indicated that inguinal lymph nodes collected lymph flow mainly from the thigh, rather than from the lower leg. Moreover, the superficial lymph flow pathways are suggested to involve dominant flow to the inguinal lymph nodes because deep pathways were dominant in the LN (−) group. However, in the LN (+) group, superficial pathways were not statistically dominant in the thigh. Therefore, although superficial lymph flow pathways are dominant to the inguinal lymph nodes, deep flow pathways still function simultaneously even when the lymph nodes are intact. However, when the inguinal lymph nodes become affected, deep pathways carry lymph flow from the lower limb to the trunk as a form of bypass.
Additionally, our results suggested that deep flow pathways become dominant as DBF type severity increases both in the LN (+) and LN (−) groups. This tendency is similar to that of secondary upper limb lymphedema. Especially from the viewpoint of DBF type severity, the ratio of deep dominant cases was higher in the type V group than in the milder types; the ratio of deep dominant was 27% in type I and II, 6% in type III and IV, and 56% in type V. However, it is interesting to note that many cases in the type I DBF group had deep dominant lymph flow pathways in both the thigh and lower leg. Additionally, type I had fewer superficial dominant cases than type II in the thigh and in the lower leg. These results are similar to those of the secondary upper limb lymphedema cases that we previously reported.12 On reviewing the cases in which lymph flow pathways were deep dominant in the thigh and lower leg, all the unaffected limbs, except one, were classified as type I DBF. However, in the unaffected side in these cases, three cases of lymph flow pathways in the thighs were classified as complex type and the other four cases were deep dominant type, whereas no case was regarded as superficial dominant. In contrast, lymph flow pathways in the lower legs were classified into three types: two cases were superficial dominant type, two other cases were complex type, and the remaining three cases were deep dominant type (Supplementary Table S6 and Supplementary Fig. S5).
Focusing on the lower legs, deep dominant cases increased as severity progressed, especially DBF types II–V in the LN (−) group. Therefore, the DBF type severity was related to the lymph flow pathway type not only in the thigh but also in the lower leg.
There were some limitations to our study. First, the lymph flow pathway type in the thigh was different from that in the lower leg in many cases. The lymph flow pathway types of secondary lower limb lymphedema are classified into nine types. Second, because we were limited to the use of 99mTc-human serum albumin owing to blood transfusion storage issues, 81 limbs were the upper limit to be included in the analysis.12 Third, given the results regarding the lymph flow pathway type, there might be room for improvement. The SPECT-CT images we used had pseudocolor added to them by relative evaluation, not by absolute scale; thus, there might have been unaccounted differences in lymph flow velocity between the cases. If we could have employed three or more evaluators to assess the lymph flow pathway type, more objective decisions could have been made. Based on this research, further objective findings will be obtained by using artificial intelligence to determine lymph flow pathway types.
Conclusion
Statistical significance was observed in the association between the inguinal lymph nodes and the DBF type using SPECT-CT images of patients with secondary lower limb lymphedema, similar to the findings of our previous report of secondary upper limb lymphedema. Thus, as the lymphedema severity increased, the lymph flow into the inguinal lymph nodes decreased. On the contrary, association between the lower limb lymph flow and the lymphedema severity was more complex when comparing the results to that obtained with upper limb lymphedema. Because the cross-sectional area of the lower limb is usually larger compared with the upper limb, the lymph flow pathways in the lower limb are thought to change between the superficial and deep layers more frequently than those in the upper limb. Furthermore, the crossovers of the lymph flow pathway around the knee were very complex. This is part of the reason for the difference between lower and upper limb lymphedema. As mentioned earlier, the lymph flow pathways came to be deep dominant as the severity worsened both in the thighs and lower legs. In addition, the pathways in the thigh tended to become deep dominant compared with those in the lower leg. Thus, detriment to the superficial pathways in the lower limb usually starts from the proximal side, and deep pathways are considered to become dominant from a compensatory perspective.
Further studies should investigate the laterality of the affected side of unilateral lower limb lymphedema and evaluate bilateral lower limb lymphedema.
Data Availability
The data and supplementary data are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
The authors thank Ms. Miho Kobayashi for collecting and managing the data and documents. They would like to thank Editage (www.editage.jp) for English language editing.
Authors' Contributions
T.F., T.M., T.K., and J.M. participated in the design of this study, assessment of the results, and drafting of the article. T.F. and T.M. participated in the assessment of the images of SPECT-CT LSG and created the database of the patients. T.F. and K.H. performed the statistical analysis and participated in the assessment of the result. Y.Y., S.Ka, S.Ki, A.I., and E.M. checked the details of the patients' backgrounds, such as past history and treatments for primary diseases. S.M., S.A., E.A., and T.I. participated in drafting the article and prepared the article's figures and movies.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010; 116:5138–5149. [DOI] [PubMed] [Google Scholar]
- 2. Pappalardo M, Lin C, Ho OA, Kuo CF, Lin CY, Cheng MH. Staging and clinical correlations of lymphoscintigraphy for unilateral gynecological cancer-related lymphedema. J Surg Oncol 2020; 121:422–434. [DOI] [PubMed] [Google Scholar]
- 3. Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 2006; 103:714–718. [DOI] [PubMed] [Google Scholar]
- 4. Ohba Y, Todo Y, Kobayashi N, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol 2011; 16:238–243. [DOI] [PubMed] [Google Scholar]
- 5. Rosian K, Stanak M. Efficacy and safety assessment of lymphovenous anastomosis in patients with primary and secondary lymphoedema: A systematic review of prospective evidence. Microsurgery 2019; 39:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pons G, Tang JB. Major changes in lymphatic microsurgery and microvascular surgery in past 20 years. Clin Plast Surg 2020; 47:679–683. [DOI] [PubMed] [Google Scholar]
- 7. Suami H, Pan WR, Mann GB, Taylor GI. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: A human cadaver study. Ann Surg Oncol 2008; 15:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suami H, Scaglioni MF. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin Plast Surg 2018; 32:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinaoka A, Koshimune S, Suami H, et al. Lower-limb lymphatic drainage pathways and lymph nodes: A CT lymphangiography cadaver study. Radiology 2020; 294:223–229. [DOI] [PubMed] [Google Scholar]
- 10. Maegawa J, Mikami T, Yamamoto Y, Satake T, Kobayashi S. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery 2010; 30:437–442. [DOI] [PubMed] [Google Scholar]
- 11. Mikami T, Hosono M, Yabuki Y, et al. Classification of lymphoscintigraphy and relevance to surgical indication for lymphaticovenous anastomosis in upper limb lymphedema. Lymphology 2011; 44:155–167. [PubMed] [Google Scholar]
- 12. Mikami T, Koyama A, Hashimoto K, et al. Pathological changes in the lymphatic system of patients with secondary upper limb lymphoedema. Sci Rep 2019; 9:8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiss M, Baumeister RG, Frick A, Wallmichrath J, Bartenstein P, Rominger A. Primary lymphedema of the lower limb: The clinical utility of single photon emission computed tomography/CT. Korean J Radiol 2015; 16:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iimura T, Fukushima Y, Kumita S, Ogawa R, Hyakusoku H. Estimating lymphodynamic conditions and lymphovenous anastomosis efficacy using (99m)Tc-phytate lymphoscintigraphy with SPECT-CT in patients with lower-limb lymphedema. Plast Reconstr Surg Glob Open 2015; 3:e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karacavus S, Yilmaz YK, Ekim H. Clinical significance of lymphoscintigraphy findings in the evaluation of lower extremity lymphedema. Mol Imaging Radionucl Ther 2015; 24:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandeel AA, Ahmed Younes J, Mohamed Zaher A. Significance of popliteal lymph nodes visualization during radionuclide lymphoscintigraphy for lower limb lymphedema. Indian J Nucl Med 2013; 28:134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and supplementary data are available from the corresponding author upon reasonable request.