Abstract
Background:
Compression therapy is an important part of the treatment of patients with lymphedema or chronic venous disease. However, there is no validated questionnaire evaluating the effect of compression and its acceptance by the patient. Therefore, the aims of this study were to construct a questionnaire evaluating the effect of compression and its acceptance by the patient, that is, the Dutch ICC Compression Questionnaire (ICC-CQ), to investigate its reliability and validity, and to translate it into English.
Methods and Results:
Eleven experts in applying compression and 51 Dutch patients with experience of using compression were involved in the construction process. One part of the ICC-CQ has to be completed by the patient and evaluates seven domains. The other part has to be completed by the health care provider and comprises three domains. Reliability and validity of the final version was investigated in a new group of 79 Dutch-speaking patients with lymphedema or chronic venous disease, wearing compression garments (N = 52) or bandages (N = 27). Except for one domain, the Intraclass Correlation Coefficients for test–rest/interrater reliability ranged from 0.55 to 0.93. Cronbach's alpha for internal consistency ranged from 0.71 to 0.97. Eighty-nine percent of the patients fully understood the questionnaire indicating good face validity, and 87% found it complete indicating good content validity. Construct validity was considered good since 10 out of 11 hypotheses were accepted.
Conclusion:
The ICC-CQ is the first reliable and valid questionnaire evaluating different kinds of compression and the experience by patients with lymphedema or chronic venous disease.
Keywords: compression, questionnaire, lymphedema, chronic venous disease
Introduction
Lymphedema is a pathological condition characterized by an abnormal collection of fluid within the interstitium. This may lead to swelling of the limbs, trunk, head/neck, or genitalia.1 Development of lymphedema has a considerable influence on quality of life. Prevalence rate of chronic lymphedema is estimated at 150 million people worldwide. Chronic venous disease manifests as telangiectasia, reticular veins, and varicose veins. In more advanced disease, there may be edema, skin fibrosis, and ulceration. This is a highly prevalent condition2 and affects millions of people worldwide.3
Compression therapy is the mainstay of lymphedema treatment and of conservative measures in chronic venous disease.4,5 Compression therapy can be applied as bandages, compression garments and as intermittent pneumatic compression.1 To evaluate the effect of compression material and systems, often the pressure under the material or system (i.e., interface pressure) is measured.6 In addition, quality of life of the subject and disease-related symptoms are evaluated. Lymphedema-specific questionnaires are the upper limb lymphedema-27,7 Lymphedema Functioning, Disability and Health questionnaire (Lymph-ICF),8,9 Lymphoedema Quality of Life Tool,10 Freiburg Life Quality Assessment for patients with lymphedema-l,11 and Lymphedema Quality of Life Inventory.12 Questionnaires specific to evaluate chronic venous disease are VEINES Quality of Life instrument for chronic venous disorders of the leg.13,14 Chronic Venous Insufficiency Questionnaire (CIVIQ),15 Tübingen Questionnaire for measuring Quality of Life in patients with Chronic Venous Insufficiency,16 Venous Leg Ulcer Quality of Life Questionnaire,17 and Wound-Qol.18 However, none of these questionnaires specifically evaluate the effect of compression therapy and the experience of patients using the compression. Besides the pressure and quality of life and symptoms, it is necessary to evaluate other aspects related to compression, such as the ease of application and removing of compression and the comfort and complications of compression. Previous studies about the effect of compression therapy used a self-developed compression-specific evaluation tool, wherein reliability or validity was not yet investigated.19–21 To our knowledge, no reliable and valid compression-specific questionnaire exists to assess different aspects of compression materials and devices applied in patients with lymphedema or chronic venous disease.
Therefore, the first aim of our study was to develop an assessment tool to evaluate the effect of different kinds of compression materials and devices applied in patients with lymphedema or chronic venous disease. The second aim was to investigate its reliability and validity. The third aim was to translate the questionnaire into English.
Material and Methods
Step 1: Development of the Dutch ICC-Compression Questionnaire
The development of the questionnaire was based on input from Dutch patients receiving compression therapy and experts in applying compression therapy. Patients with lymphedema or chronic venous disease and receiving compression therapy were recruited in the Centre for Lymphoedema and Phlebology at the University Hospitals of Leuven. The expert group was composed of individuals with a variety of professions and from different countries. Each version of the questionnaire was corrected based on the remarks from experts and patients on the previous version. A version was considered as final if consensus was reached within the expert group.
Step 2: Investigation of reliability and validity of the Dutch ICC-Compression Questionnaire
Subjects
A new group of patients was recruited in the Centre for Lymphoedema and Phlebology of the department of Vascular Surgery at the University Hospitals Leuven (Belgium) and in the Lymphology and Phlebology Clinic at Nij Smellinghe Hospital (the Netherlands). Subjects who had planned for a consultation in the hospital were contacted by phone, and the study was explained. Subjects were included if they were wearing compression stockings/garments or bandages for the treatment of lymphedema (primary and secondary) or chronic venous disease (any stage). Subjects <18 years, non-Dutch speaking and unable to fill out questionnaires independently were excluded.
Study procedure
If a subject agreed to participate, the informed consent was sent to the patient, together with four questionnaires. The following questionnaires had to be filled out 24–48 hours before the consultation:
ICC Compression Questionnaire (ICC-CQ) for patients (ICC-CQ-P).
A questionnaire for assessing face and content validity for patients (see further).
Short Form Health Survey-36 (SF-36): a reliable and valid questionnaire to assess health-related quality of life in a general population.22
For subjects with lymphedema of upper or lower limb, the Lymphoedema Functioning Disability and Health questionnaire (Lymph-ICF): a valid and reliable questionnaire for the evaluation of problems in functioning in patients with lymphedema of the lower limb8 or the upper limb.9,23
For subjects with chronic venous disease, the CIVIQ: a disease-specific reliable and valid questionnaire to evaluate the health-related quality of life in patients suffering from chronic venous insufficiency.24
All participants were asked to wear the compression material or device at the time of the consultation. During the consultation, the subject filled out the ICC-CQ-P a second time. Thereafter, two independent experienced health care providers (providing care during the consultation, such as a vascular surgeon, dermatologist, physical therapist, or nurse) filled out the ICC-CQ for health care providers (ICC-CQ-H). Each health care provider was also asked to fill out the questionnaire for assessing face and content validity for health care providers.
The questionnaire to assess face and content validity of the ICC-CQ-P and ICC-CQ-H contained questions (1) about the understandability of the questions in the different domains; (2) about the clarity of the scoring system; and (3) about the completeness of the questionnaire.
Data analysis
SPSS version 24.0 was used to analyze the quantitative outcomes.
Reliability
To investigate test–retest reliability of each domain of the ICC-CQ-P, the Intraclass Correlation Coefficient [ICC 2,1] and its 95% confidence interval was determined. To evaluate interrater reliability of each domain of the ICC-CQ-H, the ICC [1,1] was determined. To evaluate internal consistency for each domain, Cronbach's alpha coefficient was determined. To interpret the magnitude of the within-subjects variation of two scores on the different domains, the standard error of measurement (SEM) was calculated by using the following formula: SEM = SD(√1 − ICC), where SD was the mean standard deviation of the two ratings.25 For the domain scores of the ICC-CQ-P, to evaluate clinically important changes, the smallest real difference (SRD) was calculated as follows: SRD = 1.96 × SEM × √2.25 To obtain a reference range for the mean difference of the scores of the two test occasions, the 95% SRD was calculated as the mean difference between the two test occasions ±SRD.
ICCs, Cronbach's alpha coefficients, and correlation coefficients were interpreted to be weak below 0.40, moderate between 0.40 and 0.74, strong between 0.75 and 0.90, and very strong above 0.90.26
Validity
Face validity, content, and construct validity were examined. It was not possible to examine criterion validity. There is no gold standard for evaluating compression materials and devices.
Face validity was examined by asking the patients and health care providers whether the questions in the different domains of ICC-CQ-P and ICC-CQ-H, respectively, were understandable and whether the scoring system was obvious.
Content validity was examined by analyzing the answers of the patients wearing compression and their health care providers about the comprehensiveness of the questionnaire. In addition, all participants could give additional general remarks. Finally, floor and ceiling effect was investigated for the different domain scores. Floor and ceiling effects were considered to exist if more than 20% of responses reached the lowest or highest possible score.27 Face and content validity were defined as very good if >90% of the patients found the whole questionnaire understandable and complete and >90% of the domains-scores had no floor or ceiling effect. It was defined as good if the proportion was situated between 75% and 90%, as moderate if the proportion was situated between 40% and 74%, and as weak if the proportion was <40%.
Construct validity is a process in which validity is evaluated in terms of the extent to which a measure correlates with variables in a manner consistent with theory.28 In the present study, convergent validity is investigated, which is a part of construct validity. The relationship between scores on the different domains of the ICC-CQ and other disease-specific and general quality of life measures was examined. A priori, hypotheses were formulated about possible moderate to high correlations coefficients between certain domains of the ICC-CQ-P and domains of (1) the SF-36 for all participants, (2) the CIVIQ for participants with chronic venous disease, and (3) the Lymph-ICF-LL for participants with lower limb lymphedema and the Lymph-ICF-UL for participants with upper limb lymphedema (Table 1). To investigate the relationship between the domains, the Pearson correlation coefficient was used. Since a valid evaluation tool for the fitting of compression and for the skin integrity is missing, construct validity of the ICC-CQ-H was not investigated. Construct validity was defined as “very good” if more than 90% of all hypotheses were confirmed, as “good” if 75% to 90% of the hypotheses were confirmed, and as “moderate” if 40% to 74% of the hypotheses were confirmed.
Table 1.
Hypotheses Regarding Construct Validity of the ICC Compression Questionnaire for Patients
| Hypothesis | Rationale | Pearson r (p) |
|---|---|---|
| Considering all correlation coefficients for various domains of ICC-CQ-P and SF-36, moderate to high correlation coefficients would occur for: | ||
| 1. ICC-CQ-P “physical functioning” and SF-36 “physical functioning” | ICC-CQ-P physical functioning: How do you rate, your ability to move your ankle/wrist, knee/elbow, hip/shoulder, to walk, carry out job, complete household chores, practice sports, carry out leisure activities and social activities? | 0.61 (p < 0.01) |
| SF-36 physical functioning: Does your health limit you in the following activities? Vigorous activities, such as lifting heavy objects, moderate activities such as moving a table, vacuum cleaning, lifting or carrying groceries, climbing several flights of stairs, climbing one flight of stairs, bending kneeling or stooping, walking more than a mile, walking half a mile, walking 100 yards, bathing or dressing yourself? | ||
| 2. ICC-CQ-P “physical functioning” and SF-36 “role-physical” | ICC-CQ-P physical functioning: see 1. | 0.65 (p < 0.01) |
| SF-36 physical role: During the past 2 weeks, how many times have you had any of the following problems with your work or other daily activities as a result of your physical health? Cut down on the amount of time you spent on work and other activities, accomplished less than you would like, were limited in the kind of work or other activities, had difficulty performing the work or other activities. | ||
| 3. ICC-CQ-P “physical functioning” and SF-36 “social functioning” | ICC-CQ-P physical functioning: see 1. | 0.57 (p < 0.01) |
| SF-36 social functioning: During the past 2 weeks, to what extent have your physical health or emotional problems interfered with your normal social activities with family, neighbors, or groups? During the past 2 weeks, how much of the time has your physical health or emotional problems interfered with your social activities? | ||
| 4. ICC-CQ-P “disease-related symptoms” and SF-36 “bodily pain” | ICC-CQ-P disease-related symptoms: Do you experience in relation to your disease pain, loss of muscle strength, heaviness, swelling, tight skin, leakage of fluid? | −0.51 (p < 0.01) |
| SF-36 bodily pain: How much bodily pain have you had during the past 4 weeks? During the past 4 weeks, how much did pain interfere with your normal work? | ||
| Considering all correlation coefficients for various domains of ICC-CQ-P and CIVIQ, moderate to high correlation coefficients would occur for: | ||
| 5. ICC-CQ-P “physical functioning” and CIVIQ “physical impact” | ICC-CQ-P physical functioning: see 1. | −0.56 (p < 0.01) |
| CIVIQ physical impact: During the past four weeks, to what extent did your leg problems bother/limit you while doing the movements or activities listed below: climbing stairs, crouching, kneeling, walking briskly, housework? | ||
| 6. ICC-CQ-P “physical functioning” and CIVIQ “social impact” | ICC-CQ-P physical functioning: see 1. | −0.51 (p < 0.01) |
| CIVIQ social impact: During the past four weeks, to what extent did your leg problems bother/limit you while doing the movements or activities listed below: going out, travel by car, making physically strenuous efforts? | ||
| 7. ICC-CQ-P “physical functioning” and CIVIQ “general pain awareness” | ICC-CQ-P physical functioning: see 1. | −0.31 (p = 0.09) |
| CIVIQ general pain awareness: | ||
| During the past 4 weeks, to what extent did you have pain in the legs and have sleep problems? To what extent did your legs bothered/limited you in your work and while standing for a long time? | ||
| Considering all correlation coefficients for various domains of ICC-CQ-P and Lymph-ICF, moderate to high correlation coefficients would occur for: | ||
| 8. ICC-CQ-P “physical functioning” and Lymph-ICF “mobility” | ICC-CQ-P physical functioning: see 1. | −0.54 (p < 0.01) |
| Lymph-ICF-LL mobility: Due to your lymphedema, can you still sit for a prolonged time, stand for a prolonged time, kneel, walk, ride a bicycle, drive a car, take the stairs? | ||
| Lymph-ICF-UL mobility: Due to your lymphedema, can you still perform tasks with an elevated arm, lift or carry heavy objects, sleep on affected side, perform computer work (>30 minutes), sunbathe, drive a car, walk (>2 km), ride a bicycle? | ||
| 9. ICC-CQ-P “physical functioning” and Lymph-ICF “household” | ICC-CQ-P physical functioning: see 1. | −0.63 (p < 0.01) |
| Lymph-ICF-LL household: Due to your lymphedema, have you become more dependent on others, do you have problems with organizing different matters and completing household chores? | ||
| Lymph-ICF-UL household: Due to your lymphedema, can you still cook, clean, iron, garden? | ||
| 10. ICC-CQ-P “physical functioning” and Lymph-ICF “life domain/social life” | ICC-CQ-P physical functioning: see 1. | −0.64 (p < 0.01) |
| Lymph-ICF-LL life domain/social life: Due to your lymphedema, can you fulfill your job, practice sports, carry out leisure time activities, carry out social activities, wear clothes and/or shoes you like to wear and go on holiday? | ||
| Lymph-ICF-UL life domain/social life: | ||
| Due to your lymphedema, can you go on holiday, perform hobbies, practice sports, wear clothes and/or shoes you like to wear, fulfill your job, carry out social activities with friends? | ||
| 11. ICC-CQ-P “disease-related symptoms” and Lymph-ICF “physical function” | ICC-CQ-P “disease-related symptoms”: Do you experience in relation to your disease pain, loss of muscle strength, heaviness, swelling, tight skin, leakage of fluid? | 0.41 (p < 0.01) |
| Lymph-ICF-LL physical function: Do you have at the level of your leg(s) and/or foot/feet: pain, tensed skin, tingling, infections, stiffness or heaviness? | ||
| Lymph-ICF physical function: Does your arm feel heavy, stiff, swollen, feels like it has lost strength, tingle, hurt and has a tensed skin? | ||
CIVIQ, Chronic Venous Insufficiency Questionnaire; ICC-CQ-P, ICC Compression Questionnaire for Patients; Lymph-ICF-LL, Lymphedema Functioning, Disability and Health Questionnaire for Lower Limb Lymphedema; Lymph-ICF-UL, Lymphedema Functioning, Disability and Health Questionnaire for Upper Limb Lymphedema; SF-36, Short Form Health Survey-36.
Step 3: Translation of the questionnaire into English
The ICC-CQ was translated into the English language according to established international guidelines described by the World Health Organization.29,30 Therefore, a sequential approach was applied to translate the Dutch ICC-CQ into English.28,29 This was established in different stages after a standard forward–backward translation process, which has become standard in health status assessments.29,31,32 The Dutch version was translated into English by two individuals working independently. After a consensus meeting, a reconciled translation was developed. Subsequently, a third person translated the reconciled form back into Dutch. An expert committee consisting of a methodologist, the developers, a language professional, and the translators reviewed all reports and produced the final version.
Results
Step 1: Development of the Dutch ICC-CQ
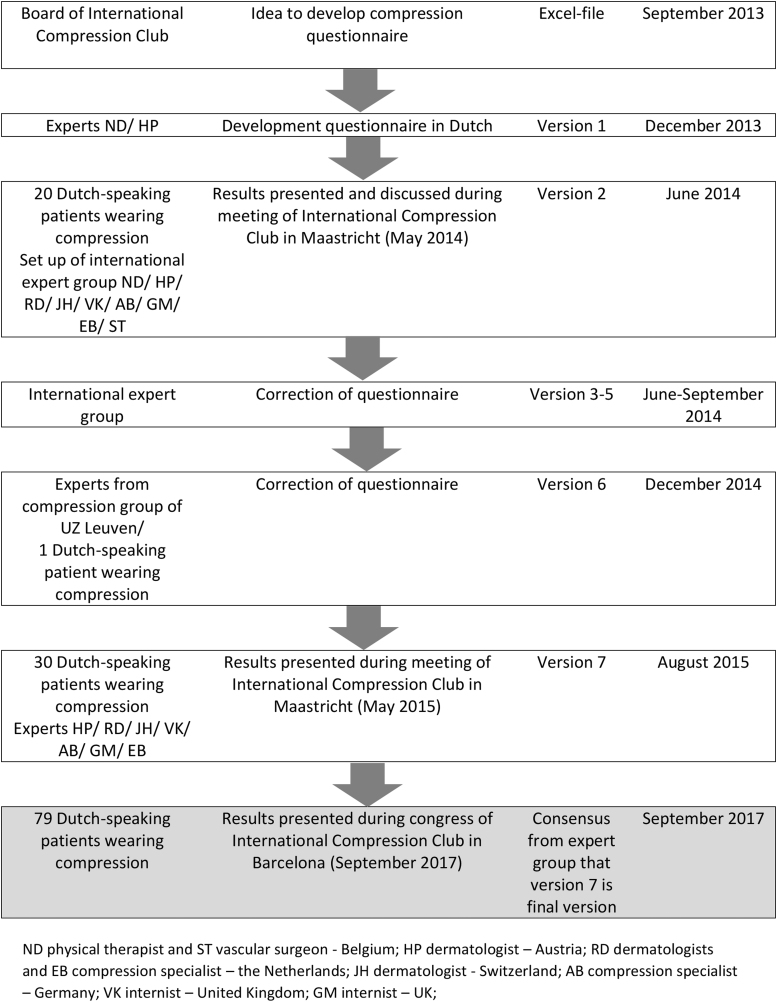
Figure 1 shows that ICC-CQ has been developed between September 2013 and August 2015. Seven different preliminary versions were developed, and the development was based on input from experts and patients using compression.
FIG. 1.
The process of development of the ICC-CQ with an overview of the kind of input from the experts/patients and the period. ICC-CQ, ICC Compression Questionnaire.
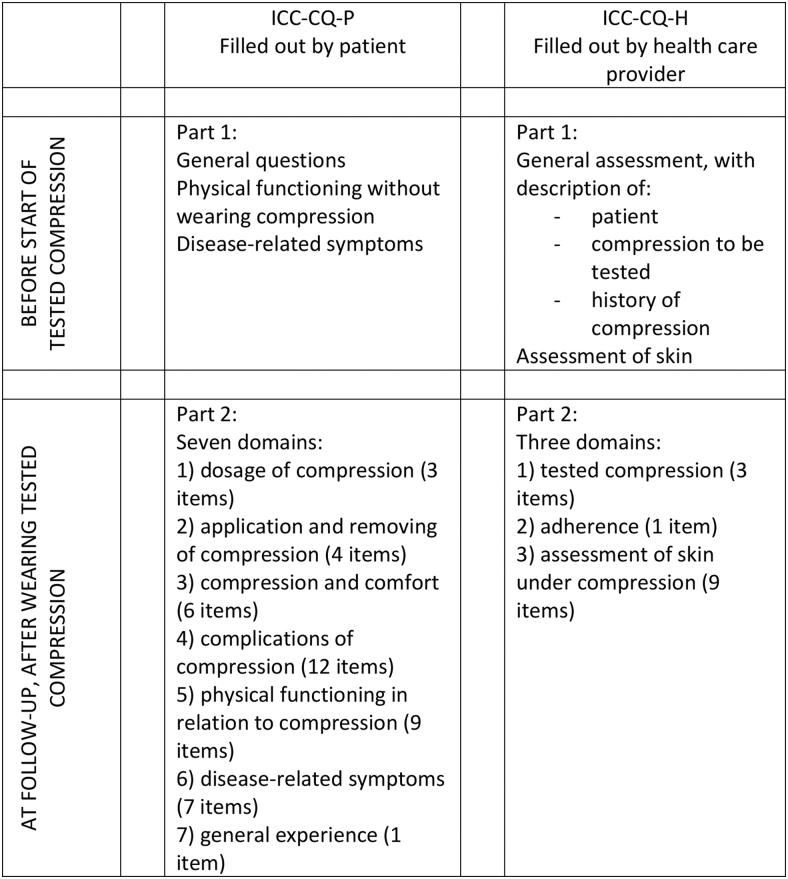
The purpose of the ICC-CQ is to evaluate the effect of different kinds of compression materials and systems and its acceptance and experience by the patient. The compression questionnaire is developed to be used for longitudinal comparative studies (see Fig. 2 for the procedure and structure). The target population are patients using compression for different causes and at different locations. As mentioned, the ICC-CQ consists of two questionnaires: ICC-CQ-P has to be filled out by the patients and the ICC-CQ-H by the health care provider. The ICC-CQ, the manual and excel file to calculate the scores can be found on the website of the International Compression Club.
FIG. 2.
Procedure of use of ICC-CQ in a clinical trial.
The ICC-CQ-P starts with a general introduction and information about the scoring system (Supplementary Appendix S1). In part 1, the patient has to score his/her physical functioning without wearing compression and has to score the disease-related symptoms before the start of the tested compression. Thereafter, the tested compression is applied. During follow-up (after wearing the compression), the patient has to fill out part 2 of ICC-CQ-P. This part starts with general questions about the patient (5 items). Thereafter seven domains are questioned (Table 2): (1) dosage of compression (3 items), (2) application and removing of compression (4 items), (3) compression and comfort (6 items), (4) complications of compression (12 items), (5) physical functioning in relation to compression (9 items), (6) disease-related symptoms (7 items), and (7) general experience (1 item).
Table 2.
Overview of the Domains of the ICC Compression Questionnaire for Patients and Health Care Providers, the Items in Each Domain, and the Formula to Calculate the Domain Scores
| Domain | Items | Calculation score |
|---|---|---|
| ICC-CQ-P | ||
| Dosage of compression (0–168) | No. of days past week; no. of hours during daytime; no. of hours at night | = question 1 × (question 2 + question 3) |
| Application and removing of compression (0–10) | Able to put on without help; able to put on with help; able to take off without help; able to take off with help | = ([question 1 × 2] + question 2b) + ([question 3 × 2] + question 4b)/6 |
| Compression and comfort (0–10) | Able to wear shoes; able to wear clothes; feeling immediately; feeling during daytime; feeling at night; appearance | = [sum of questions 1–6]/[6 – no. of questions not answered] |
| Complication of compression (0–10) | Skin irritation; tender spots; skin damage; itching; warmth; throbbing; cramps; cutting in; slippage; local swelling; bulky feeling; too tight feeling; other; other | = [sum of questions 1–14]/[14 – no. of questions not answered] |
| Physical functioning in relation to compression (0–10) | Able to move wrist/ankle; elbow/knee; shoulder/hip; use spoon/walk; carry out job; complete household chores; practice sports, carry out leisure activities; social activities | = [sum of questions 1–9]/[9 – no. of questions not answered] |
| Disease-related symptoms (0–10) | Pain; loss of muscle strength; heaviness; swelling; tight skin; tingling; leakage | = [sum of questions 1–7]/[7 – no. of questions not answered] |
| General experience (0–10) | Experience | = question 8 |
| ICC-CQ-H | ||
| Compression material (0–10) | Cover; fit; appropriate | = [sum of questions 2–4]/[3 – no. of questions not answered] |
| Adherence (0–10) | Adherence | = question 5 |
| Skin (0–10) | Dry; local swelling; general redness; local redness; strangulation; blister; erosion; ulceration; papules; other | = ([sum of questions 2.1 – 2.10]/[10 – no. of questions not answered]) × 5 |
ICC-CQ-H, ICC Compression Questionnaire for health care providers.
The ICC-CQ-H starts with an introduction about the purpose, structure, and content of the questionnaire (Supplementary Appendix S2). Before the start of the tested compression, in part 1, the health care provider performs a general assessment, that is, general information about the patient, about the compression to be tested and about the history of compression. A nominal scoring system is used. In addition, the health care provider performs an assessment of the skin that will be covered by the tested compression. After the compression has been started, in part 2, the health care provider performs an evaluation of the tested compression (3 items) and of the adherence of the patient (1 item) on an 11-point scale (Table 2). Thereafter, the health care provider performs an assessment of the skin under the compression material or device (9 items) on a 3-point scale (absent, doubtfully present, and clearly present). The questionnaire ends with an appendix with an overview of general and disease-specific quality of life questionnaires. It is recommended to use one or more of these questionnaires, in addition to the ICC-CQ.
The calculation of the score on each domain is demonstrated in Table 2. With the exception of the domain “dosage of compression” with a range of scores between 0 and 168 hours, the score on each domain ranges between 0 and 10. The general questions need an answer on a nominal scale. It takes ±7 minutes on average to complete parts 1 and 2 of the ICC-CQ-P as well as to complete both parts of the ICC-CQ-H.
Step 2: Investigation of reliability and validity of the Dutch ICC-CQ
Between October 2015 and August 2016, 79 subjects were included in this study: 62 women and 17 men. Sixty-five patients were recruited in UZ Leuven in Belgium and 14 in Nij Smellinghe Hospital in the Netherlands. Mean age was 59.6 (±13.8) years. Forty-eight patients had lymphedema, and 31 patients suffered from chronic venous disease. Fifty-nine patients had compression at the level of the upper limb, 19 patients at the level of the lower limb, and 1 of the face. Fifty-two subjects wore compression garments, and 27 wore multi-layer bandages.
Reliability
In Table 3, the results regarding reliability of the ICC-CQ-P are shown. Test–retest reliability for the domains “dosage of compression” and “physical functioning in relation to compression” is very strong and for the domains “application and removal of compression material,” “compression and comfort,” and “complications of compression” it is strong. For the domains “disease-related symptoms” and “general experience,” test–retest reliability was moderate. Internal consistency of different domain scores ranged from moderate to very strong. Scoring on the different domains had an acceptable variability (SEM between 0.4 and 10.8). The greatest variability was found for the scoring on the domain “general experience.” Subsequently, also SRD was highest for this domain.
Table 3.
Mean Scores (Min; Max) of the First and Second Rating and Reliability, Internal Consistency, Variability, and Clinically Important Change of the ICC Compression Questionnaire for Patients and Health Care Providers
| Rating 1 |
Rating 2 |
Reliabilitya |
Internal consistency |
Variability |
Clinical important change |
|
|---|---|---|---|---|---|---|
| Mean (min; max) | Mean (min; max) | ICC (95% CI) | Cronbach's α | SEM | SRD (95% CI) | |
| ICC compression questionnaire for patients | ||||||
| Dosage of compression [0–168] | 99.0 (14; 168) | 99.9 (14; 168) | 0.93 (0.90 to 0.96) | 0.97 | 10.8 | 30.0 (−30.9 to 29.2) |
| Application and removing [0–10] | 8.3 (2.7; 10.0) | 8.2 (1.7; 10.0) | 0.89 (0.83 to 0.93) | 0.94 | 0.7 | 2.0 (−2.0 to 2.0) |
| Compression and comfort [0–10] | 6.7 (0.0; 10.0) | 8.3 (1.3; 10.0) | 0.82 (0.72 to 0.88) | 0.91 | 1.0 | 2.7 (−3.1 to 2.3) |
| Complications of compression [0–10] | 2.5 (0.2; 6.3) | 2.5 (0.3; 6.2) | 0.81 (0.72 to 0.87) | 0.89 | 0.7 | 2.0 (−2.0 to 1.9) |
| Physical functioning in relation to compression [0–10] | 8.2 (2.4; 10.0) | 8.3 (4.0; 10.0) | 0.93 (0.90 to 0.96) | 0.97 | 0.4 | 1.2 (−1.3 to 1.1) |
| Disease-related symptoms [0–10] | 2.7 (0.0; 8.3) | 2.7 (0.0; 7.6) | 0.73 (0.61 to 0.82) | 0.85 | 1.1 | 3.1 (−3.0 to 3.2) |
| General experience [0–10] | 7.5 (0; 10) | 7.8 (0; 10) | 0.55 (0.31 to 0.72) | 0.71 | 1.8 | 5.0 (−5.3 to 4.7) |
| ICC compression questionnaire for health care providers | ||||||
| Compression material [0–10] | 8.6 (5.0; 10.0) | 8.6 (5.0; 10.0) | 0.71 (0.58 to 0.97) | 0.83 | 0.6 | |
| Adherence [0–10] | 9.4 (4; 10) | 9.5 (6; 10) | 0.39 (0.19 to 0.56) | 0.56 | 0.8 | |
| Skin [0–10] | 2.3 (0.0; 7.5) | 2.4 (0.0; 7.0) | 0.82 (0.73 to 0.94) | 0.90 | 0.7 | |
Test−retest reliability for ICC−CQ-P and interrater reliability for ICC-CQ-H.
CI, confidence interval; SEM, standard error of measurement; SRD, smallest real difference.
In Table 3 also, the results regarding reliability of the ICC-CQ-H are shown. Interrater reliability was strong for the “compression material” score, was moderate for the “skin” score, and was weak for the “adherence” score. Internal consistency of the domain scores was moderate to strong scores. SEM and SRD were small.
Validity
In Table 4, the remarks of the patients regarding face and content validity and details regarding floor and ceiling effect of the ICC-CQ-P are shown. All patients filled out the questionnaire for assessing face and content validity. Of all patients, 70 patients (89%) indicated that the questionnaire was completely understandable. The other 9 patients (11%) found one or more questions unclear or found the scoring system unclear. In addition, 69 patients (87%) indicated that the questionnaire was complete and had no other remarks regarding the content of the questionnaire. In none of the domain scores, floor effect was present and 3 on 7 domain scores showed a ceiling effect. Table 2 shows the hypotheses of expected moderate to high correlation coefficients between domain scores of the ICC-CQ-P and domain scores of the SF-36 questionnaire, of CIVIQ, and of Lymph-ICF questionnaires (to investigate construct validity). For 10 on 11 (or 91%) hypotheses, the Pearson correlation coefficient was interpreted as moderate to strong (r between 0.40 and 0.90) and was accepted.
Table 4.
Face and Content Validity of the ICC Compression Questionnaire for Patients and of Health Care Providers
| Remark regarding … | Face validity ( = remarks regarding comprehensibility of questions and scoring system) and content validity ( = remarks regarding completeness of questionnaire and other remarks regarding content) | Floor vs. ceiling effect ( = % score of 0 vs. 10) | Reply on remarks with consensus from expert group |
|---|---|---|---|
| ICC-CQ-P | |||
| Questionnaire in general | It judges rather the effect and experience from compression garments and not from bandages (N = 2) It is difficult to distinguish the side effects caused by compression material from the side effects caused by the disease (N = 2) Questions about durability of the compression material and system are missing (N = 2) |
Only the questions in the “application and removing of compression” domain are more specific for compression garments and this is because bandages are applied or removed without the use of material. However, a person wearing bandages can answer all questions in this domain. It is, indeed, difficult to completely distinguish the scoring of these two domains. Therefore, the separate domains only contain the typical side effects and there is no overlap. Indeed, this information is missing and has to be evaluated separately. |
|
| Scoring system | Since the anchors of the scoring system are changing, the scoring is sometimes difficult (N = 1) It is difficult to express the amount of problems in a number (N = 2) Since the effect and experience from the compression material changes from day to day, it is difficult to score the questions (N = 3) |
These remarks are typically reported when patients have to fill out a questionnaire when using a numeric scale. A numeric scale was chosen (and not a scale with words), because numbers result in more accurate estimates.33 Advise the patients to score the mean effect and experience of the last week. |
|
| Dosage of compression domain | What is daytime and what is night time (N = 1) | 1%a vs. 19% b | In the questionnaire, add between brackets the explanation “the waking hours” after the word “daytime” and the explanation “the hours sleeping in bed” after the word “night.” In fact, based on this information, we want to know the dosage of compression during 24 hours, so the exact hours during the day and at night are not necessary. |
| Application and removing of compression domain | Not possible to distinguish between help from others and help from a device (N = 1) The difference in scoring between with and without help is not obvious (N = 1) |
0% vs. 52% | Based on input from other patients, the structure of this domain has changed several times during the construction process. Finally, consensus with the expert group was reached to apply the current structure. |
| Compression and comfort domain | It is not clear what is meant with usual shoes: preferred shoes or wearable shoes (N = 1) | 1% vs. 12% | In that case, the patient has to score that she is not able to wear her usual shoes. Add between brackets the words “you like to wear” after shoes. |
| Complications of compression domain | “Development of blue toes” is missing (N = 1) “Hypoesthesia of the toes” is missing (N = 1) |
6% vs. 0% | Patients have to fill out these missing complaints in the section “other specific problems.” |
| Physical functioning in relation to compression domain | — | 0% vs. 0% | |
| Disease-related symptoms domain | Do I have to score the symptoms related to the disease or evoked by the treatment (bandaging)? (N = 2) I do not understand “tensed skin” (N = 1) |
20% vs. 0% | Emphasize here that the patient has to score the symptoms related from the disease (i.e., lymphedema or chronic venous insufficiency). The side effects from the bandaging are scored in the “complication and compression” domain. If the questionnaire is used in a randomized controlled trial comparing two compression materials/systems, the “disease-related symptom” domain has to be scored at baseline (before the start of wearing the compression) and without using the material or system. After using the material or system during a certain time interval, the patient fills out the “disease-related symptom” domain again while wearing the compression material/system. In this way, the change of symptoms related to the disease (i.e., treated by the compression) is evaluated. |
| General experience domain | — | 6% vs. 29% | |
| ICC-CQ-H | |||
| Questionnaire in general | — | ||
| Scoring system | Difficult to distinguish between present and doubtfully present (N = 1) | During the construction process, to simplify the scoring, the scoring system was changed from an 11-point scale into a 3-point scale with words. This change has led to an improvement of interrater reliability and a decrease of measurement variability. | |
| Compression material domain | — | 0% vs. 20% | |
| Adherence domain | — | 0% vs. 67% | |
| Skin domain | Global swelling is missing (N = 1) Local redness is scored identically as strangulation (N = 1) Difference between local and general redness is not obvious (N = 2) When do you report that strangulation is present: only in case of non-expected strangulation or also in case of expected strangulation that is present for instance when there is compression at the level of the knee? (N = 1) |
8% vs. 0% | Global swelling is scored in the ICC-CQ-P in the “disease-related symptoms” domain. The content of this domain has changed a lot during the construction process. To improve face validity, for instance, after each item a small explanation is provided. The health care provider has to read these explanations before starting to fill out the questionnaire. |
0–16 hours; b152–168 hours.
Table 4 also shows the remarks of the health care providers regarding face and content validity and details regarding floor and ceiling effect of the ICC-CQ-H. Nineteen health care providers filled out the questionnaire for assessing face and content validity. Of all health care providers, 14 (74%) found the questionnaire completely understandable. The other five health care providers found one question unclear (N = 4) or the scoring system unclear (N = 1). Moreover, all except one health care provider (95%) found the questionnaire complete. Neither floor nor ceiling effects of the domain scores were present.
In September 2017, the results regarding reliability and validity of the Dutch ICC-CQ were presented during the congress of the International Society of Lymphology in Barcelona. The expert group reached consensus that the seventh version is the final version.
Step 3: Translation of the questionnaire into English
Comparison of the backward translation with the original Dutch ICC-CQ was performed, and modifications were provided to the translation if needed. This resulted in the final version after unanimous agreement of all translators. Clinimetric properties of the English version of the questionnaire have yet to be investigated.
Discussion
The Dutch ICC-CQ is the first reliable and valid questionnaire to evaluate the effect of different kinds of compression materials and systems and its acceptance and experience by the patient.
The finalized ICC-CQ represents a synthesis of the available specific literature, input from an international panel of experts, and experience of expert patients with lymphedema or chronic venous disease. The questionnaire was refined over seven steps. Since there is no other tool to evaluate compression material and systems in the literature, we were not able to compare the results of our study with an existing questionnaire.
Face and content validity of the ICC-CQ was good. More than 75% of the patients and health care providers found their questionnaire understandable and complete. In Table 4, a reply on the different remarks from patients and health care providers with consensus from the expert group is shown. Construct validity was very strong. Ten of 11 (91%) hypotheses assessing construct validity were accepted. For these 10 hypotheses, moderate correlation coefficients were found between expected domain scores of the ICC-CQ-P and domain scores of other reliable and valid questionnaires that evaluate the same construct. It is important to note that we were unable to investigate construct validity of the complete ICC-CQ. Since evaluation tools to validate the other domains of ICC-CQ are missing in the literature, the hypotheses were only formulated about the “physical functioning” and “disease-related symptom” domain of ICC-CQ-P.
Except for the “general experience” domain, test–retest reliability of the ICC-CQ-P was good to very good, internal consistency was good, and measurement variability and clinical important change was small. Test–retest reliability of the score on the “disease-related symptoms” domain was borderline strong. This is unexpected, since the questions in this domain resemble the questions of the “physical function” domain of the Lymph-ICF questionnaires closely and its test–retest reliability is very strong.8,9 One of the remarks regarding face validity was that some patients did not know whether they had to evaluate their symptoms because of the disease or the side effects of the compression material/system. It may be that a number of patients scored it differently on different occasions. With the exception of the “adherence” domain, interrater reliability of the ICC-CQ-H was moderate to strong, internal consistency was strong to very strong, and measurement variability was small.
The present study had several strengths. First, the questionnaire was developed in different stages with an improvement of each version. Second, the construction was based on input from 11 experts in compression therapy and from seven different countries on the one hand and from 51 patients wearing compression on the other hand. Third, the investigation of reliability and validity of the final Dutch ICC-CQ was performed on a large group of patients (n = 79) in two different countries. Fourth, the study group was representative for all patients receiving compression (i.e., mix of patients with lymphedema or chronic venous insufficiency and of patients wearing compression garments or bandages). Finally, different aspects of reliability and validity were investigated.
The present study also had some weaknesses. First, not all types of compression therapy were applied. The included patients wore compression garments or bandages and did not receive intermittent pneumatic compression nor did they wear velcro bandages. Second, it was not possible to investigate construct validity of all domains of the questionnaire for patients and health care providers. The reason is that no evaluation tool assessing compression material and systems exists in the literature. Third, not all clinimetric properties of the Dutch ICC-CQ were investigated. Responsiveness has to be further explored. In addition, further research into the clinimetric properties of the English version of the ICC-CQ is warranted, as well as into those of other already existing translations such as the German and French language versions.
Besides the application of this questionnaire in longitudinal comparative studies, it can be used in clinical practice to make adjustments to the applied compression based on patients' feedback regarding comfort of the compression materials as well as on skin inspection as performed by the health provider, to improve patients' quality of life during treatment.
Conclusions
In conclusion, the Dutch ICC-CQ is the first reliable and valid questionnaire to evaluate different kinds of compression material and devices and its acceptance and experience by Dutch-speaking patients with lymphedema or chronic venous disease. In the future, the questionnaire can be used in longitudinal comparative studies to compare the effect and patients' views of different kinds of compression therapy. In clinical practice, this tool enables an objective evaluation of the applied compression to make adjustments to the patient-specific treatment program if needed. Further research is needed to examine responsiveness of the Dutch version and the reliability and validity of the questionnaire in other languages than Dutch.
Supplementary Material
Acknowledgments
The authors are very grateful to all the collaborators and patients who participated in this study. All authors critically revised the article for important intellectual content and approved the final article.
Ethical statement
This study obtained approval from the Ethical Committees of UZ Leuven (B322201523795) and of Nij Smellinghe Hospital (15-335/MdH/AB).
Authors' Contributions
All authors read and approved the final article. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020; 53:3–19. [PubMed] [Google Scholar]
- 2. Vuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I. An epidemiological survey of venous disease among general practitioner attendees in different geographical regions on the globe: The final results of the Vein Consult Program. Angiology 2018; 69:779–785. [DOI] [PubMed] [Google Scholar]
- 3. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F, Coordinators VCP. Epidemiology of chronic venous disorders in geographically diverse populations: Results from the Vein Consult Program. Int Angiol 2012; 31:105–115. [PubMed] [Google Scholar]
- 4. Felty CL, Rooke TW. Compression therapy for chronic venous insufficiency. Semin Vasc Surg 2005; 18:36–40. [DOI] [PubMed] [Google Scholar]
- 5. Cesarone MR, Belcaro G, Agus G, Georgiev M, Errichi BM, Marinucci R, et al. . Management of superficial vein thrombosis and thrombophlebitis: Status and expert opinion document. Angiology 2007; 58 Suppl 1:7S–14S; discussion 14S–15S. [DOI] [PubMed] [Google Scholar]
- 6. Partsch H, Clark M, Bassez S, Benigni JP, Becker F, Blazek V, et al. . Measurement of lower leg compression in vivo: Recommendations for the performance of measurements of interface pressure and stiffness: Consensus statement. Dermatol Surg 2006; 32:224–232; discussion 33. [DOI] [PubMed] [Google Scholar]
- 7. Viehoff PB, van Genderen FR, Wittink H. Upper limb lymphedema 27 (ULL27): Dutch translation and validation of an illness-specific health-related quality of life questionnaire for patients with upper limb lymphedema. Lymphology 2008; 41:131–138. [PubMed] [Google Scholar]
- 8. Devoogdt N, De Groef A, Hendrickx A, Damstra RJ, Christiaansen A, Geraerts I, et al. . Lymphedema functioning, disability and health questionnaire for lower limb lymphedema (Lymph-ICF-LL): Reliability and validity. Phys Ther 2014; 94:705–721. [DOI] [PubMed] [Google Scholar]
- 9. Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens MR. Lymphoedema functioning, disability and health questionnaire (Lymph-ICF): Reliability and validity. Phys Ther 2011; 91:944–957. [DOI] [PubMed] [Google Scholar]
- 10. Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema 2010; 5:26–37. [Google Scholar]
- 11. Augustin M, Bross F, Foldi E, Vanscheidt W, Zschocke I. Development, validation and clinical use of the FLQA-I, a disease-specific quality of life questionnaire for patients with lymphedema. Vasa 2005; 34:31–35. [DOI] [PubMed] [Google Scholar]
- 12. Klernas P, Johnsson A, Horstmann V, Kristjanson LJ, Johansson K. Lymphedema Quality of Life Inventory (LyQLI)-development and investigation of validity and reliability. Qual Life Res 2015; 24:427–439. [DOI] [PubMed] [Google Scholar]
- 13. Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, et al. . VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol 2006; 59:1049–1056. [DOI] [PubMed] [Google Scholar]
- 14. Kutlu A, Yilmaz E, Cecen D, Eser E, Ozbakkaloglu A. The Turkish validity and reliability of the venous insufficiency epidemiological and economic study-quality of life/symptoms scales. Angiology 2011; 62:329–337. [DOI] [PubMed] [Google Scholar]
- 15. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res 1996; 5:539–554. [DOI] [PubMed] [Google Scholar]
- 16. Klyscz T, Junger M, Schanz S, Janz M, Rassner G, Kohnen R. [Quality of life in chronic venous insufficiency (CVI). Results of a study with the newly developed Tubingen Questionnaire for measuring quality of life of patients with chronic venous insufficiency]. Hautarzt 1998; 49:372–381. [DOI] [PubMed] [Google Scholar]
- 17. Hareendran A, Doll H, Wild DJ, Moffatt CJ, Musgrove E, Wheatley C, et al. . The venous leg ulcer quality of life (VLU-QoL) questionnaire: Development and psychometric validation. Wound Repair Regen 2007; 15:465–473. [DOI] [PubMed] [Google Scholar]
- 18. Blome C, Baade K, Debus ES, Price P, Augustin M. The “Wound-QoL”: A short questionnaire measuring quality of life in patients with chronic wounds based on three established disease-specific instruments. Wound Repair Regen 2014; 22:504–514. [DOI] [PubMed] [Google Scholar]
- 19. Mosti G, Cavezzi A, Partsch H, Urso S, Campana F. Adjustable Velcro compression devices are more effective than inelastic bandages in reducing venous edema in the initial treatment phase: a randomized controlled trial. Eur J Vasc Endovasc Surg 2015; 50:368–374. [DOI] [PubMed] [Google Scholar]
- 20. Couzan S, Leizorovicz A, Laporte S, Mismetti P, Pouget JF, Chapelle C, et al. . A randomized double-blind trial of upward progressive versus degressive compressive stockings in patients with moderate to severe chronic venous insufficiency. J Vasc Surg 2012; 56:1344..e1–1350.e1. [DOI] [PubMed] [Google Scholar]
- 21. Benigni JP, Branchoux S, Bacle I, Taieb C. Difficulty associated with donning medical compression stockings: Results from a survey comparing two different compression stockings. Womens Health (Lond) 2013; 9:291–300. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000; 25:3130–3139. [DOI] [PubMed] [Google Scholar]
- 23. De Vrieze T, Vos L, Gebruers N, De Groef A, Dams L, Van der Gucht E, et al. . Revision of the lymphedema functioning, disability and health questionnaire for upper limb lymphedema (Lymph-ICF-UL): Reliability and validity. Lymphat Res Biol 2019; 17:347–355. [DOI] [PubMed] [Google Scholar]
- 24. Biemans AA, van der Velden SK, Bruijninckx CM, Buth J, Nijsten T. Validation of the chronic venous insufficiency quality of life questionnaire in Dutch patients treated for varicose veins. Eur J Vasc Endovasc Surg 2011; 42:246–253. [DOI] [PubMed] [Google Scholar]
- 25. Lexell JE, Downham DY. How to assess the reliability of measurements in rehabilitation. Am J Phys Med Rehabil 2005; 84:719–723. [DOI] [PubMed] [Google Scholar]
- 26. Fleiss JL. The Design and Analysis of Clinical Experiments. Chichester, New York: Wiley; 1986. [Google Scholar]
- 27. Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. . Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60:34–42. [DOI] [PubMed] [Google Scholar]
- 28. Gandek B, Ware JE Jr. Methods for validating and norming translations of health status questionnaires: The IQOLA Project approach. International Quality of Life Assessment. J Clin Epidemiol 1998; 51:953–959. [DOI] [PubMed] [Google Scholar]
- 29. Gandek B, Alacoque J, Uzun V, Andrew-Hobbs M, Davis K. Translating the short-form headache impact test (HIT-6) in 27 countries: Methodological and conceptual issues. Qual Life Res 2003; 12:975–979. [DOI] [PubMed] [Google Scholar]
- 30. WHO. Process of translation and adaptation of instruments. 2020. https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed November 20, 2020).
- 31. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J Clin Epidemiol 1993; 46:1417–1432. [DOI] [PubMed] [Google Scholar]
- 32. Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, et al. . Assessing health status and quality-of-life instruments: Attributes and review criteria. Qual Life Res 2002; 11:193–205. [DOI] [PubMed] [Google Scholar]
- 33. Buchter RB, Fechtelpeter D, Knelangen M, Ehrlich M, Waltering A. Words or numbers? Communicating risk of adverse effects in written consumer health information: a systematic review and meta-analysis. BMC Med Inform Decis Mak 2014; 14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.