Abstract
A diagnosis of lymphedema comes with a lifetime requirement for careful self-care and treatment to control skin deterioration and the consequences of excessive fluid and protein buildup leading to abnormal limb volume and an increased risk of infection. The burden of care and psychosocial aspects of physical disfiguration and loss of function are associated with compromised quality of life (QoL). The current standard therapeutic intervention is complex decongestive therapy with manual lymph drainage and frequent wearing of compression garments. With insurance limitations on therapy visits and the time and travel required, additional home treatment options are needed. Pneumatic compression pumps that mimic the manual massage pressure and pattern are sometimes prescribed, but these are bulky, difficult to apply, and require immobility during treatment. An open-label pilot study in 40 subjects was performed to evaluate the QoL and limb volume maintenance efficacy of a novel wearable compression system (Dayspring™) that is low profile, easy to use, and allows for mobility during treatment. After 28 days of use, subjects had a statistically significant 18% (p < 0.001) improvement in overall QoL as measured by the Lymphedema Quality-of-Life Questionnaire compared with baseline. Individual QoL domains, and limb volume improved with therapy. Adherence was 98% over the course of the study. Results of the clinical evaluation suggest the Dayspring wearable compression device is safe and effective and improves QoL and limb volume. The novel, low-profile device is easy to use and allows for mobility during treatment, addressing a potential barrier to adherence with pneumatic compression devices.
Keywords: lymphedema, wearable, mobility, advanced compression, quality of life
Introduction
Cancer survivorship has improved substantially over recent decades, from less than 50% in 1975 to nearly 70% in 2011.1 Lymphedema is a chronic and incurable disease that can be primary (inherited) or secondary in etiology. Secondary lymphedema is a common sequela of cancer treatment. Cancers associated with lymphedema, such as breast and prostate, have seen improved survivorship, from 75% to 91% for breast, and from 66% to 99% for prostate, respectively, from 1975 to 2011.1–3 As survivorship improves, long-term side effect risk, particularly for lymphedema also increases,4 with 5%–50% of cancer survivors developing lymphedema.5–8 Breast cancer-related lymphedema accounts for 25,000–50,000 diagnoses annually in the United States alone.9 More than 10 million Americans and hundreds of millions worldwide suffer from lymphedema, making it more prevalent than AIDS, Parkinson's disease, multiple sclerosis, muscular dystrophy, and Alzheimer's disease, combined.10,11
With lymphedema, the malformation or destruction of lymphatic pathways leads to excessive buildup of fluid and protein in interstitial spaces, and can lead to disfiguring swelling, pain, restricted range of motion, hardening of the skin (fibrosis), and recurring infections.12–15 Management of this chronic condition requires a lifetime of careful and sometimes burdensome self-care to avoid these adverse consequences. Specific treatment for lymphedema depends on the severity of the condition, which may progress if treatments are not adhered to. Complex decongestive therapy with manual lymph drainage (MLD) and frequent wearing of compression garments are considered current treatment and are used to manage symptoms.16–19 Meticulous skin care is always required, and jewelry, tight clothing, venipuncture, and other insults to the affected limb are strongly discouraged. Surgery and invasive procedures are reserved for the most severe cases, and even then, results are not maintained without ongoing home care and compression.
Pneumatic compression devices (PCDs) are a convenient self-management option for individuals with lymphedema.20,21 Advanced PCDs have been shown to stimulate lymphatic function and improve patient limb volume, self-reported symptoms and quality of life (QoL), and overall symptoms.22–25 Pump pressure profiles mimic the gentle stretch and release motions of MLD, reducing risk of damage to underlying tissue while moving lymphatic fluid out of the affected area. Recent clinical studies have demonstrated that appropriate and regular use of advanced PCDs can significantly improve the clinical outcome, including volume maintenance, reduced hospitalization, and reduced cellulitis and infection.21,24,26–28 These clinical improvements have also resulted in reduced medical costs.21,28 Advanced PCDs have significant disadvantages since they are loud, bulky, difficult to use, and must be plugged into a wall socket to operate. Advanced PCD therapy also requires the patient to be immobile for the entire duration of treatment, which consequently may be a deterrent to therapy adherence—an essential element to improve limb volume and manage lymphedema symptoms.29
With the significant burden of disease and symptom management, lymphedema is also associated with compromised QoL.30,31 Physical symptoms include pain, fatigue, decreased levels of activity, impaired range of motion, and heaviness or numbness in the affected limb.31–34 Emotional symptoms may include changes in role perception, distress, decreased self-confidence, decreased body image, anxiety, depression, anger, and grief.31,33,35 Decreased physical function, lack of information, comorbidities, and psychological and psychosocial factors have all been documented barriers to self-management of lymphedema36–38 and may exacerbate problems with work/life and ability to maintain employment.39
Reducing barriers to self-management of lymphedema symptoms and easing life impact is essential to achieving desired clinical outcomes and reducing the negative impact on QoL. Opportunities remain for improving home treatment options and adherence to beneficial therapies.
An open-label study to clinically assess a novel wearable advanced compression technology (Dayspring™, Fig. 1) was undertaken to determine if potential barriers to lymphedema self-care were effectively addressed. The Dayspring device is a novel FDA-cleared wearable sequential compression system. The following endpoints were examined:
FIG. 1.
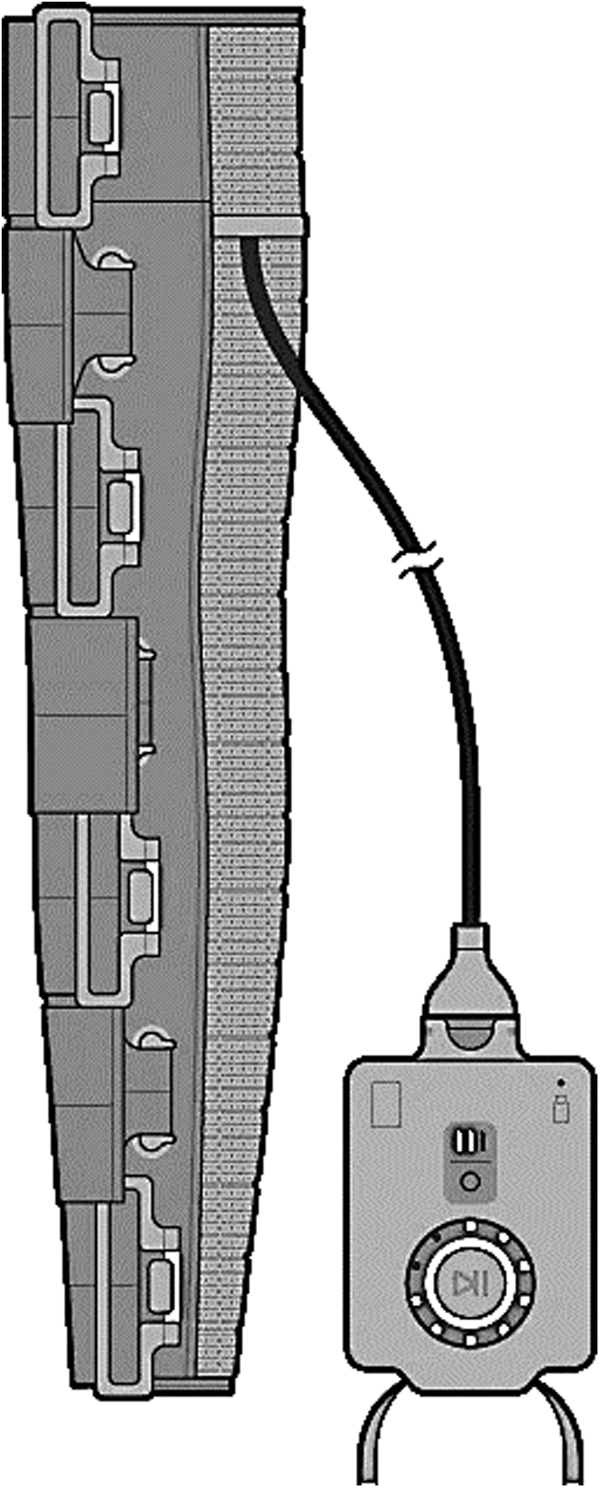
Schematic of the Dayspring—a smart wearable device—shown with the controller (right) and garment (left).
-
(1)
Improvement in QoL in subjects with unilateral upper-extremity edema after 28 days as measured by the Lymphedema Quality-of-Life Questionnaire (LYMQOL) disease-specific validated assessment tool.
-
(2)
Arm volume maintenance or improvement as measured before and after 28 days of device use.
-
(3)
Safety as assessed by reported adverse events.
-
(4)
Patient satisfaction as measured by visual analog scale (VAS) and survey at the end of the study; and
-
(5)
Adherence to therapy as measured with a smart phone app.
Methods
Setting and sample
Forty subjects with breast cancer-related lymphedema were recruited in two lymphedema clinics in the SF bay area. Inclusion criteria were: 18 years of age or older with a diagnosis of unilateral upper extremity lymphedema and capable of consenting to the study protocol. Exclusion criteria were: any contraindication for compression therapy, conditions that would prevent safe and effective use of a compression device (i.e., cellulitis, open or healing wounds), current or recurrent cancer (within 3 months of chemotherapy, radiation, or surgical treatment), acute infection, chronic kidney disease, epilepsy, pregnancy or planned pregnancy during the study period, participation in any clinical trial within the past 30 days, acute thrombophlebitis, peripheral arterial disease, pulmonary embolism or deep vein thrombosis, pulmonary edema, congestive heart failure, uncontrolled asthma, inability to consent and follow protocol requirements, and any condition where increased venous or lymphatic return is contraindicated. Subjects were instructed to continue any prescribed self-care procedures, including the use of compression garments, but to cease use of any PCDs, if applicable.
The Dayspring wearable advanced compression device consists of a programmable, segmental controller and a sleeve garment that can be sized to fit the subject. The garment contains a shape memory alloy made with Nickel/Titanium (Ni-Ti) that is programmed by a rechargeable controller to shrink in a cyclic manner, applying active gradient pressure from the distal to proximal end of the limb. This mechanistic action is similar to the motion of advanced PCDs on the market. Up to 14 independently controlled segments can be programmed to deliver 0–100 mmHg of compression pressure, with typical initial settings in a range of 30–40 mmHg. A mobile phone application can be used to program and individualize pressures, start, stop, and pause therapy and to track device usage. The quiet, low-profile device allows for mobility and range of motion during treatment. The study was approved by the WCG Western IRB.
Study design
In this prospective, multicenter, open-label, nonrandomized pilot study, subjects completed a screening visit within 90 days of study enrollment, at which time they provided consent to participate. Enrollment occurred on study day 0, which was the first use of the Dayspring device. Subjects were trained to apply the sleeve with appropriate placement, to set the controller for duration and pressure and to turn the device on and off. Baseline measurements were taken, including limb volume and the LYMQOL disease-specific QoL measurement tool, and these were repeated at the 28-day study completion visit. Subjects were instructed to use the device at least one time daily for a minimum of 45 minutes on the study arm only.
Limb volume measurement was performed by using a calibrated tape measure to measure circumference from the wrist and the ulnar styloid process and at 4 cm increments to the axilla. Measurements were taken for both upper extremities. The arm that exhibited lymphedema was designated as the study arm, while the other, the control. Measurements were taken by the same investigator throughout the study. Volume was calculated based on cylindrical segment analysis. Limb volume measurements were repeated at day 28.
The LYMQOL, a validated disease-specific QoL tool, was also administered at baseline and at day 28. The questionnaire includes 20 items related to four domains: symptoms (pain, swelling, and numbness), body image/appearance, function (activities of daily living such as eating, writing, and dressing), and mood (sleep disruption, depression, and irritability). The domains are scored from 1 (not at all) to 4 (a lot). The total score is calculated by adding all items and dividing by the total number of items. Overall QoL is scored as a single item by the patient on a scale of 1–10. The subscales (domains) indicate improvement as a lower score, whereas the overall QoL scale indicates improvement with a higher score.
A VAS study survey wase administered at the end of the study to measure patient satisfaction (day 28). The survey was used to document the time of day the device was used, if daily activities were supported during use, function, and symptoms. All were measured on a scale from 1 to 5. The VAS was administered to previous users of PCDs to assess preference and likelihood to recommend to others with lymphedema.
Device usage and therapy adherence were tracked through the mobile app linked to the Dayspring device.
Analyses
The sample size, mean, and standard deviation were calculated for continuous analysis variables or, if appropriate, the sample size, median, and range. Categorical variables were presented as the count and percentage for each category. Device and procedure-related events were summarized as count and percent of subjects experiencing an event. Pairwise comparisons were performed using a paired t-test or Wilcoxon signed-rank test, as appropriate to compare pre/post changes, as well as percent change. All statistical tests were two sided and evaluated at an α of 0.05. No adjustment for multiplicity was made. Statistical analyses were performed in SAS.
Results
Sample subject characteristics
A sample of 40 subjects completed the study. The subjects were overwhelmingly female (39/40). Average age was 65.7 ± 8.8 years, expressed as mean ± standard deviation. Of the participants, all were diagnosed with unilateral lymphedema nearly evenly split between right (n = 18) and left (n = 22) arms. A subset of 15 participants (38%) had utilized PCDs before the baseline assessment, but suspended use during the duration of the study protocol. See Table 1 for the sample demographics. No device-related adverse events were reported during the study.
Table 1.
Demographics
| Patients | 40 |
| Age (mean ± SD) | 65.7 ± 8.8 |
| Female (male) | 39 (1) |
| Race | |
| Caucasian | 32 |
| African American | 1 |
| Hispanic | 3 |
| Other | 4 |
| Affected arm | |
| Left | 22 |
| Right | 18 |
| Sites | 2 |
| Months since ALDN/RT | 26 ± 23 |
ALDN/RT, axillary lymph node dissection/radiotherapy; SD, standard deviation.
Quality of life
The LYMQOL was assessed for both overall QoL improvement and impact on the subscales of symptoms, appearance, function, and mood (Figs. 2 and 3). On average, overall QoL improved 1.3 points (18%, p < 0.001) from baseline (7.05 ± 1.41) to study completion (8.33 ± 1.22), both expressed as mean ± standard deviation. A subset (n = 15) of subjects who had used a currently on-market advanced PCD before baseline demonstrated similar positive improvement. Scores improved 1.20 points (17%, p < 0.01) from 7.07 ± 1.33 to 8.27 ± 1.62 at study end. All four subscales indicated improvement, with significantly lower scores (p < 0.001) at study end compared with baseline. Results were consistent in the subset of subjects with prior use of advanced PCD. Overall results are included in Table 2 and subset results in Table 3.
FIG. 2.
Lymphedema overall quality-of-life (LYMQOL) outcomes from subjects before and after use of the Dayspring device over a 1-month evaluation period. Horizontal bars represent mean ± 1 standard deviation. LYMQOL, Lymphedema Quality-of-Life Questionnaire; QoL, quality of life.
FIG. 3.
Lymphedema qualify-of-life (LYMQOL) functional outcomes from subjects before and after use of the Dayspring device over a 1-month evaluation period. Horizontal bars represent mean ± 1 standard deviation.
Table 2.
Lymphedema Quality-of-Life Questionnaire Results (n = 40)
| Subscale | Baseline (mean ± SD) | Posttest (mean ± SD) | p | Mean difference (from baseline) | % Difference (from baseline) |
|---|---|---|---|---|---|
| Function | 1.64 ± 0.43 | 1.42 ± 0.52 | <0.001 | 0.22 | 13 |
| Appearance | 2.26 ± 0.73 | 1.72 ± 0.65 | <0.001 | 0.54 | 24 |
| Mood | 1.73 ± 0.59 | 1.28 ± 0.35 | <0.001 | 0.45 | 22 |
| Symptoms | 2.01 ± 0.69 | 1.56 ± 0.52 | <0.001 | 0.45 | 26 |
Table 3.
Lymphedema Quality-of-Life Questionnaire Results (Prior Pneumatic Compression Device Use) (n = 15)
| Subscale | Baseline (mean ± SD) | Posttest (mean ± SD) | p | Mean difference (from PCD users) | % Difference (from PCD users) |
|---|---|---|---|---|---|
| Function | 1.59 ± 0.40 | 1.40 ± 0.40 | <0.001 | 0.19 | 12 |
| Appearance | 2.23 ± 0.58 | 1.76 ± 0.62 | <0.001 | 0.47 | 21 |
| Mood | 1.55 ± 0.44 | 1.27 ± 0.30 | <0.001 | 0.28 | 18 |
| Symptoms | 1.99 ± 0.70 | 1.60 ± 0.58 | <0.001 | 0.39 | 19 |
PCD, pneumatic compression device.
Limb volume maintenance
Limb volume decreased on average by 2% and ranged from 1% to 12% from baseline to study completion in the treatment arm (Fig. 4). This was a statistically significant reduction (p ∼ 0.042). In comparison, the nonstudy arm was stable in volume with a negligible average increase of 0.4%, which was not significant (p ∼ 0.061). The subset of subjects with previous advanced PCD use saw an average decrease in limb volume of 2.8% (range 2%–12%), which was also significant (p < 0.05). As in the overall sample, the change in the nonstudy arm was negligible (−0.7%) and not significant (p > 0.05). Contralateral limbs were used as controls in this study, because no baseline/pre-LE data were available.
FIG. 4.
Volume reduction in subjects before and after use of the Dayspring device over a 1-month evaluation period. Horizontal bars represent mean ± 1 standard deviation.
Adherence
Protocol adherence as measured by the mobile app (Fig. 5) was high at 98%. The average daily use was 43.9 minutes calculated over the 28-day study versus the prescribed use of at least 45 minutes a day as specified in the treatment protocol.
FIG. 5.

Dayspring companion app for digital interface and adherence monitoring.
Patient satisfaction and preference
Subject satisfaction with the Dayspring was high (Fig. 6). Subjects rated their preference over other devices available on the market as 92/100 (92%) on the VAS. For the subset of subject with experience using a legacy advanced PCD, preference scores were also very high at 93/100 (93%).
FIG. 6.
VAS score in preference of Dayspring over PCDs from subjects after the trial. PCDs, pneumatic compression devices; VAS, visual analog scale.
Discussion
Recently, Aldrich and colleagues demonstrated that early treatment and intervention in lymphedema patients can restore lymphatic function in cancer survivors and patients with other comorbidities.40 As a chronic condition without a cure requiring lifelong treatment, patient adherence to self-management is crucial, as failure or inability to control limb volume may lead to progression of the illness.41,42 The burden of ongoing symptoms and treatments that require time, careful attention, lifestyle disruption, and significant cost may lead to less-than-ideal adherence to beneficial treatments. Most lymphedema self-management programs consist of 3–12 treatment modalities depending on the stage and severity.43 In an assessment of the prescription for and adherence to self-care modalities for breast cancer-related lymphedema, Brown and colleagues report an average of 3.6 ± 2.1 different treatments. After 1 year, 69% of patients reported being <75% adherent to their therapies.44 The findings in the physical activity and lymphedema trial further point to less-than-optimal adherence to self-management modalities with only 31% of participants (n = 141) adhering to 1 or more self-treatment at 12 months.45 Alcorso and colleagues conducted a study to determine which psychosocial factors were associated with increased adherence to self-management. They linked the adherence levels to behaviors and found that adherence to self-management may be impacted by a reduced belief in self-efficacy, ability to follow through with prescribed treatments, perceived consequences of the diagnosis, and emotional state.41 Their findings support decreased self-efficacy and the burden of multiple treatment modalities as primary barriers to adherence.
Over the past decade, PCDs have shown beneficial clinical results and reductions in adverse events, like infection and hospitalization that serve to lower lymphedema-related costs. One study showed a 79% decrease in cellulitis infection after 12 months of advanced PCD use, and a 29% reduction in outpatient lymphedema visits.21 Another study found a similar 29% reduction in visits in as little as 12 weeks.24 Improved clinical outcomes and the subsequent reduction in health care resource use directly reduce associated costs. Studies have shown a significant 22%–50% reduction in costs associated with the management of chronic lymphedema with advanced PCDs.21,28 These devices, however, also require lifestyle disruption and are loud, bulky, and difficult to use. In one large study, advanced PCDs had the lowest adherence of all self-care modalities, with only 30% of patients using their devices, 75% or more of the prescribed amount.45
This pilot study of a novel wearable advanced compression system sought to evaluate the efficacy of Dayspring for the treatment of breast cancer-related lymphedema of the upper extremity. The study demonstrated that sequential pressure could be applied without the negative aspects associated with pneumatic devices like noise, bulkiness, difficulty using, and the need to be immobile while tethered to a wall outlet. The innovative shape memory alloy can be applied through a low-profile, quiet system controlled by a lightweight and portable programmable controller. This allows the patient to be mobile during treatment, eliminating a potential source of poor compliance with compression pumps. In the breast cancer-related lymphedema of the upper extremities, the treatment was shown to improve QoL, maintain or reduce limb volume, and to have high acceptability and compliance to therapy.
Conclusion
Results of this pilot clinical evaluation suggest the Dayspring wearable sequential compression device is safe and effective and improves QoL and maintains or improves limb volume. The low-profile and quiet device allows for mobility during treatment, addressing barriers to adherence with PCDs.
Acknowledgment
The authors would like to thank Susan Couture and Robin Johnson for their help in the study.
Author Disclosure Statement
Dr. S.G.R., Dr. P.K.-M., Dr. R.S., and Dr. J.A. serve as advisors to Koya Medical. Over the past 24 months, Dr. P.K.-M. reports providing consulting services to Precision Health Economics and Sempre Health for work unrelated to this study. The authors have no financial interests in the study.
Funding Information
This study was funded by Koya Medical, Inc.
References
- 1. Underlying incidence data are from the SEER 9 areas. http://seer.cancer.gov/registries/terms.html (accessed June 29, 2021).
- 2. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int J Cancer 2005; 114:806–816. [DOI] [PubMed] [Google Scholar]
- 3. Noone A-M, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, Penberthy L. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Prev Biomark 2017; 26:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beckjord EB, Reynolds KA, Van Londen G, Burns R, Singh R, Arvey SR, Nutt SA, Rechis R. Population-level trends in posttreatment cancer survivors' concerns and associated receipt of care: Results from the 2006 and 2010 LIVESTRONG surveys. J Psychosoc Oncol 2014; 32:125–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayes SC. Review of Research Evidence on Secondary Lymphoedema—Incidence, Prevention, Risk Factors and Treatment. NSW: National Breast and Ovarian Cancer Centre Surry Hills; 2008:1–83. [Google Scholar]
- 6. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 2007; 25:3657–3663. [DOI] [PubMed] [Google Scholar]
- 7. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013; 14:500–515. [DOI] [PubMed] [Google Scholar]
- 8. Hunter CP, Redmond CK, Chen VW, Austin DF, Greenberg RS, Correa P, Muss HB, Forman MR, Wesley MN, Blacklow RS. Breast cancer: Factors associated with stage at diagnosis in black and white women. J Natl Cancer Inst 1993; 85:1129–1137. [DOI] [PubMed] [Google Scholar]
- 9. Morrell RM, Halyard MY, Schild SE, Ali MS, Gunderson LL, Pockaj BA. Breast cancer-related lymphedema. Mayo Clin Proc 2005; 80:1480–1484. [DOI] [PubMed] [Google Scholar]
- 10. Rockson SG. Lymphedema. Am J Med 2001; 110:288–295. [DOI] [PubMed] [Google Scholar]
- 11. Szuba A, Rockson SG. Lymphedema: Anatomy, physiology and pathogenesis. Vasc Med 1997; 2:321–326. [DOI] [PubMed] [Google Scholar]
- 12. Fu MR. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J Clin Oncol 2014; 5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu MR, Rosedale M. Breast cancer survivors' experiences of lymphedema-related symptoms. J Pain Sympt Manage 2009; 38:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. International Society of Lymphology, The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology 2003; 36:84–91. [PubMed] [Google Scholar]
- 15. Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003; 52:370–379. [DOI] [PubMed] [Google Scholar]
- 16. Mayrovitz HN. The standard of care for lymphedema: Current concepts and physiological considerations. Lymphat Res Biol 2009; 7:101–108. [DOI] [PubMed] [Google Scholar]
- 17. Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: A primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin 2009; 59:8–24. [DOI] [PubMed] [Google Scholar]
- 18. International Lymphoedema Framework. Best Practice for the Management of Lymphoedema. International Consensus. London: MEP Ltd., 2006:3–52. [Google Scholar]
- 19. Cormier J, Feldman J, Askew R, Beck M, Bernas M, Francis K, Fu M, Lasinski B, Rodrick J, Stewart B. ALFP to update the best practice document. J Lymphoedema 2010; 5:68–71. [Google Scholar]
- 20. Ridner SH, Murphy B, Deng J, Kidd N, Galford E, Bonner C, Bond SM, Dietrich MS. A randomized clinical trial comparing advanced pneumatic truncal, chest, and arm treatment to arm treatment only in self-care of arm lymphedema. Breast Cancer Res Treat 2012; 131:147–158. [DOI] [PubMed] [Google Scholar]
- 21. Karaca-Mandic P, Hirsch AT, Rockson SG, Ridner SH. The cutaneous, net clinical, and health economic benefits of advanced pneumatic compression devices in patients with lymphedema. JAMA Dermatol 2015; 151:1187–1193. [DOI] [PubMed] [Google Scholar]
- 22. Adams KE, Rasmussen JC, Darne C, Tan I-C, Aldrich MB, Marshall MV, Fife CE, Maus EA, Smith LA, Guilloid R. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Optics Exp 2010; 1:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema. BMC Cancer 2006; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fife CE, Davey S, Maus EA, Guilliod R, Mayrovitz HN. A randomized controlled trial comparing two types of pneumatic compression for breast cancer-related lymphedema treatment in the home. Support Care Cancer 2012; 20:3279–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammond T. Reduction of complications and associated costs with Flexitouch® therapy for lymphedema. Open Rehabil J 2009; 2:54–57. [Google Scholar]
- 26. Ridner SH, McMahon E, Dietrich MS, Hoy S. Home-based lymphema treatment in patients with cancer-related lymphedema or non cancer-related lymphedema. Oncol Nurs Forum 2008; 35:671–680. [DOI] [PubMed] [Google Scholar]
- 27. Blumberg SN, Berland T, Rockman C, Mussa F, Brooks A, Cayne N, Maldonado T. Pneumatic compression improves quality of life in patients with lower-extremity lymphedema. Ann Vasc Surg 2016; 30:40–44. [DOI] [PubMed] [Google Scholar]
- 28. Brayton KM, Hirsch AT, Patricia J, Cheville A, Karaca-Mandic P, Rockson SG. Lymphedema prevalence and treatment benefits in cancer: Impact of a therapeutic intervention on health outcomes and costs. PLoS One 2014; 9: e114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boris M, Weindorf S. Persistence of lymphedema reduction after noninvasive complex lymphedema therapy. Cancer 1997; 11. [PubMed] [Google Scholar]
- 30. Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005; 13:904–911. [DOI] [PubMed] [Google Scholar]
- 31. Taghian NR, Miller CL, Jammallo LS, O'Toole J, Skolny MN. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit Rev Oncol Hematol 2014; 92:227–234. [DOI] [PubMed] [Google Scholar]
- 32. Burckhardt M, Belzner M, Berg A, Fleischer S. Living with breast cancer-related lymphedema: A synthesis of qualitative research. Oncol Nurs Forum 2014; 41: E220–E237. [DOI] [PubMed] [Google Scholar]
- 33. Anbari AB, Wanchai A, Armer JM. Breast cancer-related lymphedema and quality of life: A qualitative analysis over years of survivorship. Chronic Illness 2019; 0:1–12. [DOI] [PubMed] [Google Scholar]
- 34. Hormes J, Bryan C, Lytle L, Gross C, Ahmed R, Troxel A, Schmitz KH. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology 2010; 43:1–13. [PubMed] [Google Scholar]
- 35. Dominick SA, Natarajan L, Pierce JP, Madanat H, Madlensky L. The psychosocial impact of lymphedema-related distress among breast cancer survivors in the WHEL Study. Psychooncology 2014; 23:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psychooncology 2013; 22:1466–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ridner SH, Rhoten BA, Radina ME, Adair M, Bush-Foster S, Sinclair V. Breast cancer survivors' perspectives of critical lymphedema self-care support needs. Support Care Cancer 2016; 24:2743–2750. [DOI] [PubMed] [Google Scholar]
- 38. Cal A, Bahar Z. Women's barriers to prevention of lymphedema after breast surgery and home care needs: A qualitative study. Cancer Nurs 2016; 39:E17–E25. [DOI] [PubMed] [Google Scholar]
- 39. Boyages J, Kalfa S, Xu Y, Koelmeyer L, Mackie H, Viveros H, Taksa L, Gollan P. Worse and worse off: The impact of lymphedema on work and career after breast cancer. Springerplus 2016; 5:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aldrich MB, Rasmussen JC, Fife CE, Shaitelman SF, Sevick-Muraca EM. The development and treatment of lymphatic dysfunction in cancer patients and survivors. Cancers 2020; 12:2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alcorso J, Sherman KA, Koelmeyer L, Mackie H, Boyages J. Psychosocial factors associated with adherence for self-management behaviors in women with breast cancer-related lymphedema. Support Care Cancer 2016; 24:139–146. [DOI] [PubMed] [Google Scholar]
- 42. Vignes S, Porcher R, Arrault M, Dupuy A. Factors influencing breast cancer-related lymphedema volume after intensive decongestive physiotherapy. Support Care Cancer 2011; 19:935–940. [DOI] [PubMed] [Google Scholar]
- 43. Ostby PL, Armer JM, Smith K, Stewart BR. Patient perceptions of barriers to self-management of breast cancer–related lymphedema. Western J Nurs Res 2018; 40:1800–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown JC, Cheville AL, Tchou JC, Harris SR, Schmitz KH. Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Support Care Cancer 2014; 22:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown JC, Kumar A, Cheville AL, Tchou JC, Troxel AB, Harris SR, Schmitz KH. Association between lymphedema self-care adherence and lymphedema outcomes among women with breast cancer-related lymphedema. Am J Phys Med Rehabil Assoc Acad Phys 2015; 94:288. [DOI] [PMC free article] [PubMed] [Google Scholar]






