Dear editor
Patients with chronic kidney disease (CKD) are at higher risk for coronavirus disease 2019 (COVID-19)-related morbidity and mortality than general populations and, early vaccination should be prioritized for this vulnerable population. However, numerous uncertainties exist regarding the safety and efficacy of vaccination, especially in CKD patients on immunosuppressive drugs.1 Concerning safety and efficacy, a significant portion of CKD patients (1720/2509, 68.6%) showed hesitation toward vaccination in telephone survey of our center (Fig. S1). Previous studies focused on exploring immune responses to COVID-19 vaccine in patients on dialysis or receiving kidney transplant.2 , 3 Three recent studies reported the humoral response to mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in patients with CKD,4, 5, 6 yet no serial data available for inactivated SARS-CoV-2 vaccines.
We conducted a pilot, prospective study to survey the safety and humoral response to inactivated SARS-CoV-2 vaccine in CKD patients receiving a 2-dose immunization of inactivated SARS-CoV-2 vaccine (Fig. S2). We recruited 45 CKD patients, and 100 healthy controls, 100 hypertension patients and 100 diabetes patients with matched sampling time after vaccination at Peking University First Hospital, Yunnan University Affiliated Hospital and Center for Disease Control and Prevention in Hainan. Baseline characteristics of the participants are described in Table 1. All participants received a 2-dose immunization (3–5 weeks between two doses) of inactivated SARS-CoV-2 vaccine (SinoVac or Sinopharm). We collected serum samples 20–33 days after the second dose of vaccination. To assess the humoral response to inactivated SARS-CoV-2 vaccines, we measured neutralizing antibody using competitive inhibition method, and anti-SARS-CoV-2 receptor-binding domain (RBD)-specific IgG and IgM using chemiluminescence immunoassay (MCLIA, Bioscience Co., Tianjin, China). We also recruited 31 CKD patients and 67 healthy controls receiving a third dose of inactivated SARS-CoV-2 vaccine (Table S1) to evaluate the immunological enhancement in this population. This study was approved by the Peking University First Hospital (2021[441]) and Yunnan University (CHSRE2021021).
Table 1.
Demographic and clinical characteristics of study participants.
| Characteristics | Chronic kidney disease patients | Healthy controls | Hypertension disease controls | Diabetes disease controls |
|---|---|---|---|---|
| (n = 45) | (n = 100) | (n = 100) | (n = 100) | |
| Mean age (SD), y | 42.4 (16.2) | 38.4 (13.3) | 39.7 (15.4) | 56.3 (8.2) |
| Gender, n (%) | ||||
| Female | 20 (44.4) | 45 (45) | 47 (47) | 50 (50) |
| Median interval between the second dose and sampling (IQR), days | 27 (20, 33) | 30 (17, 30) | 25 (16, 38) | 28 (22, 31) |
| Applied vaccines, n (%) | ||||
| SinoVac | 21 (46.7) | 48 (48) | 59 (59) | 90 (90) |
| Sinopharm | 20 (44.4) | 43 (43) | 25 (25) | 5 (5) |
| Both | 4 (8.9) | 9 (9) | 16 (16) | 5 (5) |
| Kidney disease diagnosis, n (%) | ||||
| Chronic glomerulonephritis | ||||
| IgA nephropathy | 21 (46.7) | – | – | – |
| IgA vasculitis | 1 (2.2) | – | – | – |
| Chronic glomerulonephritis without kidney biopsy | 5 (11.1) | – | – | – |
| Podocytopathy | ||||
| Membranous nephropathy | 4 (8.9) | – | – | – |
| Focal segmental glomerular sclerosis | 1 (2.2) | – | – | – |
| Metabolic kidney disease | ||||
| Diabetic nephropathy | 4 (8.9) | – | – | – |
| Hypertensive nephropathy | 1 (2.2) | – | – | – |
| Others | ||||
| Uninephrectomy | 2 (4.4) | – | – | – |
| Fanconi syndrome | 1 (2.2) | – | – | – |
| Alport syndrome | 1 (2.2) | – | – | – |
| Acute interstitial nephritis | 1 (2.2) | – | – | – |
| Kidney amyloidosis | 1 (2.2) | – | – | – |
| Monoclonal gammopathy of renal significance | 1 (2.2) | – | – | – |
| Kidney stone | 1 (2.2) | – | – | – |
| Additional diagnosis, n (%) | ||||
| Hypertension | 12 (26.7) | – | 100 (100) | 10 (10) |
| Diabetes | 7 (15.6) | – | 4 (4) | 100 (100) |
| Obesity | 1 (2.2) | – | – | 11 |
| Gout | 3 (6.7) | – | – | – |
| Viral B hepatitis | 2 (4.4) | – | – | – |
| Fatty liver | 1 (2.2) | – | – | – |
| Coronary heart disease | 1 (2.2) | – | 2 (2) | 1 (1) |
| Asthma | 1 (2.2) | – | – | – |
| Endometrial adenomyosis | 1 (2.2) | – | – | – |
| Chronic lymphocytic leukemia | 1 (2.2) | – | – | – |
| Hypothyroidism | 1 (2.2) | – | – | 1 (1) |
| Hyperthyroidism | – | – | – | 1 (1) |
| Cerebral infarction | – | – | – | 1 (1) |
| Endometrial carcinoma of uterus | – | – | – | 1 (1) |
| Medication exposure, n (%) | ||||
| Prednisone | 4 (8.9) | – | – | – |
| Bortezomib+dexamethasone | 1 (2.2) | – | – | – |
| Hydroxychloroquine | 7 (15.6) | – | – | – |
| Cyclosporin A | 3 (6.7) | – | – | – |
| Mycophenolate mofetil | 1 (2.2) | – | – | – |
| Tripterygium wilfordii | 2 (4.4) | – | – | – |
| No immunosuppression† | 27 (60) | – | 100 (100) | 96 (96) |
indicates angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, insulin, metformin, acarbose, sodium-dependent glucose transporter inhibitor, calcium channel blockers, β-blocker, propylthiouracil, or levothyroxin sodium tablets.
Briefly, the average age of CKD patients was 42.4 years with 20 (44%) females. 21 of them received SinoVac, 20 received Sinopharm and 4 received both sequentially. The most common form of CKD was chronic glomerulonephritis (27/45, 60.0%) followed by podocytopathy (5/45, 11.1%) and metabolic kidney disease (5/45, 11.1%). There were 18 patients taking immunosuppressants during the vaccination period with 17 (94.4%) receiving monotherapy. We tightly controlled age, female ratio, types of administered vaccines and sampling time across groups, except that the average age of diabetes patients is significantly older than CKD patients. None of these participants reported severe adverse effects after vaccination.
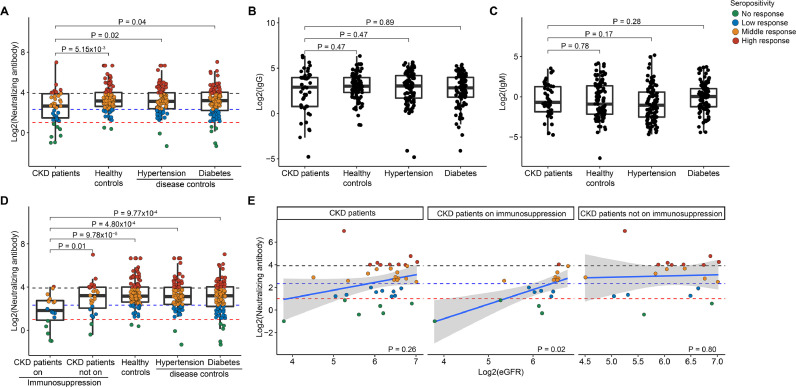
Using neutralizing antibody titer of 2 as seroconversion cutoff, we found 84% (38 of 45) of CKD patients seropositive, which was lower than that in healthy controls (98%), hypertension patients (98%) and diabetes patients (95%). The median neutralizing antibody titers in CKD patients was 6.29 (IQR, 2.78–14.62), which was significantly lower than that in healthy controls [8.91 (IQR, 6.14–16.01), P = 5.15 × 10−3], hypertension patients [8.66 (IQR, 5.24–15.68), P = 0.02], and diabetes patients [9.14 (IQR, 4.62–16.22), P = 0.04] (Fig. 1 A). Conversely, we did not observe anti-RBD IgG or IgM differences between CKD patients and controls (Fig. 1 B, C), despite of strong association between neutralizing antibody and anti-RBD IgG levels (Fig. S3). To better understand the immunogenicity of inactivated SARS-CoV-2 vaccine in CKD patients, we further stratified CKD patients into subgroups according to their disease diagnosis, estimated glomerular filtration rate (eGFR) levels and medication status (receiving immunosuppressants or not). Notably, immunosuppressive medication (Fig. 1 D) rather than eGFR levels (Fig. 1 E) or disease types (Fig. S4) showed effect on the reduction of immunogenicity. There were only 72.2% (13/18) of CKD patients receiving immunosuppressants tested seropositive after 2-dose vaccination. Moreover, we observed an immunosuppressant–dependent association between neutralizing antibody level and eGFR after adjusting for age and gender (r = 0.627, P = 0.02), suggesting that immunosuppressive agents could sensitize neutralizing antibody response to kidney function in non-dialysis kidney patients. Interestingly, a third dose significantly boosted neutralizing antibody in CKD patients while immunosuppressants impeded the boosting effects (Fig. S5).
Fig. 1.
Immune responses after 2-dose inactivated SARS-CoV-2 vaccination in patients with chronic kidney disease. (A) Neutralization antibodies response. (B) anti-SARS-CoV-2 receptor-binding domain (RBD)-specific IgG response. (C) anti-RBD IgM response. (D) Neutralization antibody responses in patients on immunosuppression. (E) The correlations between neutralizing antibody response and eGFR. Antibody titers were presented as median (IQR: interquartile range). The thresholds for neutralization antibodies is represented by the dashed lines, with <2.0 classified as no response, <5.0 as low response, <15.0 as middle response, and >=15.0 as high response. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Our initial analysis showed that majority (84%) of CKD patients acquired detectable neutralizing antibody against SARS-CoV-2 without severe adverse effects, while the antibody titers were lower than controls. In contrast, we did not observe such difference in anti-RBD IgG or IgM. The deviance between neutralizing antibody and anti-RBD IgG responses in CKD patients indicates that neutralizing antibody rather than IgG might be the most important marker reflecting humoral immune response in CKD patients. Subgroup analyses showed that immunosuppressive therapies rather than eGFR levels or disease diagnosis impair SARS-CoV-2 vaccine-induced immunity. Our analysis in non-dialysis kidney disease patients receiving inactivated SARS-CoV-2 vaccine greatly supplemented previous studies on immunosuppressive therapies,7 , 8 dialysis,9 and kidney transplantation10 impaired mRNA vaccine responses. We found that taking immunosuppressants hampers neutralizing antibody response in CKD patients and sensitizes neutralizing antibody response to kidney function. Additionally, our data demonstrates that CKD patients, even for those on immunosuppression treatment, can benefit from a third vaccination boost by improving their humoral immunity.
Disclosure statement
None declared.
Declaration of Competing Interest
Z.Z. served as a PI in a phase 4 clinical study sponsored by Sinovac Biotech Ltd. The funder has no role in study design, implementation and manuscript writing in this study.
Acknowledgments
Funding
This study was funded and supported by National Natural Science Foundation of China (91742205, 82170711, 81800636, 82070733, 82130021), the Fundamental Research Funds for the Central Universities, CAMS Innovation Fund for Medical Sciences (2019-I2M-5046), Yunnan Provincial Science and Technology Department (202102AA100051, 202003AC100010, China), and Beijing Young Scientist Program (BJJWZYJH01201910001006).
Acknowledgments
The authors thank the study participants, and clinical staff and nurses who providing help for their participation and sampling.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.05.003.
Appendix. Supplementary materials
References
- 1.Carr E.J., Kronbichler A., Graham-Brown M., et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang K., Wu X., Luo Y., Wei Z., Feng L., Wu L. Meta-analysis of immunologic response after COVID-19 mRNA vaccination in solid organ transplant recipients. J Infect. 2022;84:e73–e75. doi: 10.1016/j.jinf.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rincon-Arevalo H., Choi M., Stefanski A.L., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6:eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 4.Bouwmans P., Messchendorp A.L., Sanders J.S., et al. Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: a national prospective observational cohort study. BMC Nephrol. 2022;23:55. doi: 10.1186/s12882-022-02680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quiroga B., Soler M.J., Ortiz A., et al. Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study. Nephrol Dial Transpl. 2022;37:994–999. doi: 10.1093/ndt/gfac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchwinkler L., Solagna C.A., Messner J., et al. Antibody response to mRNA vaccines against SARS-CoV-2 with chronic kidney disease, hemodialysis, and after kidney transplantation. J Clin Med. 2021;11:148. doi: 10.3390/jcm11010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deepak P., Kim W., Paley M.A., et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr E.J., Wu M., Harvey R., et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet. 2021;398:1038–1041. doi: 10.1016/S0140-6736(21)01854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchiari D., Egri N., Bodro M., et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transpl. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.