Abstract
Background and Purpose:
The Nociceptin/Orphanin FQ (N/OFQ)-Nociceptin Opioid-like Peptide (NOP) receptor system is widely distributed in the brain and pharmacological activation of this system revealed therapeutic potential in animal models of substance use disorder. Intriguingly, studies on alcohol showed that also genetic deletion or pharmacological blockade of NOP receptors confer resilience to the development of alcohol abuse. Here, we used a genetic and pharmacological approach to evaluate the therapeutic potential of NOP antagonism in smoking cessation.
Experimental Approach:
Constitutive NOP knockout rats (NOP (−/−)) and their wild type counterparts (NOP (+/+)) were tested over a range of behaviours to characterize their motivation for nicotine. We next explored the effects of systemic administration of the NOP receptor antagonist, LY2817412 (0.0, 1.0 and 3.0 mg/kg) on nicotine self-administration. NOP blockade was further evaluated at the microcircuitry level, by microinjecting LY2817412 (0.0, 3.0 and 6.0 μg/μl) into the ventral tegmental area (VTA), nucleus accumbens (Alexander et al.) and central amygdala (CeA).
Key Results:
Genetic NOP deletion resulted in decreased nicotine intake, decreased motivation to self-administer the drug, and attenuation of cue-induced nicotine reinstatement. LY2817412 reduced nicotine intake in NOP (+/+) but not in NOP (−/−) rats, confirming that its effect is mediated by inhibition of NOP transmission. Finally, injection of LY2817412 into the VTA but not into the NAc or CeA decreased nicotine self-administration.
Conclusions and Implications:
These findings document that inhibition of NOP transmission attenuates the motivation for nicotine through mechanisms involving the VTA and suggest that NOP antagonism may represent a potential treatment for smoking cessation.
Keywords: Nicotine, Reinforcement, Reward, Relapse, NOP, VTA
Introduction
Nicotine is the major reinforcing component of tobacco responsible for addiction in cigarette smokers (Stolerman & Jarvis, 1995). Despite a general decline in cigarette smoking, more than 8 million deaths are predicted from tobacco use worldwide each year by 2030 (WHO, 2016). While wealthy countries aim for smoke-free future generations, the public health burden of tobacco persists in medium-low income countries (Jha & Peto, 2014). The introduction in the market of electronic cigarettes proposed as tool for smoking cessation, reportedly increased initiation of cigarette smoking among adolescents prompting calls for regulation of tobacco/vaping products (Hammond, Reid, Cole & Leatherdale, 2017; Leventhal et al., 2015; Primack et al., 2018). Pharmacological interventions for smoking cessation include a variety of FDA approved pharmacotherapies, including nicotine as a replacement therapy, varenicline that acts as nicotinic acetylcholine receptor (nAChR) partial agonist, and bupropion that works through blockade of the dopamine (DA) transporter (Elrashidi & Ebbert, 2014). Nevertheless, these therapies appear to be efficacious only in a proportion of patients seeking treatment and development of more efficacious cures remains an important priority. Pharmacological and genetic studies support a critical role of the endogenous opioid system in shaping the rewarding and motivational properties of nicotine among the different stages of the addiction process (for reviews, see (Berrendero, Robledo, Trigo, Martín-García & Maldonado, 2010; Hadjiconstantinou & Neff, 2011)). The Nociceptin/Orphanin FQ (N/OFQ)-Nociceptin Opioid-like Peptide (NOP) receptor system is the fourth member of the opioid family, and a growing body of evidence indicates that it has an important role in substance use disorders (for review see (Ciccocioppo et al., 2009; Schank, Ryabinin, Giardino, Ciccocioppo & Heilig, 2012; Witkin et al., 2014)). The N/OFQ peptide and the NOP receptors are highly expressed in the mesocorticolimbic system where they modulate DA, GABA and glutamate transmission (Kallupi, Varodayan, Oleata, Correia, Luu & Roberto, 2014; Roberto & Siggins, 2006). Previous work has demonstrated therapeutic potential of NOP agonists in the treatment of psychostimulant and alcohol use disorders (for review see (Ciccocioppo, Borruto, Domi, Teshima, Cannella & Weiss, 2019; Zaveri, 2011)). However, recent findings point to the possibility that not only NOP agonism but also NOP antagonism attenuates the motivation for alcohol (Borruto et al., 2020; Brunori et al., 2019; Cippitelli, Schoch, Debevec, Brunori, Zaveri & Toll, 2016; Rorick-Kehn et al., 2016). Finally, in work in which rats were trained to lever press in a model of concurrent alcohol and nicotine self-administration, the NOP antagonist SB612111 reduced nicotine consumption whereas the NOP agonist AT-202 increased it (Cippitelli, Schoch, Debevec, Brunori, Zaveri & Toll, 2016). In contrast, another study has demonstrated that mice lacking the NOP receptor show increased hippocampal acetylcholine release, higher voluntary drinking of a nicotine solution, and increased sensitivity to nicotine compared to wild-type mice (Sakoori & Murphy, 2009; Uezu et al., 2005).
To clarify the role of the N/OFQ-NOP system in nicotine abuse we examined nicotine-motivated behaviour in a line of rats (NOP (−/−)) carrying a constitutive deletion of NOP receptors in comparison with that of a wild type (NOP (+/+)) control line. We next employed these two rat lines to establish the effects of systemic administration of the selective NOP receptor antagonist, LY2817412 on nicotine self-administration. Finally, using NOP (+/+) rats, we evaluated the effects of site-specific microinjections of LY2817412 into the ventral tegmental area (VTA) the nucleus accumbens (Curtis et al.) and the central amygdala (CeA) on nicotine self-administration.
Methods
Animals
Experiments were performed in adult male NOP (−/−) and Wistar Han NOP (+/+) control rats to enable comparison with prior literature on the role of the NOP system in operant drug self-administration in the NOP (−/−) rat line (Kallupi et al., 2017). Animals were bred at the University of Camerino. The NOP (−/−) rat line was originally generated at the Hubrecht Institute (The Netherlands) by target-selected ENU-induced mutagenesis on a Brown Norway background. Heterozygous mutant were then outcrossed onto a Wistar Han line to eliminate confounding effects from other mutations that may have been introduced by the ENU mutagenesis. Biochemical characterization revealed that the NOP receptor is completely absent in homozygous knockout rats, and no adaptive changes in other opioid receptor levels and their distribution has occurred (Homberg, Mul, de Wit & Cuppen, 2009). The NOP (−/−) rats used in the experiments were obtained from mating homozygous male and female mutants. Rats (225–250 g) were housed in a temperature and humidity-controlled environment under a reverse light cycle (lights off at 8:00 AM) with food and water ad libitum. Rats were habituated to the facility and handled prior to experiments. Animals were treated in accordance with the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals (2010/63/EU). Formal approval was obtained from the Italian Ministry of Health and Internal Ethical Committee for Laboratory Animal Protection and Use of the University of Camerino (protocol n°1D580.22). All efforts were made to minimize rats’ suffering and distress. The animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson & Altman, 2010) and recommendations made by the British Journal of Pharmacology.
Catheter implantation
Animals were anesthetized with isoflurane anaesthesia: 5% induction and 2% maintenance. A single catheter made from micro-renathane tubing (ID = 0.020′′, OD = 0.037′′; Braintree Scientific) was implanted in the right jugular vein and subcutaneously positioned between the vein and the back as previously described (Shen, Deng, Ciccocioppo & Cannella, 2017). Immediately after surgery and for the subsequent three days rats were treated subcutaneously with 10 mg/kg of enrofloxacin (50 mg/mL, Baytril, Germany) and allowed 1 week of recovering before self-administration training. Catheters were flushed daily throughout the experiment with 0.1–0.2 ml of sterile saline mixed with heparin (20 U/ml; Italfarmaco S.p.A, Milan, Italy) to maintain patency that was confirmed by intravenous injection of 150 μL/rat of Sodium Pentothal (25 mg/mL, Intervet, Italy) at the end of experimental procedures.
Intracranial surgery and histological analysis
For intracranial surgery, rats were anesthetized as described above and positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Animals were bilaterally implanted with stainless steel guide cannulas (0.65 mm outside diameter) using the following coordinates (in mm) incisor bar at −3.3 mm; NAc shell, anteroposterior (AP) +1.4, lateral (L) ± 1.1, and ventral (V) −6.0; CeA, (AP) −2.5 (L) ± 4.3 and (V) −6.5; VTA, (AP) −5.6, (L) ± 2.2, and (V) −7.4 angle 12°. The guide cannulas were fixed to the skull with dental cement and three anchoring screws. All coordinates were based on the rat brain atlas (Paxinos and Watson 1998) and were adjusted for the body weight of the animals. Following surgery and for the subsequent three days animals were treated subcutaneously with 10 mg/kg of enrofloxacin (50 mg/mL, Baytril, Germany). Rats were allowed to recover 1 week after surgery. For the intracranial injections, LY2817412 was administered at a volume of 0.5μl/side via a 10-μl Hamilton syringe over 60-sec/side. The stainless-steel injector, 1.5 mm longer than the guide cannula, was allowed to remain in the brain an additional 60 sec/side before being retracted. Cannula placements were verified with injection of black India ink (0.5 μl per site) into the VTA, NAc and CeA after completion of the experiments. Histological verification of ink diffusion into the region of interest was evaluated. Animals then were deeply anesthetized with isoflurane and euthanized. The brains removed from the skull, were quickly frozen in isopentane and stored in a −80°C freezer until sectioning. Coronal sections of 40 mm obtained in the cryostat were mounted on slides and stained with cresyl violet. Placements of the injector were determined using a light microscope and mapped onto coronal sections of a rat brain stereotaxic atlas (Paxinos and Watson 1998).
Self-administration apparatus
Nicotine self-administration was performed in rat operant conditioning chambers (29.5 × 32.5 × 23.5 cm; Med Associates, St. Albans, VT) enclosed in sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with two 4-cm wide retractable levers (8 cm above the grid floor and 12 cm apart) located in the front panel with two stimulus light placed above each lever, a house light at the top of the opposite panel and a tone generator. Nicotine solution was delivered by Tygon tubing connected to a swivel and then to the catheter before the beginning of each session. A syringe pump (3.33 RPM, Med Associates, St. Albans, VT) was activated by responses on the right (active) lever and resulted in a delivery of 0.1 ml nicotine solution, while responses on the left (inactive) lever were recorded but did not result in any programmed consequences. The operant chambers were controlled and data collected with MED-PC® IV windows-compatible software.
Experimental procedures
Nicotine self-administration
Acquisition and maintenance under fixed ratio
After one week of recovery from IV surgery NOP (+/+) and NOP (−/−) rats were trained in 2 h daily nicotine (30μg/kg/0.1ml infusion) self-administration sessions (5 days/week). The response requirement for each infusion was incremented from a fixed ratio 1 (FR1) to fixed ratio 3 (FR3) until baseline was reached. Pressing the active (right) lever resulted in the infusion of 0.1ml of nicotine followed by the activation of the cue light above the lever and a 20 second time-out period during which responses at the active lever had no programmed consequences. Inactive lever presses were recorded but resulted in no reinforcer delivery.
Progressive-ratio
NOP (+/+) and NOP (−/−) rats previously trained to self-administer nicotine under a FR schedule, were subsequently challenged under a progressive ratio (PR) schedule of reinforcement. During PR sessions, the response requirements necessary to receive a single dose of nicotine increased according to the following scale: “3,6,9,12,15,20,25,32,40,50,62,77,95,118,145,178,219,268 etc” (adapted from (Richardson & Roberts, 1996)). The maximal number of responses a rat performed to obtain one infusion was referred to as the break point (BP). The PR session lasted 4 h or ended if the required ratio was not achieved within 1h.
Withdrawal and cue-induced reinstatement
Following the PR experiment NOP (+/+) and NOP (−/−) rats underwent a withdrawal period of 21 days during which they were housed in the animal facility and handled three times per week. After this period animals were returned to the self-administration boxes to be tested for cue-induced reinstatement of nicotine seeking. During 2h reinstatement sessions, responding at the active lever led to the activation of the cue light previously paired with nicotine infusion but nicotine was no longer delivered. The total number of responses at the previously active lever was considered a measure of reinstatement. Inactive lever presses were also recorded as a measure of non-specific responding.
Effect of LY2817412 on nicotine self-administration in NOP (−/−) and NOP (+/+)
After the progressive ratio test, to evaluate the effect of the NOP antagonist LY2817412 on nicotine consumption NOP (+/+) and NOP (−/−) rats were re-trained to FR3 nicotine self-administration until stable baseline of lever pressing was reached. At this point LY2817412 (0.0, 1.0 and 3.0 mg/kg) was given p.o. in a within subject Latin square using Williams’ design 1hr before the beginning of the self-administration session. The FR3 nicotine self-administration baseline was re-established between drug tests. Rats were familiarized with the intragastric gavage procedure for three consecutive days before drug tests.
Effect of LY2817412 microinjection into the VTA, NAc or CeA on nicotine self-administration in NOP (+/+) rats.
New cohorts of NOP (+/+) rats were used to test the effect of LY2817412 on FR3 nicotine self-administration following infusion into the VTA, NAc, or CeA. Rats were trained to self-administer nicotine (30μg/kg/0.1ml infusion) as previously described until a stable baseline was reached. At this point, LY2817412 (0.0, 3.0 and 6.0 μg μl−1) was microinjected into the brain areas of interest according to a Latin-square using Williamś design. Drug infusion occurred 15 min before beginning of the self-administration session. The FR3 nicotine self-administration baseline was re-established between tests. Prior to drug tests rats were familiarized with the intracranial injection procedures. Upon completion of the experiments, rats were anesthetized with isoflurane, and black India ink (0.3 μl per site) was injected into the targeted brain areas. Rats then were euthanized to remove their brains for histological verification of cannula placements. Only data from rats with correct cannula placements were included in the statistical analysis.
Materials
The (−)-Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile physiological saline and administered intravenously at a concentration of 30.0 μg/kg/0.1ml infusion. The NOP receptor antagonist LY2817412 [2’-chloro-4’,4’-difluoro-1-{[1-(3-fluoropyridin-2-yl)-3-methyl-1H-pyrazol-4-yl]methyl}−4’,5’-dihydrospiro[piperidine-4,7’-thieno[2,3-C]pyran]2,3- dihydroxybutanedioate], was synthesized at Lilly Research Laboratories and kindly provided to us. LY2817412 was dissolved in a formulation consisting of a 1:1 mixture of distilled water and 1M H3PO4 (pH 3) and was administered orally via gavage (p.o.). For intracranial microinjections the compound was dissolved in 3% DMSO, 10% Tween and 87% distilled water. Drug doses were chosen based on earlier studies in our laboratory (Borruto et al., 2020; Borruto et al., 2021).
Blinding and randomization
The laboratory animals were randomly assigned to the different experimental groups considering their nicotine responding baseline, and the treatments were assessed blindly. Correct cannula placement was assessed in a blinded manner.
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018a). In the first experiment, the same groups of NOP (−/−) and NOP (+/+) rats were subjected to FR, followed by PR (Fig.1A–B) and with systemic LY28177412 (Fig.2). The reinstatement study (Fig.1C) and brain microinjection experiments (Fig.3) were carried out in a different group of rats. Group size of n ≥ 5 was employed for statistical evaluation and using randomization the experimental groups were designed accordingly. The declared group size represents the number of independent values, and the statistical analysis was performed using these independent values. The sample sizes and animal numbers were determined by power analysis of pre-existing data. Behavioural data were analyzed by analysis of variance (ANOVA) followed by post hoc tests when appropriate. In particular, the difference between the NOP (+/+) and NOP (−/−) in the number of infusions during the acquisition and maintenance of nicotine SA were analyzed by two-way ANOVA with one factor between (rat line) and one factor within (sessions) and the lever presses by three-way ANOVA with one factor between (rat line) and two factors within (lever and sessions). The progressive ratio data were analyzed by unpaired Student t-test. Cue-induced reinstatement of nicotine seeking was analyzed by two-way ANOVA with one factor between (rat line) and one factor within (sessions). The effects of systemic and central administration of LY2817412 on nicotine SA were analyzed using one-way ANOVA with repeated measures using ‘drug dose’ as a within subject factor. Post hoc comparisons were carried out by Dunnettś or by Newman-Keuls test only when the F value attained p<0.05 and there was no significant inhomogeneity of variances. The data are presented as the mean ± SEM. Statistically significant difference was set at P< .05. Prior to ANOVA we examined for significant violations for assumptions of homogeneity of variance by using Levene’s test. Mauchly’s test of sphericity was used to test if assumption of sphericity had been violated when using repeated measures ANOVA. Data were analyzed using STATISTICA, Stat Soft 13.0 (RRID:SCR_014213). The data and statistical analyses complied with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018b).
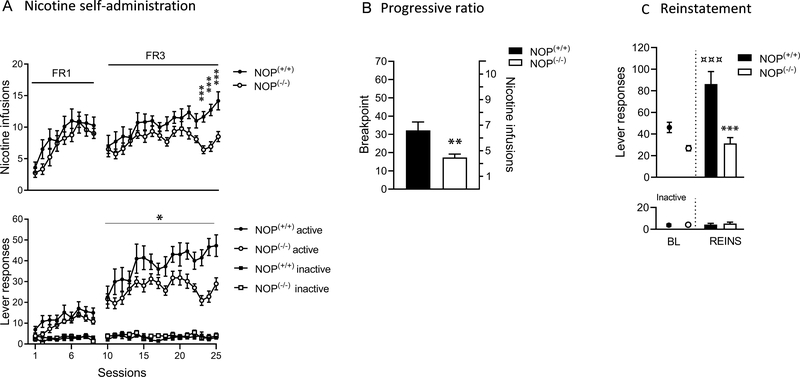
Figure 1. Nicotine addictive-behaviours in NOP (+/+) and NOP (−/−) rats.
(A) Acquisition pattern of nicotine self-administration in NOP (+/+) (n = 11) and NOP (−/−) (n = 12) rats. The number of nicotine infusions and total number of active and inactive lever presses are shown for fixed ratio-1 (FR-1; day 1–9) and fixed ratio-3 (FR-3; day 10 –25) conditions. ***p < 0.001 vs. NOP (+/+) and NOP (−/−) rats nicotine infusions on days 23, 24 and 25. *p < 0.05 vs. NOP (+/+) and NOP (−/−) rats active lever presses in FR3. (B) Motivation for nicotine as measured by the break point on a progressive-ratio (PR) schedule of reinforcement and corresponding nicotine infusions earned in NOP (+/+) (n = 11) and NOP (−/−) (n = 12) rats. **p < 0.01 vs. NOP (+/+) and NOP (−/−) rats. (C) Cue-induced reinstatement of nicotine seeking after 21 days of withdrawal in NOP (+/+) (n = 8) and NOP (−/−) (n = 9) rats compared to their respective baseline (average of active lever presses and inactive lever presses during the last 4 days of training). ***p < 0.001 vs. NOP (+/+) and NOP (−/−) rats. ***p < 0.001 vs. NOP (+/+) baseline responding and NOP (+/+) reinstatement responding. Values represent mean (± SEM).
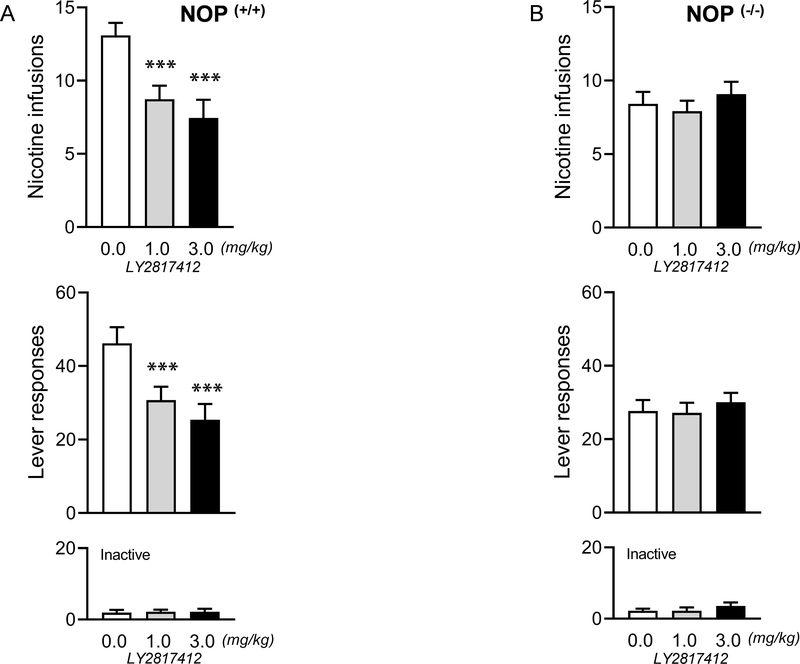
Figure 2. Systemic effects of the NOP antagonist LY2817412 on nicotine self-administration in NOP (+/+) and NOP (−/−) rats.
(A) Number of responses (infusions and total number of active and inactive lever presses) following systemic LY2817412 administration (0.0, 1.0 and 3.0 mg/kg) in NOP (+/+) (n = 11) and (B) NOP (−/−) (n = 12) rats. ***p < 0.001 vs. 0.0 mg/kg vs 1.0 and 3.0 mg/kg. Values represent mean (± SEM).
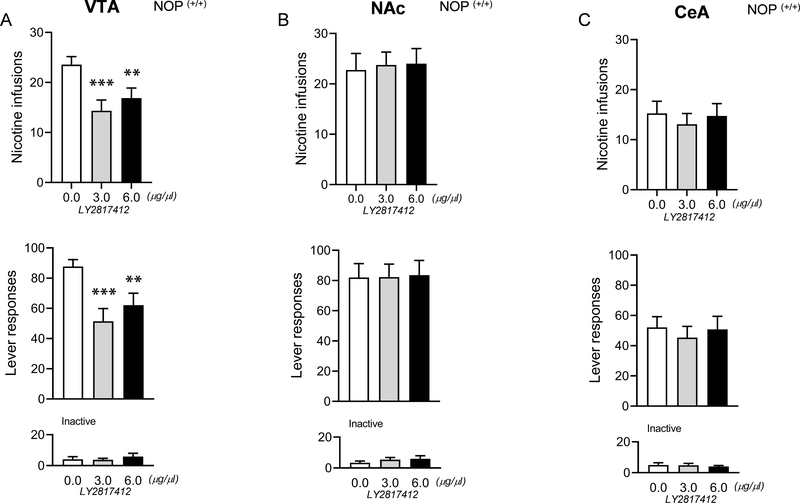
Figure 3. Central effects of the NOP antagonist LY2817412 on nicotine self-administration in VTA, NAC and CeA in NOP (+/+) rats.
(A) Number of responses (infusions and total number of active and inactive lever presses) following central LY2817412 injection (0.0, 3.0 and 6.0 μg/μl) in VTA (n = 9), (B) NAc (n = 8) and (C) CeA (n = 11) in NOP (+/+) rats. ***p < 0.001 vs. 0.0 μg/μl vs 3.0 μg/μl and **p < 0.01 vs. 0.0 μg/μl vs 6.0 μg/μl. Values represent mean (± SEM).
Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021).
Results
NOP (−/−) rats self-administer less nicotine compared to NOP (+/+) counterparts
NOP (+/+) (n=11) and NOP (−/−) (n=12) were trained to self-administer nicotine for 9 consecutive days under FR1 schedule of reinforcement followed by 16 consecutive days of FR3 (Figure 1A). Analysis of the nicotine self-administration data under the FR1 condition revealed no difference in the number of nicotine infusions and lever responding between NOP (+/+) and NOP (−/−) rats. Under the FR3 schedule of reinforcement NOP (−/−) rats administered significantly less nicotine compared to the NOP (+/+) controls and had significantly lower active lever presses. Inactive lever responses were very low and without differences between NOP (+/+) and NOP (−/−) rats on the FR1 or FR3 schedule of reinforcement.
NOP (−/−) rats show lower break point for nicotine compared to NOP (+/+) counterparts
Under the PR contingency the number of lever presses required was increased exponentially as the session progressed. The BP reached was significantly lower in NOP (−/−) (n = 11) compared to NOP (+/+) (n = 12) rats suggesting that NOP (−/−) rats have lower motivation to self-administer nicotine compared to their NOP (+/+) counterparts (figure 1B).
Environmental conditioning factors reinstate nicotine seeking in NOP (+/+) controls but not in NOP (−/−) rats
NOP (+/+) (n=8) and NOP (−/−) (n=9) rats previously trained to self-administer nicotine were tested for cue-induced reinstatement of nicotine seeking after 21 days of withdrawal. When compared to nicotine self-administration baseline (average of nicotine-related lever presses during the last 4 days of self-administration), a significant reinstatement was reached in in NOP (+/+) but not NOP (−/−) rats. When evaluating the differences in reinstatement levels NOP (+/+) rats showed higher levels of reinstatement compared to the NOP (−/−) line. Inactive lever responses were very low and no differences between NOP (+/+) and NOP (−/−) were observed (Figure 1C).
The NOP antagonist LY2817412 reduces nicotine self-administration in NOP (+/+) but not in NOP (−/−) rats
When the efficacy of LY2817412 on FR3 nicotine self-administration was tested in NOP (+/+) rats, (n=11) the compound compared to vehicle significantly reduced nicotine self-administration and the number of active lever presses at both doses (1 and 3 mg/kg) tested (Figure 2A). Analysis of the effects of LY2817412 on nicotine self-administration in NOP (−/−) rats (n=12) failed to confirm significant treatment effects (Figure 2B). Inactive lever presses were very low and not affected by the treatment neither in NOP (+/+), nor NOP (−/−) rats (Figure 2A, B).
Microinjection of LY2817412 in the VTA but not NAc or the CeA reduces nicotine self-administration in NOP (+/+) rats
The lack of efficacy of LY2817412 in NOP (−/−) after peripheral administration prompted us to probe the effects of central administration of the drug in NOP (+/+) rats only. Following LY2817412 microinjection into the VTA (n =9) the number of nicotine infusions and active lever presses were significantly reduced at both doses tested (3.0 μg/μl, and 6.0μg/μl) (Figure 3A). When the NOP antagonist was administered into the NAc (n=8) or CeA (n=11) no significant drug effects on nicotine responding were obtained (Figure 3B–C). Inactive lever pressing was very low and not affected by the drug treatment in each brain region. Following histological analysis, four rats belonging to the VTA group, one to the NAc and three to the CeA groups, were excluded from the statistical analysis due to incorrect cannula placement (Supplementary Fig.S3).
Discussion
Results demonstrated that genetic deletion or pharmacological blockade of NOP receptor by the selective antagonist LY2817412 blunted the motivation for nicotine in the rat. Following intracranial administration LY2817412 reduced nicotine self-administration when injected into the VTA but not into the CeA or the NAc.
Genetic deletion of the NOPR supresses nicotine-motivated addictive-behaviours
During the early acquisition phase of nicotine self-administration, there were no differences between the NOP (+/+) and NOP (−/−) lines when tested to an FR1 schedule of reinforcement. However, when the response requirement was increased to FR3, responding initially decreased in both genotypes, but in NOP (+/+) rats rapidly returned to FR1 levels and then further increased, an effect not observed in NOP (−/−) rats. The implication of these findings is twofold: First, genetic deletion of NOP does not affect the ability of rats to learn operant responding as suggested by the fact that under the FR1 both genotypes equally acquired nicotine-reinforced responding. This is particularly relevant because it has been shown previously that modulation of central N/OFQ-NOP transmission modifies reward-related learning. For instance, in socially defeated rats reward learning was inversely correlated with N/OFQ mRNA expression levels in the VTA, NAc, and striatum (Der-Avakian et al., 2017). Secondly, the lower rate of nicotine infusions in the NOP (−/−) line when operant responding was increased to FR3 indicates that NOP deletion blunts the motivating effects of nicotine when the effort required to obtain the drug increases. This reduced motivation for nicotine of NOP (−/−) was confirmed in the PR experiment where the BP in the knockout was significantly lower compared to wild type controls. In one of our earlier studies, we demonstrated that NOP (−/−) rats also self-administered less cocaine, heroin, and alcohol compared to the control line, while operant responding for saccharin was the same in both lines (Kallupi et al., 2017). A possible explanation of this finding is that NOP (−/−) rats have reduced motivation for substances of abuse in general while reward processing for natural reinforces is unaltered. However, this interpretation contrast with conditioned place preference data showing that NOP (−/−) are more sensitive to the rewarding effects of morphine (Rutten, De Vry, Bruckmann & Tzschentke, 2011). On the other hand, in our pilot study (see Fig S2, Supplementary Information) we found that, compared to NOP (+/+), NOP (−/−) self-administer less nicotine independently from its concentration suggesting that these two genotypes most like differ in the motivation for nicotine rather than in the sensitivity. If taken together these data may suggest that the NOP receptor system plays a different role depending on the substance of abuse under examination. Noteworthy, the fact that NOP (+/+) and NOP (−/−) did not differ in saccharin-reinforced responding indicates that the low rate of responding for drugs of abuse in the NOP (−/−) is not secondary to an impairment in motor performance. Moreover, when we evaluated drug seeking evoked by presentation of cues previously paired with nicotine self-administration, we found that NOP receptor deletion resulted in a complete loss of relapse-like behaviour. This provides additional evidence that in NOP (−/−) rats the motivation for nicotine is low and further reduced following a period of abstinence. Based on this observation it is tempting to hypothesize that high basal NOP transmission facilitates relapse. Although unproven, this hypothesis is indirectly supported by earlier findings with alcohol showing that brain expression levels of N/OFQ and NOP transcripts are higher in postdependent and genetically selected alcohol preferring rats, in which relapse propensity is higher compared to Wistar controls (Aujla, Cannarsa, Romualdi, Ciccocioppo, Martin-Fardon & Weiss, 2013; Economidou et al., 2008; Hansson et al., 2006). A possible confounding factor in the present set of experiment consists of the possibility that, not being NOP (+/+) and NOP (−/−) littermates, parental differences might have affected animals response to nicotine. To mitigate this risk we paid careful attention to maintaining the two genotypes under identical breeding and environmental conditions throughout the study.
NOP receptor antagonism attenuates nicotine self-administration in NOP (+/+) but not NOP (−/−) rats.
Having established that NOP deletion reduces the motivation for nicotine, we sought to further confirm this finding by testing the effects of LY2817412, a potent and selective NOP antagonist on nicotine-motivated behaviour. As expected, LY2817412 significantly reduced nicotine self-administration in NOP (+/+) but not NOP (−/−) rats. This provides important proof-of-concept for the potential efficacy of selective NOP antagonists as a treatment for smoking cessation. Moreover, this finding demonstrates that the effects of LY2817412 are mediated specifically by NOP. The therapeutic potential of this pharmacological approach is further supported by the results of an earlier study in which SB612111, another NOP antagonist, showed efficacy in reducing nicotine intake in rats trained to concurrently self-administer alcohol and nicotine (Cippitelli, Schoch, Debevec, Brunori, Zaveri & Toll, 2016). Moreover, NOP antagonists also have been shown to reduce alcohol drinking and relapse (Borruto et al., 2020; Borruto et al., 2021; Brunori et al., 2019; Koizumi, Midorikawa, Takeshima & Murphy, 2004; Rorick-Kehn et al., 2016). This action of NOP antagonists was observed also in a preliminary study in depressed alcoholic patients (Post et al., 2016). From the clinical perspective, the ability of LY2817412 to attenuate the motivation for both nicotine and alcohol is particularly significant as these two substances are usually co-abused (Anton et al., 2018; Domi, Barbier, Adermark & Domi, 2021; McKee, Falba, O’Malley, Sindelar & O’Connor, 2007; McKee & Weinberger, 2013).
Microinjection of LY2817412 into the VTA but not NAc or CeA reduces nicotine self-administration.
Nicotine reward is thought to be mediated by its facilitation of mesocorticolimbic DA transmission via complex mechanisms involving both postsynaptic and presynaptic modulation of VTA DA neurons (Mao, Gallagher & McGehee, 2011; Marti et al., 2011; Pontieri, Tanda, Orzi & Di Chiara, 1996; Tolu et al., 2013; Yan, Beckley, Kim & Drenan, 2019). We hypothesised therefore that the inhibitory effects of LY2817412 on nicotine self-administration, was mediated by its interference with nicotine-induced activation of VTA DA transmission. This hypothesis also was driven by our earlier findings on LY2940094, a NOP antagonist analogue to LY2817412, that reduces alcohol-induced increases of DA release in the NAc shell (Rorick-Kehn et al., 2016). These effects appear to be specific for drug reinforcers in view of reports that site specific ablation of NOP receptors in the VTA enhances the motivation for sucrose and injection of N/OFQ in the same brain region decreases binge eating (Hernandez, Perez, Soto, Le, Gastelum & Wagner, 2021; Parker et al., 2019). While selective deletion of the receptor in the CeA attenuates the hedonic value of palatable food (Hardaway et al., 2019). Moreover, we have found earlier that site specific microinjection of LY2817412 into the VTA and CeA, but not NAc, reduces alcohol drinking in genetically selected alcohol preferring rats (Borruto et al., 2020). Guided by these earlier observations we sought to establish whether the effects of LY2817412 on nicotine self-administration are mediated via blockade of NOP receptors in one of these regions. The microinjection experiments identified the VTA as the critical site for this effect in that nicotine self-administration was significantly reduced following administration of LY2817412 into this area but not when injected into the CeA or NAc. These findings raise two issues: the first is that the effects of LY2817412 on nicotine self-administration seems to be mediated selectively by VTA NOP receptors whereas, as per our previous findings with alcohol, both the VTA and the CeA seem to mediate LY2817412-induced reductions in alcohol intake. It is likely, therefore, that the mechanisms through which NOP blockade attenuates the motivation for alcohol and nicotine do not fully overlap and differ with regard to the relevance of the CeA. Secondly, considering that NOP antagonism reduces alcohol-induced DA release in the NAc one may speculate that LY2817412 attenuates nicotine reward by blunting the ability of nicotine to stimulate mesolimbic DA transmission. However, this hypothesis contrasts with earlier evidence that facilitation of VTA N/OFQ transmission acts as a stop signal to terminate reward-related responses and that activation of mesolimbic DA transmission by substances of abuse is prevented also by NOP agonists (Di Giannuario, Pieretti, Catalani & Loizzo, 1999; Parker et al., 2019; Vazquez-DeRose, Stauber, Khroyan, Xie, Zaveri & Toll, 2013).
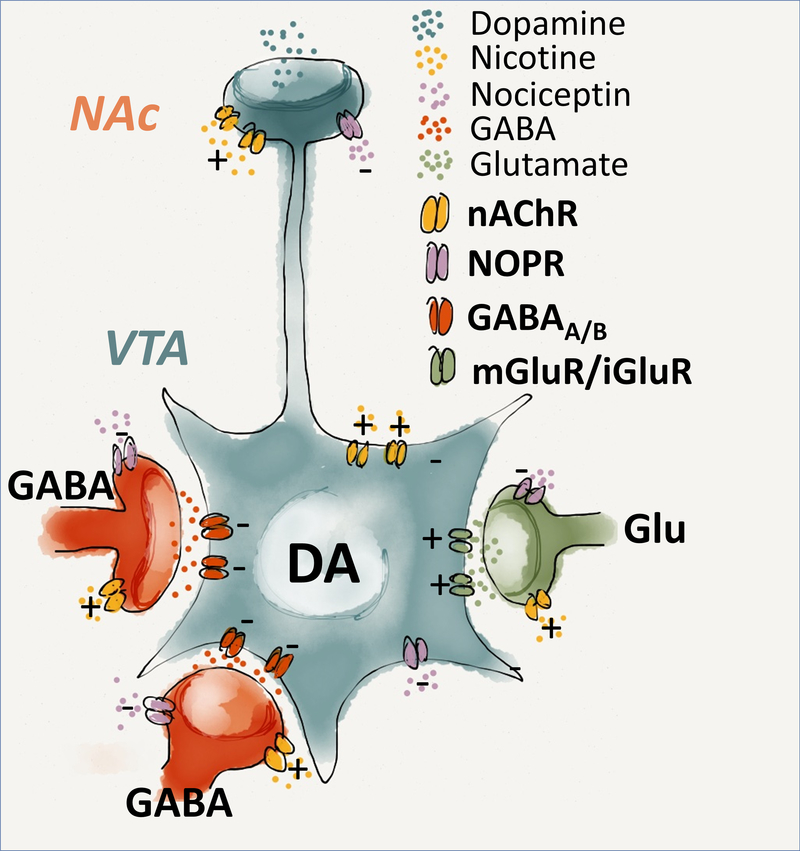
However, with regard to this inconsistency, it is important to consider that the VTA contains two populations of NOP positive neurons (Fig.4); the first one co-express TH and its activation negatively regulates DA transmission (Norton, Neal, Kumar, Akil & Watson, 2002; Zheng, Grandy & Johnson, 2002). The second population is negative to TH and are composed of GABAergic and glutamatergic cells, that are located presinaptically and, by impinging onto DA neurons, regulate the activity of the VTA DA system (Driscoll, Wallace, Mansourian, Martin & Margolis, 2020). It is known that N/OFQ acting at presynaptic level inhibits GABA release onto dopamine neurons which may potentially result in their disinhibition (Zheng, Grandy & Johnson, 2002). Conceivably, such disinhibition is prevented by simultaneous activation of NOP receptors that are located in DA cells and that hyperpolarize them. However, in condition when the role of presynaptic NOP is prevalent, receptor antagonists by blocking the inhibitory effect of N/OFQ on VTA GABAergic cells can enhance their activity consequently diminishing DA neurotransmission. Based on this conceptualization, it is possible therefore to propose a heuristic mechanism according to which modulation of N/OFQ system by NOP agonist and antagonists may both oppose the activation of VTA DA transmission by substances of abuse.
Figure 4. Schematic drawing of N/OFQ-NOP system in the ventral tegmental area (VTA) – Nucleus accumbens (Alexander et al.).
Within the VTA, NOP positive neurons are expressed in DAergic, GABAergic and Glutamatergic cells. The figure illustrates the possible mechanism through which NOP signalling influences the cellular components affecting the mesolimbic DA transmission. N/OFQ exerts an inhibitory role in the activity of both GABAergic interneurons, and DAergic neurons within the VTA. At a presynaptic lever, activation of the NOP receptors inhibits GABA release onto dopamine neurons, with subsequent disinhibition and increase in DA release. However simultaneous activation of NOP receptors that are located in DA cells prevent such disinhibition resulting in a final attenuation of the DA neurotransmission.
In conclusion, the present findings confirm that genetic deletion or pharmacological blockade of NOP attenuates the motivation for nicotine and, by extension, suggest that selective receptor antagonists such as LY2817412 may prove effective as smoking cessation agents. One analogue of LY2817412, BTRX-246040 (LY2940094), is currently under clinical development for the treatment of depression. Given the high co-occurrence of depression and nicotine abuse, it may be of particular clinical relevance to test the therapeutic potential of NOP antagonists not only in nicotine dependent patients but particularly in these comorbid patient populations.
Supplementary Material
Bullet point summary.
What is already known
NOP receptors play a pivotal role in the reinforcement and motivational aspects of drugs of abuse.
NOP receptor agonism enhances nicotine consumption in rats.
What does this study add
NOP receptor blockade attenuates the motivation for nicotine through mechanisms involving the VTA
What is the clinical significance
NOP receptors antagonism may represent a new potential approach for smoking cessation.
Acknowledgments:
This work was supported by National Institutes of Health (NIH), grant AA014351(FW, RC) from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and a grant PRIN 2017SXEXT5 (to RC). We thank Linda M. Rorick-Kehn for scientific input and thoughtful comments on the manuscript and Louise Adermark for providing us with figure 4. We also are thankful to Rina Righi, Agostino Marchi and Alfredo Fiorelli for animal breeding and expert technical assistance.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Abbreviations:
- nAChR
nicotinic acetylcholine receptor
- DA
dopamine
- N/OFQ
Nociceptin/Orphanin FQ
- NOP
Nociceptin Opioid-like Peptide
- VTA
Ventral Tegmental area
- NAc
nucleus accumbens
- CeA
Central amygdala
- FR
fixed ratio
- PR
progressive ratio
- BP
break point
- TH
Tyrosine hydroxylase
Footnotes
Conflicts of interest statement: The authors declare that they have no conflict of interest
List of Hyperlinked target reported in the same order of the revised manuscript:
Nicotine
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585
Varenicline
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5459
Bupropion
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7135
Dopamine
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940
Nociceptin/Orphanin FQ (N/OFQ)
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1681
Nociceptin Opioid-like Peptide (NOP) receptor
https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=320
GABA
Glutamate
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369
SB612111
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1693
AT-202
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8867
Isoflurane
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2505
Heparin
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4214
Sodium Pentothal
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2579
(−)-Nicotine hydrogen tartrate salt
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585
Alcohol
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2299
References
- Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein-coupled receptors. British Journal of Pharmacology 178: S27–S156. [DOI] [PubMed] [Google Scholar]
- Anton RF, Latham PK, Voronin KE, Randall PK, Book SW, Hoffman M, et al. (2018). Nicotine-Use/Smoking Is Associated with the Efficacy of Naltrexone in the Treatment of Alcohol Dependence. Alcohol Clin Exp Res 42: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, & Weiss F (2013). Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol 18: 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Robledo P, Trigo JM, Martín-García E, & Maldonado R (2010). Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev 35: 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Fotio Y, Stopponi S, Brunori G, Petrella M, Caputi FF, et al. (2020). NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharmacol 177: 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Fotio Y, Stopponi S, Petrella M, De Carlo S, Domi A, et al. (2021). NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori G, Weger M, Schoch J, Targowska-Duda K, Barnes M, Borruto AM, et al. (2019). NOP Receptor Antagonists Decrease Alcohol Drinking in the Dark in C57BL/6J Mice. Alcohol Clin Exp Res 43: 2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, & Weiss F (2019). NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol 254: 187–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, et al. (2009). Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol 43: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Schoch J, Debevec G, Brunori G, Zaveri NT, & Toll L (2016). A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci Rep 6: 26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, et al. (2018a). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, et al. (2018b). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Potter DN, Chartoff EH, Carlezon WA Jr., Pizzagalli DA, et al. (2017). Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology (Berl) 234: 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, & Loizzo A (1999). Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett 272: 183–186. [DOI] [PubMed] [Google Scholar]
- Domi A, Barbier E, Adermark L, & Domi E (2021). Targeting the Opioid Receptors: A Promising Therapeutic Avenue for Treatment in “Heavy Drinking Smokers”. Alcohol Alcohol 56: 127–138. [DOI] [PubMed] [Google Scholar]
- Driscoll JR, Wallace TL, Mansourian KA, Martin WJ, & Margolis EB (2020). Differential Modulation of Ventral Tegmental Area Circuits by the Nociceptin/Orphanin FQ System. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. (2008). Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry 64: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrashidi MY, & Ebbert JO (2014). Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert Opin Emerg Drugs 19: 243–260. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, & Neff NH (2011). Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology 60: 1209–1220. [DOI] [PubMed] [Google Scholar]
- Hammond D, Reid JL, Cole AG, & Leatherdale ST (2017). Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. Cmaj 189: E1328–e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, et al. (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A 103: 15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, et al. (2019). Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron 102: 1037–1052.e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J, Perez L, Soto R, Le N, Gastelum C, & Wagner EJ (2021). Nociceptin/orphanin FQ neurons in the Arcuate Nucleus and Ventral Tegmental Area Act via Nociceptin Opioid Peptide Receptor Signaling to Inhibit Proopiomelanocortin and A(10) Dopamine Neurons and Thereby Modulate Ingestion of Palatable Food. Physiol Behav 228: 113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Mul JD, de Wit E, & Cuppen E (2009). Complete knockout of the nociceptin/orphanin FQ receptor in the rat does not induce compensatory changes in mu, delta and kappa opioid receptors. Neuroscience 163: 308–315. [DOI] [PubMed] [Google Scholar]
- Jha P, & Peto R (2014). Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 370: 60–68. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Scuppa G, de Guglielmo G, Calò G, Weiss F, Statnick MA, et al. (2017). Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology 42: 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, & Roberto M (2014). Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology 39: 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, & Altman DG (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M, Midorikawa N, Takeshima H, & Murphy NP (2004). Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. J Neurochem 89: 257–263. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, et al. (2015). Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. Jama 314: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Gallagher K, & McGehee DS (2011). Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci 31: 6710–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti F, Arib O, Morel C, Dufresne V, Maskos U, Corringer PJ, et al. (2011). Smoke extracts and nicotine, but not tobacco extracts, potentiate firing and burst activity of ventral tegmental area dopaminergic neurons in mice. Neuropsychopharmacology 36: 2244–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, & O’Connor PG (2007). Smoking Status as a Clinical Indicator for Alcohol Misuse in US Adults. Archives of Internal Medicine 167: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, & Weinberger AH (2013). How can we use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annu Rev Clin Psychol 9: 649–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, & Watson SJ (2002). Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol 444: 358–368. [DOI] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, et al. (2019). A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell 178: 653–671.e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, & Di Chiara G (1996). Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382: 255–257. [DOI] [PubMed] [Google Scholar]
- Post A, Smart TS, Jackson K, Mann J, Mohs R, Rorick-Kehn L, et al. (2016). Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin Exp Res 40: 1935–1944. [DOI] [PubMed] [Google Scholar]
- Primack BA, Shensa A, Sidani JE, Hoffman BL, Soneji S, Sargent JD, et al. (2018). Initiation of Traditional Cigarette Smoking after Electronic Cigarette Use Among Tobacco-Naïve US Young Adults. Am J Med 131: 443.e441–443.e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, & Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Roberto M, & Siggins GR (2006). Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A 103: 9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, et al. (2016). A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res 40: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, & Tzschentke TM (2011). Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend 114: 253–256. [DOI] [PubMed] [Google Scholar]
- Sakoori K, & Murphy NP (2009). Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology 56: 896–904. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, & Heilig M (2012). Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76: 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Deng Y, Ciccocioppo R, & Cannella N (2017). Cebranopadol, a Mixed Opioid Agonist, Reduces Cocaine Self-administration through Nociceptin Opioid and Mu Opioid Receptors. Front Psychiatry 8: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, & Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–10; discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, et al. (2013). Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry 18: 382–393. [DOI] [PubMed] [Google Scholar]
- Uezu K, Sano A, Sei H, Toida K, Houtani T, Sugimoto T, et al. (2005). Enhanced hippocampal acetylcholine release in nociceptin-receptor knockout mice. Brain Res 1050: 118–123. [DOI] [PubMed] [Google Scholar]
- Vazquez-DeRose J, Stauber G, Khroyan TV, Xie XS, Zaveri NT, & Toll L (2013). Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol 699: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2016). WHO Report on the Global Tobacco Epidemic, 2011. Geneva: World Health Organization, 2011. [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, et al. (2014). The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Beckley NA, Kim VJ, & Drenan RM (2019). Differential Nicotinic Modulation of Glutamatergic and GABAergic VTA Microcircuits. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT (2011). The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem 11: 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, & Johnson SW (2002). Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol 136: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.