Abstract
Patients with sickle cell anemia (SCA) experience cerebral metabolic stress with an increase in oxygen extraction fraction (OEF) to compensate for reduced oxygen carrying capacity due to anemia. It remains unclear if anemia alone drives this metabolic stress. Using MRI, we collected voxel-wise OEF measurements to test our hypothesis that OEF would be elevated in anemic controls without SCA (AC) compared to healthy controls (HC), but OEF would be even higher in SCA compared to AC. Brain MRIs (N=159) were obtained in 120 participants (34 HC, 27 AC, 59 SCA). While hemoglobin was lower in AC versus HC (p<0.001), hemoglobin was not different between AC and SCA cohorts (p=0.459). Whole brain OEF was higher in AC compared to HC (p<0.001), but lower compared to SCA (p=0.001). Whole brain OEF remained significantly higher in SCA compared to HC (p=0.001) while there was no longer a difference between AC versus HC (p=0.935) in a multivariate model controlling for age and hemoglobin. OEF peaked within the borderzone regions of the brain in both SCA and AC cohorts, but the volume of white matter with regionally elevated OEF in AC was smaller (1.8%) than SCA (58.0%). While infarcts colocalized within regions of elevated OEF, more SCA participants had infarcts than AC (p<0.001). We conclude that children with SCA experience elevated OEF compared to AC and HC after controlling for the impact of anemia, suggesting that there are other pathophysiologic factors besides anemia contributing to cerebral metabolic stress in children with SCA.
Keywords: Sickle cell anemia, anemia, oxygen extraction fraction, stroke
Introduction
Sickle cell anemia (SCA) is an autosomal recessive disease caused by a mutation in the beta-globin gene, resulting in the production of hemoglobin S. Hemoglobin S polymerizes in the deoxygenated state, distorting the shape of the red blood cell (RBC). In addition to obstructing the microcirculation due to misshapen RBCs with aberrant rheology, hemoglobin S-containing RBCs intravascularly hemolyze, causing severe anemia, intravascular inflammation and endothelial activation throughout all organ systems.1 Specific to the brain, complications of SCA encompass silent cerebral infarctions,2 overt stroke,3 intracranial vasculopathy2,4 and cognitive decline independent of infarction or vasculopathy.5,6
Cerebral blood flow is increased in SCA to compensate for decreased arterial oxygen content in the setting of severe anemia.7–9 Furthermore, our research has shown that oxygen extraction fraction (OEF), the percent of oxygen extracted from the blood into the brain tissue, increases in patients with sickle cell anemia to meet the metabolic demands of the brain tissue.9–11 OEF peaks within the border zone of the brain, where CBF nadirs.9,12 Furthermore, the regions with a greatest increase in OEF align with regions of diminished functional connectivity13 and greatest risk for infarction in patients with SCA.9,12 Primary disease modification with an increase in arterial oxygen content via transfusion of hemoglobin A laden red blood cells relieves ongoing metabolic stress with a resultant decrease in cerebral blood flow and oxygen extraction fraction in patients with SCA.11,14
Anemia alone, in patients without SCD, has been associated with stroke15–17 and aberrant cognitive and brain development.18,19 Using a variety of techniques, including positron emission tomography (PET), MRI and near infrared reflectance spectroscopy (NIRS), CBF20–25 and OEF20,22,24–27have been shown to increase as hemoglobin decreases in healthy individuals and patients with anemia associated with chronic kidney disease, hepatic encephalopathy, post-operative blood loss and subarachnoid hemorrhage. It is well established that the severity of anemia is a primary risk factor for the neurocognitive complications of SCA,2,28,29 however it remains unclear if anemia alone drives the hemodynamic compromise experienced by patients with SCA, or if additional risk factors concomitantly contribute to metabolic stress. We undertook this MR imaging study to test our hypothesis that OEF would be elevated in anemic patients without SCA compared to controls, but OEF would be higher in patients with SCA compared to patients that were anemic for reasons other than SCA.
Methods
This study was approved by the Institutional Review Board at Washington University in St. Louis. Informed consent was obtained from all participants or legal guardian if the participant was less than 18 years of age upon enrollment. Data from three subgroups of participants from St. Louis Children’s Hospital (SLCH) were analyzed: healthy control (HC), anemic control (AC) and sickle cell anemia (SCA) participants. HC participants were siblings of a patient with sickle cell disease (SCD, any genotype), but concomitant enrollment of the sibling with SCD was not required for participation. AC participants were eligible if they did not have SCD and their hemoglobin was less than the lower limit of normal for age and sex. Enrollment within the SCA subgroup was limited to participants with hemoglobin SS or hemoglobin S beta thalassemia null genotypes. Participants that were greater than 21 years of age upon initial enrollment, unable to tolerate the brain MRI without sedation, had a contraindication to MRI due to metal (e.g., braces or metal implantation), were receiving chronic transfusion therapy, or had a past medical history including stem cell transplant, gene therapy, overt stroke, vasculopathy (defined as a narrowing of the internal carotid or middle cerebral arteries on magnetic resonance angiography) or neurologic condition that could impact cerebral hemodynamics were excluded from participation. Intermittent simple transfusion of red blood cells was not an exclusion criterion. These cross-sectional analyses were performed with a combined dataset from two longitudinal imaging studies. Hence, a subset of participants has multiple timepoints of data included in the analyses.
A brain MRI and laboratory evaluation, including a complete blood count and hemoglobin electrophoresis, were obtained on the same day at each study visit. Hemoglobin was not obtained at the time of MRI in 10 HC participants, and 21 participants (13 HC, 2 AC and 6 SCA) did not have a hemoglobin electrophoresis obtained. Eighteen participants (3 HC, 2 AC and 13 SCA) had a CBC drawn on a different day than the brain MRI, drawn a mean (standard deviation, SD) of 16.3 (±20.5) days and range of 1–84 days from the brain MRI. Nine participants (2 HC, 2 AC, 5 SCA) had hemoglobin electrophoresis drawn on a different day than the brain MRI, drawn a mean (SD) of 13.2 (±11.4) days and range 1–34 days from the MRI. White blood cell differential and reticulocyte counts were not collected prospectively. These laboratory values were abstracted from the medical record if available. Missing laboratory values were not imputed, and participants with missing values were excluded from multivariate analyses.
Imaging Data and Processing
Each participant underwent a brain MRI on a Siemens 3T Trio or Prisma without sedation. A three-dimensional magnetization prepared rapid acquisition gradient echo (MP-RAGE) T1 (TE/TR = 2.13/2,400ms or 2.94/1,810ms, inversion time = 1,000 ms, flip angle 8 degrees, and acquired 1×1×1 mm voxel resolution) and axial or coronal fluid attenuated inversion recovery (FLAIR, 94/9,000 ms, inversion time = 2500 ms, flip angle = 150 degrees and 3mm or 5mm slice thickness) were collected during each scan. Voxel-wise measurement of OEF was obtained with an asymmetric spine echo (ASE) sequence.30 A board-certified neurologist (KPG or ALF) identified and delineated infarcts on FLAIR images in anemic participants with Medical Image Processing, Analysis and Visualization software (mipav.cit.nih.gov), and voxels within infarcts were excluded from OEF processing. FLAIR images from prior clinical or research scans were used if the image acquired during the study visit was contaminated by motion artifact. Three participants (2 AC, 1 SCA) did not have a FLAIR image available, and a FLAIR image was obtained for processing from a different research or clinical scan in 15 participants with SCA.
Statistical Parametric Mapping software (SPM) was used to create T1 tissue probability maps in native (T1) space.31,32 A T1 to OEF warp was computed for each participant using Advanced Normalization Tools (ANTs), which was subsequently applied to the T1 image and probability maps.33,34 FSL MCFLIRT was used to motion correct the ASE series with a common reference frame.35 The ASE data was then decomposed into contributions from each tissue type (i.e. grey matter, white matter, CSF).36 The tissue-specific ASE signal was independently processed to compute voxel-wise OEF per participant.13 A hematocrit value is required for OEF processing, and linear regression accounting for age and sex was used to estimate hematocrit for control participants with missing lab values. ASE frames with high motion, defined as rotation > 0.04 radians in any direction or translation > 2mm in any direction, were excluded when computing OEF. Voxels with non-physiologic values (OEF<0.05 or OEF>0.95) or with high error (i.e. error value exceeding Otsu’s threshold) were excluded on a participant-specific basis, as were lesioned voxels. To create a whole brain, partial volume corrected OEF map, gray and white matter OEF maps were combined into a weighted average image, where each OEF value was weighted by the probability of the associated tissue type.
Z-score Maps with Probabilistic Threshold Free Cluster Enhancement (pTFCE)
Individual whole brain partial volume corrected OEF maps were combined to create average OEF maps for the HC, AC, and SCA cohorts. Voxels with < 80% of the participants within the cohort having a defined OEF value were excluded from the average OEF map. The AC and SCA Z-score maps were created by subtracting the mean cohort value of that voxel from the mean value of the same voxel within the HC cohort. The difference in mean OEF was divided by the standard deviation of that voxel’s value within the HC cohort. pTFCE incorporated neighborhood information to create an enhanced Z-score image. Gaussian random field theory-based Z-score thresholds were computed using the publicly available pTFCE R Package (https://github.com/spisakt/pTFCE).37,38
Infarct Density Heatmaps
For participants with infarcts identified on FLAIR images, the individuals’ FLAIR images (in native space) were aligned to an atlas space (MNI 152) through the individual’s T1. The resulting warp was subsequently applied to the lesion mask to transform the mask into atlas space using ANTs.33,34 Individual lesion masks in atlas space were combined to create the lesion density heatmap.
Statistical Analyses
Median and interquartile range were used to describe continuous variables. A Mann-Whitney U test or Kruskal- Wallis test was used to compare continuous variables, while a Chi-squared test was used to compare categorical variables. The Benjamini-Hochberg procedure was used to correct for multiple comparisons.
To investigate differences in OEF between cohorts while controlling for total hemoglobin, independent variables of age, cohort, total hemoglobin, and hemoglobin-squared (due to second-order polynomial fit) were entered into a general linear mixed model predicting whole brain OEF while adjusting for repeated subject observation.
A second model was built to explore hematologic variables associated with whole brain OEF within the SCA population. Candidate variables associated with whole brain OEF (white blood cell count, absolute neutrophil count, absolute lymphocyte count, platelet, mean platelet volume, total hemoglobin, mean corpuscular volume, reticulocyte, percent hemoglobin F, percent hemoglobin S, and percent hemoglobin A) were tested with Spearman’s Rho and Chi-square tests when appropriate to determine a univariate association. Age was forced into the model due to the developmental range within the study population, and hemoglobin-squared was included due to second-order polynomial fit. Variables with a univariate association of p < 0.20 were entered into a naïve stepwise general linear mixed model predicting whole brain OEF while adjusting for repeated subject observation.
Results
Brain MRIs were obtained in 120 participants (34 HC, 27 AC, 59 SCA). A total of 159 brain MRIs were included in the analyses, with 4 HC participants contributing 2 scans each (separated by 2.5 – 3.9 years) and 26 SCA participants contributing between 2–4 scans each (separated by 28 days – 4.5 years). Table 1 provides a description of the cohort. Hemoglobin at the time of brain MRI for baseline scans was lower in the AC subgroup compared to HC (p < 0.001), but there was not a significant difference in hemoglobin between the AC and SCA subgroup (p = 0.459, Table 1). Within the SCA cohort, 9 (9.6%) of participants received a transfusion of red blood cells at SLCH within three months of the study visit. Eighty-three percent of participants with SCA were taking hydroxyurea at a median dose of 27.9 [22.1–33.1] mg/kg/day at the time of their study visit. The AC cohort was composed of patients with heterogeneous diagnoses resulting in anemia: iron deficiency anemia (N=9), congenital dyserythropoietic anemia (N=1), aplastic anemia (N=6), hereditary spherocytosis (N=5), pyruvate kinase deficiency (N=1), loxoscelism (N=1), non-transfusion dependent beta thalassemia intermedia (N=1), hemorrhage in the setting of severe hemophilia A (N=1) and participants that were enrolled as HC participants and incidentally found to be anemic (N=2).
Table 1.
Cohort Description and OEF per Baseline Scan of Each Participant
| HC N = 34 |
AC N = 27 |
SCA N = 59 |
P-Value (HC vs. AC) |
P-Value (AC vs. SCA) |
|
|---|---|---|---|---|---|
| Scan # | 38 | 27 | 94 | - | - |
| Age (Years) | 11.0 [8.8–15.0] | 14.0 [10.0–16.0] | 10.0 [8.0–14.0] | 0.122 | 0.024* |
| Sex (M/F) | 17/17 | 9/18 | 26/33 | 0.191 | 0.347 |
| Hemoglobin (g/dL) | 12.4 [11.9–13.2] | 8.5 [7.7–10.0] | 8.1 [7.7–9.6] | < 0.001* | 0.459 |
| Hemoglobin A (%) | 82.7 [60.8–97.2] | 97.1 [92.7–97.5] | 0.0 [0.0–0.0] | 0.055 | < 0.001* |
| Hemoglobin F (%) | 0.0 [0.0–0.2] | 0.0 [0.0–0.9] | 16.5 [9.5–25.1] | 0.487 | < 0.001* |
| Hemoglobin S (%) | 13.9 [0.0–35.1] | 0.0 [0.0–0.0] | 76.8 [68.8–83.8] | 0.002* | < 0.001* |
| White Blood Cell (k/cumm) | 5.7 [4.9–7.4] | 5.7 [4.4–8.0] | 9.9 [7.5–12.9] | 0.925 | < 0.001* |
| Platelet (k/cumm) | 285.0 [246.5–327.8] | 280.0 [59.0–323.0] | 396.0 [286.0–507.0] | 0.509 | < 0.001* |
| White Matter OEF (%) | 24.9 [22.8–27.4] | 35.9 [29.6–38.4] | 39.9 [34.5–42.2] | < 0.001† | 0.003† |
| Gray Matter OEF (%) | 29.6 [27.7–30.9] | 35.7 [32.9–40.4] | 40.4 [36.2–43.2] | < 0.001† | 0.001† |
| Whole Brain OEF (%) | 28.1 [26.3–29.8] | 35.6 [31.3–39.7] | 40.6 [36.2–42.8] | < 0.001† | 0.001† |
Statistically significant
Statistically significant after correction for multiple comparisons with Benjamini-Hochberg procedure
OEF was higher in the AC cohort compared to HC across white matter (p < 0.001), gray matter (p < 0.001) and whole brain (p < 0.001), but remained lower in the AC cohort compared to SCA across tissue types (white matter (p = 0.003), gray matter (p = 0.001), whole brain (p = 0.001), Table 1, Figure 1A). Whole brain OEF within the SCA cohort remained elevated compared to HC (parameter estimate = 3.671, 95% CI 1.486, 5.857, p = 0.001) while there was no longer a significant elevation in AC compared to HC (p = 0.935) in a multivariate model controlling for age (p=0.251), hemoglobin (parameter estimate = −7.878, 95% CI −10.195, −5.561, p < 0.001), and hemoglobin-squared (parameter estimate = 0.267, 95% CI 0.148, 0.386, p < 0.001, indicating that the relationship between OEF and hemoglobin is not linear, Figure 1B).
Figure 1. OEF remains elevated in the SCA cohort after controlling for hemoglobin.
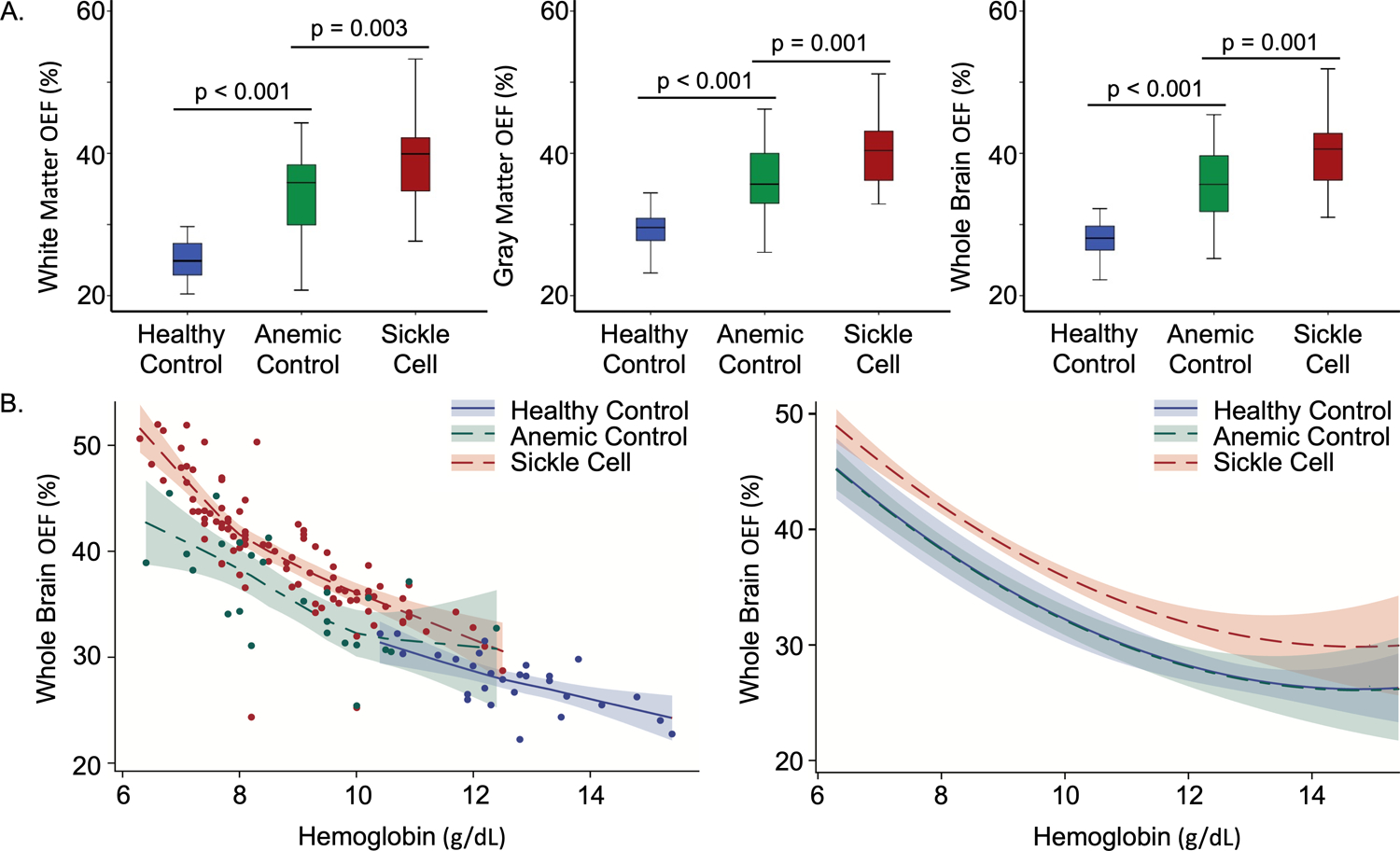
A. OEF is elevated in anemic control participants (green) compared to healthy controls (blue), but significantly lower than participants with SCA (red) in white matter (left), gray matter (middle) and whole brain (right) even though there is not a significant difference in hemoglobin between the AC and SCA cohorts (p = 0.459). B. Whole brain OEF increases as hemoglobin decreases (Spearman’s rho = −0.878, p < 0.001). Data is shown as a LOESS curve fit per cohort on the left. While controlling for age, hemoglobin, hemoglobin-squared and subject-specific effects, whole brain OEF remains significantly elevated in participants with SCA compared to HC (p = 0.001) but there is not a significant elevation in whole brain OEF in the AC participants compared to HC (p = 0.935) on the right. Figure displays hemoglobin effect plot computed at mean age of 12 years.
OEF has previously been shown to peak in the border zone regions of the brain where CBF nadirs in patients with SCA compared to HC participants.9,12 Z-score maps were created to identify the regions of white matter with significantly elevated OEF in participants with SCA versus HC and AC participants versus HC. Figure 2 illustrates that OEF peaks within the border zone regions of the brain in both the SCA and AC cohorts. However, the volume of brain with significantly elevated OEF in the AC vs HC cohorts was smaller (1.8% of white matter) than the SCA vs. HC cohorts (58.0% of white matter). Infarcts identified within the study cohort colocalized with the regions of brain with significantly elevated OEF (Figure 3). While the distribution of infarcts within the anemic cohorts co-localized with the regions of elevated OEF, a greater percentage of participants with SCA (55.2%) had infarcts identified on FLAIR imaging than anemic controls (16.0%, p < 0.001). The volume of infarction was larger in the SCA cohort (8.1 [0.0–168.4] mm3) versus the AC cohort (0.0 [0.0–0.0] mm3, p = 0.002, Figure 3). However, within the individuals with an infarct present, the volume of infarcted brain was not significantly different between the affected SCA participants (158.0 [26.6–351.7] mm3) compared to the AC participants (157.3 [64.3–335.1] mm3, p = 0.880).
Figure 2. Regions of greatest elevation in OEF in SCA and AC compared to HC.
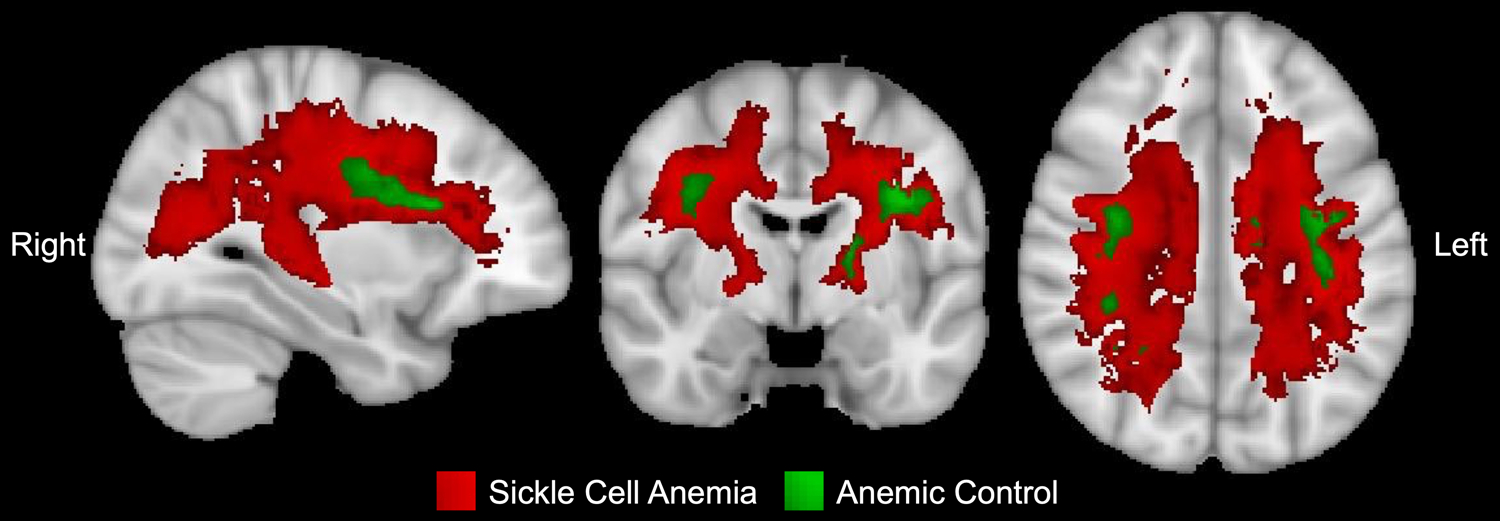
Regions of the white matter with significantly elevated OEF in the SCA (red) and AC (green) cohorts compared to HC (p<0.05 with probabilistic threshold free cluster enhancement) fall within the border zone of the brain. While the regions of elevated OEF align in both the SCA and AC cohorts, the volume of brain with elevated OEF is greater in the SCA cohort compared to AC.
Figure 3. Infarct Burden in Anemic Cohorts.
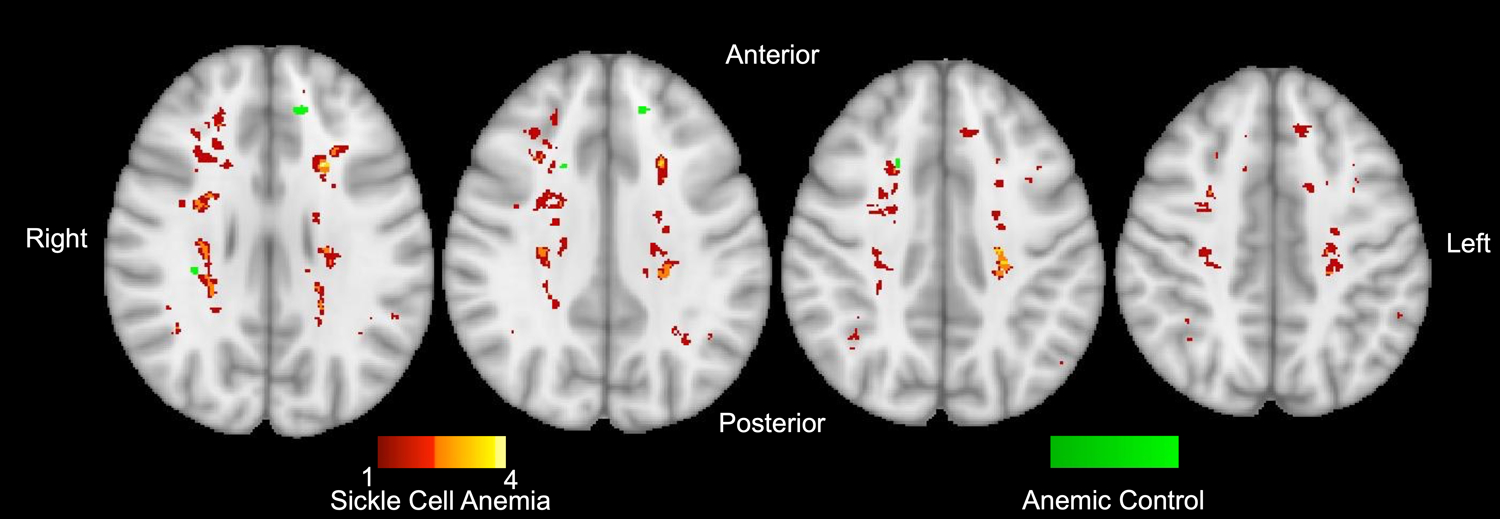
Infarct count from the SCA cohort (red, orange, yellow) and AC cohorts (green) are overlaid on axial slices of an atlas moving inferior (left) to superior (right). In contrast to the SCA cohort, none of the infarcts in the AC cohort overlap with each other. A greater percentage of participants with SCA (55.2%) had infarcts compared to AC (16%, p < 0.001), and the volume of infarcted brain tissue was larger in the SCA cohort (8.1 [0.0–168.4] mm3) versus the AC cohort (0.0 [0.0–0.0] mm3, p = 0.002).
As OEF remained elevated in the SCA cohort after controlling for hemoglobin, multivariate analyses were performed within the SCA cohort to explore potential hematologic variables associated with this increase in OEF. After univariate correlation (Supplemental Table 1), white blood cell count, absolute lymphocyte count, percent hemoglobin F, percent hemoglobin A, mean platelet volume and mean corpuscular volume met criteria for entry into the naive regression model. OEF increased as hemoglobin F (parameter estimate = 0.069, 95% CI 0.015, 0.123, p = 0.015) increased using mixed model linear regression to control for multiple timepoints per participants, age (p = 0.944), hemoglobin (parameter estimate = −11.525, 95% CI −16.950, −6.101, p < 0.001), and hemoglobin-squared (parameter estimate = 0.448, 95% CI 0.150, 0.746, p = 0.005). The associations between OEF, hemoglobin, hemoglobin-squared and percent hemoglobin F persisted in analyses limited to participants with SCA receiving hydroxyurea therapy (Supplemental Table 1).
Discussion
These data show that children with anemia for reasons other than SCA have increased whole brain, white matter and gray matter OEF, a marker of metabolic stress, compared to healthy controls. However, OEF is significantly higher in children with SCA compared to these anemic controls, even when there is not a significant difference in total hemoglobin between cohorts and hemoglobin is controlled for in multivariate analyses. Anatomically, the border zone regions of the brain have the highest OEF in both the SCA and AC cohorts when compared to HC, but the volume of brain with significantly elevated OEF is higher in SCA than AC.
Anemia, acute or chronic, is associated with neurocognitive complications across all ages. Acute silent cerebral infarcts detected with diffusion weighted imaging have been identified during severe anemic episodes in children15 and there is an increased risk of overall cognitive impairment, dementia and Alzheimer’s disease, in adults with anemia.39 Silent cerebral infarcts are seen in patients with beta thalassemia, both transfusion-independent and transfusion-dependent,40–42 and there are long-term neurocognitive consequences of iron deficiency anemia that persist after iron repletion.43–45 The mechanism of injury for these neurocognitive complications may differ by disease state. Iron deficiency directly impacts myelination, dopamine and serotonin synthesis and signaling, epigenetic regulation of gene transcription and energy metabolism via mitochondrial proteins,43,45 while infarction in patients with thalassemia could be in part due to the hypercoaguable state associated with thalassemia.46–48 However, the hemodynamic compromise associated with the decreased arterial oxygen content is common across disease states, potentially contributing to the resulting neurocognitive complications. Consistent with prior literature in anemic patient populations,20,24,26,27,49,50 our data provides evidence that pediatric patients with anemia in disease states other than SCA experience hemodynamic compromise, as measured by increased OEF. We extend these findings to show that children are impacted while at steady state and not critically ill, and that the regions of greatest OEF elevation within the AC cohort fall within the watershed distribution.
There are clinical guidelines and standards of care for treatment of patients with disorders resulting in chronic anemia,51–53 and guidelines for transfusion of patients with acute and chronic anemia.54 These treatment algorithms are primarily contingent upon total hemoglobin or hemoglobin isoforms and, for non-hereditary anemias, rely on measures of hemodynamic compromise such as tachycardia or hypotension. Here, we show that anemia has cerebral hemodynamic consequences that are more subtle than symptoms of imminent hemodynamic collapse. Utilization of biomarkers that directly assess oxygen delivery and metabolic demand may better inform such guidelines to improve neurological outcomes.24,26,49 With future investigations solidifying the link between neuroimaging biomarkers and neurocognitive outcomes of interest in patients with acute or chronic anemia, our data could potentially better define the threshold for intervention in these disease states.
The relationship between OEF and hemoglobin has been shown in anemic patients with9,10 and without SCA.20,24,26,27,49,50 However, our results demonstrate that OEF is significantly higher in patients with SCA compared to the AC cohort after controlling for total hemoglobin. This finding suggests that there are aspects of SCA physiology aside from decreased arterial oxygen content driving the compensatory increase in OEF. Prior literature has established that CBF increases as patients become more anemic, not only in SCA,7,8,14,55–59 but also in healthy controls22 and disease states other than SCA with concomitant anemia.8,20,21,24–26,56 Oxygen delivery normalizes in SCA in the setting of increased CBF,8 suggesting there is increased demand driving the elevation in OEF in the participants with SCA compared to AC. Markers of inflammation and hemolysis have been associated with increased CBF, another biomarker of hemodynamic compromise,23,60 and inflammation has been linked to poor cognitive outcomes.61,62 Anemia is an established risk factor for infarction in SCA29 and current therapies used for primary disease modification and neuroprotection improve total hemoglobin,63–65 however, an improved understanding of the mechanism driving metabolic demand in patients with SCA could provide the field with future therapeutic targets.
Albeit a smaller impact than hemoglobin, we report a positive relationship between hemoglobin F and OEF, which is consistent with our prior work.66 Fetal hemoglobin is left shifted with an increased oxygen affinity compared to hemoglobins A and S,67 but the impact of hemoglobin F alone on OEF is challenging to discern as an increase in hemoglobin F decreases polymerization of hemoglobin S and hemolysis with a resultant reduction in inflammation and increase in total hemoglobin in patients with SCA. Furthermore, the medication used to induce hemoglobin F in patients with SCA, hydroxyurea, is myelosuppressive, reducing production of white blood cells and platelets that contribute to vascular injury and endothelial activation in SCA.68 Hence, the impact of hemoglobin F induction in SCA is complex. A recent publication by Pedrosa and Lemes showed a decreased expression of hypoxia inducible factors (HIFs) in the setting of hydroxyurea.69 While the relationship between HIFs and cardiovascular disease is well-established, it is unclear whether induction of HIFs is beneficial or detrimental in the setting of cerebral hemodynamic compromise.70 Ultimately, the relationship between hemoglobin F and metrics of cerebral hemodynamic stress warrants further investigation as it not only pertains to patients with SCA in the setting of current treatment with hydroxyurea, but also with novel therapeutic options that induce hemoglobin F production or modify hemoglobin’s oxygen affinity.71,72 Furthermore, this line of investigation would potentially be relevant to neonatal patient populations and patients with ineffective erythropoiesis (e.g., thalassemia, congenital dyserythropoietic anemia) that commonly have increased hemoglobin F.
We report an increase in OEF in both anemic cohorts compared to healthy controls, which is corroborated by prior work utilizing different imaging modalities in a multitude of disease states.14,20,22,24,25,27,49,50 Contrary to our results, Vu et al. reported that OEF was significantly lower in children and adults with anemia (chronic anemias and sickle cell disease) compared to healthy controls.73 While the direction of difference differs between studies with our report of an increase in the setting of anemia and Vu et al. reporting a decrease with anemia, the two studies are in agreement that OEF in SCA differs significantly from participants with chronic anemias of other etiologies. Furthermore, we advance this area of investigation as we report a significant difference in OEF between the AC and SCA cohorts while controlling for hemoglobin while the AC cohort evaluated by Vu et al. had a significantly higher hemoglobin than the cohort with sickle cell disease.
The differences between our results and those published by Vu et al. are most likely multifactorial. First, there is variation in study populations, as Vu et al. included a heterogeneous population ranging in age from 12 to 63 years, including all sickle cell genotypes and a large percentage of both the AC and sickle cell cohorts were chronically transfused.73 Second, we measure voxel-wise OEF with an ASE sequence30 while Vu et al. utilized a TRUST sequence to obtain a flow-weighted measurement of deoxyhemoglobin in the superior sagittal sinus for a single, global assessment of OEF.74 Patients with SCA may suffer from microvascular cerebral shunting with increased arterial transit time through the microvasculature preventing adequate offloading of oxygen,75,76 resulting in a decreased deoxyhemoglobin measurement in the sagittal sinus and a decreased OEF measurement. The impact of microvascular shunting on OEF measurements via ASE and TRUST may differ due to differences in technique. While both ASE and TRUST have been evaluated and tested in the setting of hypercapnia30,74 and ASE has been validated with blood gas oximetry measurements in animal studies,77 further research will be required to best understand how the two acquisition techniques can complement each other in the study of cerebral hemodynamics in patients with SCA.
Strengths of our investigation include the inclusion of a large cohort of children with severe anemia, without a significant difference in hemoglobin between the AC and SCA cohorts, and utilization of an ASE sequence to obtain voxel-wise measurements of OEF. However, there are limitations of this study. First, the cross-sectional design limits our analyses and conclusions to understanding associations between hemoglobin and OEF. Specific markers of inflammation and hemolysis were not prospectively collected, preventing full investigation into the association of these physiologic pathways with elevated OEF. This dataset is limited to the measurement of OEF, but collection of CBF and CMRO2 will ultimately be necessary to understand consequences of elevated OEF in anemic patients. Lastly, the AC cohort consisted of a heterogeneous group of patients with different pathophysiology and both acute and chronic anemia. Longitudinal follow-up will be required to understand the impact of acute versus chronic elevation of OEF on brain development and clinical outcomes, such as stroke and cognition.
We conclude that OEF is elevated in children with non-sickle cell anemias compared to unaffected children, and that anemia is primarily driving this compensatory mechanism. However, children with SCA experience significantly higher OEF compared to children with non-sickle cell anemias after controlling for the impact of anemia, suggesting that there are additional pathophysiologic factors in SCA besides anemia that influence cerebral metabolic demand. An improved understanding of the covariates driving this increase in cerebral metabolic stress in SCA could provide future therapeutic targets for neuroprotection in this vulnerable population.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank Liam Comiskey, Rachel Shields and Luisa Gil Diaz for study set-up and coordination.
Funding Sources:
This research was supported by National Institutes of Health, National Heart, Lung and Blood Institute (K23HL136904 [MEF], R01HL157188 [MEF], R01HL129241 [ALF]), the National Institute of Neurological Disorders and Stroke (K23NS099472 [KPG], U24NS107230 [JML]), the American Society of Hematology [MEF], the Doris Duke Charitable Foundation [MEF] and The Foundation for Barnes-Jewish Hospital [MEF]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data is available upon contact of corresponding author.
Potential Conflicts of Interest: M.E.F declares equity ownership in Proclara Biociences, a biopharmaceutical company developing therapies for Alzheimer’s Disease. M.E.F., A.L.F., and M.L.H received one-time compensation for scientific advisory board participation with Bluebird Bio, who is developing gene therapy trials for sickle cell disease. M.L.H performs ongoing consulting for Bluebird Bio. M.E.F. works as a consultant for Global Blood Therapeutics, a company manufacturing Oxbryta for sickle cell disease. M.L.H receives research support from Global Blood Therapeutics and from FORMA Therapeutics, a pharmaceutical company developing potential sickle cell disease treatments. M.L.H.’s spouse is employed by Pfizer, Inc. M.M.B. is employed by OpenCell Technologies, LLC., a device manufacturer for gene-editing.
Ethics Approval and Patient Consent: This study was approved by the Institutional Review Board at Washington University in St. Louis. Informed consent was obtained from all participants or legal guardian if the participant was less than 18 years of age upon enrollment.
References
- 1.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. [DOI] [PubMed] [Google Scholar]
- 2.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653–1661. [DOI] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 4.Thangarajh M, Yang G, Fuchs D, et al. Magnetic resonance angiography-defined intracranial vasculopathy is associated with silent cerebral infarcts and glucose-6-phosphate dehydrogenase mutation in children with sickle cell anaemia. Br J Haematol. 2012;159(3):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Enos L, Gallagher D, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139(3):391–397. [DOI] [PubMed] [Google Scholar]
- 6.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89(2):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevers S, Nederveen AJ, Fijnvandraat K, et al. Arterial spin labeling measurement of cerebral perfusion in children with sickle cell disease. J Magn Reson Imaging. 2012;35(4):779–787. [DOI] [PubMed] [Google Scholar]
- 8.Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol. 2016;91(9):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fields ME, Guilliams KP, Ragan DK, et al. Regional oxygen extraction predicts border zone vulnerability to stroke in sickle cell disease. Neurology. 2018;90(13):e1134–e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(Pt 3):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilliams KP, Fields ME, Ragan DK, et al. Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia. Blood. 2018;131(9):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford AL, Ragan DK, Fellah S, et al. Silent infarcts in sickle cell anemia occur in the borderzone region and are associated with low cerebral blood flow. Blood. 2018;132(16):1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields ME, Mirro AE, Guilliams KP, et al. Functional Connectivity Decreases with Metabolic Stress in Sickle Cell Disease. Annals of Neurology. 2020;88(5):995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juttukonda MR, Lee CA, Patel NJ, et al. Differential cerebral hemometabolic responses to blood transfusions in adults and children with sickle cell anemia. Journal of Magnetic Resonance Imaging. 2019;49(2):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowling MM, Quinn CT, Plumb P, et al. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood. 2012;120(19):3891–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panwar B, Judd SE, Warnock DG, et al. Hemoglobin Concentration and Risk of Incident Stroke in Community-Living Adults. Stroke. 2016;47(8):2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramamoorthy J, Trehan A, Ahluwalia J, et al. Neuroimaging Abnormalities in Patients With Nontransfusion-dependent Thalassemia. Journal of Pediatric Hematology Oncology. 2019;41:e290–e295. [DOI] [PubMed] [Google Scholar]
- 18.Choi S, O’Neil SH, Joshi AA, et al. Anemia predicts lower white matter volume and cognitive performance in sickle and non-sickle cell anemia syndrome. American Journal of Hematology. 2019;94(10):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozoff B, Georgieff MK. Iron Deficiency and Brain Development. Seminars in Pediatric Neurology. 2006;13(3):158–165. [DOI] [PubMed] [Google Scholar]
- 20.Kuwabara Y, Sasaki M, Hirakata H, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney International. 2002;61:564–569. [DOI] [PubMed] [Google Scholar]
- 21.Floyd TF, McGarvey M, Ochroch EA, et al. Perioperative Changes in Cerebral Blood Flow after Cardiac Surgery: Influence of Anemia and Aging. Annals of Thoracic Surgery. 2003;76(6):2037–2042. [DOI] [PubMed] [Google Scholar]
- 22.Ibaraki M, Shinohara Y, Nakamura K, et al. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. Journal of Cerebral Blood Flow and Metabolism. 2010;30(7):1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borzage MT, Bush AM, Choi S, et al. Predictors of cerebral blood flow in patients with and without anemia. J Appl Physiol (1985). 2016;120(8):976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhar R, Zazulia AR, Derdeyn CP, Diringer MN. RBC Transfusion Improves Cerebral Oxygen Delivery in Subarachnoid Hemorrhage. Critical Care Medicine. 2017;45(4):653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng G, Lu H, Yu W, et al. Severity-specific alterations in CBF, OEF and CMRO2 in cirrhotic patients with hepatic encephalopathy. European Radiology. 2017;27(11):4699–4709. [DOI] [PubMed] [Google Scholar]
- 26.Neunhoeffer F, Hofbeck M, Schuhmann MU, et al. Cerebral oxygen metabolism before and after RBC transfusion in infants following major surgical procedures. Pediatric Critical Care Medicine. 2018;19(4):318–327. [DOI] [PubMed] [Google Scholar]
- 27.Morris EA, Juttukonda MR, Lee CA, et al. Elevated brain oxygen extraction fraction in preterm newborns with anemia measured using noninvasive MRI. Journal of Perinatology. 2018;38(12):1636–1643. [DOI] [PubMed] [Google Scholar]
- 28.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. British Journal of Haematology. 2009;146(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An H, Lin W. Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo- and hypercapnic conditions using an asymmetric spin echo approach. Magn Reson Med. 2003;50(4):708–716. [DOI] [PubMed] [Google Scholar]
- 31.Kiebel SJ, Ashburner J, Poline J-B, Friston KJ. MRI and PET Coregistration-A Cross Validation of Statistical Parametric Mapping and Automated Image Registration. Neuroimage. 1997;5:271–279. [DOI] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. NeuroImage. 2000;11(6 I):805–821. [DOI] [PubMed] [Google Scholar]
- 33.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tustison NJ, Cook PA, Klein A, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–179. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 36.Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med. 2008;60(6):1362–1371. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 38.Spisák T, Spisák Z, Zunhammer M, et al. Probabilistic TFCE: A generalized combination of cluster size and voxel intensity to increase statistical power. NeuroImage. 2019;185:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kung WM, Yuan SP, Lin MS, et al. Anemia and the risk of cognitive impairment: An updated systematic review and meta-analysis. Brain Sciences. 2021;11(6):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taher AT, Musallam KM, Nasreddine W, et al. Asymptomatic brain magnetic resonance imaging abnormalities in splenectomized adults with thalassemia intermedia. Journal of Thrombosis and Haemostasis. 2010;8(1):54–59. [DOI] [PubMed] [Google Scholar]
- 41.Karimi M, Haghpanah S, Pishdad P, Rachmilewitz EA. Frequency of silent cerebral ischemia in patients with transfusion-dependent β-thalassemia major compared to healthy individuals. Annals of Hematology. 2016;95(8):1387. [DOI] [PubMed] [Google Scholar]
- 42.Pazgal I, Inbar E, Cohen M, Shpilberg O, Stark P. High incidence of silent cerebral infarcts in adult patients with beta thalassemia major. Thrombosis Research. 2016;144:119–122. [DOI] [PubMed] [Google Scholar]
- 43.Bastian TW, Raghavendra R, Tran P v, Bastian Georgieff M. Neurosci insigh 2020. Neuroscience Insights. 2020;12:1–12. [Google Scholar]
- 44.Parkin PC, Koroshegyi C, Mamak E, et al. Association between Serum Ferritin and Cognitive Function in Early Childhood. Journal of Pediatrics. 2020;217:189–191.e2. [DOI] [PubMed] [Google Scholar]
- 45.Larsen B, Bourque J, Moore XTM, et al. Longitudinal development of brain iron is linked to cognition in youth. Journal of Neuroscience. 2020;40(9):1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia Thromboembolic manifestations in thalassemia. Blood. 2002;99(1):36–43. [DOI] [PubMed] [Google Scholar]
- 47.Ataga KI, Cappellini MD, Rachmilewitz EA. β-Thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. British Journal of Haematology. 2007;139(1):3–13. [DOI] [PubMed] [Google Scholar]
- 48.Cappellini MD, Motta I, Musallam KM, Taher AT. Redefining thalassemia as a hypercoagulable state. Annals of the New York Academy of Sciences. 2010;1202:231–236. [DOI] [PubMed] [Google Scholar]
- 49.Leal-Noval SR, Arellano-Orden V, Muñoz-Gómez M, et al. Red Blood Cell Transfusion Guided by Near Infrared Spectroscopy in Neurocritically Ill Patients with Moderate or Severe Anemia: A Randomized, Controlled Trial. Journal of Neurotrauma. 2017;34(17):2553–2559. [DOI] [PubMed] [Google Scholar]
- 50.Ito K, Ookawara S, Ueda Y, et al. Changes in Cerebral Oxygenation Associated with Intradialytic Blood Transfusion in Patients with Severe Anemia Undergoing Hemodialysis. Nephron Extra. 2017;7(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iolascon A, Andolfo I, Barcellini W, et al. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica. 2017;102(8):1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trompeter S, Massey E, Robinson S. Position paper on International Collaboration for Transfusion Medicine (ICTM) Guideline ‘Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline.’ British Journal of Haematology. 2020;189(3):424–427. [DOI] [PubMed] [Google Scholar]
- 53.Chou ST, Alsawas M, Fasano RM, et al. American society of hematology 2020 guidelines for sickle cell disease: Transfusion support. Blood Advances. 2020;4(2):327–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA - Journal of the American Medical Association. 2016;316(19):2025–2035. [DOI] [PubMed] [Google Scholar]
- 55.Kosinski PD, Croal PL, Leung J, et al. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: a quantitative magnetic resonance imaging study. Br J Haematol. 2017;176(2):280–287. [DOI] [PubMed] [Google Scholar]
- 56.Bush A, Chai Y, Choi SY, et al. Pseudo continuous arterial spin labeling quantification in anemic subjects with hyperemic cerebral blood flow. Magnetic Resonance Imaging. 2018;47:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Croal PL, Leung J, Kosinski P, et al. Assessment of cerebral blood flow with magnetic resonance imaging in children with sickle cell disease: A quantitative comparison with transcranial Doppler ultrasonography. Brain and Behavior. 2017;7(11):e00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Václavů L, Meynart BN, Mutsaerts HJMM, et al. Hemodynamic provocation with acetazolamide shows impaired cerebrovascular reserve in adults with sickle cell disease. Haematologica. 2019;104(4):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afzali-Hashemi L, Baas KPA, Schrantee A, et al. Impairment of Cerebrovascular Hemodynamics in Patients With Severe and Milder Forms of Sickle Cell Disease. Frontiers in Physiology. 2021;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaclavu L, Petr J, Petersen ET, et al. Cerebral oxygen metabolism in adults with sickle cell disease. Am J Hematol. 2019;95(4):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minhas PS, Latif-Hernandez A, McReynolds MR, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021;590(7844):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preau S, Vodovar D, Jung B, et al. Energetic dysfunction in sepsis: a narrative review. Annals of Intensive Care. 2021;11(104):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 64.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fields ME, Guilliams KP, Ragan D, et al. Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia. Blood. 2019;133(22):2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young RC, Rachal RE, del Pilar Aguinaga M, et al. Automated oxyhemoglobin dissociation curve construction to assess sickle cell anemia therapy. Journal of the National Medical Association. 2000;92:430–435. [PMC free article] [PubMed] [Google Scholar]
- 68.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358(13):1362–1369. [DOI] [PubMed] [Google Scholar]
- 69.Pedrosa AM, Lemes RPG. Gene expression of HIF-1a and VEGF in response to hypoxia insickle cell anaemia: Influence of hydroxycarbamide. British Journal of Haematology. 2020;190:e39–e56. [DOI] [PubMed] [Google Scholar]
- 70.Lucero García Rojas EY, Villanueva C, Bond RA. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Frontiers in Cardiovascular Medicine. 2021;8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vichinsky E, Hoppe CC, Ataga KI, et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. New England Journal of Medicine. 2019;381(6):509–519. [DOI] [PubMed] [Google Scholar]
- 72.Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. New England Journal of Medicine. 2021;384(3):252–260. [DOI] [PubMed] [Google Scholar]
- 73.Vu C, Bush A, Choi S, et al. Reduced global cerebral oxygen metabolic rate in sickle cell disease and chronic anemias. American Journal of Hematology. 2021;96(8):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-relaxation-under-spin-tagging MRI. Magnetic Resonance in Medicine. 2008;60(2):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Østergaard L, Jespersen SN, Engedahl T, et al. Capillary Dysfunction: Its Detection and Causative Role in Dementias and Stroke. Current Neurology and Neuroscience Reports. 2015;15(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juttukonda MR, Donahue MJ, Waddle SL, et al. Reduced oxygen extraction efficiency in sickle cell anemia patients with evidence of cerebral capillary shunting. Journal of Cerebral Blood Flow and Metabolism. 2021;41(3):546–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An H, Liu Q, Chen Y, Lin W. Evaluation of MR-derived cerebral oxygen metabolic index in experimental hyperoxic hypercapnia, hypoxia, and ischemia. Stroke. 2009;40(6):2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


