Abstract
Background and Purpose:
“Food addiction” is the subject of intense public and research interest. However, this nosology based on neurobehavioral similarities among obese individuals and patients with eating disorders and drug addiction remains controversial. We thus sought to determine which aspects of disordered eating are causally linked to preclinical models of drug addiction. We hypothesized that extensive drug histories, known to cause addiction-like brain changes and drug motivation in rats, would also cause addiction-like food motivation.
Experimental Approach:
Rats underwent extensive cocaine, alcohol, caffeine or obesogenic diet histories, and were subsequently tested for punishment-resistant food self-administration or “compulsive appetite”, as a measure of addiction-like food motivation.
Key Results:
Extensive cocaine and alcohol (but not caffeine) histories caused compulsive appetite that persisted long after the last drug exposure. Extensive obesogenic diet histories also caused compulsive appetite, although neither cocaine nor alcohol histories caused excess calorie intake and bodyweight during abstinence. Hence, compulsive appetite and obesity appear to be dissociable, with the former sharing common mechanisms with preclinical drug addiction models.
Conclusion and Implications:
Compulsive appetite, as seen in subsets of obese individuals and patients with binge-eating disorder and bulimia nervosa (eating disorders that do not necessarily result in obesity), appears to epitomize “food addiction”. Because different drug and obesogenic diet histories caused compulsive appetite, overlapping dysregulations in the reward circuits, which control drug and food motivation independently of energy homeostasis, may offer common therapeutic targets for treating addictive behaviors across drug addiction, eating disorders and obesity.
Keywords: Eating disorders, substance use disorders, food addiction
1. INTRODUCTION
Obesity and some eating disorders, such as binge eating disorder (BED) and bulimia nervosa (BN), are increasingly considered “food addiction” (Avena, Bocarsly, Hoebel & Gold, 2011; Gearhardt, White & Potenza, 2011). The putative validity of this nosology relies largely on neurobehavioral similarities between these forms of disordered eating and substance use disorders (drug addiction) (Serafine, O’Dell & Zorrilla, 2021; Volkow & Wise, 2005), the prototypical addictive disorder. However, the legitimacy of food addiction, based on such correlational evidence, remains controversial (Fletcher & Kenny, 2018). We have thus investigated which aspects of disordered eating are causally linked to well-established preclinical models of drug addiction.
One hallmark of drug addiction is compulsive drug craving and use that persist despite adverse consequences (punishments) (American Psychiatric Association, 2013). In animals, extensive drug histories can cause punishment-resistant “compulsive” drug self-administration and brain changes resembling those in patients with drug addiction (Vanderschuren & Ahmed, 2020). Similarly, extensive obesogenic diet histories can cause addiction-like food motivation and brain changes in animals (Wiss, Criscitelli, Gold & Avena, 2017). Since patients with drug addiction – as a universally recognized form of addiction – and people with obesity and/or eating disorders – as putative “food addiction” – share similar brain profiles (Serafine, O’Dell & Zorrilla, 2021; Volkow & Wise, 2005), we hypothesized that extensive drug histories would similarly cause punishment-resistant food self-administration or “compulsive appetite” in animals.
We tested this hypothesis in male and female rats undergoing long-term abstinence from different drug (cocaine/alcohol/caffeine) and obesogenic diet (high fat and high sugar/high fat [but not high sugar]/high sugar [but not high fat]/high protein) histories. We determined compulsive appetite based on established procedures to determine punishment-resistant drug and food self-administration in rats (e.g., Deroche-Gamonet, Belin & Piazza, 2004; Johnson & Kenny, 2010). For these procedures, along with caloric sweetened condensed milk (SCM), we used non-caloric saccharin as a food reward to isolate ‘non-homeostatic’ (hedonic/incentive) appetite regulation from ‘homeostatic’ (caloric/metabolic) regulation (Berthoud, 2006). We also used saccharin to isolate the hedonic/incentive impacts of food rewards arising from sensory processes (e.g., taste) from those arising from enteric or metabolic feedback signals from the gut (de Araujo, Schatzker & Small, 2020). Additionally, we determined the long-term effects of past drug (cocaine/alcohol/caffeine) histories on ad libitum ‘basal’ calorie intake and weight changes.
2. METHODS
All experimental procedures were conducted in accordance with the National Institutes of Health (USA) Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees at The Scripps Research Institute.
2.1. Animals
We used rats in the current study, since rats are known to replicate aspects of neurobehavioral profiles of human drug addiction and disordered eating (Serafine, O’Dell & Zorrilla, 2021; Volkow & Wise, 2005). A total of 552 Wistar rats (RRID:MGI:5657554) were used. Of these, 409 were male and 143 were female. All rats were purchased from Charles River, Inc. (Wilmington, MA). Adult male and female rats weighing 250-300g and 150-175g, respectively, at the start of experiments were used. Male and female rats were roughly the same age. All rats were housed in a temperature and humidity-controlled room and maintained on a 12 h/12 h reverse light/dark cycle. At all times, water and food were available ad libitum. All rats were maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA) and regular tap water, unless otherwise specified. For all cases, studies were designed to generate groups of equal size, using randomization and blinded analysis. However, equal group size was not always achieved because of attrition of animals due to experimental complications, including general health issues and failure to meet training criteria. Below, we report the initial numbers of animals used for each experiment as well as the final numbers retrained for statistical analyses.
2.2. Materials
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD, USA). Alcohol and caffeine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Obesogenic diets were obtained from Research Diets, Inc. (New Brunswick, NJ, USA). We used the following diets from Research Diets: #D12451 (high fat and high sugar), #D12492 (high fat), #D02022703 (high sugar) and #D08091802 (high protein).
2.3. Surgery
Rats assigned to “cocaine histories via operant self-administration” (see below) were implanted with intravenous catheters made of Micro-Renathane (Braintree Science, Braintree, MA, USA) under anesthesia, as described previously (Laque et al., 2019). Rats were allowed to recover for at least 7 days.
2.4. Behavioral Procedures
For operant conditioning procedures, rats were trained and tested during the dark (active) phases in dedicated operant conditioning chambers (Med Associates, Saint Albans, VT, USA). Each chamber was equipped with two retractable levers (one “active lever” and one “inactive lever”), a “light-cue,” a syringe pump, and either a liquid swivel system for intravenous cocaine or a drinking well for sweetened condensed milk (SCM) and saccharin. At all times, insertion of active and inactive levers into an operant conditioning chamber signaled the start of a once-daily self-administration session conducted under a fixed ratio 1 (FR1) schedule of reinforcement. Each press on the active lever resulted in a single delivery of either cocaine (1.0 mg/kg/infusion, intravenous) into the jugular vein or a non-drug reward (SCM [33% dissolved in water, 0.2 ml, oral] or saccharin [0.125% dissolved in water, 0.2 ml, oral]) into the drinking well. Each cocaine delivery was paired with 20s illumination of a light-cue, and each SCM or saccharin delivery was paired with 2s illumination of a light-cue; light-cue illumination signaled a timeout period during which presses on the active lever were recorded but had no scheduled consequences.
Rats were randomly assigned to specific experimental conditions defined by drug/diet histories and behavioral tests. We describe each experimental condition below:
2.4.1. Experiment 1: The effects of extensive cocaine histories on subsequent food motivated behavior in male rats
Male rats were randomly assigned to a specific experimental condition defined first by drug history and then behavioral test. We describe each experimental condition below (experimental timelines are schematized in Figure 1):
Figure 1:
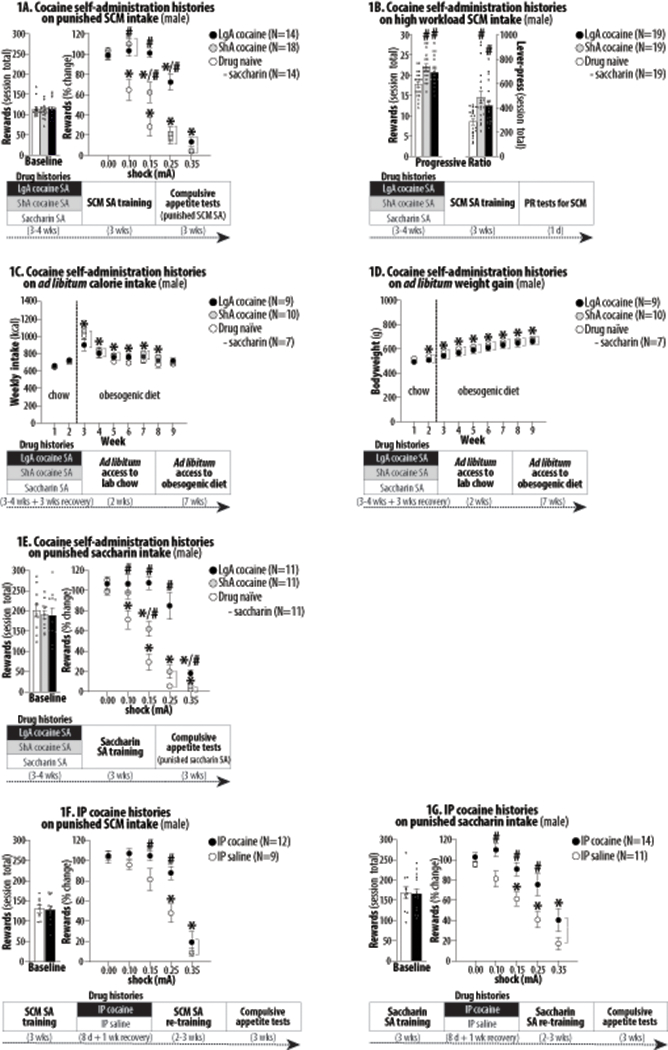
The effects of extensive cocaine histories on subsequent food motivated behavior in male rats undergoing long-term abstinence. Asterisks (*) indicate significant difference from 0.0 mA (Figures 1A/1E/1F/1G) or Week 1 (Figures 1C/1D). Pound signs (#) indicate significant difference from the drug naïve control. Abbreviations: sweetened condensed milk (SCM), self-administration (SA), long-access (LgA), short-access (ShA) and intraperitoneal (IP). 1A. Right panel depicts group averages (+SEM) of total numbers of rewards obtained (30-min totals) during the last three training sessions as “baseline”. Left panel depicts group averages (±SEM) of numbers of rewards obtained (30-min totals) during each test session as “% changes from baseline” (normalized per rat). *, P<0.001-0.05. #, P<0.001-0.05. 1B. Right and left panels depict group averages (+SEM) of total numbers of rewards and active lever-pressing under a progressive ratio (PR) schedule. #, P<0.001-0.01. 1C. Weekly food intake (kcal). *, P<0.001-0.05. 1D. Weekly bodyweight measures. *, P<0.001-0.01. 1E. *, P<0.001-0.05. #, P<0.01-0.05. 1F. *, P<0.001-0.05. #, P<0.01-0.05. 1G. *, P<0.001-0.05. #, P<0.01-0.05.
I. Cocaine histories via operant self-administration (cocaine self-administration histories):
Rats were randomly assigned to short access (ShA) cocaine, long access (LgA) cocaine or saccharin history groups. LgA cocaine history was based on procedures known to induce addiction-like escalation in drug intake and brain changes in rats (Ahmed, 2012). ShA cocaine history was based on procedures known to induce addiction-like habitual and inflexible behavior and brain changes (e.g., Lucantonio et al., 2014). Neither LgA nor ShA cocaine history is known to alter pain sensitivity in rats (Edwards et al., 2012). Since cocaine is non-caloric, we used similarly non-caloric saccharin for the control self-administration histories (drug naïve controls). Rats in Lg/ShA cocaine and saccharin history groups were further randomly assigned to the following behavioral tests and trained accordingly:
i. Compulsive appetite tests for SCM
Here we determined the animals’ willingness to work for caloric SCM in despite adverse consequences (punishments), as an indicator of addiction-like food motivation. Male (N = 54) rats were trained and tested according to the following three phases:
1. Drug histories (3–4 weeks): Rats in the ShA and LgA cocaine history groups were trained to lever-press for cocaine for 2 hrs and 6 hrs daily, respectively. Since cocaine is non-caloric, we used similarly non-caloric saccharin for the control self-administration histories. Rats in the drug naive history group were trained to lever-press for saccharin for 30 min daily. Rats were required to satisfy the following training criteria or excluded: (1) a minimum of 21 days of training and (2) a minimum of 30 cocaine (ShA), 50 cocaine (LgA) and 50 saccharin (drug naïve) deliveries for three consecutive training sessions.
2. SCM self-administration training (3 weeks): All rats were trained to lever-press for SCM – instead of cocaine or saccharin – and were required to satisfy the following training criteria or excluded: (1) a minimum of 14 days of training and (2) a minimum of 50 SCM deliveries daily for three consecutive training sessions.
3. Compulsive appetite tests for SCM (3 weeks): All rats were tested to lever-press for SCM in despite adverse consequences during the 4th-6th weeks after drug histories. Each SCM delivery was paired with an electric foot-shock punishment generated by a shocker/scrambler (Med Associates, Saint Albans, VT, USA), which was delivered contingent on a reinforced lever-press, along with SCM. Each rat underwent a total of five test sessions (30 min each) for the following 5 levels of foot-shock intensity: 0.0, 0.1, 0.15, 0.25 and 0.35 mA (0.5 s each). The shock intensities were kept constant during each test session (following a between-session design). Test sessions were separated by 2–4 days; on interim days, all rats were allowed to lever-press for SCM unpunished (0.0 mA) to re-stabilize lever-press responding (±20% of the average number of reward deliveries during the last three sessions of SCM self-administration re-training).
The current operant punishment task was adapted from previously published procedures to determine punishment-resistant “compulsive” drug and food self-administration (e.g., Deroche-Gamonet, Belin & Piazza, 2004; Johnson & Kenny, 2010; Laque et al., 2019; Spierling et al., 2020).
The final Ns retained for statistical analyses from the saccharine, ShA cocaine and LgA cocaine groups were 14/18/14 for males.
ii. Progressive ratio tests for SCM.
As another indication of addiction-like food motivation, we determined the animals’ willingness to work for caloric SCM under high workload requirements. Male rats (N = 60) underwent the following three phases:
1. Drug histories (3–4 weeks): As described in i. (Compulsive appetite tests for SCM). One rat died during surgery and was excluded.
2. SCM self-administration training (3 weeks): As described for i. Compulsive appetite tests for SCM.
3. Progressive ratio tests for SCM (1 day): Rats lever-pressed for SCM under a progressive ratio (PR) schedule of reinforcement during the 4th week after drug histories – roughly the same timeframe as compulsive appetite tests for SCM. Under the current PR schedule, the response requirements to obtain each SCM delivery increased according to the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, etc. (adapted from Richardson & Roberts, 1996). Each test session was terminated when 60 min had elapsed after the final reinforced response.
The final Ns retained for statistical analyses for the saccharin, ShA cocaine and LgA cocaine groups were 19/19/19.
iii. Ad libitum calorie intake and weight gain tests.
Here we determined the animals’ calorie intake and weight gain under unchallenged ‘basal’ conditions (i.e., continuous access to nutritionally balanced and subsequently obesogenic diets, no operant conditioning and no punishment, etc.), as an indicator of homeostatic appetite regulation. Male (N=32) rats underwent the following three phases:
1. Drug histories (3–4 weeks + 3 weeks for recovery): As described for i. (Compulsive appetite tests for SCM). Six rats that died due to surgical complications were excluded. Six rats that did not satisfy training criteria within four weeks of training were excluded. The rats then remained in their home-cages for 3 weeks; this was to match the period between addiction histories and current tests (ad libitum calorie intake and weight gain tests) to that between addiction histories and compulsive appetite tests.
2. Ad libitum access to nutritionally balanced diet (2 weeks): All rats were kept in their home cages and maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA) containing 17 kcal% fat, 58 kcal% carbohydrate (6.2 kcal% sucrose), 25 kcal% protein, and vitamins and minerals. The animals’ consumption of this nutritionally balanced diet and their weights were measured weekly during the 4th-5th weeks after drug histories – roughly the same timeframe for compulsive appetite tests for SCM.
3. Ad libitum access to obesogenic diet (7 weeks): Instead of the standard lab chow, all rats were continuously maintained on a high fat and high sugar (HFHS) diet (Product #D12451, Research Diets, Inc., New Brunswick, NJ, USA) containing 45 kcal% fat, 35 kcal% carbohydrate (17 kcal% sucrose) and 20 kcal% protein, as well as vitamins and minerals. The animals’ consumption of this obesogenic diet and their weights were measured weekly during the 6th-12th weeks after drug histories – roughly the same timeframe for compulsive appetite tests for SCM.
The final Ns retained for statistical analyses from the saccharin, ShA cocaine and LgA cocaine groups were 7/10/9 for males.
iv. Compulsive appetite tests for saccharin.
Here, we determined the animals’ willingness to work for non-caloric saccharin despite adverse consequences (i.e., punishments), as an indicator of addiction-like food motivation. Male (N=43) rats underwent the following three experimental phases:
1. Drug histories (3–4 weeks): As described in i. (Compulsive appetite tests for SCM). Seven rats that died due to surgical complications were excluded.
2. Saccharin self-administration training (3 weeks): Rats were trained to lever-press for saccharin and were required to satisfy the following training criteria: (1) a minimum of 14 days of training and (2) a minimum of 100 saccharin deliveries daily for three consecutive training sessions.
3. Compulsive appetite tests for saccharin (3 weeks): Rats were tested to lever-press for saccharin despite punishments during the 4th-6th weeks after drug histories, as with compulsive appetite tests for SCM. Each saccharin delivery was paired with an electric foot-shock, which was delivered contingent on a reinforced lever-press along with saccharin. Each rat was subjected to a total of five test sessions (30 min each) for 5 levels of foot-shock intensity. Male rats were subjected to 0.0, 0.1, 0.15, 0.25 and 0.35 mA (0.5 s each). Each shock duration was 0.5s, and shock intensities were kept constant during each test session. Test sessions were separated by 2–4 days. On interim days, rats were re-trained (30 min, daily) to lever-press for saccharin unpunished (0.0 mA) in order to re-stabilize responding (within 20% of the average number of reward deliveries during the last three sessions of saccharin operant self-administration re-training). These punished operant tests were based on well-established procedures to test punishment-resistant “compulsive” drug and food motivation in rats (Ahmed, 2012; Clemens & Holmes, 2018; Deroche-Gamonet, Belin & Piazza, 2004; Everitt, Giuliano & Belin, 2018; Johnson & Kenny, 2010; Pelloux, Everitt & Dickinson, 2007; Spierling et al., 2020; Vanderschuren & Everitt, 2004).
The finals Ns retained for statistical analyses from the saccharin, ShA cocaine and LgA cocaine groups were 11/11/11 for males.
II. Cocaine histories via intraperitoneal administration (IP cocaine histories):
In Experiment I, rats were first trained to self-administer cocaine or saccharin and then trained and tested to self-administer SCM or saccharin paired with food-shock punishments, as a measure of compulsive appetite. This design did not permit to interpret the training effects of the initial operant training with cocaine or saccharin vs. the pharmacological effects of cocaine on subsequent self-administration tests. Moreover, the use of saccharin both as the control stimulus and the stimulus representing compulsive appetite could have skewed the experimental results. We thus next determined whether the pharmacological action of cocaine is sufficient to induce punishment-resistant self-administration of SCM or saccharin.
Rats were randomly assigned to one of the following addiction history groups: IP cocaine or IP saline. The procedure for IP cocaine histories was based on procedures to induce locomotor sensitization (Schoenbaum, Saddoris, Ramus, Shaham & Setlow, 2004) – thought to model drug addiction (Robinson & Berridge, 2008) – and addiction-like brain changes (e.g., Li, Acerbo & Robinson, 2004) in rats. The current procedure is also comparable to other IP cocaine regimens to induce addiction-like habitual/inflexible behavior (e.g., Schoenbaum & Setlow, 2005) in rats. Rats in the IP saline history group served as drug naïve controls.
Rats in IP cocaine and IP saline groups were then further randomly assigned to a specific experimental condition defined by one of the following behavioral tests and trained accordingly (experimental timelines are schematized in Figure 1):
i. Compulsive appetite tests for SCM.
Male (N=21) rats were subjected to the following four experimental phases:
1. SCM self-administration training (3 weeks): Rats were trained to lever-press for SCM under FR1 and required to satisfy the following training criteria or excluded: (1) a minimum of 21 days of training and (2) a minimum of 50 SCM deliveries daily for three consecutive training sessions.
2. Drug histories (8 days): Rats were kept in their home cages and given once daily injections of IP cocaine (30 mg/kg/injection dissolved in saline, 0.5 ml) or IP saline (0.5 ml) for 8 days. Rats were then kept in their home-cages for 1 week to recover.
3. SCM self-administration re-training (2-3 weeks): Rats were re-trained to lever-press for saccharin and required to satisfy the following training criteria or excluded: (1) a minimum of 14 days of training and (2) a minimum of 50 SCM deliveries daily for three consecutive training sessions.
4. Compulsive appetite tests for SCM: As described in I. (Cocaine history via operant self-administration). These tests were conducted during the 4th-6th weeks after drug histories, as after ShA/LgA cocaine histories.
Final Ns retained for statistical analysis from the IP saline and IP cocaine groups were 9/12 for males
ii. Compulsive appetite tests for saccharin.
Male (N=25) rats were subjected to the following four phases:
1. Saccharin self-administration training (3-4 weeks): Rats were trained to lever-press for saccharin under FR1 and required to satisfy the following training criteria or excluded: (1) a minimum of 21 days of training and (2) a minimum of 50 saccharin deliveries daily for three consecutive training sessions.
2. Drug histories (8 days): Rats were kept in their home cages and given once-daily injections of IP cocaine (30 mg/kg/injection dissolved in saline, 0.5 ml) or IP saline (0.5 ml) for 8 days. Rats remained in their home-cages for 1 week to recover.
3. Saccharin self-administration retraining (3 weeks): Rats were re-trained to lever-press for saccharin and required to satisfy the following training criteria or excluded: (1) a minimum of 14 days of training and (2) a minimum of 50 saccharin deliveries daily for three consecutive training sessions.
4. Compulsive appetite tests for saccharin: As described in I. (Cocaine history via operant self-administration). These tests were conducted during the 4th-6th weeks after drug histories, as after ShA/LgA cocaine histories.
Final Ns retained for statistical analyses for the IP cocaine and IP saline groups were 11/14 for males.
2.4.2. Experiment 2: The effects of extensive cocaine histories on subsequent food motivated behavior in female rats
Female rats were randomly assigned to a specific experimental condition defined first by drug history and then behavioral test. Experimental procedures mimicked those described for Experiment 1 (male rats) unless noted otherwise. Experimental timelines are schematized in Figure 2.
Figure 2:
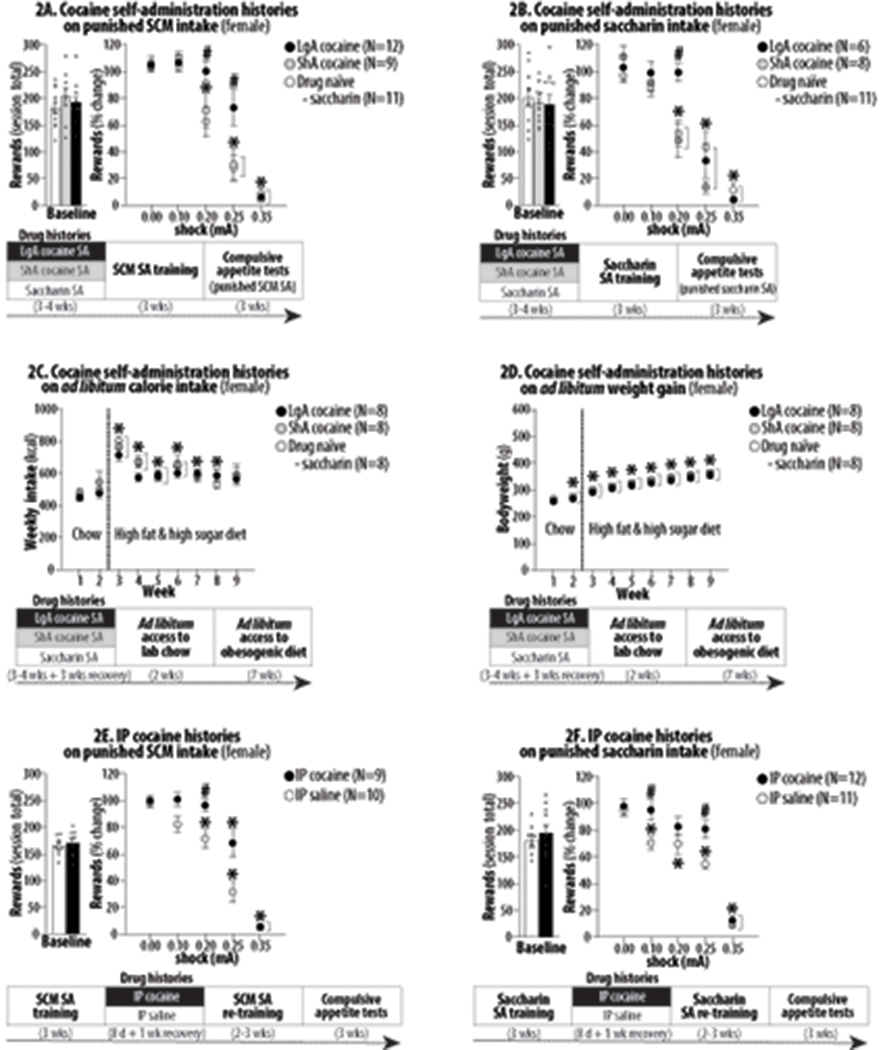
The effects of extensive cocaine histories on subsequent food motivated behavior in female rats undergoing long-term abstinence. Asterisks (*) indicate significant difference from 0.0 mA (Figures 2A/2B/2E/2F) or Week 1 (Figures 2C/2D). Pound signs (#) indicate significant difference from the drug naïve control. Abbreviations: sweetened condensed milk (SCM), self-administration (SA), long-access (LgA), short-access (ShA) and intraperitoneal (IP). 2A. *, P<0.001-0.05. #, P<0.05. 2B. *, P<0.001-0.05. #, P<0.01. 2C. *, P<0.001-0.05. #, P<0.05. 2D. *, P<0.001-0.05. #, P<0.01. 2E. *, P<0.001-0.05. 2F. *, P<0.001-0.05.
I. Cocaine histories via operant self-administration:
i. Compulsive appetite tests for SCM.
Female (N = 38) rats were trained and tested according to the following three phases:
1. Drug histories (3-4 weeks).
2. SCM self-administration training (3 weeks).
3. Compulsive appetite tests for SCM (3 weeks). As described for Experiment 1, except that female rats were subjected to 0.0, 0.15, 0.2, 0.25 and 0.35 mA foot-shocks. This was because a pilot experiment – where we tested 0.0, 0.1, 0.15, 0.2, 0.25, and 0.35 mA in drug naïve rats – revealed that female rats were less sensitive to electric foot-shock than male rats; 0.1 mA was sufficient to start reducing responding in male rats (Experiment 1) but not in female rats (pilot experiment). We thus used 0.15 mA as the second shock intensity (after 0.0 mA) to be tested in female rats, rather than 0.1 mA for male rats. In the pilot study, we also observed a pronounced reduction at 0.2 mA in female rats, which was not tested in male rats (Experiment 1), while both 0.25 and 0.35 mA were effective to reduce responding in female rats, as in male rats (Experiment 1). Based on this, we decided to test 0.0, 0.15, 0.2, 0.25 and 0.35 mA in female rats, as opposed to 0.0, 0.1, 0.15, 0.25, 0.35 mA in male rats (Experiment 1) while keeping the total number of tests for both male and female rats at 5 tests per animal.
The final Ns retained for statistical analyses from the saccharine, ShA cocaine and LgA cocaine groups were 11/9/12 for females.
ii. Compulsive appetite tests for saccharin.
Female (N=32) rats underwent the following three experimental phases:
1. Drug histories (3-4 weeks).
2. Saccharin self-administration training (3 weeks).
3. Compulsive appetite tests for saccharin (3 weeks): As described for Experiment 1 (male rats), except that female rats were subjected to foot-shocks at 0.0, 0.15, 0.2, 0.25 and 0.35 mA (as for Compulsive appetite tests for SCM).
The finals Ns retained for statistical analyses from the saccharin, ShA cocaine and LgA cocaine groups were 11/8/6 for females.
iii. Ad libitum calorie intake and weight gain tests.
Female (N=30) rats underwent the following three phases:
1. Drug histories (3-4 weeks + 3 weeks for recovery).
2. Ad libitum access to nutritionally balanced diet (2 weeks). These tests were conducted during the 4th-5th weeks after drug histories, as after ShA/LgA/IP cocaine histories in male rats.
3. Ad libitum access to obesogenic diet (7 weeks). These tests were conducted during the 6th-12th weeks after drug histories, as after ShA/LgA/IP cocaine histories in male rats.
The final Ns retained for statistical analyses from the saccharin, ShA cocaine and LgA cocaine groups were 8/8/8 for females.
II. Cocaine histories via intraperitoneal administration:
i. Compulsive appetite tests for SCM.
Female (N=20) rats were subjected to the following four experimental phases:
1. SCM self-administration training (3 weeks).
2. Drug histories (8 days).
3. SCM self-administration re-training (2-3 weeks).
4. Compulsive appetite tests for SCM: As described for Experiment 1 (male rats), except that female rats were subjected to foot-shocks at 0.0, 0.15, 0.2, 0.25 and 0.35 mA. These tests were conducted during the 4th-6th weeks after drug histories, as after ShA/LgA/IP cocaine histories in male rats.
Final Ns retained for statistical analysis from the IP saline and IP cocaine groups were 10/9 for females.
ii. Compulsive appetite tests for saccharin.
Female (N=23) rats were subjected to the following four phases:
1. Saccharin self-administration training (3-4 weeks).
2. Drug histories (8 days).
3. Saccharin self-administration retraining (3 weeks).
4. Compulsive appetite tests for saccharin. As described for Experiment 1 (male rats), except that female rats were subjected to foot-shocks at 0.0, 0.15, 0.2, 0.25 and 0.35 mA. These tests were conducted during the 4th-6th weeks after drug histories, as after ShA/LgA/IP cocaine histories in male rats.
Final Ns retained for statistical analyses for the IP cocaine and IP saline groups were 11/12 for females.
2.4.3. Experiment 3: The effects of other histories on subsequent food motivated behavior.
We lastly determined the effects of extensive alcohol, caffeine and obesogenic diet histories in male rats. Rats (N = 174) were randomly assigned to one of the following histories: 1. Alcohol history via vapor exposure, 2. Alcohol history via liquid diet, 3. Caffeine, 4. Obesogenic diet I – high fat and high sugar (HFHS) diet, and 5. Obesogenic diet II – high fat (HF), high sugar (HS) or high protein (HP) diet. Rats were trained and subjected to i. Compulsive appetite tests for saccharin or ii. Ad libitum calorie intake and weight gain tests. Experimental timelines are schematized in Figure 3.
Figure 3:
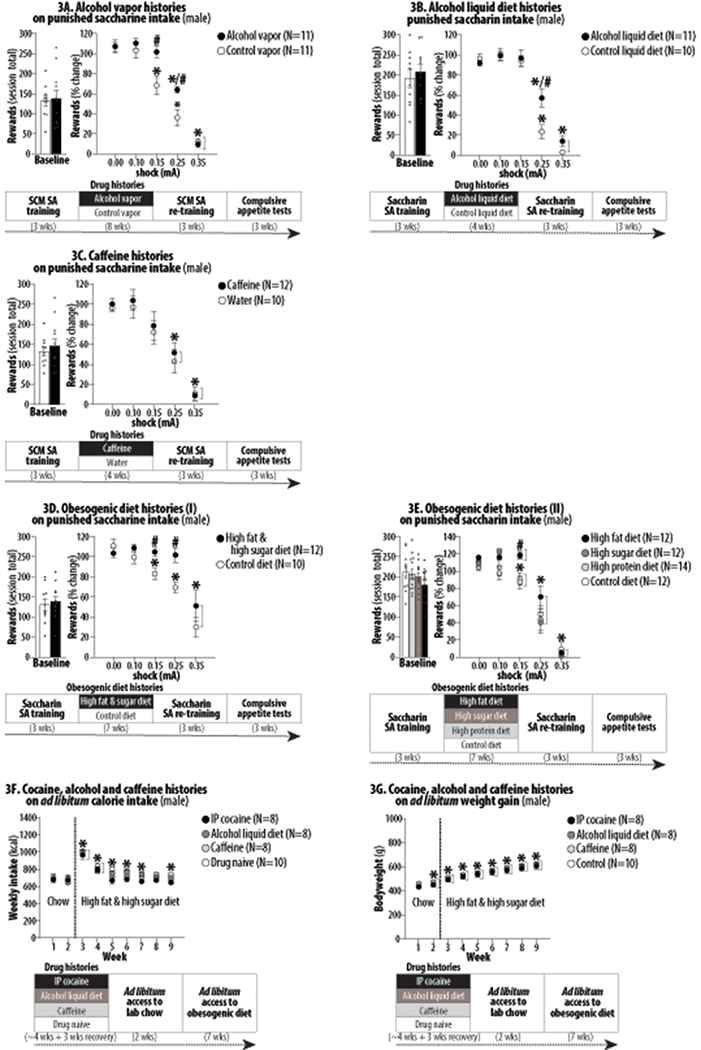
The effects of extensive alcohol, caffeine and obesogenic diet histories on subsequent food motivated behavior in male rats undergoing long-term abstinence. Asterisks (*) indicate significant difference from 0.0 mA (Figures 3A/3B/3C/3D/3E) or Week 1 (Figures 3F/3G). Pound signs (#) indicate significant difference from the control group. Abbreviation: intraperitoneal (IP). 3A. *, P<0.001-0.05. #, P<0.01-0.05. 3B. *, P<0.01. #, P<0.01-0.05. 3C. *, P<0.001-0.05. 3D. *, P<0.001-0.05. #, P<0.01. 3E. *, P<0.001-0.05. #, P<0.05. 3F. *, P<0.001-0.05. 3G. *, P<0.001-0.01.
i. Compulsive appetite tests for saccharin.
Male rats (N=140) underwent the following four experimental phases:
1. Saccharin self-administration training: As described in II. (cocaine histories via intraperitoneal administration).
2. Drug/diet histories: Rats were randomly assigned to one of the following four addiction histories (see below). For all cases, rats were randomly further assigned to experimental or control groups. As described above, rats were maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA) and tap water, unless otherwise specified.
2.1. Alcohol histories via vapor exposure (8 weeks). Rats were housed in airtight plexiglass chambers, randomly assigned to alcohol vapor or control vapor groups, and exposed to chronic intermittent alcohol vapor or continuous fresh air for 8 weeks. Rats in the alcohol vapor group underwent a daily cycle of alcohol vapor (14 hrs) and fresh air (10 hrs). This alcohol history was based on procedures to induce alcohol dependence in rats marked by somatic signs of withdrawal, addiction-like brain changes and excessive alcohol intake (e.g., McBride & Li, 1998). Tail blood samples were collected daily at the 14th hr of alcohol vapor exposure to determine blood alcohol levels (BALs); evaporated alcohol concentration (22-27 mg/l) in the chamber was adjusted as necessary to maintain BALs of 150 to 250 mg% (mg/dl). Rats in the control group were exposed to continuous fresh air (24 hrs). Rats were then kept in their home-cages for 2 weeks to recover.
2.2. Alcohol histories via liquid diet (4 weeks). Rats were randomly assigned to alcohol or control liquid diet groups and kept in their home cages. Instead of standard lab chow, rats in the alcohol liquid diet group were maintained on a continuous (24 hrs; 7 days/week) liquid diet consisting of alcohol (10%, v/v) and Boost® nutritional supplement (Nestle USA, Rosslyn, VA, USA) fortified with a vitamin and mineral mix (adapted from Serrano et al., 2018) for 4 weeks. This alcohol history was based on procedures to induce alcohol dependence marked by somatic signs of withdrawal, addiction-like brain changes and excessive alcohol intake (McBride & Li, 1998). Rats in the control liquid diet group were maintained on continuous non-alcohol liquid diet, consisting of Boost® nutritional supplement fortified with a vitamin and mineral mix, with a caloric content (cal/ml) equal to that of the alcohol liquid diet described above. Rats then were kept in their home-cages and maintained on standard lab chow for 2 weeks to recover.
2.3. Caffeine histories (4 weeks). Rats were randomly assigned to caffeinated or non-caffeinated water groups and kept in their home cages. Instead of tap water, rats in the caffeinated water group were continuously (24 hours; 7 days/week) maintained on caffeine (1.0 mg/ml) added to drinking water for 4 weeks. This caffeine history was based on procedures to potentiate nicotine rewards (e.g., Shoaib, Swanner, Yasar & Goldberg, 1999). Rats in the non-caffeinated water (control) group were continuously maintained on regular tap water. Rats were then kept in their home-cages and maintained on regular tap water for 2 weeks to recover.
2.4. Obesogenic diet histories I – high fat and high sugar (HFHS) diet (7 weeks). Rats were randomly assigned to obesogenic or control diet groups and kept in their home cages. Instead of standard lab chow, rats in the experimental group were continuously (24 hrs; 7 days/week) maintained on a HFHS diet (Product #D12451, Research Diets, Inc., New Brunswick, NJ, USA) containing 45 kcal% fat, 35 kcal% carbohydrate (17 kcal% sucrose), 20 kcal% protein and vitamins and minerals for 7 weeks. This procedure was based on procedures to promote diet-induced obesity and metabolic changes (e.g., Desai et al., 2007) and is comparable to diet regimens known to induce compulsive eating (Johnson & Kenny, 2010; Spierling et al., 2020). Rats in the control group were maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA). All rats completed this phase. Rats were then kept in their home-cages and maintained on standard lab chow for 2 weeks to recover.
2.5. Obesogenic diet histories II – high fat (HF), high sugar (HS) or high protein (HP) diets (7 weeks). Rats were randomly assigned to HF, HS, HP or control diet groups, and kept in their home cages. Rats were maintained on their assigned diet type continuously (24 hrs; 7 days/week) for 7 weeks. Rats in the HF diet group were maintained on a high fat diet (Product #D12492, Research Diets, Inc., New Brunswick, NJ, USA), containing 60 kcal% fat, 20 kcal% carbohydrate (6.8 kcal% sucrose), 20 kcal% protein and vitamins and minerals. This diet has been used to induce an obese phenotype and non-alcoholic fatty liver in rodents (e.g., Kirpich et al., 2011). Rats in the HS diet group were maintained on a high sugar diet (Product #D02022703, Research Diets, Inc., New Brunswick, NJ, USA) containing 10 kcal% fat, 70 kcal% carbohydrate (60 kcal% sucrose) and 20 kcal% protein, as well as vitamins and minerals. This diet has been used to induce an obese phenotype and non-alcoholic fatty liver in rodents (e.g., Kirpich et al., 2011). Rats in the HP diet group were maintained on a high protein diet (Product #D08091802, Research Diets, Inc., New Brunswick, NJ, USA) containing 10 kcal% fat, 30 kcal% carbohydrate (6.7 kcal% sucrose) and 60 kcal% protein, as well as vitamins and minerals. This HP diet is not known to cause diet-induced obesity. Rats in the control group were maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA). Rats were then kept in their home-cages and maintained on standard lab chow for 2 weeks to recover.
3. Saccharin self-administration re-training (3 weeks): As described in II (Cocaine histories via intraperitoneal administration).
4. Compulsive appetite tests for saccharin: As described in I. (Cocaine histories via operant self-administration). These tests were conducted during the 4th-6th weeks after drug/diet histories, as after ShA/LgA/IP cocaine histories.
Final Ns retained for statistical analyses for alcohol and control vapor history groups were 11/11, alcohol and control liquid diet history groups were 11/10, caffeinated and non-caffeinated water history groups were 12/10, HFHS and control diet history groups were 12/10, and HF, HS, HP and control diet history groups were 12/12/14/12.
ii. Ad libitum calorie intake and weight gain tests.
Rats underwent the following three experimental phases:
1. Drug/diet histories. Male rats (N=34) were randomly assigned to one of the following groups: i. Cocaine history via intraperitoneal administration, ii. Alcohol history via liquid diet, iii. Caffeine history and iv. Control history. All groups were run simultaneously. For all cases, rats were maintained on standard lab chow (Product #7012, Teklad Diets-Envigo, Madison, WI, USA) and regular tap water, unless otherwise specified.
1-i. Cocaine histories via intraperitoneal administration (4 weeks). Rats were kept in their home cages and maintained on standard lab chow and regular tap water for 4 weeks. During the initial 3 weeks, rats were left undisturbed. During the fourth week, rats were given once daily injection of IP cocaine (30 mg/kg/injection dissolved in saline, 0.5 ml) for 8 days.
1-ii. Alcohol histories via liquid diet (4 weeks). Rats were kept in their home cages and maintained on the alcohol (10% v/v) liquid diet described above – instead of standard lab chow – and regular tap water for 4 weeks.
1-iii. Caffeine histories (4 weeks).Rats were kept in their home cages and maintained on standard lab chow and caffeine (1.0 mg/ml) added to drinking water – instead of regular tap water – for 4 weeks.
1-iv. Control histories (4 weeks). Rats were kept in their home cages and maintained on standard lab chow and regular tap water for 4 weeks.
Rats were then kept in their home-cages for 3 weeks to recover; this was done to match the period between drug/diet histories and ad libitum calorie intake and weight gain tests to that between drug/diet histories and compulsive appetite tests.
2. Ad libitum access to nutritionally balanced diet (2 weeks): All rats were kept in their home cages and maintained on standard lab chow. The animals’ consumption of this nutritionally balanced diet and their weight gains were measured on a weekly basis during the 4th-5th weeks after drug/diet histories, as with ShA/LgA cocaine histories.
3. Ad libitum access to obesogenic diet (7 weeks): Instead of standard lab chow, all rats were maintained on a high fat and high sugar diet (Product #D12451, Research Diets, Inc., New Brunswick, NJ, USA). The animals’ consumption of this obesogenic diet and their weight gains were measured on a weekly basis during the 6th-12th weeks after drug/diet histories, as with ShA/LgA cocaine histories.
Final Ns retained for statistical analyses from the cocaine, alcohol, caffeine and control history groups were 8/8/8/10.
2.5. Data analysis
Statistical analyses were undertaken only for studies where each group size was at least n=5. The declared group size is the number of independent values, and statistical analysis was done using these independent values. Data were analyzed by Student’s t-test and parametric statistical analyses (ANOVA). For all cases, differences were considered significant when P<0.05 (two-tailed). In multigroup studies with parametric variables, post-hoc tests were conducted using Holm-Sidak tests only if F in ANOVA achieved P<0.05 and there was no significant variance in homogeneity. For all cases, we did not observe statistical outliers and thus no outlier was included in the Results. We used GraphPad Prism 8.4.3. (GraphPad, San Diego, CA, USA; RRID:SCR_002798) and IBM SPSS Statistics 25 (IBM Corporation, Armok, NY, USA; RRID:SCR_002865).
We describe the statistical methods used for different experimental conditions below:
2.5.1. Experiment 1: The effects of extensive cocaine histories on subsequent food motivated behavior in male rats
I. Cocaine histories via operant self-administration:
i. Compulsive appetite tests for SCM.
We first calculated the average number of rewards obtained (30-min totals) during the last 3 “SCM self-administration training” sessions for each rat as a measurement of basal SCM intake (baseline scores). These baseline scores were analyzed using one-way ANOVA with drug histories (3 levels: drug naïve [saccharin], ShA and LgA) as between-subjects factor. We observed large individual differences in these baseline scores. For example, the range of baseline scores in the drug naïve control group was between 96.6 and 166.7. To control for this within-group variability, we used the baseline score of each rat to convert the number of rewards obtained (30-min totals) by the same animal during each test session as “% changes from baseline,” a normalized measurement of punishment-resistance. Based on this normalization, 50 rewards obtained by a rat with the baseline score of 100 is expressed as “50% of baseline”, while the same 50 rewards obtained by a rat with the baseline score of 150 is expressed as “33% of baseline.” The test scores were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (3 levels) as between-subjects factor.
ii. Progressive ratio tests for SCM.
Total numbers of active lever-presses and rewards obtained were separately analyzed using one-way ANOVA with addiction history (3 levels: saccharin, ShA and LgA) as between-subjects factor.
iii. Ad libitum calorie intake and weight gain tests.
Weekly calorie intake and bodyweight gain were separately analyzed using two-way repeated measures ANOVA with week (9 levels: standard lab chow for 2 weeks and then obesogenic diet for 7 weeks) as within-subjects factor and addiction history (3 levels: drug naive, ShA and LgA) as between-subjects factor.
iv. Compulsive appetite tests for saccharin.
We first calculated the average number of rewards obtained (30-min totals) during the last 3 “saccharin self-administration re-training” sessions for each rat as a measurement of basal saccharin intake (baseline score). These baseline scores were analyzed using one-way ANOVA with addiction history (3 levels: drug naive, ShA and LgA) as between-subjects factor. As with basal SCM intake, we observed large individual differences in these baseline scores. To control for this within-group variability, we used the baseline score of each rat to convert the number of rewards obtained (30-min totals) by the same animal during each test session as “% changes from baseline,” a measurement of punishment-resistance. These test scores were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (3 levels) as between-subjects factor.
II. Cocaine histories via intraperitoneal administration (IP cocaine histories).
i. Compulsive appetite tests for SCM.
As for cocaine histories via operant self-administration, we first calculated the average number of rewards obtained (30-min totals) during the last 3 “SCM self-administration re-training” sessions for each rat, as a measurement of basal SCM intake (baseline scores). These baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: IP saline and IP cocaine) as between-subjects factor. We used the baseline score of each rat to convert the number of rewards obtained (30-min totals) during each test session as “% changes from baseline,” a measurement of punishment-resistance. These test scores were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subject factor.
ii. Compulsive appetite tests for saccharin.
As for cocaine histories via operant self-administration, we first calculated the average number of rewards obtained (30-min totals) during the last 3 “saccharin self-administration re-training” sessions for each rat, a measurement of basal SCM intake (baseline scores). These baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: IP saline and IP cocaine) as between-subjects factor. We used the baseline score of each rat to convert the number of rewards obtained (30-min totals) during each test session as “% changes from baseline,” a measurement of punishment-resistance. These test scores were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subjects factor.
2.5.2. Experiment 2: The effects of extensive cocaine histories on subsequent food motivated behavior in female rats
Statistical procedures were as described for Experiment 1 (male rats).
2.5.3. Experiment 3: The effects of other histories on subsequent food motivated behavior.
i. Compulsive appetite tests for saccharin
As for cocaine histories, we used the average number of rewards obtained (30-min totals) during the last 3 “saccharin self-administration re-training” sessions as the “baseline” score to calculate and depict the number of rewards obtained (30-min totals) during each test session as “% changes from baseline”.
i-1. Alcohol histories via vapor exposure
The baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: control vapor [air] and alcohol vapor) as between subject factor. The test scores (% changes from baseline) were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subjects factor.
i-2. Alcohol histories via liquid diet.
The baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: control liquid diet and alcohol liquid diet) as between subject factor. The test scores (% changes from baseline) were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subjects factor.
i-3. Caffeine histories.
The baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: non-caffeinated water [water] and caffeinated water [caffeine]) as between subject factor. The test scores (% changes from baseline) were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subjects factor.
i-4. Obesogenic diet histories I – high fat and high sugar (HFHS) diet.
The baseline scores were analyzed using one-way ANOVA with addiction history (2 levels: control diet and HFHS diet) as between-subjects factor. The test scores (% changes from baseline) were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (2 levels) as between-subjects factor.
i-5. Obesogenic diet histories II – high fat (HF), high sugar (HS) or high protein (HP) diet.
The baseline scores were analyzed using one-way ANOVA with addiction history (4 levels: HF, HS, HP and control diet) as between-subjects factor. The test scores (% changes from baseline) were analyzed using two-way repeated measures ANOVA with foot-shock intensity (5 levels) as within-subjects factor and addiction history (4 levels) as between-subjects factor.
ii. Ad libitum calorie intake and weight gain tests.
Weekly calorie intake and bodyweight gain were separately analyzed using two-way repeated measures ANOVA with week (9 levels: standard lab chow for 2 weeks and then obesogenic diet for 7 weeks) as within-subjects factor and addiction history (4 levels: lab chow [control], IP cocaine [cocaine], alcohol liquid diet [alcohol] and caffeinated water [caffeine]) as between-subjects factor.
3. RESULTS
For all cases, we report statistical results most relevant to the interpretation of results in the text below. Detailed statistical results are described in Tables 1/2/3. Detailed justifications for each experiment are described in Discussion.
Table 1:
Statistical results for the effects of extensive cocaine histories on subsequent food motivated behavior in male rats undergoing long-term abstinence (see Figure 1).
| Figure number | Factor name | F-value | p-value |
|---|---|---|---|
| 1A (left panel) | History | F(2,43) = 0.2640 | NS |
| 1A (right panel) | History Shock History x Shock |
F(2,43) = 25.47 F(4,43) = 127.3 F(8,172) = 9.279 |
<0.001* <0.001* <0.001* |
| 1B (left panel) | History | F(2,54) = 7.641 | <0.01* |
| 1B (right panel) | History | F(2,54) = 6.819 | <0.01* |
| 1C | History Week History x Week |
F(2,23) = 0.747 F(8,23) = 54.435 F(16,184) = 1.625 |
NS <0.001* NS |
| 1D | History Week History x Week |
F(2,23) = 0.761 F(8,23) = 530.44 F(16,184) = 0.44 |
NS <0.001* NS |
| 1E (left panel) | History | F(2,30) = 0.2197 | NS |
| 1E (right panel) | History Shock History x Shock |
F(2,30) = 25.11 F(4,30) = 143.5 F(8,120) = 12.09 |
<0.001* <0.001* <0.001* |
| 1F (left panel) | History | t(19) = 0.214 | NS |
| 1F (right panel) | History Shock History x Shock |
F(1, 19) = 6.804 F(4,19) = 70.67 F(4,76) = 2.789 |
<0.05* <0.001* <0.05* |
| 1G (left panel) | History | t(23) = 0.168 | NS |
| 1G (right panel) | History Shock History x Shock |
F(1,23) = 13.15 F(4,23) = 37.06 F(4,92) = 1.293 |
<0.01* <0.001* NS |
Table 2:
Statistical results for the effects of extensive cocaine histories on subsequent food motivated behavior in female rats undergoing long-term abstinence (see Figure 2).
| Figure number | Factor name | t-value and F-value | p-value |
|---|---|---|---|
| 2A (left panel) | History | F(2,29) = 0.759 | NS |
| 2A (right panel) | History Shock History x Shock |
F(2,29) = 4.297 F(4,29) = 105.9 F(8,116) = 3.065 |
<0.05* <0.001* <0.01* |
| 2B (left panel) | History | F(2,22) = 0.690 | NS |
| 2B (right panel) | History Shock History x Shock |
F(2,22) = 1.903 F(4,22) = 81.45 F(8,88) = 3.321 |
0.17 (NS) <0.001* <0.01* |
| 2C | History Week History x Week |
F(2,21) = 0.402 F(8,21) = 32.133 F(16,168) = 1.137 |
NS <0.001* NS |
| 2D | History Week History x Week |
F(2,21) = 0.508 F(8,21) = 325.831 F(16,168) = 0.067 |
NS <0.001* NS |
| 2E (left panel) | History | t(17) = 0.558 | NS |
| 2E (right panel) | History Shock History x Shock |
F(1,17) = 18.51 F(4,17) = 89.40 F(4,68) = 3.440 |
<0.001* <0.001* <0.05* |
| 2F (left panel) | History | t(21) = 0.8620 | NS |
| 2F (right panel) | History Shock History x Shock |
F(1,21) = 7.183 F(4,21) = 87.94 F(4,84) = 2.577 |
<0.05* <0.001* <0.05* |
Table 3:
Statistical results for the effects of extensive alcohol, caffeine and obesogenic diet histories on subsequent food motivated behavior in male rats undergoing long-term abstinence (see Figure 3).
| Figure number | Factor name | t-value and F-value | p-value |
|---|---|---|---|
| 3A (left panel) | History | t(20) = 0.214 | NS |
| 3A (right panel) | History Shock History x Shock |
F(1,20) = 6.489 F(4,20) = 112.7 F(4,80) = 4.375 |
<0.05* <0.001* <0.01* |
| 3B (left panel) | History | t(19) = 0.601 | NS |
| 3B (right panel) | History Shock History x Shock |
F(1,19) = 3.121 F(4,76) = 110.3 F(4,76) = 3.820 |
0.09 (NS) <0.001* <0.01* |
| 3C (left panel) | History | t(20) = 0.6677 | NS |
| 3C (right panel) | History Shock History x Shock |
F(1,20) = 0.307 F(4,20) = 41.68 F(4,80) = 0.116 |
NS <0.001* NS |
| 3D (left panel) | History | t(20) = 0.459 | NS |
| 3D (right panel) | History Shock History x Shock |
F(1,20) = 9.599 F(4,20) = 24.06 F(4,80) = 1.863 |
<0.001* <0.001* 0.12 (NS) |
| 3E (left panel) | History | F(3,46) = 1.509 | NS |
| 3E (right panel) | History Shock History x Shock |
F(3,46) = 3.149 F(4,46) = 216.7 F(12,184) = 1.98 |
<0.05* <0.001* <0.05* |
| 3F | History Week History x Week |
F(3,30) = 1.027 F(8,30) = 89.31 F(30,240) = 1.527 |
NS <0.001* NS |
| 3G | History Week History x Week |
F(3,30) = 0.116 F(8,30) = 514.3 F(30,240) = 1.335 |
NS <0.001* NS |
3.1. Experiment 1: The effects of extensive cocaine histories on subsequent food motivated behavior in male rats.
Detailed statistical results are described in Table 1.
I. Cocaine histories via operant self-administration:
i. Compulsive appetite tests for SCM.
Compared to the control (saccharin) histories, neither ShA nor LgA cocaine histories significantly altered the average number of rewards obtained during the last three SCM self-administration training sessions, as a measure of “baseline” SCM intake (Figure 1A, left panel). One-way ANOVA on SCM rewards obtained revealed no significant effect of History (F2,43 = 0.26, NS). In contrast, ShA and LgA histories significantly increased rats’ punishment-resistance to obtain SCM (Figure 1A, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of History (F2,43 = 25.47, P<0.001), Shock (F4,43 = 127.30, P<0.001) and History X Shock interaction (F8,172 = 9.28, P<0.001). Post-hoc Holm-Sidak tests further revealed that LgA histories produced greater effects than ShA histories.
ii. Progressive ratio tests for SCM.
Compared to the control (saccharin) histories, ShA and LgA cocaine histories significantly increased the number of rewards obtained (Figure 1B, left) and lever-presses (Figure 1B, right). One-way ANOVA on SCM rewards obtained revealed significant effects of History (F2,54 = 7.64, P<0.01). Similarly, one-way ANOVA on lever-pressing revealed significant effects of History (F2,54 = 6.82, P<0.01).
iii. Ad libitum calorie intake and bodyweight tests.
Compared to the control (saccharin) histories, ShA and LgA cocaine histories did not significantly alter weekly kcal intake (Figure 1C) and bodyweights (Figure 1D). Two-way ANOVA for repeated measures on weekly kcal intake revealed only significant effects of Week (F8,23 = 54.44, P<0.001). Similarly, two-way ANOVA for repeated measures on bodyweights revealed only significant effects of Week (F8,23 = 530.44, P<0.001).
iv. Compulsive appetite tests for non-caloric saccharin.
Compared to the control (saccharin) histories, neither ShA nor LgA cocaine histories significantly altered “baseline” saccharin intake (Fig 1E, left panel). One-way ANOVA on rewards obtained revealed no significant effects of History (F2,30 = 0.22, NS). In contrast, ShA and LgA histories significantly increased rats’ punishment-resistance to obtain saccharin (Figure 1E, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of History (F2,30 = 25.11, P<0.001), Shock (F4,30 = 143.50, P<0.001) and History X Shock interaction (F8,120 = 12.09, P<0.001). Post-hoc Holm-Sidak tests revealed that LgA histories produced greater effects than ShA cocaine histories.
II. Cocaine histories via intraperitoneal administration:
Compared to the control (IP saline) histories, IP cocaine histories did not significantly alter “baseline” SCM and saccharin intake (Figure 1F/1G, left panels). Student’s t-test on SCM rewards obtained revealed no significant effect of History (t19 = 0.21, NS). Student’s t-test on saccharin rewards obtained revealed no significant effect of History (t23 = 0.17, NS). In contrast, IP cocaine histories significantly increased rats’ punishment-resistance to obtain SCM and saccharin (Figure 1F/1G, right panels). Two-way ANOVA for repeated measures on active lever-pressing for SCM revealed significant effects of History (F1,19 = 6.80, P<0.05), Shock (F4,19 = 70.67, P<0.001) and History X Shock interaction (F4,76 = 2.76, P<0.05). Two-way ANOVA for repeated measures on active lever-pressing for saccharin revealed significant effects of History (F1,23 = 13.15, P<0.01) and Shock (F4,23 = 37.06, P<0.001).
3.2. Experiment 2: The effects of extensive cocaine histories on subsequent food motivated behavior in female rats
Detailed statistical results are described in Table 2.
I. Cocaine histories via operant self-administration:
Compared to the control (saccharin) histories, neither ShA nor LgA cocaine histories significantly altered “baseline” SCM and saccharin intake (Figure 2A/2B, left panels). However, compared to the control histories, LgA, but not ShA, histories significantly increased rats’ punishment-resistance to obtain SCM and saccharin (Figure 2A/2B, right panels). Two-way ANOVA for repeated measures on active lever-pressing for SCM revealed significant effects of History (F2,29 = 4.30, P<0.05), Shock (F4,29 = 105.90, P<0.001) and History X Shock interaction (F8,116 = 3.07, P<0.05). Two-way ANOVA for repeated measures on active lever-pressing for saccharin revealed significant effects of Shock (F4,22 = 81.45, P<0.001) and History X Shock interaction (F8,88 = 3.32, P<0.01). Compared to the control histories, ShA and LgA cocaine histories did not significantly alter weekly calorie intake (Figure 2C) and bodyweights (Figure 2D). Two-way ANOVA for repeated measures on weekly kcal intake revealed only significant effect of Week (F8,21 = 32.13, P<0.001) but not History (F2,21 = 0.40, NS) or History X Week interaction (F16,168 = 1.14, NS). Similarly, two-way ANOVA for repeated measures on weekly kcal intake revealed only significant effect of Week (F8,21 = 325.83, P<0.001) but not History (F2,21 = 0.51, NS) or History X Week interaction (F16,168 = 0.07, NS).
II. Cocaine histories via intraperitoneal administration:
Compared to the control (IP saline) histories, IP cocaine histories did not significantly alter “baseline” SCM and saccharin intake (Figure 2E/2F, left panels). However, IP cocaine histories significantly increased rats’ punishment-resistance to obtain SCM and saccharin (Figure 2E/2F, right panels). Two-way ANOVA for repeated measures on active lever-pressing for SCM revealed significant effects of History (F1,17 = 18.51, P<0.001), Shock (F4,17 = 89.40, P<0.001) and History X Shock interaction (F4,68 = 3.44, P<0.05). Two-way ANOVA for repeated measures on active lever-pressing for saccharin revealed significant effects of History (F1,21 = 7.18, P<0.05), Shock (F4,21 = 87.94, P<0.001), and History X Shock interaction (F4,84 = 2.58, P<0.05).
3.3. Experiment 3: The effects of other histories on subsequent food motivated behavior in male rats.
Detailed statistical results are described in Table 3.
i. Compulsive appetite tests for saccharin.
For all cases, no significant group difference was found in baseline saccharin intake (Figure 3A/3B/3C/3D/3E, left panels). Compared to the control vapor (air) histories, alcohol vapor histories significantly increased rats’ punishment-resistance to obtain saccharin (Figure 3A, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of History (F1,20 = 6.49, P<0.05), Shock (F4,20 = 112.70, P<0.001), and History X Shock interaction (F4,80 = 4.38, P<0.01). Compared to the control diet histories, alcohol liquid diet histories significantly increased rats’ punishment-resistance to obtain saccharin (Figure 3B, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of Shock (F4,76 = 110.30, P<0.001) and History X Shock interaction (F4,76 = 3.82, P<0.01). Compared to the control histories, caffeine histories did not significantly alter and rats’ punishment-resistance to obtain saccharin (Figure 3C, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed only the significant effects of Shock (F4,20 = 41.68, P<0.001). Compared to the control diet histories, high fat and high sugar diet histories significantly increased rats’ punishment-resistance to obtain saccharin (Figure 3D, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of History (F1,20 = 9.59, P<0.001) and Shock (F4,20 = 24.06, P<0.001). Compared to the control diet histories, high fat and high sugar, but not high protein, diet histories, significantly increased rats’ punishment-resistance to obtain saccharin (Figure 3E, right panel). Two-way ANOVA for repeated measures on active lever-pressing revealed significant effects of History (F3,46 = 3.15, P<0.05), Shock (F4,46 = 216.70, P<0.001), and History X Shock interaction (F12,184 = 1.98, P<0.05).
ii. Ad libitum calorie intake and bodyweight tests
Compared to the control histories, IP cocaine, alcohol liquid diet and caffeine histories did not significantly alter weekly calorie intake (Figure 3F) and bodyweights (Figure 3G). Two-way ANOVA for repeated measures on weekly kcal intake revealed only the significant effect of Week (F8,30 = 89.31, P<0.001) but not History (F2,23 = 1.03, NS) or History X Week interaction (F30,240 = 1.53, NS). Similarly, two-way ANOVA for repeated measures on weekly bodyweights revealed only the significant effect of Week (F8,30 = 514.30, P<0.001) but not History (F8,30 = 0.12, NS) or History X Week interaction (F30,240 = 1.34, NS).
4. DISCUSSION
We hypothesized that extensive drug histories, which cause addiction-like brain changes and punishment-resistant “compulsive” drug self-administration in rats (Vanderschuren & Ahmed, 2020), would similarly cause punishment-resistant food self-administration or “compulsive appetite”. We first tested this hypothesis in male rats with 6-hr daily (LgA) cocaine self-administration histories known to cause addiction-like neurobehavioral profiles (Ahmed, 2012).
Male rats with LgA cocaine histories endured greater shock-intensities to obtain SCM than drug naïve rats (Figure 1A, right panel). Rats with less extensive 2-hr daily (ShA) cocaine self-administration histories, also known to cause addiction-like neurobehavioral profiles (e.g., Lucantonio et al., 2014), showed smaller but significant resistance (Figure 1A, right panel). In contrast, no significant group difference was found in unpunished “baseline” SCM intake (Figure 1A, left panel). Since LgA and ShA cocaine histories do not alter pain sensitivity (Edwards et al., 2012), this punishment-resistance likely reflects enhanced motivation (Singer, Fadanelli, Kawa & Robinson, 2018) or craving (Chao, Grilo & Sinha, 2016) – rather than reduced pain or aversion sensitivity (Jean-Richard-Dit-Bressel, Ma, Bradfield, Killcross & McNally, 2019). Accordingly, when tested under a PR schedule (an increasing work-load paradigm not involving pain), rats with LgA and ShA histories worked more to obtain SCM (Figure 1B). Since the enhanced responding for SCM was apparent against at least two “cost” measures (foot-shock and increasing workload), these effects could also reflect insensitivity to punishment contingency (Jean-Richard-Dit-Bressel, Lee, Liew, Weidemann, Lovibond & McNally, 2021; Jean-Richard-Dit-Bressel, Ma, Bradfield, Killcross & McNally, 2019) and/or the development of habitual behavior (Everitt, Giuliano & Belin, 2018).
In the current study, rats with ShA/LgA cocaine histories – as a group – developed punishment-resistant SCM self-administration (Figure 1A, right panel). However, only a minority of rats with extensive cocaine histories developed punishment-resistant cocaine self-administration in previous studies (e.g., Deroche-Gamonet, Belin & Piazza, 2004; Pelloux, Everitt & Dickinson, 2007). This apparent contradiction is likely due to differences in the experimental design: while these studies used a single strong shock intensity (e.g., 0.8 mA, 2 sec, two shocks per reinforcer in Deroche-Gamonet, Belin & Piazza, 2004) to detect ‘individual differences’ within rats with the same drug histories, we used multiple milder intensities (0.1-0.35 mA, 0.5 sec, one shock per reinforcer) to detect ‘group differences’ among rats with different drug histories. Consistent with this view, using a single strong shock intensity (0.8 mA) with a shorter duration (1 sec) and only one shock per reinforcer, we found that rats with extensive cocaine histories – as a group – could develop punishment-resistant cocaine self-administration (Laque et al., 2019). Using a different procedure (conditioned suppression), another study has also found that rats with extensive cocaine histories – as a group – develop compulsive cocaine self-administration (Vanderschuren & Everitt, 2004).
In the current study, punishment-resistant SCM self-administration (Figure 1A, right panel) was evident during long-term (3-5 weeks) abstinence from cocaine self-administration, suggesting prolonged changes in appetite regulation. However, when unpunished, rats with (LgA/ShA) or without (drug naïve) cocaine self-administration histories all responded similarly for SCM (Figure 1A, left panel). We thus next investigated the long-term effects of past cocaine histories on unpunished ‘basal’ calorie intake and bodyweight change. When male rats with (LgA/ShA) or without (drug naïve) cocaine self-administration histories were given unchallenged ad libitum access to nutritionally balanced diet and then obesogenic diets high in fat and sugar during long-term (3-11 weeks) abstinence, all showed similar patterns of calorie intake and bodyweight change (Figures 1C/1D).
Current cocaine users are known to exhibit altered fat metabolism and storage – physiological changes thought to prevent them from becoming overweight and obese (Billing & Ersche, 2015; Ersche, Stochl, Woodward & Fletcher, 2013). Thus, similar metabolic alterations may explain why rats with past cocaine histories failed to show altered bodyweights beyond drug naïve rats during abstinence (Figures 1D). However, this interpretation is challenged by the fact that unlike current stimulant users who report increased preference for fatty foods and increased calorie intake (Billing & Ersche, 2015; Ersche, Stochl, Woodward & Fletcher, 2013), rats with past cocaine histories did not consume extra calories beyond drug naïve controls even when given unchallenged ad libitum access to diets high in fat and sugar (Figures 1C). Since calorie intake was not altered (beyond control rats), metabolic alterations due to past cocaine histories do not likely account for the ‘unaltered’ bodyweight of rats with LgA/ShA cocaine histories. Indeed, current stimulant users with active metabolic alterations are known to maintain reduced weights despite their increased calorie intake (Billing & Ersche, 2015; Ersche, Stochl, Woodward & Fletcher, 2013). Nevertheless, future studies should determine the long-term effects of past cocaine histories on energy metabolism and storage.
Food motivation is thought to operate under both ‘homeostatic’ (caloric/metabolic) and ‘non-homeostatic’ (hedonic/incentive) control (Berthoud, 2006). Via visceral afferent and endocrine connections, adipose tissue and gastrointestinal signals engage hypothalamic and hindbrain circuits that control calorie intake to maintain energy balance (homeostatic control). We found that neither ShA nor LgA cocaine histories significantly altered this homeostatic regulation (as reflected in unaltered basal calorie intake and bodyweight beyond control histories). In contrast, the reward circuits, including the ventral tegmental area and the nucleus accumbens, regulate food and drug motivation independently of caloric needs (non-homeostatic control). Extensive cocaine histories cause aberrant neuroadaptations in the reward circuits, thereby dysregulating motivation for this non-caloric reward and ultimately manifesting as compulsive drug craving and use (Volkow, Koob & McLellan, 2016). We thus hypothesized that extensive cocaine histories would similarly cause compulsive appetite via non-homeostatic dysregulation.
We tested this hypothesis by determining male rats’ willingness to lever-press for saccharin, a metabolically and gastro-intestinally inert reward, paired with foot-shock punishment during long-term (3–5 weeks) abstinence. Rats with LgA cocaine histories exhibited greater punishment-resistance for saccharin than drug naïve controls (Figure 1E, right panel). Rats with ShA cocaine histories exhibited smaller but significant resistance (Figure 1E, right panel). As with baseline SCM intake, no significant group difference was found in baseline saccharin intake (Figure 1E, left panel). Since saccharin is non-caloric, its intake in well-trained animals is presumably not under homeostatic control. Hence, like compulsive drug craving and use, compulsive appetite likely reflects non-homeostatic hedonic/incentive dysregulation.
We originally observed punishment-resistant SCM and saccharin self-administration in rats trained to lever-press for cocaine before being tested to press the same lever for food rewards. We thus determined whether extensive operant training – rather than cocaine itself – caused punishment-resistant self-administration. We here tested IP cocaine histories based on procedures to induce addiction-like neurobehavioral profiles in rats (e.g., Li, Acerbo & Robinson, 2004; Schoenbaum & Setlow, 2005). Like rats with cocaine self-administration histories, rats with IP cocaine histories endured greater foot-shocks to obtain SCM and saccharin (Figures 1F/1G, left panels), while no significant group difference was found in baseline SCM and saccharin intake (Figures 1F/1G, right panels). These results parallel previous findings that similar IP cocaine histories facilitate operant responding for sucrose under a high workload condition (FR30) (Klein, Gehrke, Green, Zentall & Bardo, 2007) and indicate that cocaine’s pharmacological action is sufficient to induce compulsive appetite. These results also indicate that the development of punishment-resistance is independent from the shift from initial cocaine to subsequent SCM or saccharin self-administration or the “negative/positive contrast” effects which can result in compulsive appetite (Heyne et al., 2009). Finally, the use of saccharin both as the control stimulus and the stimulus representing compulsive appetite does not appear to have skewed the experimental results.
Drug addiction is more common among men, but eating disorders disproportionately affect women (Whiteford et al., 2013). We thus investigated whether sex plays a role in the long-term effects of cocaine on food motivation. Like male rats, female rats showed increased punishment-resistance to obtain SCM or saccharin during long-term abstinence from voluntary (LgA) or passive (IP) cocaine administration (Figures 2A/2B/2D/2E, right panels). In contrast, female rats with ShA cocaine histories failed to show such resistance, suggesting sex differences in drug sensitivity. Like male rats, no significant group difference was found in baseline SCM and saccharin intake (Figures 2A/2B/2D/2E, left panels). Like male rats, female rats with ShA/LgA cocaine histories also showed neither excess calorie intake nor weight gain when given ad libitum food access (Figures 2C/2D). Thus, extensive cocaine histories appear to induce compulsive appetite via non-homeostatic dysregulation, irrespective of sex.
Extensive exposures to different drugs and obesogenic diets cause similar brain changes (Serafine, O’Dell & Zorrilla, 2021; Volkow & Wise, 2005). We thus investigated the generalizability of compulsive appetite in male rats with different drug and diet histories: alcohol (via ‘vapor’ or ‘liquid diet’ known to cause physical dependence and excess alcohol intake in rats (McBride & Li, 1998)), caffeine (thought to provide anti-obesity action (Heckman, Weil & Gonzalez de Mejia, 2010)) and obesogenic diets (known to cause physiological changes resembling obese individuals and punishment-resistant food self-administration in rats (e.g., Johnson & Kenny, 2010)). Like rats with extensive cocaine histories, rats given alcohol via vapor or liquid diet endured greater foot-shocks for saccharin during long-term abstinence (Figures 3A/3B). Since alcohol exposures increase – rather than decrease – pain sensitivity (Edwards et al., 2012), this punishment-resistance likely reflects enhanced motivation rather than reduced pain sensitivity. Accordingly, a recent study has shown that rats undergoing acute (~6 hrs) withdrawal from alcohol work more to obtain sucrose under a PR schedule (Alaux-Cantin et al., 2021). These effects could also reflect punishment-insensitivity (Jean-Richard-Dit-Bressel, Lee, Liew, Weidemann, Lovibond & McNally, 2021; Jean-Richard-Dit-Bressel, Ma, Bradfield, Killcross & McNally, 2019) and/or habitual behavior (Everitt, Giuliano & Belin, 2018).
Unlike rats with cocaine and alcohol histories, rats with caffeine histories did not show increased punishment-resistance to obtain saccharin (Figure 3C). In contrast, extending previous reports (e.g., Johnson & Kenny, 2010; Rossetti, Spena, Halfon & Boutrel, 2014; Spierling et al., 2020), rats given obesogenic diets high in both fat and sugar endured greater foot-shocks to obtain saccharin (Figure 3F). Similar punishment-resistance was observed in rats given diets high in either fat or sugar but not diets high in protein (Figure 3G). These results are consistent with previous findings that highly processed diets high in refined fat and carbohydrates – but not those high in protein – are most strongly associated with addictive behaviors (Gearhardt & Hebebrand, 2021b; Small & DiFeliceantonio, 2019).
As with cocaine histories, punishment-resistant self-administration of saccharin was evident during long-term abstinence from alcohol and obesogenic diets. Previous studies have shown that rats with past opioid (morphine) histories endure greater foot-shock punishments to obtain sucrose (Li, Zheng, Xu, Zhang, Liu & Bai, 2017), while rats with past nicotine histories work more to obtain sucrose under a PR schedule (LeSage, Burroughs & Pentel, 2006). Thus, both drug and obesogenic diet histories appear to produce prolonged changes in appetite regulation.
However, when given unchallenged ad libitum food access, male rats with IP cocaine, alcohol vapor or caffeine histories showed no excess calorie intake or bodyweight gain while undergoing long-term abstinence (Figures 3H/3I). Cocaine and alcohol histories thus appear to cause compulsive appetite via non-homeostatic dysregulation. In contrast, previous studies have found that rats with obesogenic diet histories maintain significantly higher bodyweights after being placed on nutritionally balanced diets for 5-10 weeks and exhibit altered calorie intake (in some cases reducing calorie intake) (e.g., Holtrup et al., 2017; Uriarte, Paternain, Milagro, Martinez & Campion, 2013). Taken together with our findings (Figures 3D/3E), obesogenic diet histories, unlike drug histories, may cause compulsive appetite via homeostatic as well as non-homeostatic appetite dysregulation. Future studies should determine this possibility.
Given that current drug users often prefer meals high in fat (Billing & Ersche, 2015; Ersche, Stochl, Woodward & Fletcher, 2013), future studies should also determine the effects of combined or altered drug and obesogenic diet histories on subsequent food motivated behaviors in rats. Future studies should also explore the relevance of the current results to ‘cross-addiction transfer’ where individuals with drug use histories are more prone to gain weight, develop binge eating and prefer more intensely sweet foods during abstinence despite often reporting significant weight concerns (e.g., Cowan & Devine, 2008; Hodgkins, Frost-Pineda & Gold, 2007). However, when given unchallenged ad libitum access to diets high in fat and sugar, drug naïve rats consumed as many calories (Figures 1C/2C/3F) and gained as much weight (Figures 1D/2D/3G) as rats with drug histories. Future studies thus should further determine the effects of drug histories on subsequent diet preference under a challenged condition.
5. CONCLUSIONS
In summary, extensive drug histories known to cause addiction-like neurobehavioral changes in rats also caused compulsive appetite without causing excess calorie intake and bodyweight gain. Compulsive appetite and obesity thus appear to be dissociable (Boswell, Potenza & Grilo, 2021; Gearhardt & Hebebrand, 2021a). Indeed, BED and BN are marked by compulsive appetite but not necessarily obesity (Udo & Grilo, 2018). However, mechanisms leading to obesity may also manifest as compulsive appetite, as subsets (~30%) of obese individuals exhibit addiction-like compulsive eating (Gearhardt, Corbin & Brownell, 2016; Minhas, Murphy, Balodis, Samokhvalov & MacKillop, 2021). Nevertheless, like drug addiction, “food addiction” is perhaps better defined as a disorder of compulsive appetite, as seen in subsets of eating disorder patients and obese individuals, than as a disorder of obesity in general, which is a heterogeneous, multifaceted condition.
Beyond similar behavioral profiles, drug addiction, BED and BN share similar brain profiles (Boswell, Potenza & Grilo, 2021; Serafine, O’Dell & Zorrilla, 2021) as well as genetic risk factors (Munn-Chernoff et al., 2020) and higher-than-expected comorbidities (e.g., the rates of drug addiction in BED patients [20-40%] are higher than those in the general population [~10%]) (e.g., Udo & Grilo, 2018). Similarly, “food addiction” as defined by the Yale Food Addiction Scale is characterized by addiction-like compulsive eating but not necessary obesity and shares higher-than-expected comorbidities with BED, BN and drug addiction (e.g., Horsager, Faerk, Gearhardt, Lauritsen & Ostergaard, 2021; Jimenez-Murcia et al., 2019). These findings suggest common etiologies for compulsive behaviors in drug addiction, eating disorders and putative “food addiction”.
The current results provide an animal model to investigate the common neurobiological mechanisms that mediate compulsive behaviors motivated by drugs and obesogenic diets. Overlapping neuroadaptations in the reward circuits, which regulate drug and food motivation independently of energy homeostasis, may serve as common targets for treating compulsive behaviors across drug addiction, eating disorders and obesity. Our findings reveal a need for systematic investigations of this possibility.
BULLET POINT SUMMARY.
‘What is already known’
The validity of “food addiction” is largely based on correlational evidence and remains controversial.
‘What this study adds’
Disordered eating is causally linked to preclinical drug addiction models via compulsive appetite.
‘Clinical significance’
Like drug addiction, “food addiction” is better defined as a disorder of compulsive behavior.
ACKNOWLEDGEMENT
This work was supported by Extramural and Intramural funding from National Institute on Drug Abuse as well as National Institute of Alcohol Abuse and Alcoholism, National Institute of Health, USA: R01DA037294 (N.S.), R01AA023183 (N.S.), R01DA08467 (F.W.), R01AA021549 (F.W.) and K99/R00DA035865 (M.W.B.). A.L. and H.N. were supported by Ruth L. Kirschstein Institutional National Research Service Award from National Institute of Alcohol Abuse and Alcoholism, National Institute of Health, USA: T32AA007456 (PIs, Drs. Loren “Larry” Parsons, Michael Taffe and Marisa Roberto). This is the manuscript #30130 published by The Scripps Research Institute.
8. DECLARATION OF TRANSPARENCY AND SCIENTIFIC REGOUR
THE Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the British Journal of Pharmacology guidelines for Design & Analysis and Animal Experimentation and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Abbreviations:
- BED
Binge-eating disorder
- BN
bulimia nervosa
- SA
self-administration
- LgA
long access
- ShA
short access
- SCM
sweetened condensed milk
Footnotes
Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://guidetopharmacology.org.
CONFLICT OF INTEREST STATEMENT: The authors declare no competing interests.
9. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
10. REFERENCES
- Ahmed SH (2012). The science of making drug-addicted animals. Neuroscience 211: 107–125. [DOI] [PubMed] [Google Scholar]
- Alaux-Cantin S, Alarcon R, Audegond C, Simon O’Brien E, Martinetti MP, Ahmed SH, et al. (2021). Sugar, a powerful substitute for ethanol in ethanol postdependent rats: Relevance for clinical consideration? Addict Biol: e13023. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. [Google Scholar]
- Avena NM, Bocarsly ME, Hoebel BG, & Gold MS (2011). Overlaps in the nosology of substance abuse and overeating: the translational implications of “food addiction”. Curr Drug Abuse Rev 4: 133–139. [DOI] [PubMed] [Google Scholar]
- Berthoud HR (2006). Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity (Silver Spring) 14 Suppl 5: 197S–200S. [DOI] [PubMed] [Google Scholar]
- Billing L, & Ersche KD (2015). Cocaine’s appetite for fat and the consequences on body weight. Am J Drug Alcohol Abuse 41: 115–118. [DOI] [PubMed] [Google Scholar]
- Boswell RG, Potenza MN, & Grilo CM (2021). The Neurobiology of Binge-eating Disorder Compared with Obesity: Implications for Differential Therapeutics. Clin Ther 43: 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Grilo CM, & Sinha R (2016). Food cravings, binge eating, and eating disorder psychopathology: Exploring the moderating roles of gender and race. Eat Behav 21: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, & Holmes NM (2018). An extended history of drug self-administration results in multiple sources of control over drug seeking behavior. Prog Neuropsychopharmacol Biol Psychiatry 87: 48–55. [DOI] [PubMed] [Google Scholar]
- Cowan J, & Devine C (2008). Food, eating, and weight concerns of men in recovery from substance addiction. Appetite 50: 33–42. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Schatzker M, & Small DM (2020). Rethinking Food Reward. Annu Rev Psychol 71: 139–164. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, & Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305: 1014–1017. [DOI] [PubMed] [Google Scholar]
- Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. (2007). Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A 104: 11766–11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. (2012). Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology 62: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, & Fletcher PC (2013). The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite 71: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, & Belin D (2018). Addictive behaviour in experimental animals: prospects for translation. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, & Kenny PJ (2018). Food addiction: a valid concept? Neuropsychopharmacology 43: 2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2016). Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav 30: 113–121. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, & Hebebrand J (2021a). The concept of “food addiction” helps inform the understanding of overeating and obesity: Debate Consensus. Am J Clin Nutr 113: 274–276. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, & Hebebrand J (2021b). The concept of “food addiction” helps inform the understanding of overeating and obesity: YES. Am J Clin Nutr 113: 263–267. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, White MA, & Potenza MN (2011). Binge eating disorder and food addiction. Curr Drug Abuse Rev 4: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman MA, Weil J, & Gonzalez de Mejia E (2010). Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75: R77–87. [DOI] [PubMed] [Google Scholar]
- Heyne A, Kiesselbach C, Sahun I, McDonald J, Gaiffi M, Dierssen M, et al. (2009). An animal model of compulsive food-taking behaviour. Addict Biol 14: 373–383. [DOI] [PubMed] [Google Scholar]
- Hodgkins C, Frost-Pineda K, & Gold MS (2007). Weight gain during substance abuse treatment: the dual problem of addiction and overeating in an adolescent population. J Addict Dis 26 Suppl 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Holtrup B, Church CD, Berry R, Colman L, Jeffery E, Bober J, et al. (2017). Puberty is an important developmental period for the establishment of adipose tissue mass and metabolic homeostasis. Adipocyte 6: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsager C, Faerk E, Gearhardt AN, Lauritsen MB, & Ostergaard SD (2021). Food addiction comorbid to mental disorders in adolescents: a nationwide survey and register-based study. Eat Weight Disord. [DOI] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, Lee JC, Liew SX, Weidemann G, Lovibond PF, & McNally GP (2021). Punishment insensitivity in humans is due to failures in instrumental contingency learning. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, Ma C, Bradfield LA, Killcross S, & McNally GP (2019). Punishment insensitivity emerges from impaired contingency detection, not aversion insensitivity or reward dominance. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Murcia S, Aguera Z, Paslakis G, Munguia L, Granero R, Sanchez-Gonzalez J, et al. (2019). Food Addiction in Eating Disorders and Obesity: Analysis of Clusters and Implications for Treatment. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, & Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Gobejishvili LN, Bon Homme M, Waigel S, Cave M, Arteel G, et al. (2011). Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J Nutr Biochem 22: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ED, Gehrke BJ, Green TA, Zentall TR, & Bardo MT (2007). Repeated Cocaine Experience Facilitates Sucrose-Reinforced Operant Responding in Enriched and Isolated Rats. Learn Motiv 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laque A, G LDN, Wagner GE, Nedelescu H, Carroll A, Watry D, et al. (2019). Anti-relapse neurons in the infralimbic cortex of rats drive relapse-suppression by drug omission cues. Nat Commun 10: 3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, & Pentel PR (2006). Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav 83: 585–591. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, & Robinson TE (2004). The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci 20: 1647–1654. [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng X, Xu N, Zhang Y, Liu Z, & Bai Y (2017). The consummatory and motivational behaviors for natural rewards following long-term withdrawal from morphine: no anhedonia but persistent maladaptive behaviors for high-value rewards. Psychopharmacology (Berl) 234: 1277–1292. [DOI] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-Chaudhary S, Shaham Y, et al. (2014). Orbitofrontal activation restores insight lost after cocaine use. Nat Neurosci 17: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, & Li TK (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12: 339–369. [DOI] [PubMed] [Google Scholar]
- Minhas M, Murphy CM, Balodis IM, Samokhvalov AV, & MacKillop J (2021). Food addiction in a large community sample of Canadian adults: prevalence and relationship with obesity, body composition, quality of life and impulsivity. Addiction. [DOI] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Johnson EC, Chou YL, Coleman JRI, Thornton LM, Walters RK, et al. (2020). Shared genetic risk between eating disorder- and substance-use-related phenotypes: Evidence from genome-wide association studies. Addict Biol: e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, & Dickinson A (2007). Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 194: 127–137. [DOI] [PubMed] [Google Scholar]
- Richardson NR, & Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363: 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti C, Spena G, Halfon O, & Boutrel B (2014). Evidence for a compulsive-like behavior in rats exposed to alternate access to highly preferred palatable food. Addict Biol 19: 975–985. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, & Setlow B (2004). Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci 19: 1997–2002. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, & Setlow B (2005). Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex 15: 1162–1169. [DOI] [PubMed] [Google Scholar]
- Serafine KM, O’Dell LE, & Zorrilla EP (2021). Converging vulnerability factors for compulsive food and drug use. Neuropharmacology: 108556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Pavon FJ, Buczynski MW, Schlosburg J, Natividad LA, Polis IY, et al. (2018). Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology 43: 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, & Goldberg SR (1999). Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology (Berl) 142: 327–333. [DOI] [PubMed] [Google Scholar]
- Singer BF, Fadanelli M, Kawa AB, & Robinson TE (2018). Are Cocaine-Seeking “Habits” Necessary for the Development of Addiction-Like Behavior in Rats? J Neurosci 38: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, & DiFeliceantonio AG (2019). Processed foods and food reward. Science 363: 346–347. [DOI] [PubMed] [Google Scholar]
- Spierling S, de Guglielmo G, Kirson D, Kreisler A, Roberto M, George O, et al. (2020). Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacology 45: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, & Grilo CM (2018). Prevalence and Correlates of DSM-5-Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol Psychiatry 84: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte G, Paternain L, Milagro FI, Martinez JA, & Campion J (2013). Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem 69: 601–611. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, & Ahmed SH (2020). Animal Models of the Behavioral Symptoms of Substance Use Disorders. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, & Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Wise RA (2005). How can drug addiction help us understand obesity? Nat Neurosci 8: 555–560. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- Wiss DA, Criscitelli K, Gold M, & Avena N (2017). Preclinical evidence for the addiction potential of highly palatable foods: Current developments related to maternal influence. Appetite 115: 19–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


