Abstract
Purpose/Objectives:
Dosimetric and technical challenges often limit radiotherapy (RT) target coverage for breast cancer patients who require bilateral breast/chest wall and regional nodal irradiation (RNI). We evaluated the feasibility of using volumetric modulated arc therapy (VMAT) to administer bilateral comprehensive RNI including the internal mammary nodes.
Materials/Methods:
We analyzed all patients treated at our institution with bilateral RNI using VMAT from 2017–2020. Medical records were reviewed to ascertain clinicopathologic features, radiotherapeutic parameters and treatment-related adverse events.
Results:
The cohort comprised 12 patients who underwent VMAT for bilateral RNI, with a median follow-up of 14.5 months. Median V5Gy for bilateral lungs was 96.1% (range: 84.5%−99.8%). Median V20Gy for each lung was 27.5% (range: 14.9–38.1%). Cardiac mean dose was a median of 699 cGy (range: 527–1117 cGy).
Five patients (41%) developed grade 1 cough/dyspnea, with one patient developing grade 3 dyspnea. Of note, three of these (60%) were current or former smokers. No patient received glucocorticoid therapy or required respiratory intervention and none developed longer term pulmonary complaints. Decline in ejection fraction occurred in one patient with a pre-existing cardiac condition who also received anthracycline-based chemotherapy and trastuzumab. Only one patient experienced locoregional recurrence with synchronous distant progression and subsequently succumbed to her disease. No secondary cancers have been noted to date.
Conclusion:
VMAT appears to be a feasible and tolerable RT modality for breast cancer patients who require bilateral comprehensive adjuvant RT with RNI obtaining excellent target coverage. No patients required medical intervention for pulmonary complaints despite a median bilateral V5 approaching 100%, providing further evidence that V5 is not predictive for complications.
Introduction
Patients with locally advanced breast cancer often require RT to the affected breast or chest wall (CW) and regional lymph nodes to reduce the risk of recurrent disease. In two similarly-designed prospective randomized studies, the addition of regional nodal irradiation (RNI) to whole breast or chestwall RT resulted in reduced rates of locoregional recurrence and improved disease-free survival.4–6. As a result, RNI has seen increased use in contemporary practice, occasionally leading to treatment planning challenges. In the MA.20 phase III randomized study, the addition of RNI, inclusive of targeting the supraclavicular and infraclavicular fossae, and internal mammary lymph node chains, to whole-breast radiation therapy significantly increased rates of pneumonitis, dermatitis, and lymphedema, although absolute rates of these events were low6. Cardiotoxicity from breast radiation also remains a significant concern when treating patients with left-sided disease involvement9. Contemporary approaches have sought to reduce cardiac exposure using a variety of novel approaches. A recent study published in 2020 showed the dosimetric advantages of multi-beam IMRT, which increased coverage of targeted tissue while reducing the volume of adjacent organs receiving high doses of RT in patients with unilateral node-positive breast cancer10.
With the continued advent of modern radiation therapy techniques, such as volumetric modulated arc therapy (VMAT), enhanced sparing of critical organs can be achieved while adequately covering adjacent areas of concern. VMAT has been shown to improve conformality and homogeneity compared to conventional radiation therapy techniques with reduced dose to the heart and lungs11. However, because VMAT may increase low dose exposure to surrounding non-target structures, concern regarding its widespread adoption in breast cancer persists. In this study, we sought to evaluate the feasibility and safety of VMAT in breast cancer patients requiring bilateral comprehensive breast/chestwall radiation therapy with RNI.
Materials and Methods
Patient Selection
All patients treated at our institution with bilateral RNI using VMAT from 2017–2020 were identified. Patients were diagnosed with either synchronous bilateral breast cancer or had evidence of unilateral disease with contralateral nodal involvement. Medical records were reviewed to ascertain relevant clinicopathologic features.
Dosimetric Parameters
Institutional guidelines for VMAT treatment planning have been previously reported12. Planning target volume (PTV) was inclusive of the bilateral breast or chestwall, supraclavicular and infraclavicular fossae, axillae levels I/II/III (regardless of type of axillary surgery), and bilateral internal mammary lymph node chains. As certain studies have suggested that the volume of lung receiving 5Gy (V5), 20Gy (V20), and mean lung dose may predict for rates of pulmonary toxicity13, individual and total lung dosimetric parameters were analyzed. Mean and maximum doses received by the heart and left anterior descending artery were also recorded to evaluate cardiotoxicity. Respiratory motion management with deep inspiration breath hold was utilized in 10/12 (83%) of patients.
Treatment-related toxicity
Presence and severity of pulmonary or cardiovascular morbidity were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE, v5.0). Other common side effects from breast RT, including lymphedema and skin toxicity, were recorded. Recurrence rates and secondary malignancies were documented as determined by advanced imaging or date of last clinic visit.
Results
Patient Characteristics
This study included 12 evaluable patients (median age 53.5; range 39–67), all of whom received bilateral VMAT to the breast/chest wall with bilateral RNI to a total dose of 5000 cGy. Select patient characteristics are detailed in Table 1. Median follow-up was 14.5 months (range: 1–24 months). Patients were evaluated by their care team at routine intervals between 3–6 months or sooner if clinically indicated. Of the total cohort, 4 (33%) patients were diagnosed with synchronous bilateral breast cancers while the remaining had unilateral cancers with contralateral regional nodal involvement. Three patients (25%) underwent breast conserving surgery, while seven (58%) underwent mastectomy (5 bilateral, 2 unilateral). One patient was not considered a surgical candidate, and one was treated with unilateral local excision only. All but one patient had axillary assessment performed, with 6 patients (50%) treated with bilateral axillary lymph node dissection (ALND). All patients received systemic therapy, of which 11 (92%) were anthracycline-based. Three patients (25%) also received HER-2 directed therapy. Of note, three patients had received prior breast RT and two (17%) were carriers of pathogenic germline BRCA1 or BRCA2 variants. Of these three patients that underwent re-irradiation, two were treated comprehensively to the unilateral affected breast and regional nodes to a dose of approximately 5000 cGy followed by a sequential boost to the surgical cavity. The average time -interval between prior and current treatment was 10.6 years. Seven (58%) reported being current or former smokers, and one had ongoing chronic obstructive pulmonary disease.
Table 1.
Patient Characteristics
| N | |
|---|---|
| Age | |
| <50 | 4 |
| >50 | 8 |
| Disease status | |
| Synchronous bilateral breast cancer | 4 |
| Unilateral cancer with contralateral spread | 8 |
| Axillary surgery | |
| None | 1 |
| Bilateral ALND | 6 |
| Unilateral ALND | 1 |
| ALND/SLN | 3 |
| SLN only | 1 |
| Chemotherapy | |
| ACT | 11 |
| Other | 1 |
| BRCA mutation positive | |
| Yes | 2 |
| Smoking history | |
| Nonsmoker | 5 |
| Former | 6 |
| Current | 1 |
| Prior breast RT | |
| Yes | 3 |
| No | 9 |
Abbreviations: ALND, axillary lymph node dissection. SLN, sentinel lymph node assessment. ACT, Adriamycin, Cyclophosphamide, Paclitaxel. RT, radiation therapy.
Dosimetric Analysis
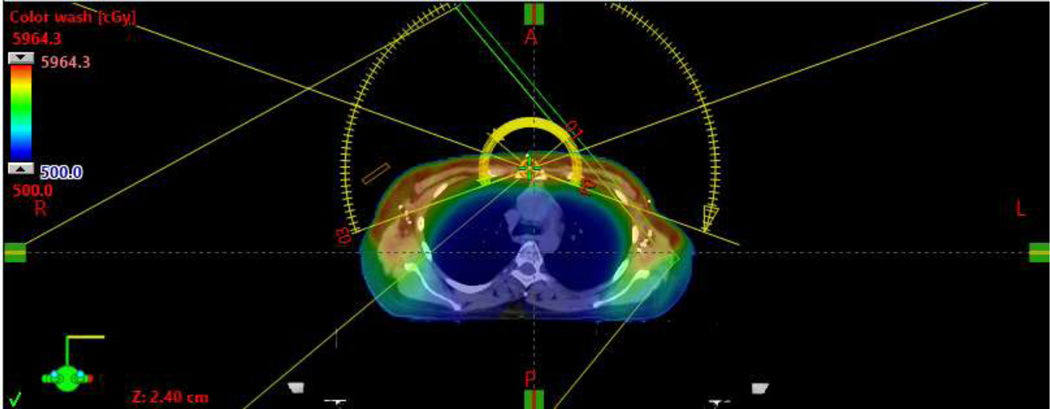
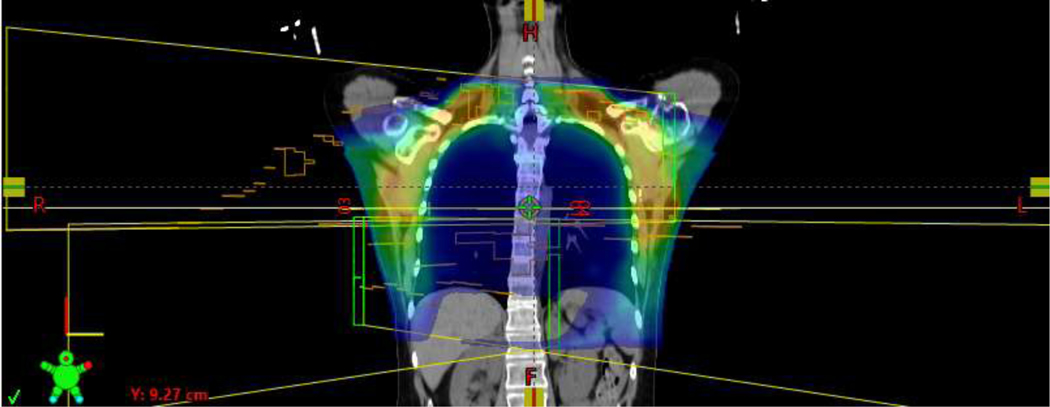
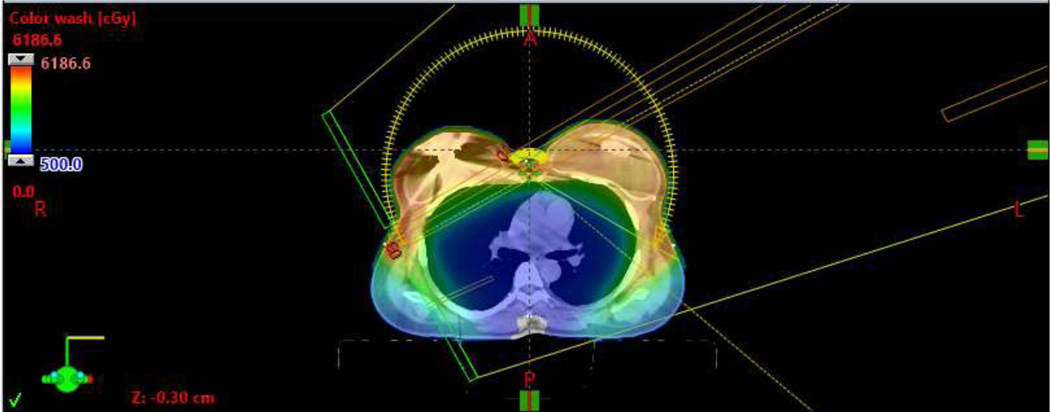
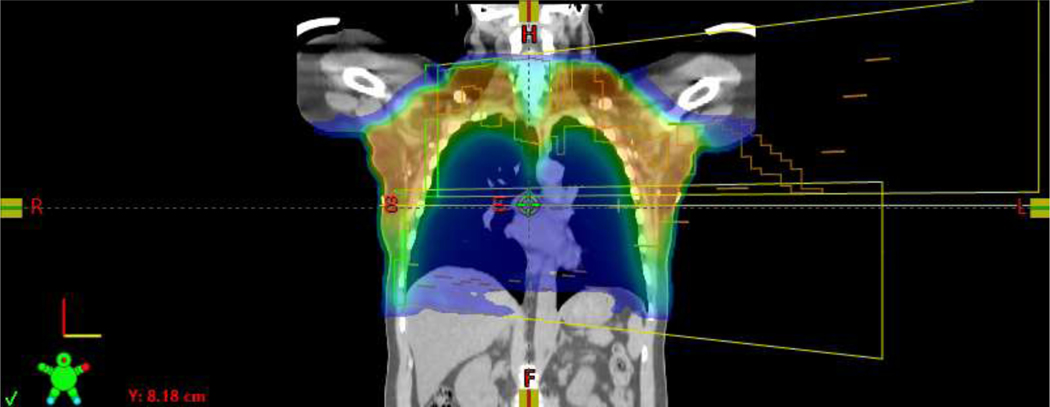
Representative bilateral VMAT plans, without [Figure 1A/B] and with reconstruction [Figure 1C/D], are shown in Figure 1. Mean V95% of the PTV was 99.2%. Median IMN D95% was 99.1%, with minimum D95% of 85.6% [Table 2].
Figure 1A.
Representative axial view of a bilateral VMAT plan without reconstruction.
Figure 1B.
Representative coronal view of a bilateral VMAT plan without reconstruction.
Figure 1C.
Representative axial view of a bilateral VMAT plan with tissue expander reconstruction.
Figure 1D.
Representative coronal view of a bilateral VMAT plan with reconstruction
Table 2.
Dosimetric Analysis
| Median | Range | |
|---|---|---|
| PTV V95% | 99.2% | 94.1–101.6% |
| IMN D95% | 99.1% | 85.6–103.3% |
| Right Lung V5 | 97.65% | 82.3–99.7% |
| Left Lung V5 | 94.85% | 80–100% |
| Right Lung V20 | 26.75% | 16.8–38.1% |
| Left Lung V20 | 28.15% | 14.9–35.8% |
| Heart Mean (cGy) | 699 | 484–1128 |
For the lungs, each lung considered independently per the standard breast RT approach, the median V5 was 96.2% (range: 80%−100%). Median V5 for the combined bilateral lung volume was 96.1% (range 84.5%−99.8%). Median V20 for each lung analyzed separately was 27.5% (range: 14.9%−38.1%) [Table 2]. Taken together, the total mean lung dose ranged from 1336 cGy to 1948 cGy (median 1567 cGy).
Mean dose to the heart ranged from 484 cGy to 1128 cGy (median: 699 cGy) [Table 2]. The maximum dose to the heart ranged from 2365 cGy to 4793 cGy (median: 3023 cGy) and median maximum dose to the lower anterior descending artery was 2320 cGy.
Toxicity
Four patients (33%) developed grade 1 cough following therapy. Only one patient (8%) developed grade 3 dyspnea at rest [Table 3]. No patient received glucocorticoid therapy or required respiratory invention as a result of these symptoms, and none developed longer term pulmonary complaints.
Table 3.
Toxicity Outcomes
| N | |
|---|---|
| Pulmonary | |
| None | 5 |
| Grade 1 Cough | 4 |
| Grade 1 Dyspnea | 2 |
| Grade 3 Dyspnea | 1 |
| Cutaneous | |
| Grade 1 Dermatitis | 9 |
| Grade 2 Dermatitis | 2 |
| Grade 3 Dermatitis | 1 |
| Lymphedema | |
| Present | 2 |
| Absent | 10 |
| Cardiac | |
| None | 11 |
| Grade 3 Left Ventricular Dysfunction | 1 |
Cardiac toxicity was rare with a decline in ejection fraction noted in only one patient with pre-existing cardiac co-morbidities who had also received anthracycline-based chemotherapy and Her-2 directed therapy.
Clinically significant lymphedema was noted in two patients, one of whom underwent bilateral ALND. Three patients (25%) had evidence of skin toxicity, reaching the threshold of grade 2–3 dermatitis [Table 3]. Patients utilized appropriate skin care following therapy without long-term sequelae.
Disease Outcomes and Secondary Malignancies
Albeit short follow-up, 11 out of 12 patients (92%) had no evidence of locoregional recurrence as determined by advanced imaging or date of last clinical visit. One patient, who presented with cT4N3 disease with contralateral axillary nodal involvement, had PET scan evidence of synchronous local, regional, and distant recurrence three months after treatment completion. She succumbed to her disease less than a year after radiation therapy. To date, no secondary malignancies have been documented.
Discussion
Here, we demonstrate the safety and feasibility of bilateral VMAT for patients diagnosed with synchronous bilateral breast cancers or with contralateral spread of a locally advanced breast cancer breast cancer, with dosimetric cardiac and pulmonary limits that largely exceed established unilateral constraints yet yield limited subsequent toxicity.
Recently, several randomized studies have detailed the benefit of RNI. MA.20 randomized 1800 women with node-positive or high risk node-negative breast cancer to whole breast irradiation therapy alone or whole breast irradiation with RNI6. This study showed an absolute 5% improvement in disease-free survival in the nodal-irradiation group compared to women that received breast only treatment. In a parallel study, EORTC 22922 showed a 15-year reduction of breast cancer mortality and any breast cancer recurrence in patients with stage I-III breast cancer treated with internal mammary and supraclavicular irradiation5. A similar third study specifically analyzed the potential benefit of adding IMN irradiation in patients with early-stage breast cancer. With a median follow-up of nearly 9 years, there was a 3.7% absolute improvement in overall survival in the patients with right-sided breast cancer receiving IMN irradiation14. Thus, with these studies all proving improved disease outcomes with the addition of RNI, a technique that successfully treats these nodal basins while protecting nearby OARs is imperative.
Previous literature has shown certain dosimetric parameters, such as mean lung dose, V5, V20, to predict for radiation pneumonitis15, 16. In our cohort, using VMAT, median V20 of each lung was 27.5%. Among studies with V20 <30%, rates of grade 1–2 radiation pneumonitis were only 6% with no reports of grade 3 and 4 toxicity 17–19. The highest V20 in any patient in our cohort remained <40%, which is quoted as the upper limit in the national NSABP-B51 trial for patients treated with RNI. Interestingly, despite V5 approaching 100%, only one patient had CTCAE grade 3 pulmonary toxicity, which responded to conservative management. Thus, we propose that V5 in these cases should not be considered a limiting factor when designing a plan to adequately cover bilateral breast/chest wall and regional nodal basins. We acknowledge retrospective scoring and documenting treatment toxicities raises the possibility of unanticipated bias and confounding, particularly in this small cohort of patients. Larger and longer term studies will be needed to evaluate the true risk profile of this treatment paradigm.
An important point is the increasing use of immunotherapy in breast cancer patients. Immune checkpoint inhibitors (ICIs) have resulted in reports of increased risk of pneumonitis in patients treated for melanoma and non-small cell lung cancer 20, 21. A recent study also demonstrated that patients with prior ICI-associated immune-related events were at high risk for clinically significant radiation pneumonitis from thoracic RT 22. Thus, with the publication of KEYNOTE-522 showing significantly higher pathologic complete response rates in patients with triple negative breast cancer receiving pembrolizumab23, RT following or concurrent with immunotherapy will likely become more common in breast cancer therapy. Physicians should exercise caution as data in this domain are limited - these patients may require close monitoring if a comprehensive bilateral RNI approach is recommended.
An oft-cited landmark study by Darby et al showed that rates of major coronary events increased linearly with mean dose to the heart by 7.4% per gray9. In our study, VMAT plans achieved mean heart doses ranging from 484–1128 cGy. To date, only one patient had evidence of a decline in ejection fraction after combined modality treatment, which also included receipt of anthracycline-based chemotherapy and trastuzumab.
Although longer follow-up is warranted, 92% of our patient cohort displayed no clinical or radiographic evidence of locoregional recurrence.
In conclusion, for patients requiring bilateral RT to the breast/chestwall and regional nodes, VMAT appears to be a feasible and well-tolerated option in this small cohort. Longer follow-up among larger cohorts will be needed to further evaluate the safety and efficacy of this technique.
Acknowledgements:
The authors would like to thank Roberto Adsuar, Anthony Abaya, and Michael Sullivan for data management and administrative support.
This work was partially supported by the NIH/NCI Cancer Center Support Grant (P30 CA008748)
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. [DOI] [PubMed] [Google Scholar]
- 4.Dodwell D, Taylor C, McGale P, et al. Abstract GS4–02: Regional lymph node irradiation in early stage breast cancer: An EBCTCG meta-analysis of 13,000 women in 14 trials. Cancer Research. 2019;79:GS4–02-GS04–02. [Google Scholar]
- 5.Poortmans PM, Weltens C, Fortpied C, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21:1602–1610. [DOI] [PubMed] [Google Scholar]
- 6.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015;373:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109:650–657. [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 10.Ho AY, Ballangrud A, Li G, et al. Long-Term Pulmonary Outcomes of a Feasibility Study of Inverse-Planned, Multibeam Intensity Modulated Radiation Therapy in Node-Positive Breast Cancer Patients Receiving Regional Nodal Irradiation. Int J Radiat Oncol Biol Phys. 2019;103:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. [DOI] [PubMed] [Google Scholar]
- 12.Dumane VA, Saksornchai K, Zhou Y, Hong L, Powell S, Ho AY. Reduction in low-dose to normal tissue with the addition of deep inspiration breath hold (DIBH) to volumetric modulated arc therapy (VMAT) in breast cancer patients with implant reconstruction receiving regional nodal irradiation. Radiat Oncol. 2018;13:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen LB, Offersen BV, Dano H, et al. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol. 2016;34:314–320. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Kumar S, Lawrence A, Das MK, Kumar S. Ipsilateral lung dose volume parameters predict radiation pneumonitis in addition to classical dose volume parameters in locally advanced NSCLC treated with combined modality therapy. South Asian J Cancer. 2014;3:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Lee J, Ha B, Lee R, Lee KJ, Suh HS. Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer. Radiat Oncol J. 2011;29:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blom Goldman U, Anderson M, Wennberg B, Lind P. Radiation pneumonitis and pulmonary function with lung dose-volume constraints in breast cancer irradiation. J Radiother Pract. 2014;13:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind PA, Wennberg B, Gagliardi G, Fornander T. Pulmonary complications following different radiotherapy techniques for breast cancer, and the association to irradiated lung volume and dose. Breast Cancer Res Treat. 2001;68:199–210. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol. 1985;4:9–14. [DOI] [PubMed] [Google Scholar]
- 20.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Fu Y, Zhu B, Zhang B, Wang J. Pneumonitis Induced by Immune Checkpoint Inhibitors: From Clinical Data to Translational Investigation. Front Oncol. 2020;10:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaverdian N, Beattie J, Thor M, et al. Safety of thoracic radiotherapy in patients with prior immune-related adverse events from immune checkpoint inhibitors. Ann Oncol. 2020;31:1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 24.Dumane VA, Bakst R, Green S. Dose to organs in the supraclavicular region when covering the Internal Mammary Nodes (IMNs) in breast cancer patients: A comparison of Volumetric Modulated Arc Therapy (VMAT) versus 3D and VMAT. PLoS One. 2018;13:e0205770. [DOI] [PMC free article] [PubMed] [Google Scholar]