Abstract
Objective:
Despite their effectiveness in preventing the transmission of HIV among people who inject drugs (PWID), syringe services programs (SSPs) in many settings are hampered by social and political opposition. We aimed to estimate the impact of closure and temporary interruption of SSP on the HIV epidemic in a rural US setting.
Methods:
Using an agent-based model calibrated to observed surveillance data, we simulated HIV risk behaviors and transmission in adult populations who inject and do not inject drugs in Scott County, Indiana. We projected HIV incidence and prevalence between 2020-2025 for scenarios with permanent closure, delayed closure (one additional renewal for 24 months before closure), and temporary closure (lasting 12 months) of an SSP in comparison to persistent SSP operation.
Results:
With sustained SSP operation, we projected an incidence rate of 0.15 per 100 person-years among the overall population [95% simulation interval: 0.06-0.28]. Permanently closing the SSP would cause an average of 58.4% increase in the overall incidence rate during 2021-2025, resulting in a higher prevalence of 60.8% [50.9%-70.6%] (18.7% increase) among PWID by 2025. A delayed closure would increase the incidence rate by 38.9%. A temporary closure would cause 12 (35.3%) more infections during 2020-2021.
Conclusions:
Our analysis suggests that temporary interruption and permanent closure of existing SSPs operating in rural US may lead to “rebound” HIV outbreaks among PWID. To reach and sustain HIV epidemic control, it will be necessary to maintain existing and implement new SSPs in combination with other prevention interventions.
Keywords: HIV/AIDS, syringe services program, people who inject drugs, agent-based model
Introduction
Syringe service programs (SSPs) are an effective and cost-effective measure in curbing the transmission of HIV and other bloodborne infections, reducing the risk of overdose, and improving linkage to HIV care among people who inject drugs (PWID) [1-4]. Nonetheless, rural communities in the United States (US) often face unique obstacles for implementing SSPs. Such obstacles frequently include stigmatization of drug use, inadequate access to HIV testing and subsequent care, and limited political and social support [5]. As of August 1st, 2019, SSPs were operating in 41 states and the District of Columbia, concentrated primarily in coastal urban areas [6].
One of the largest outbreaks of HIV among PWID in the US occurred in Scott County, Indiana in 2015. Out of a fewer than 24,000 residents, 181 persons were diagnosed with HIV infection within a year, corresponding to an incidence rate over 50 times that of the national average [7, 8]. The outbreak raised concerns about vulnerability to rapid HIV transmission in this rural county, which had historically recorded only a total of five HIV diagnoses during 2004 to 2013 [7, 9, 10]. Within a week of the declaration of the public health emergency in April 2015, the Indiana General Assembly authorized the first legal SSP to operate in the state [7]. In addition to anonymous, needs-based syringe exchange, the program also provided integrated and comprehensive harm reduction services. Two modeling studies have examined the dynamics of the Scott County outbreak, concluding that a more rapid public health response could have substantially reduced the total number of HIV infections, particularly if an SSP program had been established prior to the outbreak [11, 12].
In Indiana, the bill authorizing the operation of SSPs in nine of its counties (including Scott County) is subject to a two-year sunset clause. Consequently, every two years the program may be jeopardized due to a lack of social and political support. Given the expansion of HIV incidence during the outbreak and the potential shortage of sterile injection equipment, questions remain regarding the impact of the SSP closure [13, 14].
While there is a paucity of literature for the repercussions of SSP interruptions on HIV incidence, the limited evidence available has demonstrated impacts that would be expected to exacerbate risks of HIV acquisition. Following the closure of SSP in Windham, Connecticut, there were significant rises in the percentage of PWID receiving syringes from an unreliable source, the frequency of reusing syringes, and the percentage of PWID who reported syringe sharing [15]. Ivsins et al. showed a marked increase in the probability of syringe and needle sharing after the closure of the city’s only fixed-site SSP in Victoria, British Columbia, despite continuous operation through mobile and satellite programs [16]. A recent qualitative study in Kanawha County, West Virginia showed that PWID described more frequently injecting with used syringes and engaging in a range of less safe injection practices after the suspension of an SSP [5].
However, none of these studies have quantified how the closure of SSP might affect the risk for HIV transmission among PWID. As a rural community which has recently experienced an HIV outbreak among PWID and is facing imminent threat of closure of the only SSP in operation [17], Scott County, Indiana provides an ideal context for assessing the potential impact of SSP closure where transmission network data are readily available as a result of prior intensive outbreak investigations [7, 9]. Using an agent-based model (ABM), we aimed to examine the impact of the permanent or temporary closure of a rural SSP on the HIV epidemic among PWID in Scott County, Indiana, and to determine to what extent there could be a rebound outbreak after the termination of an SSP in a setting with elevated HIV prevalence among PWID.
Methods
Study design
We adapted and extended the TITAN (Treatment of Infection and Transmission in Agent-Based Networks) modeling framework (https://www.titanmodel.org), a previously published ABM [18], to simulate the transmission of HIV among adult population in Scott County, Indiana and examine the impact of SSPs [12]. Our model simulated the adult population of 14,573 individuals (aged 18-64) in Scott County between 2010 and 2025 at monthly time steps. We accounted for the heterogeneity of HIV infection risk and access to HIV health services, including HIV testing, antiretroviral therapy (ART) and SSP utilization [9]. With this design, we estimated both the preventative benefits of SSP among PWID as well as the potential spillover effects that SSP might have on HIV transmission among people who do not inject drugs. Model parameters were informed by local evidence from Scott County where possible, as well as by estimates from existing literature [12]. More details concerning model structure, parameter values, uncertainty ranges and process of model calibration can be found in the Appendix.
Model description
The age and gender distributions of the 14,573 adult individuals in our model were parameterized by demographic data from the US Census for Scott County [19]. The prevalence of injection drug use within the population (3.1% [range: 1.9%-4.2%]) was based on a capture-recapture study in a similar rural setting in Cabell County, West Virginia [20], and was adjusted to the defined age range. Without considering the transitions between PWID and non-PWID, we considered a dynamic population where agents left the model at death or by aging out at 65 years and were replaced by new agents with the same initial characteristics to maintain a constant size of PWID and non-PWID populations.
We modeled dynamic networks in which HIV transmission can occur through sexual contact and syringe sharing. To construct the syringe sharing network and population heterogeneity, the annual target number of injection partners and the number of inject acts per month for each PWID agent was randomly drawn from predefined distributions (Appendix Table A1). Prior to SSP implementation, syringe sharing occurred in 32.1% [17.6%-46.6%] of all injection acts [21]. Similarly, we also created a concurrent network for sexual transmission assuming all agents were able to engage in sexual behavior. Each agent was assigned an annual target number of sex partners and a number of condomless sexual acts per month. The base per-act probabilities of transmission associated with different injection and sexual risk behaviors were derived from a meta-analysis [22]. To improve computational efficiency, only behaviors occurring within serodiscordant dyads (sexual, injection, or both) were explicitly simulated, and depending on the type of dyad, the corresponding transmission probabilities were used to estimate the risk of transmission.
Following seroconversion, agents first experienced a two-month acute stage of HIV infection with five-fold per-act probability of transmission [23], followed by a chronic stage and a monthly probability of progression to AIDS based on the use of ART and achievement of viral suppression [24]. Infected agents were modeled with a monthly probability to be diagnosed via HIV testing, use ART, and achieve viral suppression. HIV-uninfected agents and HIV-infected agents unaware of their infection status had a yearly probability of HIV testing of 20.2% [17.8%-22.6%] [25]. Upon diagnosis, an agent initiated antiretroviral treatment in the next time step with a probability of 65% and achieved viral suppression with a probability of 91.5%, according to the statewide HIV care continuum data reported by the Indiana State Department of Health [26]. Here we did not explicitly model treatment dropout but assumed constant coverage of treatment and viral suppression. We also assumed no change to injection behavior but 27% fewer condomless sex acts following HIV diagnosis [27].
Following model initialization, a single agent engaging in injection drug use was selected to spontaneously seroconvert, representing the initial source of HIV within the dynamic network. Consistent with our prior study, the selected agent was required to have at least four injection partners, according to the possible range of injection contacts reported by the phylogenetic cluster within the inferred transmission network in the Scott County outbreak [9, 12]. A full list of model parameters is presented in Appendix Table A1.
Syringe Services Program
Based on the Scott County Health Department data [28], the SSP was implemented in the model in April 2015 and was assumed to scale up linearly to reach a total of 290 PWID in March 2016 and maintain at this level thereafter. PWID enrolled in the SSP were less likely to share syringes with their partners (2.0% [0.4%-4.8%] versus 32.1% [17.6%-46.6%] among those not enrolled in the SSP) [21]. Meanwhile, we also accounted for additional HIV testing performed alongside SSP services (19.5% per month) among PWID agents enrolled in the SSP based on local data [28], which was disabled when SSP was closed. The determination of SSP enrollments and HIV testing alongside SSP services are detailed in Appendix p10. To replicate the contact tracing investigation conducted in Scott County during the outbreak, we modeled contact tracing testing practices in 2015 (disabled in 2016 and after) where the monthly probability of HIV screening increased to 87.3% [84.2%-90.2%] in the time-step immediately following the diagnosis of an agent’s partner [7].
Modeling scenarios
Before assessing the impact of SSP closure, we ran the baseline model over a 10-year time period that comprised a period with few to no HIV transmissions (2011-2014), a rapidly growing HIV outbreak in 2015, a period of declining incidence following SSP implementation (2015-2016), and a period of returning to low new HIV diagnoses and stable HIV prevalent cases (2017-2020).
We modeled and compared four distinct scenarios to estimate the impact of SSP closure on the rebound of HIV infection over an evaluation period between 2020 and 2025: (1) persistent operation, representing a scenario where an SSP will persist at current service levels throughout 2021-2025; (2) permanent closure, representing a scenario where SSP is fully suspended from 2021-2025, during which injection behaviors for the current SSP enrollees will resume as in the pre-SSP period; (3) delayed closure, representing a scenario where the SSP will be extended for an additional two years (2021-2022) and then be closed for the remaining period (2023-2025); (4) temporary closure, representing temporary SSP service interruption caused by a major incident for the period between April 2020 to March 2021 [29], with conditions returning to pre-interruption levels thereafter.
The primary outcome was HIV incidence, expressed as the cumulative number of HIV infections over the five-year study period (2021-2025), annual number of new HIV infections per 100,000 adult population, and the average incidence rate per 100 person-years during the evaluation period. We also estimated HIV prevalence at the end of 2025 as our secondary outcome. These outcomes were calculated for the overall population and stratified by engagement in injection drug use.
Model calibration and sensitivity analysis
We calibrated our model using data from the Indiana State Department of Health on the number of new HIV diagnoses and number of persons living with HIV/AIDS in each year between 2015-2018 in Scott County [30]. We applied a random calibration approach to repeatedly sample from estimated uncertainty ranges for relevant model parameters (N=11) and then fit against the selected calibration targets. We used a Latin hypercube sampling method [31] to draw 100,000 random parameters sets to inform the simulation model and derive 1,000 calibrated subsets providing the best fit (measured by the mean percentage deviation). We performed evaluation analyses over these calibrated subsets that also captured parameter uncertainty, and we presented the mean value over these sets with 95% simulation intervals (SIs) where appropriate. More details regarding model calibration can be found in Appendix p14.
We performed additional sensitivity analysis on a scenario representing the SSP service interruption (15% reduction in SSP enrollment and 56% reduction in HIV testing probability) due to the COVID-19 pandemic using local health department data collected before and during 2020 (Appendix p15). To examine the relative contributions of SSP and contact tracing to the HIV incidence reduction in 2015 and after, we constructed three counterfactual scenarios: (1) no contact tracing; (2) no SSP; and (3) neither SSP nor contact tracing. The differences in the number of new HIV infections during 2015 to 2019 between scenario (1) and (3) and between scenario (2) and (3) were used to decompose the independent effects of SSP and contact tracing, respectively. Given the existing high HIV prevalence among PWID due to the previous outbreak (48% in 2019 based on our estimates), in another sensitivity analysis, we lowered the prevalence of HIV by 85% (to match the national average HIV prevalence among PWID [32]) in 2019 to estimate the potential impact of SSP closure for settings with lower baseline HIV prevalence.
Results
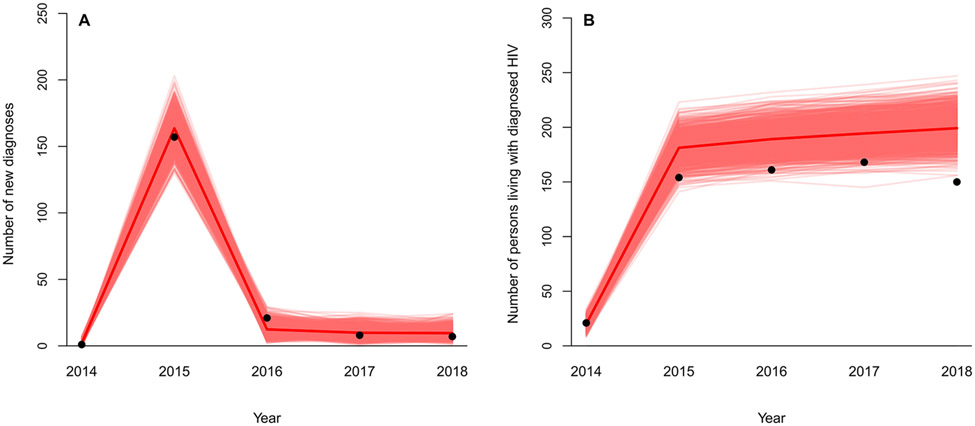
Following calibration, our model demonstrated excellent fit to the selected targets of new HIV diagnoses and prevalent cases (Fig. 1). The steep decline in new diagnoses from 2015 to 2016 after SSP implementation was captured. We estimated that contact tracing alone accounted for a slightly larger percentage in incident HIV cases averted (70% versus 63% attributable to SSP) in 2015, while the SSP played a greater role in long-term epidemic control (82%-84%) in 2016 and after (Appendix Fig A3).
Fig. 1. Model calibration to HIV new diagnoses and prevalent diagnosed cases in Scott County, Indiana.
Dots represent observed data in Scott County, IN between 2014 and 2018.[30] Red lines represent simulated model estimates following calibration from 1,000 runs (the solid red line represents the mean). Panel A: number of new HIV diagnoses. Panel B: number of persons living with diagnosed HIV.
With persistent SSP operation, our model projected a total of 109 incident infections [95% SI: 46-205] among the entire adult population between 2021-2025 (Fig. 2), resulting in an incidence rate of 0.15 [0.06-0.28] per 100 person-years and a prevalence of 1.7% [1.4%-2.2%] in 2025. Stratified analysis also demonstrated a relatively low level of HIV incidence among both PWID and people who do not inject drugs, with 37 [16-70] and 71 [17-163] infections over five years with persistent SSP operation, respectively. The incidence rate also remained relatively low at 2.08 [1.12-3.18] and 0.10 [0.02-0.23] per 100 person-years, respectively. Prevalence among PWID remained at 51.2% [41.0%-61.8%] in 2025, similar to that in prior years (2017-2020, Fig. 3).
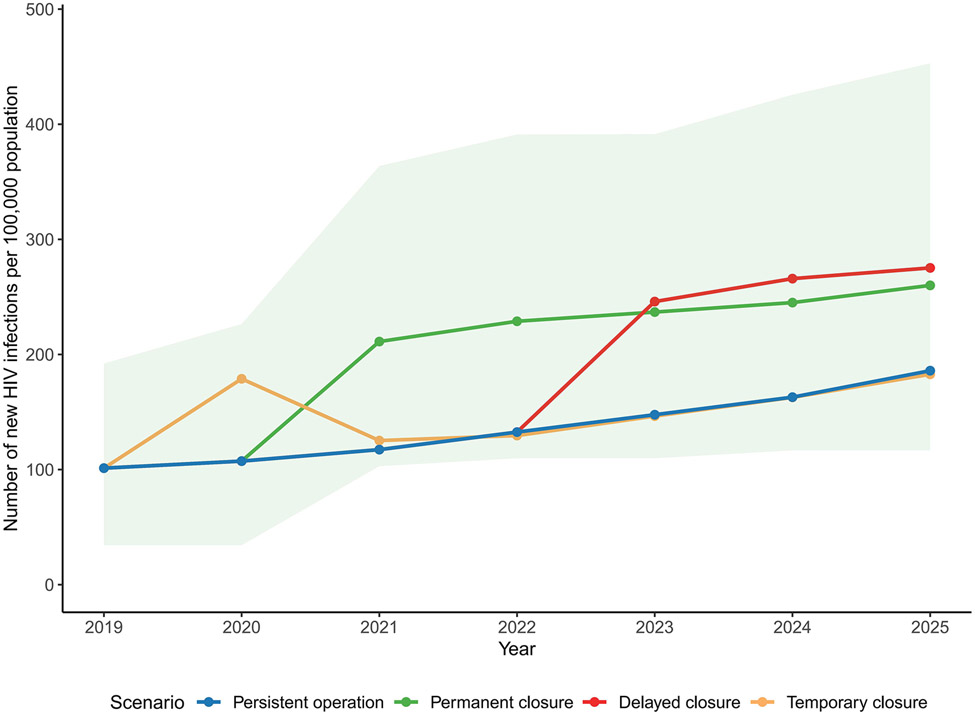
Fig. 2. Number of new HIV infections per 100,000 population under different modeling scenarios representing syringe service program (SSP) closure in a rural US county.
Solid lines represent the mean of estimated number of new HIV infections per 100,000 population. Green shade represents 95% simulation interval of estimated incidence under SSP permanent closure scenario. Other simulations intervals are not shown but were of similar magnitude.
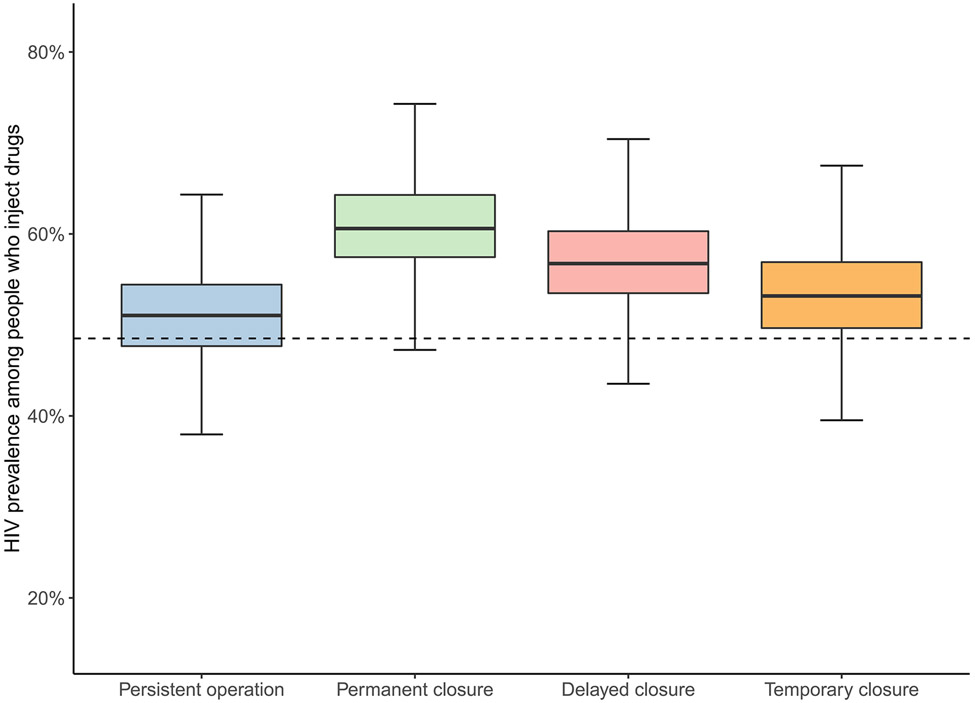
Fig. 3. Estimated HIV prevalence among people who inject drugs under different scenarios representing syringe service program closure at the end of 2025.
Dashed line represents baseline estimated HIV prevalence among people who inject drugs at the end of 2019
Suspending the SSP permanently would result in an average increase of 63.5 HIV infections over a five-year period (2021-2025) (a 58.4% increase) compared with persistent operation. This corresponded to a substantially higher incidence rate of 0.24 [0.14-0.37] infections per 100 person-years between 2021-2025 among the total population. We estimated an average of 60.2 more infections (a 161% increase) among PWID, elevating the incidence rate to 5.51 [3.88-7.21] per 100 person-years. Among PWID, HIV prevalence increased by an average of 18.6 percent to 60.8% [50.9%-70.6%].
If SSP closure is delayed by two years (i.e., the program is renewed once), we estimated 42.4 additional HIV infections (a 38.9% increase) compared with sustaining SSP operation for 5 years (2021-2025). This could help maintain the overall incidence rate to a level of 0.21 [0.11-0.34] per 100 person-years and a prevalence of 56.9% [47.0%-67.7%] among PWID in 2025, although still higher than the persistent operation scenario. In this scenario, an average of 39.9 more infections (107% increase) were estimated for PWID over a five-year period.
In modeling a temporary SSP closure during April 2020 and March 2021, we estimated that this interruption would lead to a temporary spike in HIV incidence, but new infections soon returned to its original trend when SSP was resumed. An average of 11.6 (35.3%) more infections were estimated during 2020-2021 in comparison with no interruption and persistent SSP operation. Under the estimated impact of COVID-19 interruptions, we estimated a relatively more moderate spike in HIV incidence, with an average of 0.9 (2.6%) more infections during 2020-2021.
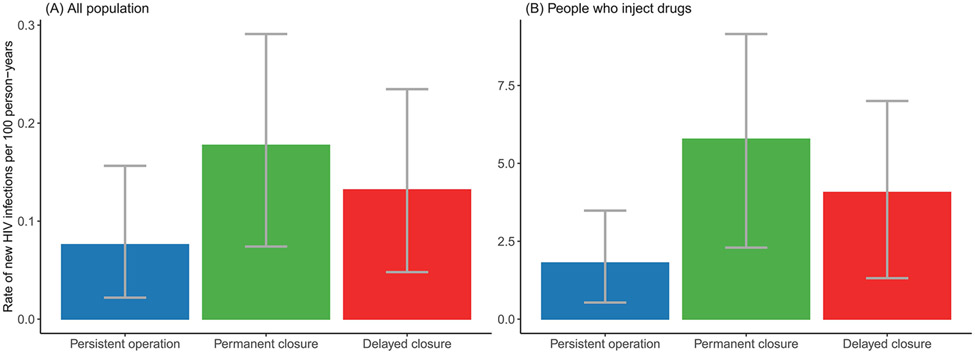
Alternatively, when reducing baseline HIV prevalence by 85%, we found permanent and delayed closure would lead to greater relative increase in overall incidence rate per 100 person-years by an average of 133.4% and 73.6% (219.8% and 125.2% among PWID) during the five-year evaluation period, respectively (Fig. 4, Appendix Fig. A4).
Fig. 4. Estimated rate of new HIV infections per 100 person-years during 2021 to 2025 under different syringe service program closure scenarios if HIV prevalence is reduced by 85%: (A) among all population; (B) among people who inject drugs.
Discussion
This analysis presents the first study, to our knowledge, to quantitively examine the impact of SSP closure on HIV incidence using a modeling approach. In a rural American setting that had previously experienced an HIV outbreak among PWID, our modeling results suggest that closing an existing SSP would likely lead to a rebound HIV outbreak, with a 2.6-fold increase in incident infections among PWID in five years relative to SSP sustainment. The potential impact of SSP closure was found to be substantially greater for other settings with lower baseline HIV prevalence (in which a larger share of the population is susceptible to HIV infection). Although delaying SSP closure with another renewal was found to reduce the size of the rebound, sustaining SSP operation and associated health services will be imperative to maintain long-term epidemic control.
The ongoing COVID-19 pandemic is placing an increased burden on the health care system while creating unprecedented challenges in delivering routine medical services, including SSPs [29, 33]. In this analysis, we simulated scenarios with temporary interruption to SSP provision due to the current COVID-19 pandemic and other major incidents, and our results show that even such a short-term service disruption may cause a spike in HIV incidence. The economic fallout caused by a “big event” such as the COVID-19 pandemic may also create vulnerability by increasing in the size of the population of PWID and proportions of people engaged in high-risk behaviors [34]. If so, the consequences of SSP closure would be expected to be more severe, although we did not explicitly account for these possible impacts on the risk levels in Scott County for purposes of this study. Nevertheless, our conservative estimates underline the importance of sustaining the continuous operation of SSPs during public health emergencies.
The public health impact of SSP closure as assessed in this study should not be considered exhaustive. First, prior evidence has shown that the implementation of SSP in Scott County was also associated with declining risks for other bloodborne infections such as hepatitis C virus (HCV) [13, 21]. Second, the SSP in Scott County is integrated with many other health services, including overdose prevention, referral to substance use disorder treatment, and referrals to ART and preexposure prophylaxis (PrEP) [21]. Benefits associated with the scale-up of these services would also be forfeited if the SSP is suspended. Therefore, our estimates for the impact of SSP closure should be considered conservative since we did not explicitly account for changes in these services following SSP closure.
Despite scientific consensus on its public health benefits [4], SSPs face substantial barriers to ongoing operation in rural areas in the US, including laws prohibiting syringe exchange or syringe possession, lack of federal funding or community support, and stigma [7]. In a comprehensive analysis after the outbreak in Indiana, the CDC identified 220 other counties, overwhelmingly rural, that were vulnerable to rapid HIV/HCV transmission. Prior phylogenetic analyses following the outbreak revealed large networks of syringe sharing among PWID in Scott County, despite it being sparsely populated. Such networks are likely present in other rural communities [7, 35]. Therefore, proactively implementing SSPs will be critical to prevent the spread of HIV into rural networks of PWID. To maximize preventative benefits or limit future HIV outbreaks, SSP implementation should also be supported by other public health interventions as a comprehensive prevention portfolio, such as expanded HIV/HCV screening, ART and PrEP use, and medications for opioid use disorder [36]. A more recent HIV outbreak among PWID in Kanawha County, West Virginia [37], which occurred soon after an SSP was closed, may provide real-world evidence for the importance of continuous operation of SSPs. This modeling framework, although developed for the setting of Scott County, can be adapted to other vulnerable communities at risk of HIV outbreaks and be applied to answer various policy questions beyond the impact of SSP closure, such as how implementing new or expanding existing interventions may help mitigate the devastating impact if an SSP is forced to close.
Several limitations may be present for this analysis. First, we did not explicitly simulate durations of injection drug use or transitions to non-injecting states; however, given the relatively short time-frame of the evaluation, we expect the overall effect of this model simplification to be small. We also used a stochastic process to assign behavior parameters upon creating each agent to capture the heterogeneity in their risk behaviors. Second, we assumed no change to injection behavior (except for the probability of syringe sharing and HIV testing rate among SSP clients) following SSP closure, given no evidence was available. Third, we characterized the uptake and population-level effectiveness of the SSP by modeling a fixed number of PWID enrolled in the SSP, yet detailed data regarding program engagement and retention were unavailable. Lastly, findings of our additional sensitivity analysis on reduced HIV prevalence may not be generalizable to all other settings or representative of the US average since the actual impact of SSP closure is also dependent on underlying risk behaviors of the population, access to HIV services, and uptake of existing SSPs.
Our analysis demonstrates the necessity of maintaining existing SSPs as part of a comprehensive prevention package for PWID in rural American settings, particularly those with elevated HIV prevalence. When sociopolitical forces subvert these programs, the most vulnerable suffer. To reach the goals set by the ‘Ending the HIV Epidemic’ initiative [38], it will be necessary to overcome the social and structural barriers that are challenging the implementation and sustained operation of SSPs and other harm reduction strategies in rural settings in US.
Supplementary Material
Acknowledgement
We acknowledge Lisa Webber, Non-Medical Case Manager of the Scott County Health Department, for providing local data for SSP enrollment and the impact of COVID-19 on SSP service interruption. XZ and BDLM conceptualized the study. XZ wrote the first draft of the article. XZ and SEB conducted analyses. WCG assisted with analyses and contributed to manuscript development. WCG, SEB, MNL, SG, APG, SRF, BN and BDLM helped to interpret results and critically revise the manuscript. BDLM secured funding for the study. All authors approved the final draft.
Conflicts of Interest and Source of Funding
This study was funded by the National Institutes of Health (grant number DP2DA040236 to B.D.L.M and grant number R25MH083620 to W.C.G.). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
All authors declare no competing interests.
References
- 1.Ruiz MS, O’Rourke A, Allen ST. Impact evaluation of a policy intervention for HIV prevention in Washington, DC. AIDS and behavior 2016; 20(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen TQ, Weir BW, Des Jarlais DC, Pinkerton SD, Holtgrave DR. Syringe exchange in the United States: a national level economic evaluation of hypothetical increases in investment. AIDS and behavior 2014; 18(11):2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarlais DCD, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas—United States, 2013. Morbidity and mortality weekly report 2015; 64(48):1337–1341. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. International journal of epidemiology 2014; 43(1):235–248. [DOI] [PubMed] [Google Scholar]
- 5.Allen ST, Grieb SM, O’Rourke A, Yoder R, Planchet E, White RH, et al. Understanding the public health consequences of suspending a rural syringe services program: a qualitative study of the experiences of people who inject drugs. Harm reduction journal 2019; 16(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Viña MH, Prood NE, Herpolsheimer A, Waimberg J, Burris S. State laws governing syringe services programs and participant syringe possession, 2014–2019. Public Health Reports 2020; 135(1_suppl):128S–137S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. New England Journal of Medicine 2016; 375(3):229–239. [DOI] [PubMed] [Google Scholar]
- 8.Linley L, Johnson AS, Song R, Wu B, Hu S, Gant Z, et al. Estimated HIV incidence and prevalence in the United States, 2014–2018. 2020. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- 9.Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, et al. Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J Infect Dis 2017; 216(9):1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strathdee SA, Beyrer C. Threading the needle—how to stop the HIV outbreak in rural Indiana. New England Journal of Medicine 2015; 373(5):397–399. [DOI] [PubMed] [Google Scholar]
- 11.Gonsalves GS, Crawford FW. Dynamics of the HIV outbreak and response in Scott County, IN, USA, 2011–15: a modelling study. The Lancet HIV 2018; 5(10):e569–e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedel WC, King MR, Lurie MN, Galea S, Townsend JP, Galvani AP, et al. Implementation of syringe services programs to prevent rapid human immunodeficiency virus transmission in rural counties in the United States: a modeling study. Clinical Infectious Diseases 2020; 70(6):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser H, Zibbell J, Hoerger T, Hariri S, Vellozzi C, Martin NK, et al. Scaling-up HCV prevention and treatment interventions in rural United States—model projections for tackling an increasing epidemic. Addiction 2018; 113(1):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerner AM, Fauci AS. Opioid injection in rural areas of the United States: A potential obstacle to ending the HIV epidemic. Jama 2019; 322(11):1041–1042. [DOI] [PubMed] [Google Scholar]
- 15.Broadhead RS, Van Hulst Y, Heckathorn DD. The impact of a needle exchange's closure. Public Health Reports 1999; 114(5):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivsins A, Chow C, Macdonald S, Stockwell T, Vallance K, Marsh DC, et al. An examination of injection drug use trends in Victoria and Vancouver, BC after the closure of Victoria's only fixed-site needle and syringe programme. International journal of drug policy 2012; 23(4):338–340. [DOI] [PubMed] [Google Scholar]
- 17.Legan M. Indiana Needle Exchange That Helped Contain A Historic HIV Outbreak To Be Shut Down. 2021, Available at: https://www.npr.org/sections/health-shots/2021/06/01/1001278712/indiana-needle-exchange-that-helped-contain-an-hiv-outbreak-may-be-forced-to-clo. [Google Scholar]
- 18.Bessey S, McGrath M, King M. marshall-lab/TITAN: v1.2.4. Zenodo. 2020, Available at: https://zenodo.org/record/4266540#.YV95JtrMIdU. [Google Scholar]
- 19.United States Census Bureau. American Community Survey: Demongraphic and Housing Estiamtes. Available at: https://data.census.gov/cedsci/table?d=ACS%205-Year%20Estimates%20Data%20Profiles&table=DP05&tid=ACSDP5Y2015.DP05&g=0400000US18_0500000US18143. [Google Scholar]
- 20.Allen ST, O’Rourke A, White RH, Schneider KE, Kilkenny M, Sherman SG. Estimating the number of people who inject drugs in a rural county in Appalachia. American journal of public health 2019; 109(3):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel MR, Foote C, Duwve J, Chapman E, Combs B, Fry A, et al. Reduction of injection-related risk behaviors after emergency implementation of a syringe services program during an HIV outbreak. JAIDS Journal of Acquired Immune Deficiency Syndromes 2018; 77(4):373–382. [DOI] [PubMed] [Google Scholar]
- 22.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS (London, England) 2014; 28(10):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellan SE, Dushoff J, Galvani AP, Meyers LA. Reassessment of HIV-1 acute phase infectivity: accounting for heterogeneity and study design with simulated cohorts. PLoS medicine 2015; 12(3):e1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Ledergerber B, Tilling K, Weber R, Sendi P, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. The Lancet 2005; 366(9483):378–384. [DOI] [PubMed] [Google Scholar]
- 25.Henderson ER, Subramaniam DS, Chen J. Rural-urban differences in human immunodeficiency virus testing among US adults: findings from the behavioral risk factor surveillance system. Sexually transmitted diseases 2018; 45(12):808–812. [DOI] [PubMed] [Google Scholar]
- 26.Indiana State Department of Health. The State of Indiana Integrated Prevention and Care Plan 2017-2021. 2016, Available at: https://www.in.gov/isdh/23725.htm.
- 27.Doyle JS, Degenhardt L, Pedrana AE, McBryde ES, Guy RJ, Stoové MA, et al. Effects of HIV antiretroviral therapy on sexual and injecting risk-taking behavior: a systematic review and meta-analysis. Clinical infectious diseases 2014; 59(10):1483–1494. [DOI] [PubMed] [Google Scholar]
- 28.Scott County Health Department. Scott County Syringe Service Program (SSP), 2015-2021. 2021, Available at: https://www.scottcounty.in.gov/egov/documents/1620403692_24795.pdf; 2021(Dec 20).
- 29.Bartholomew TS, Nakamura N, Metsch LR, Tookes HE. Syringe Services Program (SSP) Operational Changes During the COVID-19 Global Outbreak. The International Journal on Drug Policy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indiana State Department of Health. HIV/STD/Viral Hepatitis Semi-Annual Reports. In.
- 31.Helton JC, Davis FJ. Latin hypercube sampling and the propagation of uncertainty in analyses of complex systems. Reliability Engineering & System Safety 2003; 81(1):23–69. [Google Scholar]
- 32.Strathdee SA, Kuo I, El-Bassel N, Hodder S, Smith LR, Springer SA. Preventing HIV outbreaks among people who inject drugs in the United States: plus ça change, plus ça même chose. Aids 2020; 34(14):1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glick SN, Prohaska SM, LaKosky PA, Juarez AM, Corcorran MA, Des Jarlais DC. The Impact of COVID-19 on Syringe Services Programs in the United States. AIDS and behavior 2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman SR, Sandoval M, Mateu-Gelabert P, Rossi D, Gwadz M, Dombrowski K, et al. Theory, measurement and hard times: some issues for HIV/AIDS research. AIDS and behavior 2013; 17(6):1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young AM, Rudolph AE, Havens JR. Network-based research on rural opioid use: an overview of methods and lessons learned. Current HIV/AIDS reports 2018; 15(2):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. The Lancet Infectious diseases 2013; 13(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuehn BM. Restrictive Policies Threaten Efforts to Stop 2 West Virginia HIV Outbreaks. JAMA 2021; 325(22):2238–2240. [DOI] [PubMed] [Google Scholar]
- 38.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. Jama 2019; 321(9):844–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.