Abstract
Spaceflight-associated neuro-ocular syndrome (SANS) has been well documented in astronauts both during and after long-duration spaceflight and is characterized by the development of optic disc edema, globe flattening, choroidal folds, and hyperopic refractive error shifts. The exact mechanisms underlying these ophthalmic abnormalities remain unclear. New findings regarding spaceflight-associated alterations in cerebrospinal fluid spaces, specifically perivascular spaces, may shed more light on the pathophysiology of SANS. The preliminary results of a recent brain magnetic resonance imaging study show that perivascular spaces enlarge under prolonged microgravity conditions, and that the amount of fluid in perivascular spaces is linked to SANS. The exact pathophysiological mechanisms underlying enlargement of perivascular spaces in space crews are currently unclear. Here, we speculate that the dilation of perivascular spaces observed in long-duration space travelers may result from impaired cerebral venous outflow and compromised cerebrospinal fluid resorption, leading to obstruction of glymphatic perivenous outflow and increased periarterial cerebrospinal fluid inflow, respectively. Further, we provide a possible explanation for how dilated perivascular spaces can be associated with SANS. Given that enlarged perivascular spaces in space crews may be a marker of altered venous hemodynamics and reduced cerebrospinal fluid outflow, at the level of the optic nerve and eye, these disturbances may contribute to SANS. If confirmed by further studies, brain glymphatic dysfunction in space crews could potentially be considered a risk factor for the development of neurodegenerative diseases, such as Alzheimer’s disease. Furthermore, long-duration exposure to microgravity might contribute to SANS through dysregulation of the ocular glymphatic system. If prolonged spaceflight exposure causes disruption of the glymphatic systems, this might affect the ability to conduct future exploration missions, for example, to Mars. The considerations outlined in the present paper further stress the crucial need to develop effective long-term countermeasures to mitigate SANS-related physiologic changes during long-duration spaceflight.
Keywords: astronaut, cerebrospinal fluid, glymphatic system, optic disc edema, perivascular spaces, spaceflight-associated neuro-ocular syndrome
Introduction
Spaceflight-associated neuro-ocular syndrome (SANS), previously known as visual impairment and intracranial pressure syndrome, has been well documented in astronauts both during and after long-duration spaceflight (LDSF).1,2 The neuro-ocular findings of LDSF include optic disc edema (ODE), globe flattening (GF), choroidal folds (CF), cotton-wool spots (CWS), hyperopic refractive error shifts, and retinal nerve fiber layer thickening.1,2 An increased understanding of factors contributing to this syndrome is currently a top priority for space agencies. At present, the precise mechanisms underlying these ophthalmic abnormalities are still unclear. New findings regarding spaceflight-associated alterations in cerebrospinal fluid (CSF) spaces, specifically perivascular spaces (PVS), may shed more light on the pathophysiology of SANS.3,4 The preliminary results of a recent brain magnetic resonance imaging (MRI) study show that PVS enlarge under prolonged microgravity conditions, and that the amount of fluid in PVS is linked to SANS.3,4 Dilated PVS have previously been associated with aging and cerebral small vessel disease.5–8 PVS are key anatomical components of the recently discovered glymphatic system, and some studies have speculated that dilated PVS may be a manifestation of dysfunction of this perivascular pathway.7–9 Therefore, disturbances of the glymphatic pathway, reflected by concomitant PVS enlargement, may play an important role in the development of SANS. In the present paper, we speculate on possible mechanisms that may be involved in the reported structural changes in PVS. Our hypothesis is that the dilation of PVS observed in the brains of long-duration space travelers may result from altered venous hemodynamics and reduced CSF outflow, leading to obstruction of glymphatic perivenous outflow and increased periarterial CSF inflow, respectively. We further provide a possible explanation for how dilated PVS can be associated with SANS. Further elucidation of the mechanisms underlying PVS enlargement may provide more insight into the pathophysiology of SANS as well as into the physiology of cerebral fluid drainage in astronauts.4
Discussion
Previously Proposed SANS Hypotheses
In a 2011 report, the National Aeronautics and Space Administration (NASA) first documented neuro-ophthalmic findings in seven astronauts following LDSF on the International Space Station (ISS).1 These unique findings included ODE, GF, CF and hyperopic shifts in refraction. Although the precise mechanism causing these neuro-ophthalmic anomalies is unclear and may result from multiple sources, we will very briefly describe several hypotheses that have been offered to account for them. First, SANS findings were initially hypothesized to occur as a direct result of increased intracranial pressure (ICP) similar to terrestrial idiopathic intracranial hypertension (IIH).1 The classical view of ICP regulation proposes that CSF is largely produced in the choroid plexus and drainage is largely dependent upon the pressure difference between the relatively high-pressure cranial CSF and the lower pressure cervical venous system. It is hypothesized that cephalad fluid shifts, caused by exposure to microgravity, lead to cervical venous congestion and impairment of CSF outflow as well as cerebral venous congestion both of which could cause a rise in ICP.1 Second, it has been proposed that SANS findings may result from localized events occurring at the level of the orbital optic nerve sheath (ONS) with or without a rise in ICP. This hypothesis proposes that the tightly confined and densely septated cul-de-sac-like anatomic connection between the intracranial and orbital subarachnoid space (SAS) may create a fragile flow equilibrium that may be impacted by the cephalad fluid shifts that occur during prolonged microgravity exposure.1 Thus, CSF within the SAS of the orbit may become partially or completely sequestered producing a type of ONS compartment syndrome that, coupled with a possible one-way ball valve like mechanism, could produce elevated pressures within the ONS.
Strangman and colleagues10 further propose that because of the incompressible nature of CSF and brain tissue, enhanced vascular pulsatility in large vessels passing through the SAS can be directly transmitted from the cranial CSF compartment to the orbital SAS. Thus, pulsatility waves within the cranial SAS may travel down the orbital ONSs and potentially result in a water hammer effect on the posterior ocular structures as well as perhaps augmenting the one-way ball valve mechanism leading to a further rise in ONS pressure. Furthermore, Shinojima and colleagues,11 using a mathematical model of the ONS, proposed that the optic nerve and globe are retracted posteriorly because of brain upward shift and the consequent uplifting of the optic chiasm during LDSF. They suggested that this posterior pull on the optic nerve and globe compresses the CSF within the ONS resulting in pressure elevation, expansion, and subsequent ODE. Wåhlin and colleagues,12 in a 2020 MRI study, documented that LDSF caused lengthening of the optic nerve and a concurrent anterior movement of the optic nerve head which again suggested that SANS is caused by an altered pressure difference between the brain and the eye and a resulting forward push to the globe. It has also been suggested that the magnitude of SANS changes may be at least partly determined by the elastic properties of brain ventricles and ONS such that the brain and ONS may act as buffers that impede the development of SANS.13,14 Defects in the vitamin B12-dependent 1-carbon pathways, elevated carbon dioxide levels, high-sodium diets and resistive exercise may also play a role in this process.15–18 Lawley and colleagues19 suggested that the complete removal of gravity may not pathologically elevate ICP but may prevent the normal terrestrial lowering of ICP when the subject is upright. Finally, we believe that SANS-related changes may also be explained by dysregulation of the ocular glymphatic system.
The Brain Glymphatic System
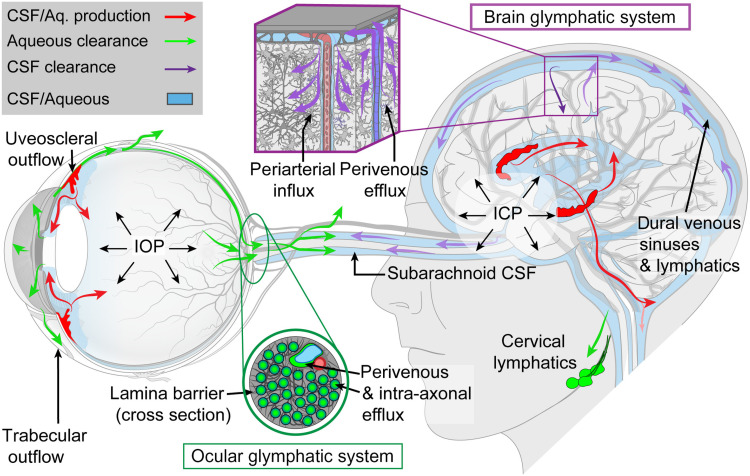
PVS are part of the recently discovered “glymphatic system”, which is a brain-wide, glia-dependent perivascular network that plays an important role in the elimination of interstitial metabolic waste products, including amyloid-β (Aβ), from the brain.20 From the SAS, CSF enters the periarterial spaces to exchange with interstitial fluid (ISF) within the parenchyma (Figure 1).20,21 The CSF/ISF mixture is subsequently cleared along perivenous spaces, and drains out of the brain via meningeal and cervical lymphatic vessels as well as along cranial nerves (Figure 1).20,21 CSF within the SAS is driven into the perivascular space by the combined effects of arterial pulsatility, respiration, slow vasomotion, and CSF pressure gradients.22 The subsequent transport of CSF into the brain parenchyma from the perivascular space is facilitated by aquaporin-4 (AQP4) water channels.22 These channels are expressed in a highly polarized manner in astrocytic vascular endfeet that create an outer wall around the cerebral vasculature.22 This arrangement creates the PVS that differs from the rest of the neuropil by its low resistance towards fluid flow. It was originally reported that deletion of AQP4 reduces post-stroke edema.23 However, this finding is controversial as later studies found that deletion of AQP4 aggravated tissue swelling.24 AQP4 loses its polarized expression in astrocytic endfeet in pathological conditions, such as increased ICP, traumatic brain injury, neuroinflammation and even in aging. The loss of AQP4 polarization towards the vascular endfeet of astrocytes has in turn been linked to a reduction in glymphatic fluid flow in a number of neurological disorders.25 The glymphatic system has been described in both animal models and humans.20,26 An intriguing finding regarding the glymphatic system is that this pathway is primarily active during sleep and anesthesia, while largely disengaged during wakefulness.27 Furthermore, it has been shown that several factors, such as body mass index, time of day and genetics, affect PVS in the brain under physiological conditions.28 Glymphatic system dysfunction has been associated with various neurodegenerative disorders, such as Alzheimer’s disease (AD).29,30
Figure 1.
The brain and ocular glymphatic systems. Macroscopic overview of the brain and ocular glymphatic systems, emphasizing the role played by pressure gradients, hydrostatic barriers, and lymphatic drainage, shown in the context of known pathways for aqueous humour and cerebrospinal fluid (CSF) efflux. ICP, intracranial pressure; IOP, intraocular pressure. Figure reproduced from Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance – new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021;99(2):e283–e284..21
Impact of Long-Duration Spaceflight on Perivascular Spaces
Barisano and colleagues3,4 assessed the spaceflight-associated alterations detectable in multiple CSF spaces, including PVS, of NASA astronauts, Roscosmos (ROS) cosmonauts, and European Space Agency (ESA) astronauts, and investigated their relation to SANS. The authors performed a comparative, joint analysis of brain MRI scans in 23 NASA astronauts, 13 ROS cosmonauts, and 4 ESA astronauts before and within the first two weeks after LDSF on the ISS. They used the same analysis pipeline, thereby eliminating investigators bias. An additional follow-up scan was performed in 4 ESA astronauts and 10 ROS cosmonauts seven months after return to Earth. Brain MRI data from 13 age- and education-matched male volunteers acquired with a time interval similar to the preflight-postflight and preflight-follow-up (n=8) intervals and from 7 NASA astronauts acquired before and after missions of short duration in the Space Shuttle Program were used as controls. PVS volume increased after LDSF, with significantly greater percent enlargements in NASA astronauts compared to ROS cosmonauts (25.5% and 12.4%, respectively, p=0.006).4 The NASA Shuttle group showed no significant pre- to postflight increases in PVS volume.3 PVS enlargement was positively correlated with upward brain shift (rs=0.33, p=0.04). Further, mission duration was positively correlated with changes in PVS volume (rs=0.32, p=0.04) and negatively correlated with volume changes of the SAS at the vertex (rs=−0.53, p<0.001). Both pre- and postflight PVS volumes were significantly higher in NASA astronauts who developed SANS compared with NASA astronauts who did not. Additionally, the NASA astronauts who developed SANS had a significantly larger percentage increase in PVS than the ROS group (p=0.02). SANS data for ROS cosmonauts and ESA astronauts were not available in the abstract of the oral presentation of the study results.4 The authors concluded that long-duration missions to the ISS are associated with PVS volume increases in space crews, while not equally affecting NASA astronauts and ROS cosmonauts, which could be related to several factors including differences in countermeasures and/or training protocols. Furthermore, the amount of fluid in PVS was linked to SANS.
Proposed Mechanisms Underlying Enlargement of Perivascular Spaces in Space Crews
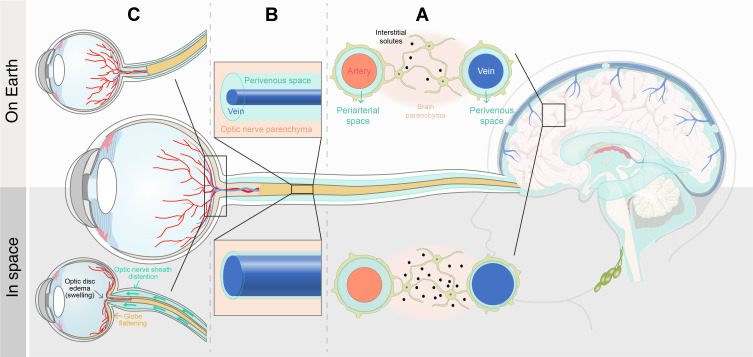
The exact pathophysiological mechanisms underlying PVS enlargement in space crews are currently unclear. Here, we propose two main potential mechanisms: increased periarterial CSF inflow and obstruction of glymphatic perivenous outflow. We hypothesize that altered venous hemodynamics during spaceflight may cause disruption of the glymphatic system. A prospective cohort study by Marshall-Goebel and colleagues31 analysed internal jugular vein flow in 11 ISS crew members participating in LDSF missions. In that study, the authors reported stagnation and even reversal of the internal jugular vein cerebral venous outflow in 6 of 11 astronauts, suggesting that spaceflight may cause venous congestion and even some degree of retrograde blood flow from the central veins through the internal jugular vein into the brain. We speculate that such stagnant or retrograde flow patterns may lead to restriction of normal glymphatic flow. Indeed, due to impaired cerebral venous outflow, blood volume in the cerebral veins increases and veins become more distended, which in turn, may lead to closure of the perivenous spaces resulting in diminished glymphatic clearance of metabolites from the brain (Figure 2A). This would result in higher concentrations of Aβ and other neuronally derived metabolites lingering in the ISF rather than being transported into the CSF or lymphatic vessels (Figure 2A). Because of the obstruction of glymphatic perivenous outflow, CSF-ISF stagnates in the interstitium. As the CSF-ISF load is elevated, the fluid flux is blocked, and the interstitial pressure increases. As a result, the CSF, which is driven into the periarterial spaces by a combination of arterial pulsatility and CSF pressure gradients, stagnates and accumulates at the periarterial site with simultaneous dilation of the periarterial spaces (Figure 2A). Furthermore, spaceflight-induced headward fluid shifts, and a possible increase in ICP, may alter the distribution of CSF to different outflow pathways. Indeed, given that the venous and lymphatic CSF outflow pathways may be compromised due to the microgravity-induced cephalad fluid shifts,1 and given the chronic, mildly elevated ICP in space,2 the question is whether these factors could further drive CSF into the periarterial spaces, causing further fluid accumulation and enlargement of periarterial spaces.
Figure 2.
Proposed mechanisms underlying dilated perivascular spaces in space crews in relation to spaceflight-associated neuro-ocular syndrome. (A) Blood volume in cerebral veins may increase as a result of a microgravity-induced decrease in cerebral venous outflow, which in turn, may lead to closure of the perivenous spaces, thereby compromising the glymphatic CSF-ISF outflow from the interstitial tissue, and resulting in diminished glymphatic clearance of metabolites from the brain. Consequently, periarterial spaces may dilate due to CSF accumulation. A reduced CSF resorption and mildly elevated ICP in microgravity may further drive CSF into the periarterial spaces, causing further fluid accumulation and PVS dilation. (B) If the cephalad venous fluid shift during spaceflight is associated with increased resistance to venous drainage from the eye, veins in the optic nerve may distend and perivenous spaces may close, resulting in diminished ocular glymphatic outflow. (C) Compromised CSF resorption and impaired cerebral venous outflow, reflected by dilated PVS, may contribute to globe flattening and optic disc edema through overflow of CSF along the optic nerve sheath and dysregulation of the ocular glymphatic system, respectively.
It should be noted that other mechanisms could also contribute to the spaceflight-associated enlargement of PVS. In microgravity, in addition to the cephalad fluid shifts secondary to the removal of the normal gravity-induced hydrostatic pressure gradients, tissues no longer have weight, and tissue compressive forces are lost.32 Tissue weight exerts a compressive force on the outside of vessels reducing their ability to dilate.33 The elimination of these forces could help explain the enlargement of PVS in space crews.
With regard to the hypothesis proposed in the present article, it would be interesting to know whether the dilated PVS observed in space crews were arteriolar or venular. If the majority of enlarged PVS were periarteriolar rather than perivenular, this would support our hypothesis. The postulated increase in periarterial CSF inflow and obstruction of glymphatic perivenous outflow may become increasingly important under conditions of prolonged microgravity exposure, where the body fluids redistribute cranially and where the ICP may be chronic, mildly elevated. This could explain the reported correlation between mission duration and changes in PVS volume. If confirmed by further studies, glymphatic dysfunction and enlargement of PVS could increase the astronaut’s risk of developing neurodegenerative diseases such as AD. Furthermore, as discussed below, glymphatic dysregulation due to weightlessness could predispose to the development of SANS-associated ODE.
Retrograde Glymphatic Cerebrospinal Fluid Inflow into the Optic Nerve
The optic nerve, a white matter tract of the central nervous system, is ensheathed in all three meningeal layers and surrounded by CSF in the SAS.34,35 Normally, the SAS of the optic nerve is contiguous with the SAS of the brain, allowing CSF circulation between the intracranial and optic nerve SASs. Recent studies have provided evidence for CSF entry into the optic nerve via a glymphatic pathway.36–38 After fluorescent tracer injection into the CSF of live mice, Mathieu and colleagues36 found that CSF enters the optic nerve through spaces immediately surrounding blood vessels, bordered by AQP4-positive astrocytic endfeet. Wang and colleagues37 confirmed and extended these findings by demonstrating that CSF tracer influx occurs along the periarterial and pericapillary spaces in the optic nerve by use of reporter mice in which arteries can be distinguished by their abundance of smooth muscle cells compared with veins. Jacobsen and colleagues38 performed an MRI study of human visual pathway structures following intrathecal administration of gadobutrol serving as a CSF tracer. CSF tracer enrichment was observed within the optic nerve, optic chiasm, optic tract, and primary visual cortex. In the vitreous body, only a nonsignificant signal increase was evident. Based on their observations, the authors hypothesized that a glymphatic system exists within the human visual pathway. Interestingly, substantial CSF tracer enrichment was observed in the retrobulbar part of the optic nerve, as compared to the middle and posterior sections.38 As this finding corresponds to the entrance of the central retinal artery, the authors suggested that, analogous to the main entry routes of CSF tracer molecules into the human brain parenchyma along large artery trunks at the brain surface, this area could function as a major periarterial route facilitating the entry of CSF tracer from the SAS into the optic nerve interstitium.
Anterograde Ocular Glymphatic Transport Blockage Under Microgravity Conditions
Given that ODE is one of the key clinical features of SANS, the question is whether, similarly to the brain, ocular fluids are cleared from the eye, specifically from the optic nerve head, towards perivenous drainage pathways in the optic nerve. A recent study by Wang and colleagues37 seems to support this possibility. The authors identified a novel “ocular glymphatic clearance system” for removal of fluid and metabolites from the intraocular space via the proximal optic nerve in rodents (Figure 1). Intraocularly administered tracers (eg, Aβ) entered retinal ganglion cell axons and the perivenous spaces of the retina and optic nerve head before being cleared by the anterograde glymphatic pathway. After traversing the lamina barrier, intra-axonal tracers were further cleared via perivenous spaces in the optic nerve and subsequently drained through dural lymphatics around the optic nerve into the cervical lymph nodes. The authors further demonstrated that efflux via this pathway is driven by the high-to-low pressure gradient between the intraocular pressure (IOP) and ICP.37 A rise in ICP was shown to inhibit the ocular glymphatic outflow, whereas an increase in IOP accelerated outflow of small tracers delivered into the vitreous body. These findings also suggest that a rise in orbital CSF pressure, which is assumed to occur in microgravity due to mildly elevated ICP and/or CSF compartmentalization,39 would directly inhibit ocular glymphatic outflow. This lends support to the hypothesis, originally proposed by Wostyn and colleagues,40–44 that blockage of anterograde ocular glymphatic transport may contribute towards ODE development in SANS. In addition, ocular glymphatic outflow could also be reduced as a result of the cephalad venous fluid shift during spaceflight, causing the veins in the optic nerve to distend, which in turn may close the perivenous spaces (Figure 2B). The resulting impairment of ocular fluid efflux along perivenous spaces, in combination with an increased capillary filtration at the optic nerve head as proposed by Macias and colleagues,45 might further contribute to the ODE seen in astronauts.
Dilated Perivascular Spaces and SANS
Given that PVS volumes were significantly greater in NASA astronauts who developed SANS than those who did not,3,4 the question is how the accumulation of fluid in PVS could be linked to SANS. As discussed above, we speculate that the enlargement of PVS observed in the brains of long-duration space travelers may result from impaired cerebral venous outflow and compromised CSF resorption, leading to obstruction of glymphatic perivenous outflow and increased periarterial CSF inflow, respectively. Thus, enlarged PVS in space crews may be a marker of altered venous hemodynamics and reduced CSF outflow. At the level of the optic nerve and eye, these disturbances may contribute to SANS through several mechanisms. First, CSF outflow impairment may result in overflow of CSF along the sheath of the optic nerve, leading to a rise in orbital CSF pressure within the ONS and anteriorly directed forces on the posterior globe (Figure 2C). This may result in flattening of the posterior globe and hyperopic refractive error shifts because of the shortened axial length. The relatively high frequency of CF in astronauts compared to patients with terrestrial IIH suggests that these changes may occur as a result of CSF accumulation within the orbital ONS with locally elevated ONS pressures, with or without a rise in CSF pressure in the entire CSF system, in conjunction with choroidal expansion that occurs during LDSF. This choroidal expansion may lead to a more rigid choroid that is more susceptible to mechanical folding from posterior GF.46 Moreover, elevation of ICP may inhibit ocular glymphatic outflow, as reported by Wang and colleagues,37 possibly causing optic disc swelling (Figure 2C), which demonstrates a pattern of retinal nerve fiber layer thickening on optical coherence tomography (OCT). Second, assuming that the decreased cerebral venous outflow during spaceflight31 may correlate with an increased resistance to venous drainage from the eye, the latter may result in obstruction of perivenous outflow along the veins in the optic nerve (Figure 2B). This may further compromise the glymphatic efflux of ocular fluid into the optic nerve, leading to fluid stasis within the optic nerve head, further contributing to ODE (Figure 2C). We hypothesize that CWS may occur largely as a result of space radiation.47 Additionally, reduced glymphatic ocular outflow may result in the accumulation of toxic metabolites causing axonal damage and obstruction of axoplasmic transport. These sequestered metabolites could also cause or at least lower the threshold for CWS formation. Based on the above considerations, we conclude that dilated PVS observed in the brains of long-duration space travelers might indirectly reflect the astronaut’s risk of developing SANS.
SANS Study Techniques Past, Present and Future
Given our proposal that glymphatic dysregulation may contribute to SANS, it is reasonable to ask how we might further evaluate this hypothesis. Pre- and post-LDSF MRI studies have been performed on Earth and the results of these studies have proven extremely valuable. However, due to the large size of the MRI device, this methodology can only be performed at established locations on Earth. Thus, the MRI device cannot be used during LDSF on the ISS, when anatomical changes may be taking place. Similarly, although ICP measurements were performed using Ommaya technology during parabolic flight,19 no ICP measurements have been performed during LDSF. Lumbar puncture opening pressures have also been performed on Earth following LDSF but due to technical and safety constraints they have not, thus far, been done during actual LDSF. Thus, the post-mission time delay associated with both procedures has limited their usefulness in the study of SANS and specifically the possible impact of the glymphatic system.
Inflight studies performed on the ISS have included IOP measurements, auto-refractions, retinal photography, retinal and optic nerve head OCT as well as ocular and orbital ultrasound. Each of these studies has provided valuable information regarding inflight changes in the retina, optic disc, eye contour and orbital ONS. However, none of these procedures can examine crucial changes that may occur in the human brain glymphatic system during LDSF. Thus, a non-invasive, precise brain examination technique is needed that has the capacity to be flown on the ISS for long-term functional brain monitoring. One technique, near-infrared spectroscopy (NIRS), was recently described by Myllylä and colleagues.48 This technique utilizes NIRS to non-invasively measure long-term water dynamics in the human brain. Specifically, this device measures fluctuations in water content, especially in the CSF, that are assumed to correlate the dynamics of glymphatic circulation. Since this is a portable, non-invasive device, this technique could potentially be utilized to measure the glymphatic circulation of the human brain during LDSF on the ISS.
Given the limited number of astronauts available for study during LDSF on the ISS, and the lack of practical on-board large instrumentation, we will briefly discuss the potential use of terrestrial SANS analog studies to evaluate glymphatic function. Such analog studies include dry immersion and head-down tilt (HDT) studies. For many years, HDT studies have been utilized as a terrestrial spaceflight analog and have provided researchers with valuable data regarding ocular and cerebral changes that may occur during prolonged exposure to microgravity. Marshall-Goebel and colleagues31 quantified the internal jugular vein cross-sectional area using ultrasound, measured the pressure and characterized the Doppler flow velocity profile to describe cerebral venous outflow during spaceflight compared to various postures on Earth to include 15-degree HDT. Strangman and colleagues,10 in the SPACECOT study, used NIRS in subjects exposed to 12-degree HDT to examine cerebral blood volume pulsatility. These studies exemplify the potential for using HDT studies in conjunction with ultrasound, Doppler, NIRS and other devices for the study of the glymphatic circulation in a controlled terrestrial laboratory environment.
Countermeasures
Finally, we will briefly discuss several long-term countermeasures to impaired glymphatic flow and SANS that could be considered for future space travelers. First, although the required structural engineering and cost may be prohibitive, an effective theoretical countermeasure would involve construction of a large, spinning, centrifuge-like portion of a space station that could provide Earth-like G forces that would counter the cephalad fluid shift and PVS enlargement. Second, thigh cuffs have been used by Russian cosmonauts to limit the cephalad fluid shift induced by spaceflight. Third, breathing against inspiratory resistance has also been hypothesized as a countermeasure to increased ICP and central venous pressure (CVP). Recently, a study by Hansen and colleagues,49 using Ommaya reservoirs to measure ICP and peripherally inserted central catheters to measure CVP, showed that acute thigh cuff inflation had little effect on ICP or CVP. In contrast, impedance threshold breathing acutely reduced ICP via a reduction in CVP. Fourth, lower body negative pressure (LBNP) sequesters fluid volume, mainly venous blood, in the lower extremities thus helping to mitigate the cephalad fluid shift during LDSF. It is used by cosmonauts on the ISS toward the end of their mission as a proposed countermeasure for postflight orthostatic intolerance. As noted previously, cosmonauts have less of an increase in PVS expansion during LDSF compared to astronauts who do not routinely use LBNP. It is possible that the difference in PVS dilation between NASA astronauts and cosmonauts may occur at least partly as a result of the use of LBNP countermeasures in cosmonauts. This suggests that such countermeasures may prove to reduce the risk of PVS volume increases in space crews. Furthermore, an ISS study by Marshall-Goebel and colleagues31 demonstrated that LBNP can restore vascular physiology to a state similar to that seen in upright and supine positions on Earth. The authors suggested that, although further research is warranted, LBNP may be a promising countermeasure to blood flow stasis and thrombosis associated with spaceflight.31
Conclusions
In this review, we have described the findings of a recent study demonstrating enlargement of PVS in the brains of long-duration space travelers and showing that the amount of fluid in PVS is linked to SANS. We have speculated about possible mechanisms underlying PVS enlargement, and provided a possible explanation for how dilated PVS can be associated with SANS. According to our hypothesis, the PVS enlargement observed in space crews may result from impaired cerebral venous outflow and compromised cerebrospinal fluid resorption, leading to obstruction of glymphatic perivenous outflow and increased periarterial CSF inflow, respectively. From this point of view, enlarged PVS may be a marker of altered venous hemodynamics and reduced CSF outflow. This may clarify the reported link between PVS volume and SANS. A reduced CSF resorption in microgravity may lead to GF and hyperopic refractive error shifts as a result of excess CSF accumulation along the ONS compressing the posterior globe. Moreover, elevated ICP and an increase in resistance to venous drainage from the eye may compromise the glymphatic efflux path along the perivenous space in the optic nerve. The resulting impairment of ocular fluid efflux, in combination with an increased capillary filtration at the optic nerve head, might contribute to the optic disc swelling seen in astronauts.
Dysfunction of glymphatic pathways in astronauts may have long-term consequences for brain health, and may potentially increase the risk of developing neurodegenerative diseases such as AD, though this requires a further understanding of the role of the glymphatic system on these conditions in both ground-based and spaceflight settings. If prolonged spaceflight exposure causes disruption of the glymphatic systems, this might affect the ability to conduct future exploration missions, for example to Mars. Obviously, future research is required to provide additional insight regarding the role of glymphatic dysregulation due to weightlessness. This could be challenging given that the small number of subjects that participate in LDSF poses an inevitable obstacle to studying spaceflight-associated changes. The considerations outlined in the present paper further stress the crucial need to develop effective long-term countermeasures to mitigate SANS-related physiologic changes during long-duration spaceflight.
Acknowledgments
We thank Dan Xue for providing us with Figure 2.
Disclosure
Dr Peter Wostyn reports personal fees from P&X Medical, outside the submitted work; In addition, Dr Peter Wostyn is the inventor of patent US 10,980,437 B2 issued. The authors report no other conflicts of interest in this work.
References
- 1.Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058–2069. [DOI] [PubMed] [Google Scholar]
- 2.Lee AG, Mader TH, Gibson CR, Tarver W. Space flight-associated neuro-ocular syndrome. JAMA Ophthalmol. 2017;135(9):992–994. [DOI] [PubMed] [Google Scholar]
- 3.Barisano G, Sepehrband F, Tomilovskaya E, et al. Spaceflight-associated changes in the perivascular spaces of astronauts and cosmonauts. Paper (paper ID: 66383) presented at: 72nd International Astronautical Congress 2021; 2021; Dubai, United Arab Emirates. [Google Scholar]
- 4.Wuyts FL Technical keynotes: 8.1 Seemingly different impact of spaceflight on NASA, ESA and Roscosmos space crew regarding the perivascular space. Abstract of the Global Space Exploration Conference 2021; St. Petersburg, Russia. [Google Scholar]
- 5.Duperron MG, Tzourio C, Sargurupremraj M, et al. Burden of dilated perivascular spaces, an emerging marker of cerebral small vessel disease, is highly heritable. Stroke. 2018;49(2):282–287. [DOI] [PubMed] [Google Scholar]
- 6.Bouvy WH, Zwanenburg JJM, Reinink R, et al. Perivascular spaces on 7 Tesla brain MRI are related to markers of small vessel disease but not to age or cardiovascular risk factors. J Cereb Blood Flow Metab. 2016;36(10):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114(11):1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding J, Sigurðsson S, Jónsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility-Reykjavik study. JAMA Neurol. 2017;74(9):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue Y, Liu N, Zhang M, Ren X, Tang J, Fu J. Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res Bull. 2020;161:78–83. [DOI] [PubMed] [Google Scholar]
- 10.Strangman GE, Zhang Q, Marshall-Goebel K, et al. Increased cerebral blood volume pulsatility during head-down tilt with elevated carbon dioxide: the SPACECOT Study. J Appl Physiol. 2017;123(1):62–70. [DOI] [PubMed] [Google Scholar]
- 11.Shinojima A, Kakeya I, Tada S. Association of space flight with problems of the brain and eyes. JAMA Ophthalmol. 2018;136(9):1075–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wåhlin A, Holmlund P, Fellows AM, Malm J, Buckey JC, Eklund A. Optic nerve length before and after spaceflight. Ophthalmology. 2021;128(2):309–316. [DOI] [PubMed] [Google Scholar]
- 13.Wostyn P, Mader TH, Gibson CR, et al. The possible role of elastic properties of the brain and optic nerve sheath in the development of spaceflight-associated neuro-ocular syndrome. AJNR Am J Neuroradiol. 2020;41(3):E14–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wostyn P, Mader TH, Gibson CR, De Deyn PP. The buffering capacity of the brain and optic nerve against spaceflight-associated neuro-ocular syndrome. Proc Natl Acad Sci U S A. 2019;116(32):15770–15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwart SR, Gibson CR, Gregory JF, et al. Astronaut ophthalmic syndrome. FASEB J. 2017;31(9):3746–3756. [DOI] [PubMed] [Google Scholar]
- 16.Zwart SR, Laurie SS, Chen JJ, et al. Association of genetics and B vitamin status with the magnitude of optic disc edema during 30-day strict head-down tilt bed rest. JAMA Ophthalmol. 2019;137(10):1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurie SS, Vizzeri G, Taibbi G, et al. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep. 2017;5(11):e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurie SS, Christian K, Kysar J, et al. Unchanged cerebrovascular CO2 reactivity and hypercapnic ventilatory response during strict head-down tilt bed rest in a mild hypercapnic environment. J Physiol. 2020;598(12):2491–2505. [DOI] [PubMed] [Google Scholar]
- 19.Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance – new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021;99(2):e283–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: a Beginner’s Guide. Neurochem Res. 2015;40(12):2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. [DOI] [PubMed] [Google Scholar]
- 24.Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci. 2015;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringstad G, Valnes LM, Dale AM, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018;3(13):e121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barisano G, Sheikh-Bahaei N, Law M, Toga AW, Sepehrband F. Body mass index, time of day and genetics affect perivascular spaces in the white matter. J Cereb Blood Flow Metab. 2021;41(7):1563–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-Alps) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–178. [DOI] [PubMed] [Google Scholar]
- 31.Marshall-Goebel K, Laurie SS, Alferova IV, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2(11):e1915011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckey JC, Phillips SD, Anderson AP, et al. Microgravity-induced ocular changes are related to body weight. Am J Physiol Regul Integr Comp Physiol. 2018;315(3):R496–R499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan M, Phillips SD, Archambault-Leger V, et al. Proposed mechanism for reduced jugular vein flow in microgravity. Physiol Rep. 2021;9(8):e14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London A, Benhar I, Schwartz M. The retina as a window to the brain - from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. [DOI] [PubMed] [Google Scholar]
- 35.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129(Pt 4):1027–1030. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci. 2017;58(11):4784–4791. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Lou N, Eberhardt A, et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12(536):eaaw3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen HH, Ringstad G, Jørstad ØK, Moe MC, Sandell T, Eide PK. The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci. 2019;60(7):2773–2780. [DOI] [PubMed] [Google Scholar]
- 39.Wostyn P, Gibson CR, Mader TH. The Odyssey of the ocular and cerebrospinal fluids during a mission to Mars: the “ocular glymphatic system” under pressure. Eye. 2022;36(4):686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wostyn P, De Winne F, Stern C, Mader TH, Gibson CR, De Deyn PP. Potential involvement of the ocular glymphatic system in optic disc edema in astronauts. Aerosp Med Hum Perform. 2020;91(12):975–977. [DOI] [PubMed] [Google Scholar]
- 41.Wostyn P, De Deyn PP. Intracranial pressure-induced optic nerve sheath response as a predictive biomarker for optic disc edema in astronauts. Biomark Med. 2017;11(11):1003–1008. [DOI] [PubMed] [Google Scholar]
- 42.Wostyn P, Killer HE, De Deyn PP. Why a one-way ticket to Mars may result in a one-way directional glymphatic flow to the eye. J Neuroophthalmol. 2017;37(4):462–463. [DOI] [PubMed] [Google Scholar]
- 43.Wostyn P, De Deyn PP. The “ocular glymphatic system”: an important missing piece in the puzzle of optic disc edema in astronauts? Invest Ophthalmol Vis Sci. 2018;59(5):2090–2091. [DOI] [PubMed] [Google Scholar]
- 44.Wostyn P, De Winne F, Stern C, De Deyn PP. Dilated prelaminar paravascular spaces as a possible mechanism for optic disc edema in astronauts. Aerosp Med Hum Perform. 2018;89(12):1089–1091. [DOI] [PubMed] [Google Scholar]
- 45.Macias BR, Patel NB, Gibson CR, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol. 2020;138(5):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mader TH, Gibson CR, Lee AG. Choroidal folds in astronauts. Invest Ophthalmol Vis Sci. 2016;57(2):592. [DOI] [PubMed] [Google Scholar]
- 47.Mader TH, Gibson CR, Otto CA, et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017;37(2):133–139. [DOI] [PubMed] [Google Scholar]
- 48.Myllylä T, Harju M, Korhonen V, Bykov A, Kiviniemi V, Meglinski I. Assessment of the dynamics of human glymphatic system by near-infrared spectroscopy. J Biophotonics. 2018;11(8):e201700123. [DOI] [PubMed] [Google Scholar]
- 49.Hansen AB, Lawley JS, Rickards CA, et al. Reducing intracranial pressure by reducing central venous pressure: assessment of potential countermeasures to spaceflight-associated neuro-ocular syndrome. J Appl Physiol. 2021;130(2):283–289. [DOI] [PubMed] [Google Scholar]