Abstract
We describe outcomes with posttransplantation cyclophosphamide and non-myeloablative conditioning based allogeneic blood or marrow transplantation for myelofibrosis using matched or mismatched, family or unrelated donors. The conditioning regimen consisted of fludarabine, cyclophosphamide and total body irradiation. Forty-two patients, with a median age of 63 years, were included, of whom 19% had intermediate-1, 60% had intermediate-2, and 21% had high-risk DIPSS-plus disease, and 60% had atleast one high-risk somatic mutation. Over 90% patients engrafted neutrophils at a median of 19.5 days and 7% had graft failure. At 1 and 3-years, respectively, the overall survival was 65% and 60%, relapse-free survival was 65% and 31%, relapse was 5% and 40%, and non-relapse mortality was 30% and 30%. Acute graft versus host disease grade 3–4 was noted in 17% at 1 year and chronic graft versus host disease requiring systemic therapy in 12% patients. Spleen size ≥ 17 cm or prior splenectomy was associated with inferior relapse-free survival (HR 3.50, 95% CI 1.18–10.37, P=0.02) and higher relapse rate (SDHR not calculable, P=0.01). Age > 60 years (SDHR 0.26, 95% CI: 0.08–0.80, P=0.02) and peripheral blood graft (SDHR 0.34, 95% CI 0.11–0.99, P=0.05) was associated with lower risk of relapse. In our limited sample, the presence of a high-risk mutation was not statistically significantly associated with an inferior outcome although ASXL1 was suggestive of inferior survival (SDHR 2.36. 95% CI 0.85–6.6, P=0.09). Overall, this approach shows comparable outcomes as previously reported and underscores the importance of spleen size in evaluation of transplant candidates.
Keywords: post-transplantation cyclophosphamide, myelofibrosis, non-myeloablative conditioning
Introduction
Growing evidence supports the use of high dose posttransplantation cyclophosphamide (PTCy) to prevent graft versus host disease (GVHD) following allogeneic blood or marrow transplantation (BMT)1–6. PTCy is not toxic to donor stem cells as these are likely quiescent and express high level of the detoxifying enzyme aldehyde dehydrogenase that confers resistance to cyclophosphamide7–9. Additionally, the specific timing of PTCy, preceding calcineurin inhibitors, selectively targets proliferating alloreactive T cells and preserves regulatory T cells9,10. Many reports have now shown that nonmyeloablative HLA-haploidentical donor BMT, with PTCy GVHD prophylaxis is associated with low rates of both acute and chronic GVHD6,11,12. Moreover, relapse rates and survival are similar to those seen in matched donor BMT. Accordingly, haploidentical donor BMT has become a standard approach for patients in need of BMT but lacking a matched donor. PTCy has also been shown to be effective GVHD prophylaxis for non-myeloablative BMT1.
These studies, however, are not informative for the use of non-myeloablative conditioning or PTCy for GVHD prophylaxis in BMT for myelofibrosis, as these patients were not included in most of the above referenced studies. BMT remains the only potential cure in myelofibrosis. However, with a median age above 60, most affected patients are too old to be considered for myeloablative conditioning13. Hence, a non-myeloablative conditioning regimen allows for transplantation in patients with complex comorbidities resulting from the chronic myeloid malignancy or older age. We aim to address this gap in knowledge by describing clinical outcomes of patients who underwent non-myeloablative conditioning PTCy based BMT for myelofibrosis.
Materials and Methods
Patient selection and overall plan
All patients of age ≥ 18 years who underwent non-myeloablative BMT for myelofibrosis between January 1, 2010 and August 1, 2020 using PTCy based GVHD prophylaxis and nonmyeloablative conditioning regimen at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University were included in this analysis. BMT using matched or mismatched related or unrelated donors were included. A diagnosis of myelofibrosis was made using WHO criteria14. Patients who had progressed to acute myeloid leukemia (> 20% blasts in the peripheral blood or bone marrow) at any time prior to BMT were not included. Clinical data were obtained by conducting a retrospective chart review. The study was approved by the Johns Hopkins Institutional Review Board. Primary outcomes of interest included overall survival (OS) and relapse free survival (RFS). Additional outcomes of interest were hematopoietic recovery following BMT, cumulative incidence of relapse, non-relapse mortality (NRM), and acute as well as chronic GVHD.
Treatment regimen and supportive care
The non-myeloablative conditioning regimen consisted of cyclophosphamide 14.5 mg/kg/day intravenously (IV) on days −6 and −5, fludarabine 30 mg/m2/day IV on days −6 through −2 with total body irradiation of 200 or 400 cGy on day −1 delivered as single fraction4,6. On day 0, the donor marrow or peripheral blood graft was infused. Cell dose is capped at 10×106/kg for patients receiving peripheral blood grafts from haploidentical donors. GVHD prophylaxis included administration of PTCy 50 mg/kg IV on days +3 and +4 along with mesna, following which mycophenolate mofetil along with tacrolimus or sirolimus was started on day +5.
Mycophenolate mofetil was stopped at day +35 in all patients. In the absence of GVHD, tacrolimus or sirolimus was continued up to day +180 until around 2018 when our center determined that the duration could be shortened to 60 days15,16. Granulocyte colony stimulating factor was given starting day +5 until absolute neutrophil count was greater than 1 × 109/L for 3 days. Supportive care included antimicrobial prophylaxis against Pneumocystis jirovecii, Candida albicans, and herpes zoster/simplex infections, administered according to the institutional practice guidelines. Supportive care with anti-pyretics and intravenous fluids is also used for management, as appropriate, in patients who develop cytokine release syndrome. No prophylactic therapy is used to prevent cytokine release syndrome. Ultrasonography for spleen size assessment is now routinely done prior to BMT in these patients. In patients in whom imaging was not available, measurement recorded by physical exam was used for analysis and palpable spleen size was added to a baseline normal size of 11 cm.
Definitions
Dynamic International Prognostic Scoring System (DIPSS)-plus scoring was calculated as previously described17. High-risk cyogenetics were defined as +8, −7/7q-, i(17q), −5/5q-, 12p-, inv(3) or 11q23 rearrangement17. High-risk somatic mutations were pathogenic mutations in ASXL1, EZH2, IDH1, IDH2, SRSF2, U2AF1 Q157, TP53 based on published data on myelofibrosis18–20. Mutation-enhanced international prognostic scoring system Version 2.0 (MIPSS70+ v2.0) was calculated using published data21.
Neutrophil engraftment was defined as first of the 3 consecutive days when absolute neutrophil count was ≥0.5 × 109/L and platelet engraftment was first of the 3 consecutive days when platelet count was ≥20 × 109/L, without transfusion support. Complete donor chimerism refers to > 95% donor chimerism. Graft failure was defined as failure to engraft with donor chimerism following BMT, without evidence of relapse22. NRM was defined as death from any cause in the absence of disease relapse. Acute GVHD was graded based on previously published standard criteria23,24. The initial date for time-to-event outcomes was date of BMT. OS was defined from date of transplantation to death from any causes, or censored at the last follow-up date for alive patients. Relapse was defined as recurrence of disease related cytopenias (identified in a setting of lack of donor chimerism or marrow findings and after ruling out infections or medications as the etiology of cytopenias), and/or reappearance of molecular or genetic markers of disease. The event of RFS included relapse and death, whichever occurred first. Alive patients who never relapsed were censored at the last of follow-up date for RFS.
Somatic mutations testing
Most patients underwent molecular mutation testing using 63-gene panel testing established by Johns Hopkins Molecular Pathology laboratory, certified by the Clinical Laboratory Improvement Amendments25. Variants were identified and filtered using institutional algorithm as previously published26. Testing was done on peripheral blood or bone marrow specimens during the disease course or from the pre-BMT sample from archived DNA per institutional protocol.
Statistical analysis
Patient characteristics were summarized via descriptive statistics including median (range) for continuous variables and number (percentage) for categorical variables. Kaplan-Meier method was applied for point estimators of OS and RFS. Cumulative incidence of NRM, GVHD, and relapse were estimated accounting for the corresponding competing events. When estimating the cumulative incidence of relapse, NRM was a competing event and vice versa. The competing events for estimating cumulative incidence of GVHD included graft failure and death event without GVHD/graft failure. Associations between risk factors and outcomes were assessed via Cox proportional hazards model for OS and RFS27 and via Fine and Gray’s proportional subdistribution hazards model for relapse and NRM28. Specifically for spleen size, the nonlinearity association with RFS was evaluated first by constructing restricted cubic spline function of spleen size with three knots placed at 25th, 50th, and 75th percentile. For convenience of interpretability, the spleen size was then categorized based on median estimate and the estimators of log relative hazards by spleen size. Due to limited sample size, the associations were conducted univariately. P-values were two-sided with significant threshold of 0.05 for hypothesis generating. All analyses were conducted in R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient and disease characteristics
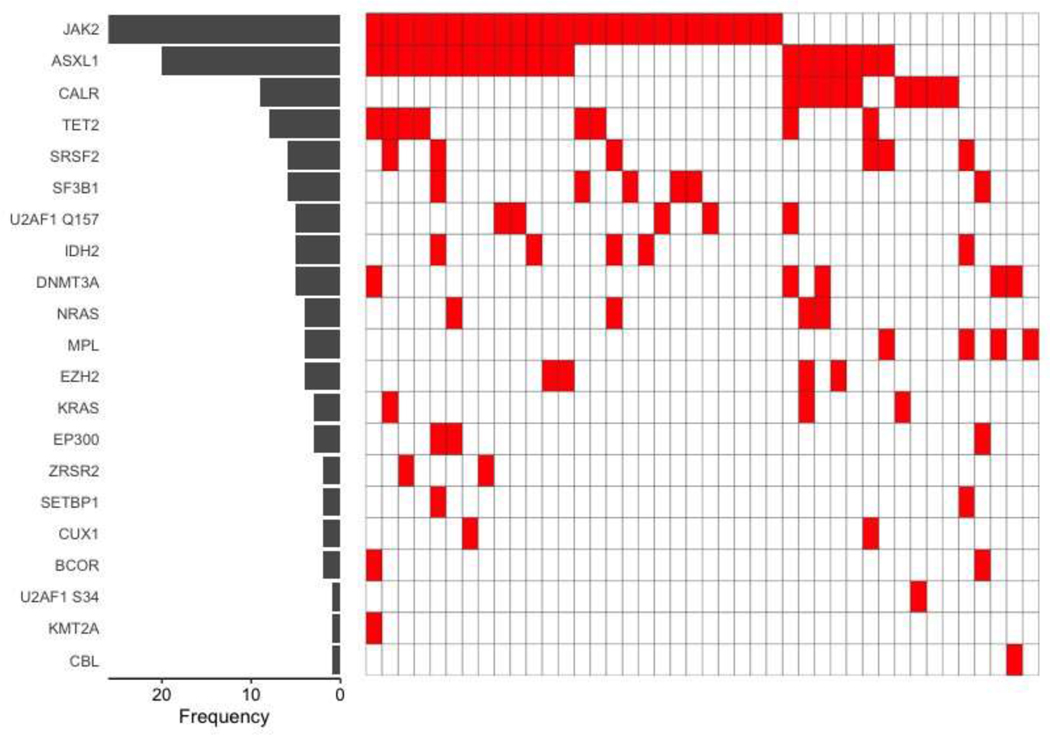
Forty-two consecutive patients underwent BMT with non-myeloablative conditioning and PTCy for myelofibrosis at our center between January 1, 2010 and August 1, 2020. Patient, disease and BMT related details for these patients are shown in Table 1. Twenty-six (62%) were older than 60 years and 15 (36%) were ≥ 65 years of age at the time of BMT. JAK2 mutation was the most common driver mutations in 26 (62%) patients while CALR was the driver mutation in 9 (21%) patients, of which 5 (56%) were type 1 CALR mutation. Somatic mutation testing was obtained on bone marrow sample from pre-BMT marrow biopsy in 20 (48%), prior to initiation of treatment in 14 (33%) and during treatment in 8 (19%) patients. High-risk somatic mutations were identified in 25 (60%) patients and were dominated by the presence of ASXL1 frameshift or nonsense mutations in 20 (48%) patients. Co-occurrence of somatic mutations, including the driver mutations, is shown in Figure 1.
Table 1:
Baseline patient and disease characteristics
| Characteristic | N = 42 |
|---|---|
| Age at BMT -median (range) | 63 (32, 74) |
| Gender (%) | |
| Male | 25 (60) |
| Female | 17 (40) |
| BMT Year (%) | |
| 2010–2016 | 14 (33) |
| 2017–2019 | 28 (67) |
| HCT-CI (%) | |
| 0–1 | 20 (48) |
| ≥ 2 | 22 (52) |
| Diagnosis (%) | |
| Primary myelofibrosis | 19 (45) |
| Post ET/PV | 23 (55) |
| Median time (in months) from original MPN diagnosis to BMT (range) | 78.8 (6.2 – 321.7) |
| Median time (in months) from myelofibrosis diagnosis to BMT (range) | 39.8 (6.2 – 321.7) |
| Driver mutation (%) | |
| JAK2 | 26 (62) |
| CALR Type 1 | 5 (12) |
| Non-Type 1 CALR | 4 (10) |
| MPL | 4 (10) |
| Triple negative | 3 (7) |
| DIPSS-Plus at BMT (%) | |
| Intermediate 1 | 8 (19) |
| Intermediate 2 | 25 (60) |
| High risk | 9 (21) |
| MIPSS70+ v2.0 at BMT (%) | |
| Low | 2 (5) |
| Intermediate | 8 (19) |
| High | 19 (45) |
| Very high | 13 (31) |
| High-risk karyotype (%)* | 7 (17) |
| High-risk somatic mutations** | 25 (60) |
| Number of high-risk mutations: | (n=25) |
| One | 9 (36) |
| Two | 14 (56) |
| Three | 2 (8) |
| ASXL1 mutation present | 20 (48) |
| Prior JAK inhibitor use (%) | 34 (81) |
| Prior splenectomy (%) | 6 (14) |
| Median spleen size at BMT in patients with intact spleen in cm, n=36 (range) | 16.75 (11–28) |
| Donor platform (%) | |
| Haploidentical family | 33 (79) |
| MRD/MUD | 6 (14) |
| MMUD | 3 (7) |
| Graft source (%) | |
| Bone marrow | 7 (17) |
| PB | 35 (83) |
| TBI (%) | |
| 200 cGy | 19 (45) |
| 400 cGy | 23 (55) |
| Median cell dose x 106/kg (range) | 7.85 (3.2 – 14.6) |
| GVHD prophylaxis (%) | |
| Tacrolimus MMF PTCy | 28 (67) |
| Sirolimus MMF PTCy | 14 (33) |
| Recipient CMV serostatus (%) | |
| Negative | 24 (57) |
| Positive | 18 (43) |
High-risk karyotype includes complex karyotype or abnormalities including +8, −7/7q-, i(17q), −5/5q-, 12p-, inv(3) or 11q23 rearrangement.
High-risk non-driver somatic mutations include pathogenic mutations in ASXL1, EZH2, IDH1, IDH2, SRSF2, U2AF1 Q157, TP53
(BMT, blood or marrow transplantation; CMV, cytomegalovirus; DIPSS, dynamic international prognostic scoring system; GVHD, graft versus host disease; HCT-CI, hematopoietic cell transplantation-comorbidity index; MMUD, mismatched unrelated donor; MRD, matched related donor; MIPSS, Mutation Enhanced International Prognostic Scoring System; MUD, matched unrelated donor; PB, peripheral blood; TBI, total body irradiation)
Figure 1:
Mutational landscape of all patients
Thirty-four (81%) patients received prior JAK inhibitor therapy, all of whom had received ruxolitinib while one had also received pacritinib and momelotinib on respective clinical trials. A hypomethylating agent was used along with ruxolitinib in 18 (43%) patients, for immunosuppression and promoting engraftment following BMT or for spleen size reduction as previously published in chronic phase myelofibrosis29. All patients had <5% blasts in the bone marrow by immunohistochemistry at the time of BMT. One patient had 16% blasts on the flow cytometry of the aspirate but was included in the analysis as aspirate was hemodiluted, blasts were <5% on immunohistochemistry on marrow and never reported to be >20%. The dose of total body irradiation was 200cGy in 19(45%) and 400cGy in 22(55%) patients.
Engraftment and chimerism
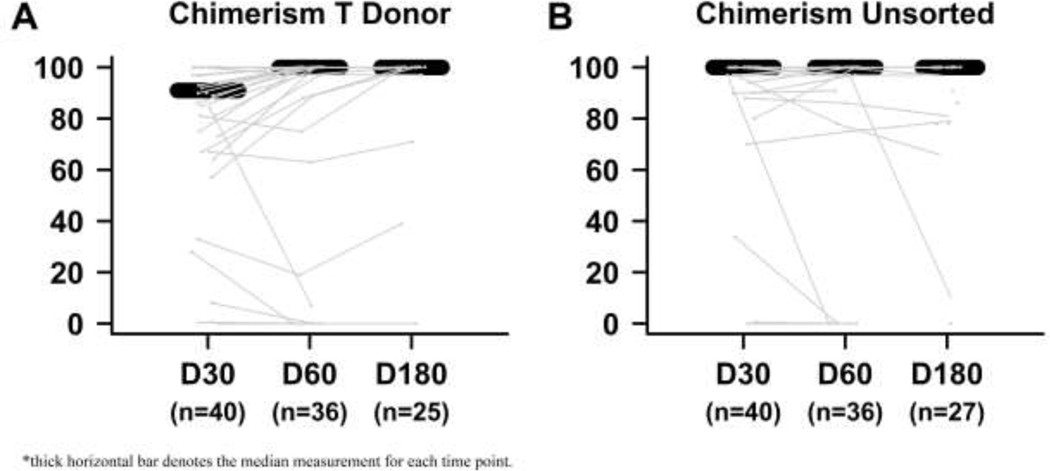
Table 2 summarizes clinical outcomes following BMT. Thirty-eight (91%) patients had engraftment of neutrophils at a median of 19.5 (IQ range 17, 26.75 days) and 32 (76%) patients had platelet engraftment at a median 31 days (IQ range, 23–44 days). Four (10%) patients died prior to neutrophil engraftment and 7 (17%) died without achieving platelet engraftment. Three (10%) additional patients had primary graft failure with subsequent restoration of autologous hematopoiesis and are still alive at last follow up (994, 2134, and 2337 days post-BMT, respectively) with chronic phase disease and improvement in symptoms. Two (67%) of these three had received a peripheral blood graft while one (33%) received a marrow graft. All three received 200cGy total body irradiation. Peripheral blood chimerism of both whole blood and T cell is depicted over time in Figure 2.
Table 2:
Summary of clinical outcomes
| Outcomes | N = 42 |
|---|---|
| Median days to neutrophil engraftment, n= 38 (IQR) | 19.5 (17, 27) |
| Median days to platelet engraftment, n =32 (IQR) | 31 (23, 44) |
| Graft failure (%) | 3 (7) |
| Overall survival, probability (95% CI) | |
| 1 year | 0.65 (0.52 – 0.82) |
| 3 year | 0.60 (0.46 – 0.79) |
| Relapse free survival, probability (95% CI) | |
| 1 year | 0.65 (0.52 – 0.82) |
| 3year | 0.31 (0.17 – 0.56) |
| Cumulative incidence of NRM, probability (95% CI) | |
| Day +100 | 0.17 (0.05 – 0.28) |
| 1 year | 0.30 (0.15 – 0.44) |
| 3 year | 0.30 (0.15 – 0.44) |
| Cumulative incidence of relapse, probability (95% CI) | |
| 1 year | 0.05 (−0.02 – 0.12) |
| 3 year | 0.40 (0.20 – 0.60) |
| Cumulative incidence of acute GVHD grade 2–4, probability (95% CI) | |
| Day +100 | 0.12 (0.02 – 0.22) |
| 1 year | 0.32 (0.17 – 0.47) |
| Cumulative incidence of acute GVHD grade 3–4, probability (95% CI) | |
| Day +100 | 0.10 (0– 0.19) |
| 1 year | 0.17 (0.05 – 0.29) |
| Cumulative incidence of chronic GVHD, probability (95% CI) | |
| 1 year | 0.20 (0.07 – 0.34) |
| 3 year | 0.22 (0.08 – 0.36) |
(CI, confidence interval; GVHD, graft versus host disease; IQR, interquartile range)
Figure 2:
Peripheral blood T cell and unsorted chimerism over time
OS and RFS
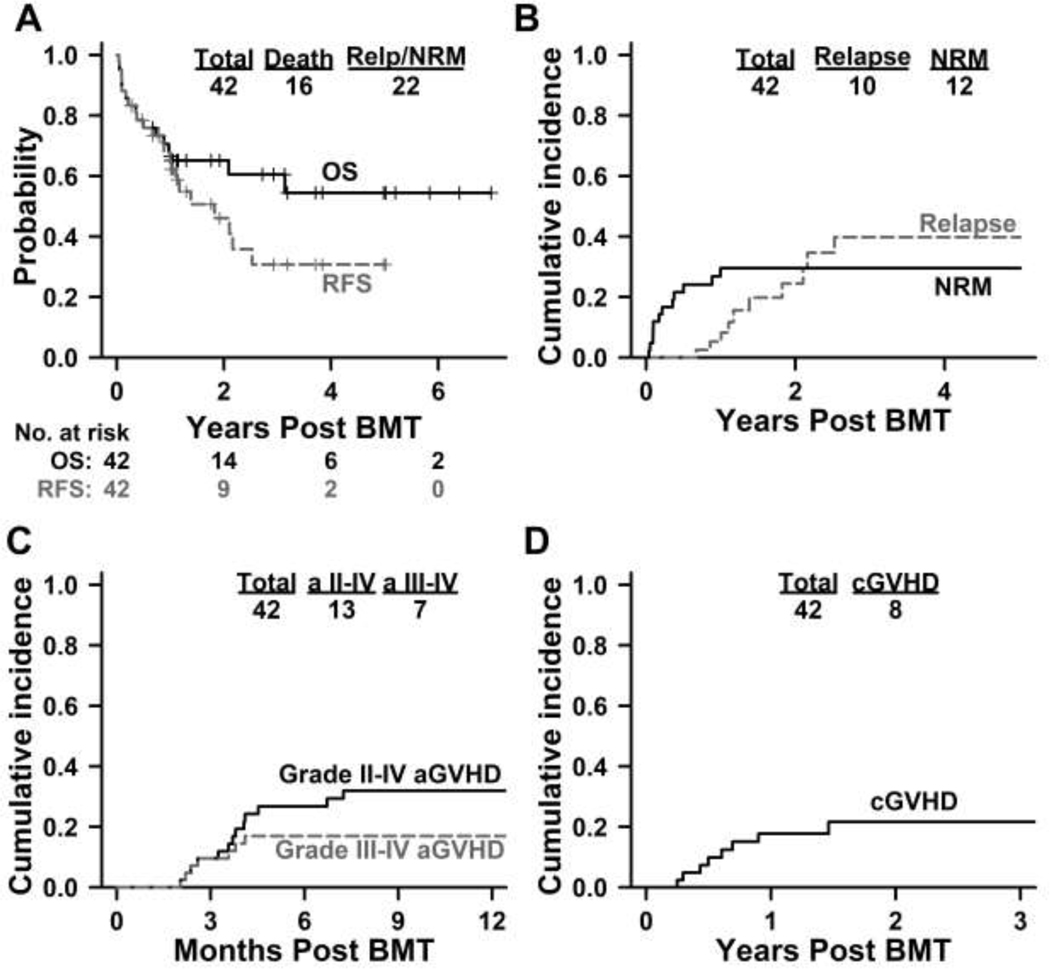
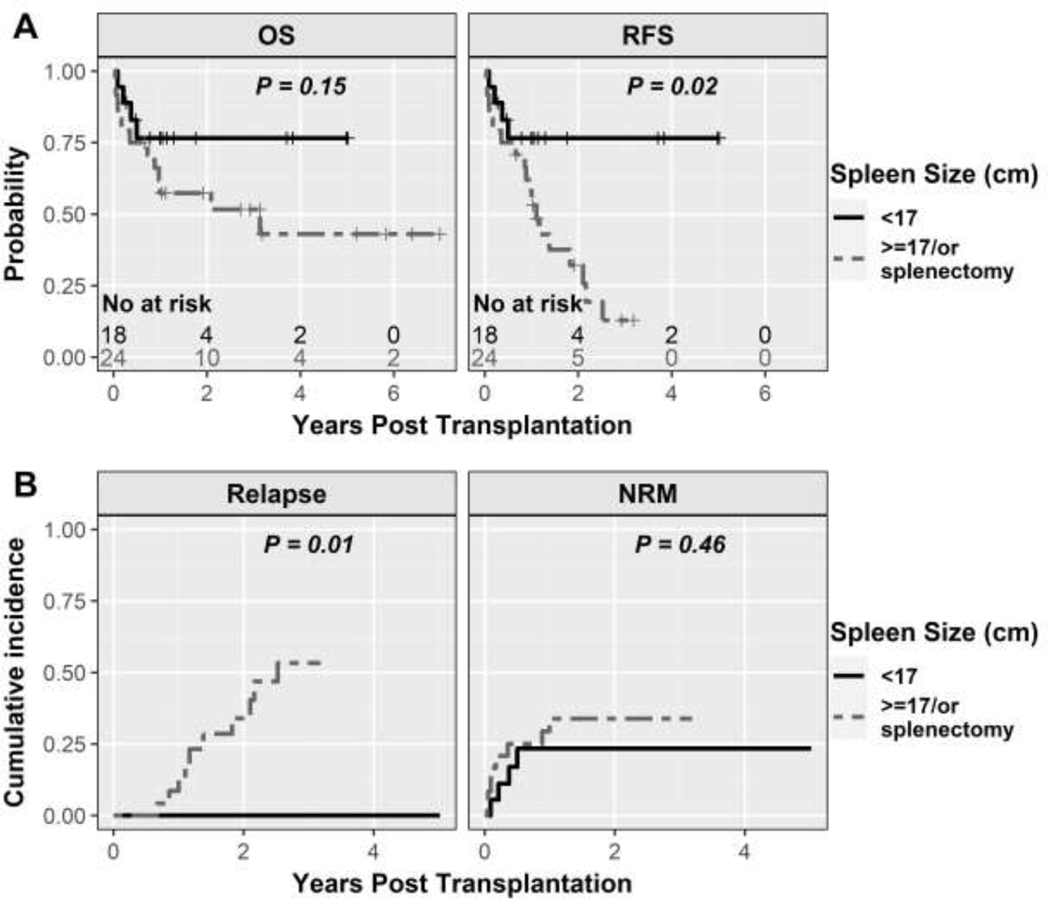
Median follow-up, in this study, was 2.72 years (range, 14 days – 6.99 years) after BMT based on reversed Kaplan-Meier method. The probability of OS was 65% (95%CI: 52–82%) and 60% (95%CI: 46–79%) at 1-year and 3-years respectively, while RFS was 65% (95%CI: 52–82%) and 31% (95%CI: 17–56%), respectively (Figure 3A). In the univariate analysis, spleen size of ≥ 17 cm and those who had undergone a splenectomy had an inferior RFS (HR 3.50, 95% CI 1.15–10.65, P=0.03 for spleen size ≥ 17cm and HR 3.53, 95%CI 0.86 – 14.33, P=0.08).
Figure 3:
Clinical outcomes with PTCy based non-myeloablative conditioning BMT in myelofibrosis
NRM and relapse
In the entire cohort, the cumulative incidence of NRM was 17% (95%CI: 5–28%) at day +100 and 30% (95% CI: 15–44%) at 1 year. NRM in the cohort of age ≤ 60 years was 6% (1/16) and in age > 60 years was 42% (11/26). Univariate analysis demonstrated association of age > 60 years at BMT [subdistribution hazard (SDHR) 9.46, 95%CI: 1.37–65.16, P=0.02] or increase in age per decade (SDHR 3.18, 95%CI: 1.05–9.62, P=0.04) with a worse NRM. All clinical outcomes for patients for age > 60 years at BMT, who composed a major proportion of the study, are depicted in Supplementary Figure 1 in comparison with the younger cohort (age ≤ 60 years).
The cumulative incidence of relapse was 5% (95%CI: 2–12%) at 1 year and 40% (95%CI: 20–60%) at 3 years (Figure 3B). In univariate analysis, age > 60 years (SDHR 0.26, 95%CI: 0.08–0.80, P=0.02) and peripheral blood graft (SDHR 0.34, 95%CI 0.11–0.99, P=0.05) were associated with lower risk of relapse; while spleen size ≥ 17 cm or prior splenectomy (HR not calculable, P=0.01) and CALR driver mutation (HR 5.41, 95%CI 1.68–17.47, P=0.005) were associated with higher risk of relapse. Cumulative incidence of relapse at 3-years in patients with spleen <17 cm was 0%, ≥ 17 cm was 40%, and in those with prior splenectomy was 80%. Other patient-, disease-, and BMT-related characteristics studied were not statistically significantly associated with these clinical outcomes.
The causes of the 16 deaths were relapse in 4 (25%), GVHD in 2 (12%), infection in 7 (44%) and organ toxicity in 1 (6%) patients. One patient had diffuse large B cell lymphoma before myelofibrosis which was in remission at the time of myelofibrosis diagnosis and at BMT. This patient had a relapse of lymphoma 364 days after BMT and died of relapsed lymphoma, while myelofibrosis remained in remission at the time of death. The cause of death is unknown in one patient. Infections were bacterial in one patient, fungal in 4 patients and viral in 2 patients. No patients who died of infection had a prior splenectomy and 6/7 had received prior ruxolitinib treatment.
Acute and chronic GVHD
Any grade (grades 1–4) acute GVHD was noted in 20 (48%) patients. Skin was the most common organ involved in 15/20 (75%) patients – skin alone in 7/16 (44%), skin and gastrointestinal tract in 4/16 (25%), skin and liver in 2/16 (13%), while all 3 organs were involved in 2/16 (13%). Isolated gastrointestinal tract was noted in 2/20 (10%) while liver the only organ in 1/20 (5%) patient. Both gastrointestinal tract and liver were involved in 2/20 (10%) patients. At day +100, the incidence of grade 2–4 acute GVHD was 12% (95%CI 2–22%) and grade 3–4 acute GVHD was 10% (95%CI 9–19%%), while at 1 year, grade 2–4 acute GVHD was 32% (95%CI 17–29%) and grade 3–4 acute GVHD was 17% (95%CI 5–29%) (Figure 3C). The cumulative incidence of chronic GVHD at 1 year was 20% (95%CI 7–34%) and at 3 years was 22% (95%CI 8–36%) (Figure 3D). Chronic GVHD requiring systemic therapy was noted in 5/42 (12%) patients.
There was no statistically significant difference in grade 3–4 acute GVHD or chronic GVHD between patients who received tacrolimus versus sirolimus. However, there was a suggestion of higher grade 2–4 acute GVHD in patients who received sirolimus (Supplementary Figure 2).
Spleen size and prior splenectomy
Of patients with intact spleen (n=36), the median spleen size was 16.75 cm (range, 11 – 28 cm). Spleen size was available by imaging (CT or ultrasound) in 27 out of 36 patients with an intact spleen. With spleen as a continuous variable, an increase in spleen size beyond 11 cm was linearly associated with an increased log relative hazard of RFS up to a spleen size of 17 cm (Supplementary Figure 3). With an increase in spleen size beyond 17 cm, the log relative hazard RFS remained relatively stable.
Five patients had a prior splenectomy due to absence of response in spleen size with JAK-inhibitors or advanced disease. Another patient had a splenectomy during surgical debulking for pseudomyxoma peritonei. Patients with a prior splenectomy (n=6) underwent BMT with marrow graft in 1/6 (17%) patients and peripheral blood graft in 5/6 (83%) patients. These 6 patients engrafted neutrophils at a median of 18 (range, 15–30) days and platelets at a median 30 (range, 16–47) days. All had full donor chimerism in both T cell fraction and unsorted samples at day +60. However, 4/6 (67%%) of these relapsed at days +244, +314, +428 and +667, respectively, and 3/6 (50%) have died at the time of last follow-up from relapse disease. Three (50%) are alive at days +244, +376 and +1146 from BMT, respectively.
Pre-BMT driver and non-driver somatic mutations
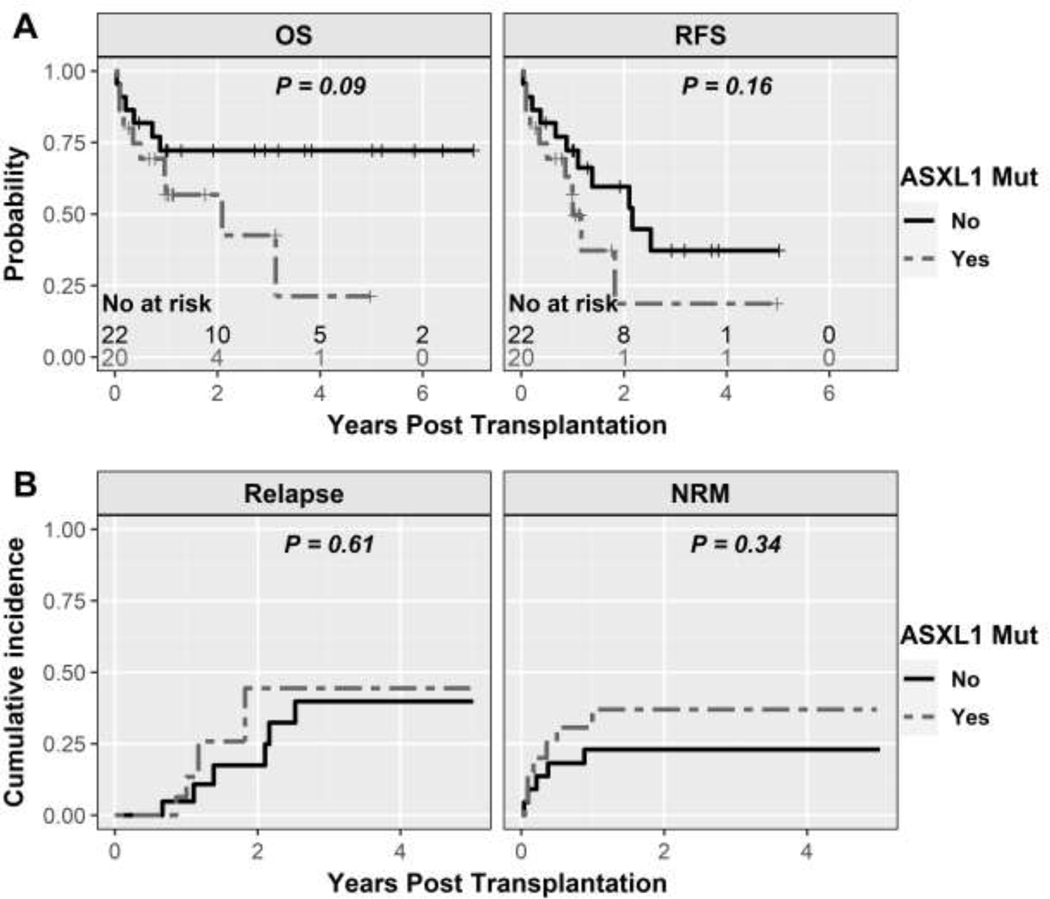
The type of driver mutation (JAK2 vs CALR vs MPL/triple negative) was not statistically significantly associated with OS, RFS or NRM while CALR was associated with higher relapse (SDHR 5.41, 95%CI 1.68–17.47, P=0.005; Table 3). The presence of high-risk somatic mutations and a higher number of high-risk mutations was also evaluated with these clinical outcomes and no statistically significant association was noted (Table 3 and Supplementary Figure 4). Since ASXL1 was the most prevalent high-risk mutation, we analyzed outcomes with ASXL1 separately. While not statistically significant in this relatively small sample size, patients with ASXL1 had an inferior OS (HR 2.36, 95%CI 0.85–6.60, P=0.09), RFS (HR 1.85, 95% CI 0.77–4.43, P=0.17), and NRM (HR 1.73, 95%CI 0.57–5.3, P=0.34; Table 3 and Figure 5). Relapse rates were statistically similar (HR 1.09, 95%CI 0.37–3.7, P=0.89). To integrate genomic and non-mutational risk factors, we further analyzed by MIPSS70+ version 2.0 at the time of BMT. Patients with low/intermediate MIPSS70+ version 2.0 did not have statistically different clinical outcomes compared to patients with high/very high MIPSS70+ version 2.0 score as depicted in Table 3 and Supplementary Figure 5.
Table 3:
Univariate analysis for OS, RFS, NRM, and relapse
| OS | RFS | NRM | Relapse | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | #evt/n | HR(95 %CI) | p-value | #evt | HR(95 %CI) | p-value | #evt | SDHR(95 %CI) | p-value | #evt | SDHR(95 %CI) | p-value |
| Age at BMT | ||||||||||||
| ≤ 60 | 4/16 | 7 | 1 | 6 | ||||||||
| > 60 | 12/2 6 | 2.58(0.83–8.04) | 0.10 | 15 | 1.60(0.65–3.99) | 0.31 | 11 | 9.46(1.37–65.16) | 0.02 | 4 | 0.26(0.08–0.80) | 0.02 |
| Age increase per decade | 16/42 | 1.31 (0.67–2.55) | 0.43 | 22 | 1.20 (0.68–2.14) | 0.53 | 12 | 3.18 (1.05–9.62) | 0.04 | 10 | 0.48 (0.28–0.82) | 0.007 |
| Gender | ||||||||||||
| Male | 10/25 | 14 | 6 | 8 | ||||||||
| Female | 6/17 | 0.98(0.35–2.69) | 0.97 | 8 | 0.95(0.40–2.26) | 0.90 | 6 | 1.55(0.52–4.68) | 0.43 | 2 | 0.37(0.08–1.58) | 0.18 |
| BMT Year | ||||||||||||
| 2010–2016 | 5/14 | 10 | 4 | 6 | ||||||||
| 2017–2020 | 11/28 | 1.36 (0.46–4.05) | 0.56 | 12 | 0.78(0.33–1.83) | 0.57 | 8 | 0.98(0.30–3.18) | 0.97 | 4 | 0.48(0.16–1.47) | 0.20 |
| Years from myelofibrosi s to BMT | 16/42 | 1.01(0.94–10.9) | 0.70 | 22 | 1(0.94–1.07) | 0.96 | 12 | 0.99(0.87–1.13) | 0.92 | 10 | 1.02(0.95–1.09) | 0.62 |
| HCT-CI | ||||||||||||
| 0–1 | 7/20 | 11 | 6 | 5 | ||||||||
| ≥ 2 | 9/22 | 1.30(0.48–3.50) | 0.60 | 11 | 1.14(0.49–2.65) | 0.76 | 6 | 0.96(0.32–2.87) | 0.94 | 5 | 1.23(0.36–4.22) | 0.74 |
| Diagnosis | ||||||||||||
| Primary myelofi brosis | 6/19 | 8 | 5 | 3 | ||||||||
| Post ET/PV | 10/23 | 1.57(0.57–4.33) | 0.38 | 14 | 1.85(0.75–4.34) | 0.19 | 7 | 1.31(0.43–3.98) | 0.63 | 7 | 2.23(0.62–0.81) | 0.22 |
| Prior JAK inhibitor use | ||||||||||||
| No | 1/8 | 2 | 1 | 1 | ||||||||
| Yes | 15/34 | 4.69(0.61–36.18) | 0.14 | 20 | 3.87(0.88–16.98) | 0.07 | 11 | 2.70(0.31–23.48) | 0.37 | 9 | 3.01(0.35–26.10) | 0.32 |
| DIPSS-Plus at BMT | ||||||||||||
| Intermediate 1/2 risk | 12/33 | 16 | 9 | 7 | ||||||||
| High risk | 4/9 | 1.25(0.40–3.90) | 0.70 | 6 | 1.29(0.51–3.31) | 0.59 | 3 | 1.25(0.35–4.45) | 0.73 | 3 | 1.36(0.43–4.25) | 0.60 |
| MIPSS70 v2.0 at BMT | ||||||||||||
| Low/Intermediate | 4/10 | 5 | 2 | 3 | ||||||||
| High/Very high | 12/32 | 1.10(0.35–3.42) | 0.87 | 17 | 1.36(0.50–3.71) | 0.54 | 10 | 1.76(0.40–7.83) | 0.46 | 7 | 0.84(0.22–3.15) | 0.79 |
| Spleen size | ||||||||||||
| at BMT | ||||||||||||
| 11–17cm | 4/18 | 4 | 4 | 0 | ||||||||
| >17cm/Prior splenect omy | 12/24 | 2.24(0.72–6.96) | 0.16 | 18 | 3.5(1.18–10.37) | 0.02 | 8 | 1.56(0.49–4.99) | 0.46 | 10 | – | 0.01 |
| Driver mutation | ||||||||||||
| JAK2 | 10/26 | 13 | 9 | 4 | ||||||||
| CALR | 4/9 | 0.91(0.28–2.91) | 0.87 | 6 | 1.28(0.48–3.43) | 0.62 | 1 | 0.28(0.03–2.38) | 0.24 | 5 | 5.41(1.68–17.47) | 0.00 5 |
| MPL/ Triple negative | 2/7 | 0.62(0.13–2.85) | 0.54 | 3 | 0.74(0.21–2.61) | 0.64 | 2 | 0.78(0.17–3.64) | 0.76 | 1 | 0.85(0.09–7.92) | 0.88 |
| High-risk cytogenetics | ||||||||||||
| No | 16/35 | 20 | 12 | 8 | ||||||||
| Yes | 0/7 | Not evaluable | 2 | 0.33(0.08–1.41) | 0.14 | 0 | Not evaluable | 2 | 1.21(0.29–5) | 0.80 | ||
| High-risk somatic mutations | ||||||||||||
| No | 5/17 | 9 | 4 | 5 | ||||||||
| Yes | 11/25 | 1.81(0.63–5.24) | 0.27 | 13 | 1.42(0.60–3.36) | 0.43 | 8 | 1.52(0.47–4.92) | 0.49 | 5 | 0.93(0.29–3.02) | 0.90 |
| ASXL1 | ||||||||||||
| Absent | 6/22 | 11 | 5 | 6 | ||||||||
| Present | 10/20 | 2.36(0.85–6.6) | 0.09 | 11 | 1.85(0.77–4.43) | 0.17 | 7 | 1.73(0.57–5.3) | 0.34 | 4 | 1.09(0.32–3.7) | 0.89 |
| Number of high-risk mutations | ||||||||||||
| 0 | 5/17 | 9 | 4 | 5 | ||||||||
| 1 | 5/9 | 2.16(0.62–7.52) | 0.23 | 6 | 1.5(0.53–4.21) | 0.45 | 4 | 2.07(0.55–7.79) | 0.28 | 2 | 0.73(0.14–3.7) | 0.70 |
| >=2 | 6/16 | 1.6(0.48–5.26) | 0.44 | 7 | 1.35(0.49–3.72) | 0.56 | 4 | 1.20(0.31–4.69) | 0.79 | 3 | 1.14(0.3–4.41) | 0.85 |
| Donor platform | ||||||||||||
| Haploidentical family | 13/33 | 19 | 10 | 9 | ||||||||
| MRD/MUD | 2/6 | 0.63(0.14–2.78) | 0.54 | 2 | 0.39(0.09–1.69) | 0.21 | 1 | 0.44(0.07–2.86) | 0.39 | 1 | 0.50(0.06–4.41) | 0.53 |
| MMUD | 1/3 | 0.62(0.08–4.78) | 0.65 | 1 | 0.41(0.05–3.04) | 0.38 | 1 | 0.86(0.16–4.60) | 0.86 | 0 | 0(0–0) | – |
| Graft source | ||||||||||||
| Bone marrow | 2/7 | 5 | 1 | 4 | ||||||||
| PB | 14/35 | 2.06(0.46–9.31) | 0.35 | 17 | 0.92(0.34–2.50) | 0.86 | 11 | 2.54(0.40–16.29) | 0.33 | 6 | 0.34(0.11–0.99) | 0.05 |
| TBI | ||||||||||||
| 200 cGy | 6/19 | 12 | 5 | 7 | ||||||||
| 400 cGy | 10/23 | 2.22(0.72–6.85) | 0.17 | 10 | 1.46(0.57–3.78) | 0.43 | 7 | 1.24(0.41–3.76) | 0.71 | 3 | 0.78(0.24–2.53) | 0.68 |
(BMT, blood or marrow transplantation; DIPSS, dynamic international prognostic scoring system; #evt, number of events; MIPSS, Mutation Enhanced International Prognostic Scoring System; MRD, matched related donor; MUD, matched unrelated donor; n, number of patients; NRM, non-relapse mortality; OS, overall survival; PB, peripheral blood; RFS, relapse free survival; TBI, total body irradiation)
Figure 5:
Kaplan-Meier curves for clinical outcomes by presence of ASXL1 mutation
Outcomes with peripheral blood graft and TBI 400cGy
Twenty three (55%) underwent BMT with our current standard regimen using 400cGy TBI along with fludarabine and cyclophosphamide as conditioning chemotherapy and peripheral blood grafts. The median follow up is 355 days (range, 33 −764 days) from BMT based on reversed Kaplan-Meier method. Nineteen (83%) of these were done using a haploidentical donor, 2 (9%) with matched unrelated donor BMT and 1 (4%) each with matched unrelated and mismatched unrelated donor. No graft failure occurred in this cohort. Although a smaller sample size and shorter follow-up than the entire group, 6-month estimate of OS was 69% (95%CI 53%−91%) and RFS was 69% (95%CI 53%−91%), as shown in Supplementary Figure 6. Three (13%) patients have relapsed so far and have died as a result of progressive disease. Five (22%) had grade 3–4 acute GVHD and 2 (9%) had developed chronic GVHD requiring systemic immunosuppression.
Discussion
While the role of BMT as a potentially curative option is well-established in myelofibrosis, the variability in BMT platforms could influence outcomes30,31. Our study adds to the growing data on the use of PTCy based regimens for GVHD prophylaxis, specifically using non-myeloablative conditioning in older patients with myelofibrosis. Clinical outcomes with this platform are at least comparable to other registry and institutional data, with acceptable levels of adverse events32–36. Registry data from Center for International Blood and Marrow Transplant Research reported BMT outcomes of 233 myelofibrosis patients using reduced intensity conditioning and calcineurin inhibitor based GVHD prophylaxis32. In this study, only 38% had intermediate-2 or high risk disease per DIPSS scoring and 88% received a peripheral blood graft. At 3 years, they reported an OS of 52%, RFS 32%, NRM 22%, and relapse 47%. GVHD was grade 2–4 in 37% and grade 3–4 in 19% patients by day +100 and chronic GVHD was seen in 42% patients at 1 year. Of note, patients were much younger at BMT in this study compared to our cohort, with a median age of 55 years and only 27% patients over the age of 60 years. For older patients, which constitutes a majority of patients in our study, an EBMT study in patients of age ≥65 years, predominantly receiving matched unrelated donor graft and busulfan based reduced intensity conditioning demonstrated a 5-year OS of 50%, NRM 37% and incidence of relapse 25%35. Studies demonstrating the role of reduced intensity conditioning using fludarabine-busulfan or fludarabine-melphalan, have shown lower rates of relapse at 9–29% but this was at the cost of a higher NRM of 30–40%37,38.
As stands out in this study and others, relapse of primary disease continues to be a challenge in this patient population and also a major cause of death (around 40% at 3 years in our study), while NRM and GVHD have improved due to superior supportive care over the years. In a larger study with a longer follow up using Human Mortality Database, relapse was reported as a major cause of death in 41% myelofibrosis patients at 2–5 years and 61% patients over 5 years from BMT39. Persistence of transfusion dependence and molecular evidence of disease at day +100 have previously been identified as risk factors for poorer OS and RFS in patients with myelofibrosis40. Using this available information, prevention of relapse following BMT in myelofibrosis, especially in patients with these high-risk features at day +100, becomes an area of unmet need and studies directed to improve relapse rates are urgently needed. Based on an early suggestion of improved disease control with peripheral blood graft and better engraftment with higher dose of TBI, we recently changed our standard practice to include peripheral blood grafts and 400cGy radiation for all patients with myelofibrosis. While follow-up on this cohort is limited, the RFS appears encouraging.
Spleen size has long been considered a poor prognostic factor in myelofibrosis although the size cut-offs have never been defined. Additionally, spleen size is not commonly included in most risk stratifications for myelofibrosis including the recently developed clinical-molecular transplant scoring system for patients undergoing BMT17,41,42. An Italian study established a spleen size of > 22cm as a risk factor for inferior survival along with transfusion dependence and donor other than HLA-identical sibling43. In another analysis from CIBMTR, enlarged spleen size was associated with delayed engraftment compared to patients with normal spleen size, while OS was similar44. Data from EBMT has also shown that palpable spleen size <5cm below costal margin had statistically significantly superior OS and NRM compared to patients with 5–14 cm and ≥15 cm while relapse rate was statistically similar45. In our study, an increase in spleen size especially up to the size of 17 cm was linearly associated with increased relapses and inferior RFS. Beyond a spleen size of 17 cm, the association of spleen size with outcomes tapered off. Splenectomy did not appear to improve these outcomes in our study, suggesting that spleen size may be a marker for advanced disease rather than an independent prognostic variable; hence, suggesting the need for inclusion in prediction models for BMT in this disease.
The understanding of the role of somatic genomic alterations, both driver and other somatic mutations, in myelofibrosis prognostication has markedly improved in recent years. Mutations in JAK-STAT pathway, including JAK2, CALR and MPL, are the hallmark of MPN pathogenesis. In preclinical models, additional mutations such as TET2, EZH2, TP53 suggest features of progression18,46,47. In retrospective studies, mutations in ASXL1, EZH2, SRSF2, IDH1, IDH2 and U2AF1 Q157 are associated with inferior prognosis19,20. Such an impact of genomic alterations is not well established in the context of BMT as different studies have shown conflicting results. Furthermore, no studies have reported the impact of somatic mutations on BMT outcomes with the non-myeloablative PTCy platform. Kroger et al demonstrated an association of lower NRM and improved RFS in patients with CALR mutation while ASXL1 and IDH2 were associated with lower RFS48. Subsequently a study by Tamari et al showed no impact of ASXL1 or IDH2; however, U2AF1 and DNMT3A were associated with inferior OS49. In yet another study by Ali et al, CBL mutations were associated with lower OS and higher NRM while ASXL1, IDH or CALR mutations were not associated with inferior outcomes50. In our study with the PTCy platform, patients with high-risk non-driver somatic mutations, particularly ASXL1, appeared to have an inferior overall survival but this was not statistically significant in this limited sample size. In contrast to the study by Ali et al, we did not find a difference in outcomes by MIPSS70+ version 2.0 which is likely related to a different BMT platform and a smaller sample size in our study50. Taken together, it is evident from the above discrepant results that the impact of specific genomic alterations is not the same in the non-BMT and BMT contexts. Such an association of somatic mutations with BMT outcomes warrants a dedicated larger study addressing this specific question.
In our study, older age (> 60 years) was associated with higher NRM which is consistent with prior reports35,39 but was also with lower relapse. The high SDHR (9.46) for NRM in patients with age > 60 years at BMT underscores the need for careful selection of older patients for BMT. At the same time, the wide 95% confidence interval (1.37–65.16) suggests the uncertainty of this estimate which is likely due to low number of NRM events in the cohort of age ≤ 60 years (1 NRM event in age ≤ 60 years). The lower relapse incidence in patients with age > 60 years is likely in part due to the higher NRM in this cohort. Such associations may reflect on some of the limitations of this retrospective study with a small sample size and limited number of events likely contributing to these effects. Another unexpected finding is higher relapse in patients with CALR mutation, which is likely related to the fact that around half of these were not the type 1 mutations which are associated with better prognosis51. Furthermore, 3 out of 5 patients with CALR type 1 had a concurrent ASXL1 mutation which in turn is known to have a poorer prognosis. This is potentially also a consequence of a limited sample size. Nevertheless, we describe the successful use of PTCy based BMT platform in myelofibrosis for the first time to our knowledge, with a suggestion that peripheral blood grafts and 400 cGy of TBI may be beneficial for engraftment. This data aligns with the growing interest in PTCy as GVHD prophylaxis in various disease, donor and conditioning regimen settings.
Supplementary Material
Figure 4:
Kaplan-Meier curves for clinical outcomes by spleen size category (11–16 cm versus ≥ 17 cm/splenectomy)
Highlights.
PTCy with non-myeloablative conditioning results in acceptable clinical outcomes with BMT in myelofibrosis
Older age had higher NRM, while increased spleen size or marrow graft had higher relapse
No graft failure has been noted with peripheral blood graft using 400cGy total body irradiation with fludarabine and cyclophosphamide
Acknowledgements:
This work was supported by the National Institutes of Health, National Cancer Institute (P01 CA225618 and P30 CA06973).
Abbreviations
- BMT
Allogeneic blood or marrow transplantation
- DIPSS
Dynamic international prognostic scoring system
- GVHD
Graft versus host disease
- HLA
Human leukocyte antigen
- HR
hazard ratio
- IV
intravenously
- MIPSS70+ v2.0
Mutation-enhanced international prognostic scoring system Version 2.0
- NRM
Non-relapse mortality
- OS
Overall survival
- PTCy
Post-transplantation cyclophosphamide
- RFS
Relapse free survival
- SDHR
Subdistribution hazard ratio
Footnotes
Conflict of interests/Disclosures:
TJ: Institutional research support from CTI Biopharma and Syneos Health, Consultancy with Targeted Healthcare Communications, Advisory board participation with Care Dx, Bristol Myers Squibb, Incyte and CTI Biopharma
H-LT, AED, LPG, AA, HE, JFBM, LL, RA, DG, PI, JW, GP, GG, BDG, SAA, AA, WBD, CBG, CAH, IG, LS, NWJ, IB, RV, ML, RJJ: No relevant disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolanos-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of posttransplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25):3817–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as singleagent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote grafthost tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47(13):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and highdose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6):3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 9.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imus PH, Tsai HL, Luznik L, et al. Haploidentical transplantation using posttransplant cyclophosphamide as GVHD prophylaxis in patients over age 70. Blood Adv. 2019;3(17):26082616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster JA, Luznik L, Tsai HL, et al. Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv. 2020;4(20):5078–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61(1):10–15. [DOI] [PubMed] [Google Scholar]
- 14.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood.2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 15.DeZern AE, Elmariah H, Zahurak M, et al. Shortened-Duration Immunosuppressive Therapy after Nonmyeloablative, Related HLA-Haploidentical or Unrelated Peripheral Blood Grafts and Post-Transplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2020;26(11):2075–2081. [DOI] [PubMed] [Google Scholar]
- 16.Kasamon YL, Fuchs EJ, Zahurak M, et al. Shortened-Duration Tacrolimus after Nonmyeloablative, HLA-Haploidentical Bone Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24(5):1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397. [DOI] [PubMed] [Google Scholar]
- 18.Rampal R, Ahn J, Abdel-Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111(50):E5401–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, Finke CM, Lasho TL, et al. U2AF1 mutation types in primary myelofibrosis: phenotypic and prognostic distinctions. Leukemia. 2018;32(10):2274–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–1869. [DOI] [PubMed] [Google Scholar]
- 21.Tefferi A, Guglielmelli P, Lasho TL, et al. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis. J Clin Oncol. 2018;36(17):1769–1770. [DOI] [PubMed] [Google Scholar]
- 22.Robert Lowsky HM. Mechanisms and Treatment of Graft Failure. Thomas’ Hematopoietic Cell Transplantation: Stem Cell Transplantation, I, Fifth Edition. 2015:944–958.
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 24.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. [DOI] [PubMed] [Google Scholar]
- 25.Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karantanos T, Gondek LP, Varadhan R, et al. Gender-related differences in the outcomes and genomic landscape of patients with myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Br J Haematol. 2021. [DOI] [PMC free article] [PubMed]
- 27.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society 1972;34(2):187–220. [Google Scholar]
- 28.Jason P Fine RJG. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 29.Masarova L, Verstovsek S, Hidalgo-Lopez JE, et al. A phase 2 study of ruxolitinib in combination with azacitidine in patients with myelofibrosis. Blood. 2018;132(16):1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gowin K, Ballen K, Ahn KW, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4(9):1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain T, Mesa RA, Palmer JM. Allogeneic Stem Cell Transplantation in Myelofibrosis. Biol Blood Marrow Transplant. 2017;23(9):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2014;20(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raj K, Eikema DJ, McLornan DP, et al. Family Mismatched Allogeneic Stem Cell Transplantation for Myelofibrosis: Report from the Chronic Malignancies Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(3):522–528. [DOI] [PubMed] [Google Scholar]
- 34.Rondelli D, Goldberg JD, Isola L, et al. MPD-RC 101 prospective study of reducedintensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124(7):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Boluda JC, Pereira A, Kroger N, et al. Allogeneic hematopoietic cell transplantation in older myelofibrosis patients: A study of the chronic malignancies working party of EBMT and the Spanish Myelofibrosis Registry. Am J Hematol. 2021;96(10):1186–1194. [DOI] [PubMed] [Google Scholar]
- 36.Kunte S, Rybicki L, Viswabandya A, et al. Allogeneic blood or marrow transplantation with haploidentical donor and post-transplantation cyclophosphamide in patients with myelofibrosis: a multicenter study. Leukemia. 2021. [DOI] [PMC free article] [PubMed]
- 37.Jain T, Kunze KL, Temkit M, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2019;54(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robin M, Porcher R, Wolschke C, et al. Outcome after Transplantation According to Reduced-Intensity Conditioning Regimen in Patients Undergoing Transplantation for Myelofibrosis. Biol Blood Marrow Transplant. 2016;22(7):1206–1211. [DOI] [PubMed] [Google Scholar]
- 39.Robin M, de Wreede LC, Wolschke C, et al. Long-term outcome after allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica. 2019;104(9):1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain T, Kunze KL, Mountjoy L, et al. Early post-transplantation factors predict survival outcomes in patients undergoing allogeneic hematopoietic cell transplantation for myelofibrosis. Blood Cancer J. 2020;10(3):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gagelmann N, Ditschkowski M, Bogdanov R, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133(20):2233–2242. [DOI] [PubMed] [Google Scholar]
- 42.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J Clin Oncol. 2018;36(4):310–318. [DOI] [PubMed] [Google Scholar]
- 43.Bacigalupo A, Soraru M, Dominietto A, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45(3):458–463. [DOI] [PubMed] [Google Scholar]
- 44.Akpek G, Pasquini MC, Logan B, et al. Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(6):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polverelli N, Mauff K, Kroger N, et al. Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: A retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96(1):69–79. [DOI] [PubMed] [Google Scholar]
- 46.Chen E, Schneider RK, Breyfogle LJ, et al. Distinct effects of concomitant Jak2V617F expression and Tet2 loss in mice promote disease progression in myeloproliferative neoplasms. Blood. 2015;125(2):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu T, Kubovcakova L, Nienhold R, et al. Loss of Ezh2 synergizes with JAK2V617F in initiating myeloproliferative neoplasms and promoting myelofibrosis. J Exp Med. 2016;213(8):1479–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroger N, Panagiota V, Badbaran A, et al. Impact of Molecular Genetics on Outcome in Myelofibrosis Patients after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(7):1095–1101. [DOI] [PubMed] [Google Scholar]
- 49.Tamari R, Rapaport F, Zhang N, et al. Impact of High-Molecular-Risk Mutations on Transplantation Outcomes in Patients with Myelofibrosis. Biol Blood Marrow Transplant. 2019;25(6):1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali H, Aldoss I, Yang D, et al. MIPSS70+ v2.0 predicts long-term survival in myelofibrosis after allogeneic HCT with the Flu/Mel conditioning regimen. Blood Adv. 2019;3(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89(8):E121–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.