Abstract
Objective:
To investigate the association of infertility with atherosclerotic cardiovascular disease (ASCVD) in the Women’s Health Initiative. We hypothesized that nulliparity and pregnancy loss may reveal more extreme phenotypes of infertility, allowing further understanding of the association of infertility with ASCVD.
Design:
Prospective cohort study
Setting:
U.S.
Patients:
Women’s Health Initiative Cohort
Exposures:
Infertility, parity, pregnancy loss
Main Outcome Measures:
Primary outcome was risk of ASCVD among women with and without a history of infertility, stratified by history of live birth and pregnancy loss. Cox proportional hazards models were adjusted for demographics and ASCVD risk factors.
Results:
Among 158,787 women, 25,933 (16.3%) reported a history of infertility, among whom 20,427 (80%) had at least one live birth and 9,062 (35%) experienced at least one pregnancy loss. There was a modest overall association between infertility and ASCVD (aHR 1.02, 95% CI 0.99–1.06) over 19 years of follow up. Among nulliparous women, infertility was associated with a 13% higher risk of ASCVD (95% CI 1.04–1.23). Among nulliparous women who experienced a pregnancy loss, infertility was associated with a 36% higher risk of ASCVD (95% CI 1.09–1.71).
Conclusions:
Women with a history of infertility overall experienced a modestly higher risk of ASCVD compared to women without a history of infertility. ASCVD risk was much higher among nulliparous infertile women and among nulliparous infertile women who also experienced a pregnancy loss, suggesting that in these more extreme phenotypes, infertility may be associated with ASCVD risk.
Keywords: infertility, pregnancy, gravidity, parity, cardiovascular disease
Capsule:
Infertility is associated with increased risk of cardiovascular disease among nulliparous women in the Women’s Health Initiative.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death among women in the U.S. (1). Compared with men, women generally present with different symptoms of ASCVD, are more likely to be mis-diagnosed, and experience worse outcomes, including a higher risk of death after a major cardiovascular event (2–4). Innovative strategies are needed to improve ASCVD prevention and treatment among women.
Infertility, defined as the inability to conceive after attempting for more than a year, affects 15% of reproductive-age women in the U.S. (5). The consequences of an infertility diagnosis extend beyond the pursuit of family building, because women with infertility also face increased risks for severe maternal morbidity, cancer, chronic disease, and death (6–9). Underlying mechanisms remain unclear, including whether infertility itself or underlying conditions drive the increased morbidity risk. Several studies have reported an association between infertility and ASCVD, primarily in pre-menopausal women of reproductive age (7, 10, 11). Studies that determine later-life ASCVD risk among infertile women are needed. Current studies also consider infertility as a single homogenous exposure, when in fact there is significant variation in cause and duration of infertility with varied biologic pathways to ASCVD (10–13). The inability to achieve live birth, manifested either as pregnancy loss or nulliparity, can reveal more extreme phenotypes of infertility. Nulliparity, defined as a state of not having given birth to a child, is more frequent among women with a history of infertility(14) and women with infertility who never achieve live birth may represent a more severe form of the underlying disease process leading to infertility. While pregnancy loss is distinct from infertility (15), infertile women who conceive have a 2.6-fold higher risk of pregnancy loss compared to fertile women (16). Infertility and pregnancy loss have shared risk factors, the most significant of which is female age, and are both common events of reproductive failure (17). The objective of this study is to explore the association of infertility with ASCVD among 158,787 postmenopausal participants in the Women’s Health Initiative (WHI) Cohort. We hypothesized that nulliparity and pregnancy loss may reveal more extreme phenotypes of infertility, allowing further understanding of the association of infertility with ASCVD.
Methods
Study Cohort
The WHI enrolled 161,808 postmenopausal women, aged 50–79 years at baseline (1993–1998), as described previously (18, 19). WHI participants were included in the present study if they responded to questions regarding their reproductive history at the time of enrollment in the WHI; those with missing information on infertility status (N=1,644) or number of live births or pregnancy losses (N=1,377) were excluded. The current analysis includes outcomes through September 2020. The WHI was reviewed and approved by the IRB of the Fred Hutchinson Cancer Research Center in accordance with U.S. Department of Health and Human Services regulations at 45 CFR 46 (approval number: 3467-EXT).
Definition of Study Exposures
Infertility was self-reported at the time of enrollment in the WHI. Participants were asked: “Have you ever tried to become pregnant for more than 1 year without becoming pregnant?”. Those who marked “yes” were designated as infertile. Number of live births and spontaneous pregnancy losses, defined in the questionnaire as occurring before 6 months gestation, were also self-reported at enrollment. Women with no live births were considered nulliparous and women with at least one live birth were considered parous. Pregnancy loss was considered as both a binary outcome for the primary analysis and categorical variable (0,1,2,3+) for a subgroup analysis. Additional pregnancy outcomes collected in WHI including stillbirth (defined in the questionnaire as loss of pregnancy after 6 months gestation), pregnancy termination, and ectopic pregnancy were not included in this study due to small sample sizes of these outcomes.
Definition of Study Outcome
The primary outcome was any first-time ASCVD event, as defined by the American Heart Association, including any one of the following phenotypes: clinical myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial disease, carotid artery disease, and CVD death (due to definite coronary heart disease, cerebrovascular disease, pulmonary embolism, or other or unknown cardiovascular cause) (20). ASCVD events were prospectively ascertained by review of medical records between study entry and 2020, and adjudicated using standard criteria (21). ASCVD was considered as both a composite outcome as well as individual disease phenotypes.
Covariates
We analyzed baseline characteristics from the data collected at enrollment into the WHI. Data on age, race, ethnicity, education level, and family income were recorded at study entry. We additionally examined ASCVD risk factors identified from the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease (20). The ASCVD risk factors included age at menopause, hyperlipidemia (defined as self-reported use of cholesterol-lowering drugs), hypertension (defined as self-reported treated or untreated hypertension), diabetes (self-reported and not pregnancy related), body mass index (BMI) as kg/m2 (based on clinical measures at baseline of weight and height), and self-reported smoking status (smokers defined as ever having smoked 100 cigarettes over their lifetime) and were also recorded at study entry.
Statistical Analyses
We first compared baseline characteristics, including ASCVD risk factors and pregnancy histories, between women with and without a history of infertility using Student’s T-test for continuous outcomes and Chi-square test for categorical outcomes. We then calculated the distribution of ASCVD outcomes (composite, defined as having at least one of the CVD phenotypes, as well as individual phenotypes) among women with and without a history of infertility. We additionally stratified the prevalence of ASCVD outcomes by parity and pregnancy loss based on differences hypothesized a priori that infertile women who are also nulliparous or experience a pregnancy loss have a more severe infertility phenotype. We then conducted multivariable time-to-event analyses for the composite ASCVD outcome using Cox proportional hazards models to compare women with and without a history of infertility. We additionally stratified infertility-ASCVD regression models by parity and pregnancy loss based on differences hypothesized a priori. For each subgroup analysis, the reference group was restricted to women with the pregnancy-related exposure (parity or pregnancy loss) and without a history of infertility. All regression models were adjusted for age at enrollment, income, education, smoking status and race/ethnicity as potential confounders. The model was then adjusted for ASCVD clinical risk factors including age at menopause, hyperlipidemia, hypertension, diabetes and BMI (linear and quadratic). Sequential adjustment was performed because ASCVD risk factors were collected at the time of enrollment in WHI, therefore the temporality in relation in infertility diagnosis cannot be ascertained. Person-months at risk were calculated from entry into the analytic cohort at study baseline until independently confirmed death, loss to follow-up, or ASCVD diagnosis. Multiple imputation using 10 imputations by fully conditional specification methods was used in all multivariable models to account for missing values of the following covariates: family income, education level, smoking history, race/ethnicity, age at menopause, hypertension, diabetes, and hyperlipidemia. Missingness of imputed covariates for the models ranged from 0–7%. Multiple imputation was used for covariates, but not for exposures (infertility, pregnancy loss, and parity) due to controversy in imputing values for the exposure based on the covariates and outcome, and then using those values to estimate the effect of the exposure on the outcome (22). A sensitivity analysis was performed excluding women with ASCVD risk factors to determine their influence on effect estimates. Statistical analyses were conducted in SAS version 9.4. A two-sided P-value less than 0.05 was considered statistically significant.
Results
Of the 161,808 women enrolled in the WHI, 158,787 were eligible for this analysis (Figure 1). Among these participants, 25,933 (16.3%) reported a history of infertility. On average, women were 63 years of age at the time of enrollment and subsequently followed for an average of 19 years (Table 1), with no differences by history of infertility. Average age at primary ASCVD event was 81.4 years in both groups. Compared to women with no history of infertility, women with history of infertility were more likely to be White (Hispanic and Non-Hispanic), to have received a college degree or higher level of education, and have an annual family income over $75,000. Women with a history of infertility were also more likely to report hyperlipidemia and smoking history, while age at menopause and the prevalence of hypertension and diabetes were similar between the groups. 1.9% of WHI participants (N=3,021) were excluded from the analysis because they did not respond to questions about infertility or prior pregnancy history. These participants were more likely to be Black or Hispanic and had lower SES indicators than included participants, but did not differ in prevalence of ASCVD (Supplementary Tables 7 and 8).
Figure 1.
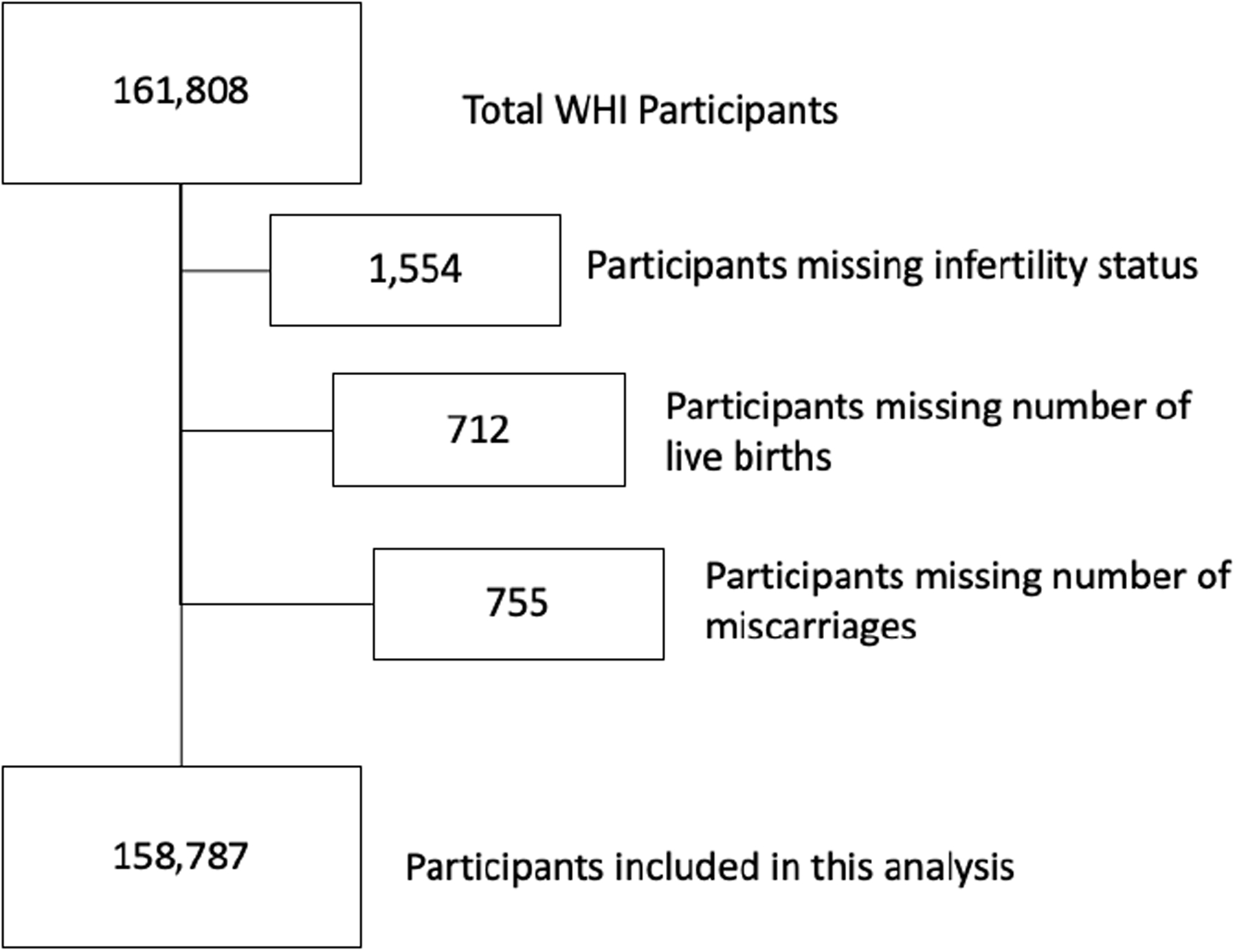
Flow Diagram for the Selection of Study Participants from the Women’s Health Initiative (WHI)
Table 1.
Baseline Characteristics of Study Population from the Women’s Health Initiative
| Variable* | History of Infertility N=25,933 (16.3%) | No History of Infertility N=132,854 (83.7%) | P-value** |
|---|---|---|---|
| Age at enrollment, mean (SD), yrs | 63.2 (7.4) | 63.2 (7.2) | 0.88 |
| Duration of follow up, mean (SD), yrs | 19.3 (5.1) | 19.3 (5.2) | 0.43 |
| Race, N (%) | <0.01 | ||
| Asian | 573 (2.2) | 2,585 (1.9) | |
| Black | 1,773 (6.8) | 11,904 (9.0) | |
| Native American/Alaska Native | 56 (0.2) | 231 (0.2) | |
| Pacific Islander | 19 (0.1) | 97 (0.1) | |
| White | 22,047 (85.0) | 109,217 (82.2) | |
| >1 Race | 277 (1.1) | 1,352 (1.0) | |
| Ethnicity, N (%) | <0.01 | ||
| Hispanic | 928 (3.6) | 6,067 (4.6) | |
| Non-Hispanic | 25,005 (96.4) | 126,787 (95.4) | |
| Highest Level of Education | <0.01 | ||
| High school degree or less | 5,243 (20.4) | 30,103 (22.8) | |
| Vocational or training school | 2,559 (9.9) | 13,548 (10.3) | |
| College or higher level education | 17,945 (69.7) | 88,220 (66.9) | |
| Annual Family income (USD) | <0.01 | ||
| <20,000 | 3,374 (14.0) | 21,343 (17.2) | |
| 20,000–75,000 | 15,617 (64.6) | 80,328 (64.7) | |
| >75,000 | 5,191 (21.5) | 22,404 (18.1) | |
| Atherosclerotic Cardiovascular Disease Risk Factors | |||
| Age at menopause, mean (SD), yrs | 47.4 (6.8) | 48.2 (6.4) | <0.01 |
| BMI (kg/m2) | <0.01 | ||
| <18.5 | 222 (0.86) | 1,132 (0.86) | |
| 18.5–24.9 | 9,222 (35.9) | 44,736 (34.0) | |
| 25.0–29.9 | 8,963 (34.9) | 45,688 (34.7) | |
| 30.0–34.9 | 4,524 (17.6) | 24,631 (18.7) | |
| >35 | 2,785 (10.8) | 15,387 (11.7) | |
| Hypertension, N (%) | 8,270 (33.6) | 42,815 (34.0) | 0.28 |
| Diabetes, N (%) | 1,525 (5.9) | 7,844 (5.9) | 0.89 |
| Ever smoker, N (%) | 13,197 (51.3) | 64,545 (48.9) | <0.01 |
| Hyperlipidemia, N (%) | 3,623 (14.8) | 17,455 (13.9) | <0.01 |
| Age at primary atherosclerotic cardiovascular disease event, mean (SD), yrs | 81.4 (7.6) | 81.4 (7.5) | 0.97 |
| Number of pregnancies, N (%) | <0.01 | ||
| 0 | 4,066 (15.7) | 10,714 (8.1) | |
| 1 | 3,419 (13.2) | 7,658 (5.8) | |
| 2 | 5,540 (21.4) | 25,002 (18.9) | |
| 3 | 2,192 (20.1) | 30,230 (22.8) | |
| 4 | 3,462 (13.4) | 24,003 (181) | |
| 5+ | 4,206 (16.3) | 35,021 (26.4) | |
| Number of live births, N (%) | <0.01 | ||
| 0 | 5,478 (21.1) | 13,690 (10.3) | |
| 1 | 4,492 (17.3) | 9,905 (7.5) | |
| 2 | 7,249 (28.0) | 33,407 (25.2) | |
| 3 | 4,965 (19.2) | 33,690 (25.4) | |
| 4 | 2,283 (8.8) | 21,562 (16.2) | |
| 5+ | 1,466 (5.7) | 20,600 (15.5) | |
| Number of pregnancy losses, N (%) | <0.01 | ||
| 0 | 16,842 (65.0) | 93,624 (70.5) | |
| 1 | 5,596 (21.6) | 26,100 (19.7) | |
| 2 | 2,051 (7.9) | 8,601 (6.5) | |
| 3+ | 1,444 (5.6) | 4,529 (3.4) | |
Percentage is column percentage. The percentage of missing observations for each covariate was as follows: 0% for age at screening, 1% for race and ethnicity, 0.7% for education, 6.6% for income, 5.7% for age at menopause, 0.9% for BMI, 5.2% for hypertenstion, 0.1% for diabetes, 0.7% for smoking status and 5.7% for hyperlipidemia.
Student’s T-test for continuous outcomes and Chi-square test for categorical outcomes.
Number of pregnancies, pregnancy losses and live birth differed between groups. Among women with a history of infertility, 21,867 (84.3%) became pregnant at least once, 20,455 (79.9%) had at least one live birth, and 9,091 (35.0%) experienced at least one pregnancy loss. Among women with no history of infertility, 122,140 (91.9%) became pregnant at least once, 119,164 (89.7%) had at least one live birth and 39,230 (29.5%) experienced at least one pregnancy loss.
The prevalence of any ASCVD outcome was 15.4% among all women with a history of infertility, and 14.9% among all women without a history of infertility (Table 2). The prevalence of peripheral arterial disease (1.2 vs 1.0%, respectively) and carotid artery disease (1.2 vs 1.0%, respectively) was higher among women with a history of infertility compared to women without a history of infertility. We replicated the comparisons by infertility status after stratifying by parity and pregnancy loss history (Supplementary Tables 1–6). Among all nulliparous women and nulliparous women with at least one pregnancy loss, a history of infertility was associated with a higher prevalence of any ASCVD outcome and higher prevalence of several individual ASCVD phenotypes.
Table 2.
Prevalence of Atherosclerotic Cardiovascular Disease inclusive of individual clinical phenotypes, stratified by fertility status.
| History of Infertility N=25,933 | No History of Infertility N=132,854 | |
|---|---|---|
| N (%) | N (%) | |
| Atherosclerotic Cardiovascular Disease (composite)ψ | 4,000 (15.4) | 19,860 (14.9) |
| Clinical myocardial infarction | 1,084 (4.2) | 5,330 (4.0) |
| Cardiac procedure (i.e., stent) | 1,523 (5.9) | 7,507 (5.7) |
| Percutaneous transluminal coronary angioplasty | 1,132 (4.4) | 5,471 (4.1) |
| Ischemic stroke | 863 (3.3) | 4,420 (3.3) |
| Peripheral arterial disease | 305 (1.2) | 1,335 (1.0) |
| Carotid artery disease | 297 (1.2) | 1,309 (1.0) |
| Death due to Cardiovascular Disease | 1,629 (6.3) | 8,181 (6.2) |
Includes any one of the following phenotypes: clinical myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial disease, carotid artery disease, or cardiovascular disease death.
Using time-to-event analysis, infertility was associated with a modest risk of ASCVD overall (aHR 1.02, 95% CI 0.99–1.06, Table 3). Based on a priori hypotheses, we modeled the association between infertility and ASCVD stratified by the other reproductive factors (Table 3). Among all nulliparous women, women with a history of infertility had a 21% higher risk of ASCVD in the crude model (HR 1.21, 95% CI 1.11–1.31), which attenuated to 13% higher risk after adjustment for covariates (HR 1.13, 95% CI 1.04–1.23). Among nulliparous women who also experienced a pregnancy loss, women with a history of infertility had a 33% higher risk of ASCVD in the crude model (HR 1.33, 95% CI 1.07–1.65) and 36% higher risk after adjustment for covariates (HR 1.36, 95% CI 1.09–1.71). Among nulliparous women without a history of pregnancy loss, infertility was weakly associated with ASCVD risk after covariate adjustment (HR 1.09, 95% CI 0.99–1.20).
Table 3.
Association of Fertility Status with Atherosclerotic Cardiovascular Disease using Cox proportional hazards models, stratified by pregnancy -related exposures.
| N | Composite Atherosclerotic Cardiovascular Disease Prevalence, N (%) | Crude HR (95% CI) | Adjusted HR1 (95% CI) | Adjusted HR2 (95% CI) | |
|---|---|---|---|---|---|
| All women, No History of Infertility | 132,854 | 19,814 (14.9) | -- (Ref) | ||
| All women, History of Infertility | 25,933 | 3,992 (15.4) | 1.03 (0.99–1.07) | 1.04 (1.00–1.07) | 1.02 (0.99–1.06) |
| Stratified by history of livebirth and pregnancy loss | |||||
| Among all nulliparous | |||||
| No History of Infertility | 13,690 | 1,742 (12.7) | -- (Ref) | ||
| History of Infertility | 5,478 | 831 (15.2) | 1.21 (1.11–1.31) | 1.17 (1.08–1.27) | 1.13 (1.04–1.23) |
| Among nulliparous with ≥ 1 pregnancy loss | |||||
| No History of Infertility | 1,152 | 150 (13.1) | -- (Ref) | ||
| History of Infertility | 986 | 171 (17.5) | 1.33 (1.07–1.65) | 1.47 (1.18–1.84) | 1.36 (1.09–1.71) |
| Among nulliparous without pregnancy loss | |||||
| No History of Infertility | 12,538 | 1,593 (12.7) | |||
| History of Infertility | 4,492 | 661 (14.7) | 1.17 (1.07–1.28) | 1.12 (1.02–1.23) | 1.09 (0.99–1.20) |
| Among all parous | |||||
| No History of Infertility | 119,164 | 18,072 (15.2) | -- (Ref) | ||
| History of Infertility | 20,455 | 3,161 (15.5) | 1.02 (0.98–1.05) | 1.03 (0.99–1.07) | 1.01 (0.97–1.05) |
| Among parous with ≥ 1 pregnancy loss | |||||
| No History of Infertility | 38,078 | 6,329 (16.6) | -- (Ref) | ||
| History of Infertility | 8,105 | 1,310 (16.2) | 0.96 (0.91–1.02) | 0.99 (0.94–1.06) | 0.98 (0.92–1.04) |
| Among parous without pregnancy loss | |||||
| No History of Infertility | 81,086 | 11,785 (14.5) | |||
| History of Infertility | 12,350 | 1,856 (15.0) | 1.03 (0.98–1.08) | 1.04 (0.99–1.09) | 1.03 (0.98–1.08) |
Adjusted for age at enrollment, family income, highest level of education attained, smoking status, race, and ethnicity.
Additionally adjusted for Atherosclerotic Cardiovascular Disease risk factors assessed at enrollment including age at menopause, hyperlipidemia (defined as use of cholesterol-lowering drugs), hypertension (including use of antihypertensive drugs), and diabetes (not pregnancy related), and BMI
Among all parous women, a history of infertility was not associated with ASCVD risk before or after covariate adjustment (aHR 1.01, 95% CI 0.97–1.05). Similarly, a history of infertility was not associated with ASCVD risk among parous women with a history of pregnancy loss (aHR 0.98, 95% CI 0.92–1.04) or parous women without a history of pregnancy loss (aHR 1.03, 95% CI 0.98–1.08).
In a subgroup analysis among nulliparous women with a history of infertility, the association of number of pregnancy losses and ASCVD was ascertained using time-to-event analysis. While the results are limited by small sample size, ASCVD risk was non-significant but higher after 2 pregnancy losses (aHR 1.28, 95% CI 0.92–1.77) and significantly higher after 3 or more pregnancy losses (aHR 1.51, 95% CI 1.10–2.06) but not increased after a single pregnancy loss (aHR 1.12, 95% CI 0.90–1.39) compared to women with no pregnancy loss (Supplementary Table 9).
Finally, we performed a sensitivity analysis excluding women with ASCVD risk factors, including age at menopause < 40 years, hyperlipidemia, hypertension, diabetes, and BMI > 30 kg/m2 with similar results to the overall analysis (Supplementary Table 10).
Discussion
In this large, multiracial cohort of postmenopausal women followed for up to 26 years, women with infertility experienced a modestly higher risk of ASCVD compared to women without a history of infertility. Among nulliparous women, particularly those with a history of pregnancy loss, the risk of ASCVD associated with infertility was much higher. These findings suggest that in these more extreme phenotypes, infertility may be associated with ASCVD risk. Our findings were robust to adjustment for sociodemographic characteristics and ASCVD risk factors. The exploration of nulliparity and pregnancy loss as more extreme infertility phenotypes and their relation to ASCVD risk among infertile women is a novel aspect of our study. We have previously reported among 64,000 infertile women (median age 34 years during 2003–2016) a 14% higher risk of cardiovascular disease compared to fertile women (7). These findings are complementary to a small study using the National Health and Nutrition Examination Survey data, which identified an 83% increased risk of cardiovascular disease among 111 women with a history of infertility (median age 40 years) (11) as well as a Swedish cohort of 80,000 women (median age 50 years), among whom infertility was associated with 20% increased risk of incident CVD compared to women without a history of infertility (10). Among older cohorts of women, however, infertility has not been consistently associated with increased risk of ASCVD (23, 24), suggesting that as women age, they may develop risk factors for cardiovascular disease that are stronger than infertility, such as metabolic syndrome.
Population studies of infertility are currently limited by considering infertility as a binary exposure, and not accounting for variation in severity or heterogenous underlying causes. The results of our study suggest significant associations of infertility and ASCVD among women who experience infertility and do not achieve live birth. This group may represent a more severe form of infertility. Alternative explanations for increased risk of ASCVD among nulliparous women include biological or social risk factors not captured by the current study design (25). For example, breastfeeding has a protective effect on ASCVD risk among women in the WHI, suggesting that hormonal exposures which are also affected by parity may play a significant role in ASCVD risk (24).
The highest risk group for ASCVD in our study was nulliparous women with a history of infertility who also experienced a pregnancy loss. Pregnancy loss is more common among women with a history of infertility and may be a more extreme phenotype of infertility, linked by common risk factors, including female age (26). Pregnancy loss has also been independently associated with an 11% higher ASCVD risk later in life among women in the WHI (27). In a subgroup analysis among nulliparous women with a history of infertility, we found non-significant but higher ASCVD risk among women with 2 or more pregnancy losses, but no increase in risk among with a single pregnancy loss. Our findings suggest that both infertility and pregnancy loss may be associated with ASCVD risk, and further investigation of these exposures is warranted in other cohorts.
Among women who become pregnant and achieve live birth, evidence is accumulating that pregnancy outcomes can be used to improve cardiovascular disease risk prediction. The physiologic demands of pregnancy exacerbate subclinical metabolic and vascular susceptibilities, such that pregnancy is viewed as a maternal stress test that can unmask future risk of cardiovascular disease(13). As over 80% of women with infertility eventually become pregnant (28), further studies are needed to understand the relation between infertility and adverse pregnancy outcomes, which are independently associated with ASCVD risk (29).
The biological mechanisms underlying the association of infertility and later life ASCVD are not addressed by our study and warrant evaluation. Perturbations at any level of the female reproductive system, ranging from hypothalamic/pituitary regulation and ovarian function to anatomic reproductive tract differences, can also cause female infertility (30). Infertility may be a cause or consequence of chronic inflammation, which has been strongly associated with ASCVD risk (31, 32). Reproductive disorders including ovulatory dysfunction, menstrual irregularity, polycystic ovarian syndrome (33, 34), and endometriosis(35) can cause infertility and also have been associated with increased risk of ASCVD (36).
Study Strengths
The WHI is a rare and unique source of longitudinal data on reproductive factors including infertility. A significant advantage of our study is that participants were followed for an average of 19 years after study enrollment (average age at enrollment=63 years). The median age for onset of ASCVD in women is 73 years (37) and prior studies of infertility (30) and ASCVD risk are primarily in younger cohorts of women (median age 34–40 years) (7, 11). Additional strengths of our study include the size of our cohort and utilization of adjudicated ASCVD outcomes.
An additional advantage of this study is our ability to study the natural history of infertility as it relates to ASCVD risk. Due to the age of WHI participants at time of enrollment (50–79 years between 1993–1998), very few participants would have had access to IVF. The first successful birth from IVF took place in 1981 in the U.S. (38), and access to IVF was not widely available until 1990 (39) when the youngest WHI participants were beyond their reproductive years and approaching menopause. Future studies are warranted in contemporaneous cohorts to compare ASCVD risk among women receiving infertility treatment from women with an infertility history.
Study Limitations
There are several limitations to our study design and analysis. Infertility, pregnancy loss and conditions associated with infertility including menstrual cycle irregularity have all been associated with premature mortality including premature cardiovascular mortality (40–43). Women with earlier onset or more severe ASCVD, as well as women with reproductive exposures that increase risk of premature mortality, may have been excluded from enrollment in the WHI. This possibility of selection bias may limit the conclusions of our study, and potentially result in underestimating the association of infertility with ASCVD. In addition, it is possible that the study included women with a history of cardiovascular disease and thus events that occurred prior to study entry were not captured. While we did adjust for cardiovascular disease risk factors in our analysis, we did not adjust for history of cardiovascular disease in accordance with the principles put forth by Howards et al(44).
While infertility is a heterogenous disease process and individual causes of infertility may have varied biologic pathways to ASCVD (45), a limitation of our work is that underlying causes of infertility are not investigated. For example, women with male factors as the only cause of infertility would not be expected to face an increased risk of ASCVD. By including heterogenous causes of infertility as a single exposure, we may be under or over-estimating associations with ASCVD risk. Duration of infertility has also been associated with ASCVD risk (10, 46), which we were unable to account for using the WHI.
Infertility is a highly memorable experience for women and self-report has been validated in studies compared to medical records (47) and the prevalence of infertility among WHI participants is similar to the prevalence of infertility in the US (30). Nevertheless, a potential for recall bias resulting in misclassification of infertility could have attenuated associations. The characteristics of women with infertility in the WHI also differ from previous findings from ethnically diverse cohorts of US women (48) including a lower proportion of infertile women who identify as Black, low income and with a lower prevalence of ASCVD risk factors including hypertension, obesity and hyperlipidemia. Because these characteristics are also independently associated with ASCVD risk (20, 49–51), their under-representation in the WHI may limit generalizability of our results. Furthermore, it is not possible to ascertain whether ASCVD risk factors developed before or after a woman’s reproductive years (at the time of her infertility diagnosis), as these data were collected at the time of enrollment into the WHI cohort. Because of this concern, we conducted two adjusted models, with the second containing covariates that could have preceded infertility including hypertension, BMI, dyslipidemia. A sensitivity analysis was also performed excluding women with ASCVD risk factors, with similar results to the overall analysis.
Conclusion
In conclusion, in this large, diverse cohort of women followed for nearly two decades, we report that infertility overall was modestly associated with risk of ASCVD; however, more extreme phenotypes of infertility among women provide significant insight into a woman’s cardiovascular health. Infertile women who did not achieve live birth or who also experienced a pregnancy loss faced a significantly higher ASCVD risk compared to women without a history of infertility. Given that infertility and pregnancy loss occur during a woman’s reproductive years, our findings suggest this period may present a unique opportunity for early identification and modification of ASCVD risk factors.
Supplementary Material
Funding:
Funding for this work was provided by a grant from the Stanford Maternal and Child Health Research Institute (MCHRI) and support from the Eunice Kennedy Shriver NICHD of the National Institutes of Health under award number 1K12HD103084. The WHI program is funded by NHLBI, NIH, US DHHS through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Murugappan is a Scientific Advisor to Hannah Life.
References
- 1.Heron M Deaths: Leading Causes for 2017. National Vital Statistics Reports 2017;68. [PubMed] [Google Scholar]
- 2.McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003;108:2619–23. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Benjamin E, Berra K, Bezanson J, Dolor R, Lloyd-Jones D et al. American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin TJ, Soumerai SB, Willison DJ, Gurwitz JH, Borbas C, Guadagnoli E et al. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Arch Intern Med 1996;156:799–805. [PubMed] [Google Scholar]
- 5.Wright VC, Schieve LA, Reynolds MA, Jeng G. Assisted reproductive technology surveillance--United States, 2000. MMWR Surveill Summ 2003;52:1–16. [PubMed] [Google Scholar]
- 6.Murugappan G, Li S, Alvero RJ, Luke B, Eisenberg ML. Association between infertility and all-cause mortality: analysis of US claims data. Am J Obstet Gynecol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of incident chronic medical conditions in infertile women: analysis of US claims data. Am J Obstet Gynecol 2019;220:473 e1–e14. [DOI] [PubMed] [Google Scholar]
- 8.Murugappan G, Li S, Lathi RB, Baker VL, Luke B, Eisenberg ML. Increased risk of severe maternal morbidity among infertile women: analysis of US claims data. Am J Obstet Gynecol 2020;223:404 e1–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Risk of cancer in infertile women: analysis of US claims data. Hum Reprod 2019;34:894–902. [DOI] [PubMed] [Google Scholar]
- 10.Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod 2012;27:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertil Steril 2019;111:138–46. [DOI] [PubMed] [Google Scholar]
- 12.Senapati S Infertility: a marker of future health risk in women? Fertil Steril 2018;110:783–9. [DOI] [PubMed] [Google Scholar]
- 13.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J 2010;159:215–21 e6. [DOI] [PubMed] [Google Scholar]
- 14.Lepkowski JM MW, Davis KE, Groves RM, Van Hoewyk J. . The 2006–2010 National Survey of Family Growth: Sample design and analysis of a continuous survey. National Center for Health Statistics. Vital Health Stat 2010;150. [PubMed] [Google Scholar]
- 15.Practice Committee of the American Society for Reproductive Medicine. Electronic address aao. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2020;113:533–5. [DOI] [PubMed] [Google Scholar]
- 16.Hakim RB, Gray RH, Zacur H. Infertility and early pregnancy loss. Am J Obstet Gynecol 1995;172:1510–7. [DOI] [PubMed] [Google Scholar]
- 17.Agenor A, Bhattacharya S. Infertility and Miscarriage: Common Pathways in Manifestation and Management. Women’s Health 2015;11:527–41. [DOI] [PubMed] [Google Scholar]
- 18..Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 2003;13:S18–77. [DOI] [PubMed] [Google Scholar]
- 20.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e563–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 2003;13:S122–8. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 2019;48:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cairncross ZF, Ahmed SB, Dumanski SM, Nerenberg KA, Metcalfe A. Infertility and the Risk of Cardiovascular Disease: Findings From the Study of Women’s Health Across the Nation (SWAN). CJC Open 2021;3:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane-Cordova AD, Gunderson EP, Greenland P, Catov JM, Lewis CE, Gabriel KP et al. Life-Course Reproductive History and Cardiovascular Risk Profile in Late Mid-Life: The CARDIA Study. Journal of the American Heart Association 2020;9:e014859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H-Y, Qiao J, Sun X-X, Wang S-Y, Liang X-Y, Sun Y et al. Epidemiological Survey and Risk Factor Analysis of Recurrent Spontaneous Miscarriages in Infertile Women at Large Infertility Centers. Chin Med J (Engl) 2017;130:2056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV et al. Relation of Pregnancy Loss to Risk of Cardiovascular Disease in Parous Postmenopausal Women (From the Women’s Health Initiative). Am J Cardiol 2019;123:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan DA, Goldman MB, Koniares KG, Nesbit CB, Toth TL, Fung JL et al. Long-term reproductive outcomes in patients with unexplained infertility: follow-up of the Fast Track and Standard Treatment Trial participants. Fertility and Sterility. [DOI] [PubMed] [Google Scholar]
- 29.Sondergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M et al. Association of Adverse Pregnancy Outcomes With Risk of Atherosclerotic Cardiovascular Disease in Postmenopausal Women. JAMA Cardiol 2020;5:1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbieri RL. Female Infertility. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe’s Reproductive Endocrinology. Philadelphia: Elsevier, 2019:556–81.e7. [Google Scholar]
- 31.Lorenzatti AJ, Servato ML. New evidence on the role of inflammation in CVD risk. Current Opinion in Cardiology 2019;34:418–23. [DOI] [PubMed] [Google Scholar]
- 32.Moore KJ. Targeting inflammation in CVD: advances and challenges. Nature Reviews Cardiology 2019;16:74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 2002;87:2013–7. [DOI] [PubMed] [Google Scholar]
- 34.Gast GC, Grobbee DE, Smit HA, Bueno-de-Mesquita HB, Samsioe GN, van der Schouw YT. Menstrual cycle characteristics and risk of coronary heart disease and type 2 diabetes. Fertil Steril 2010;94:2379–81. [DOI] [PubMed] [Google Scholar]
- 35.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and Risk of Coronary Heart Disease. Circ Cardiovasc Qual Outcomes 2016;9:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 2008;93:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Crimmins EM, Hayward MD, Ueda H, Saito Y, Kim JK. Life with and without heart disease among women and men over 50. J Women Aging 2008;20:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederberger C, Pellicer A, Cohen J, Gardner DK, Palermo GD, O’Neill CL et al. Forty years of IVF. Fertil Steril 2018;110:185–324 e5. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J, Trounson A, Dawson K, Jones H, Hazekamp J, Nygren KG et al. The early days of IVF outside the UK. Hum Reprod Update 2005;11:439–59. [DOI] [PubMed] [Google Scholar]
- 40.Wang YX, Arvizu M, Rich-Edwards JW, Stuart JJ, Manson JE, Missmer SA et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. Bmj 2020;371:m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YX, Mínguez-Alarcón L, Gaskins AJ, Missmer SA, Rich-Edwards JW, Manson JE et al. Association of spontaneous abortion with all cause and cause specific premature mortality: prospective cohort study. Bmj 2021;372:n530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murugappan GLS, Alvero R, Luke B, Eisenberg ME. Association of infertility and all-cause mortality: analysis of US claims data. Am J Obstet Gynecol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y-X, Farland LV, Wang S, Gaskins AJ, Wang L, Rich-Edwards JW et al. Association of infertility with premature mortality among US women: Prospective cohort study. The Lancet Regional Health - Americas 2022;7:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. American journal of epidemiology 2012;176:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Group ECW. Diagnosis and management of the infertile couple: missing information. Hum Reprod Update 2004;10:295–307. [DOI] [PubMed] [Google Scholar]
- 46.Magnus MC, Fraser A, Rich-Edwards JW, Magnus P, Lawlor DA, Håberg SE. Time-to-pregnancy and risk of cardiovascular disease among men and women. Eur J Epidemiol 2021;36:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dick ML, Bain CJ, Purdie DM, Siskind V, Molloy D, Green AC. Self-reported difficulty in conceiving as a measure of infertility. Hum Reprod 2003;18:2711–7. [DOI] [PubMed] [Google Scholar]
- 48.Wellons MF, Lewis CE, Schwartz SM, Gunderson EP, Schreiner PJ, Sternfeld B et al. Racial differences in self-reported infertility and risk factors for infertility in a cohort of black and white women: the CARDIA Women’s Study. Fertil Steril 2008;90:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham G Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGruder HF, Malarcher AM, Antoine TL, Greenlund KJ, Croft JB. Racial and ethnic disparities in cardiovascular risk factors among stroke survivors: United States 1999 to 2001. Stroke 2004;35:1557–61. [DOI] [PubMed] [Google Scholar]
- 51.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation 2005;111:1233–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


