Abstract
Objective:
To determine trends in amputations and revascularizations for peripheral arterial disease in a well-defined population.
Patients and methods:
Population-based cohort study of Olmsted County, MN residents with peripheral arterial disease undergoing amputation or revascularization between January 1, 1990 and December 31, 2009. Population-level 5-year incidence trends for endovascular, open surgical and hybrid revascularizations and major and minor amputations were determined. Limb-specific outcomes following revascularization including major adverse limb events and amputation-free survival were compared between initial surgical and endovascular/hybrid revascularization groups using Kaplan-Meier analysis.
Results:
We identified 773 residents who underwent 1906 limb-procedures, including 689 open revascularizations, 685 endovascular/hybrid revascularizations and 220 major amputations. Over the 20-year study period, the incidence of endovascular and hybrid revascularizations increased while the incidence of open surgical revascularizations and major amputations decreased. Incidence of revascularizations for chronic limb-threatening ischemia did not change. Among residents with chronic limb-threatening ischemia undergoing their first revascularization on a limb, endovascular revascularization was associated with more major adverse limb events and major amputations compared to surgical revascularization over the ensuing 15 years.
Conclusions:
A rising incidence of endovascular/hybrid revascularizations and decreasing incidence of open surgical revascularizations for peripheral arterial disease was associated with a decreasing incidence of major amputations in this population between 1990 and 2009, despite a stable incidence of revascularizations for chronic limb-threatening ischemia. With more major adverse limb events and major amputations following endovascular revascularization, these trends suggest that additional emphasis should be placed on improving limb salvage efforts beyond just mode of revascularization.
Introduction:
Atherosclerotic peripheral arterial disease (PAD) affects 12% of adults and 20% of persons over age 70 1. Chronic limb-threatening ischemia (CLTI) due to PAD can lead to major amputation, which remains a common occurrence with significant associated morbidity, mortality and financial costs 2–4. Claudication, though not a major limb threat, can cause significant functional impairment.
The efficacy of endovascular (ENDO) vs open surgical (OPEN) revascularization on prevention of limb loss has been debated extensively 2. Generally, ENDO is considered less morbid but less durable compared to OPEN. With a paucity of level 1 data on the subject, the BASIL Trial is the most frequently cited randomized trial and demonstrated improved amputation-free survival with bypass surgery as opposed to angioplasty 5. Specialty societies followed suit in assigning Class IIa recommendations for autogenous vein bypass as the initial revascularization for CLTI patients expected to survive two or more years and angioplasty for the rest 6. Subsequent comparative effectiveness studies by AHRQ, however, found no difference in amputation-free survival or relief of claudication symptoms between ENDO and OPEN, though the level of evidence supporting these conclusions was low 7,8.
Population-based studies have generally shown an increase in the incidence of ENDO over the past few decades and a decrease in OPEN 9–18. Most showed associations with reductions in major amputations 9,11,13,15,16,18, though others have not 10,12,14,17. Most of these studies were limited in the amount of individual-level data, such as laterality or ability to account for multiple procedures on an individual over an extended time period. Our study was conducted to assess the impact of endovascular technology by evaluating population-level trends in ENDO and OPEN among residents of a single county (Olmsted County, MN) in the United States between 1990 and 2009 as well as concomitant trends in major and minor amputations. Our study is largely an update of the study by Hallett et al 19 on the Olmsted County population from 1973–1992, which showed that an increase in revascularizations (primarily OPEN) was associated with a decrease in major amputations.
Methods:
Subject Identification, Data Acquisition and Follow-up:
This study was a population-based cohort study of Olmsted County, MN residents undergoing procedures for atherosclerotic PAD. Utilizing the Rochester Epidemiology Project (REP) 20, patients were identified through an electronic search of CPT-4 and ICD-9 procedure codes for all lower extremity revascularization (endovascular and open), lumbar sympathectomy and amputation procedures performed between January 1, 1990 and December 31, 2009 (Supplemental Methods). Manual review of the complete medical records from Mayo Clinic, Olmsted Medical Center and other REP providers was performed to identify patients with atherosclerotic PAD (as diagnosed by their physician at the time) as the indication for the procedure and confirm Olmsted County residency at the time of the procedure using REP criteria 20. Demographic, clinical and procedural data were then collected via manual chart review and logged in REDCap 21. Chart review of all surviving patients was updated in August, 2012. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and supported by funds from the Mayo Clinic Gonda Vascular Center and Division of Vascular and Endovascular Surgery. Details about the REP records-linkage system have been previously described 20.
Chart abstraction utilized methods and terminology similar to Hallett et al 19, with the addition of comorbidity data and newer endovascular procedure/classification terminology (Supplemental Methods). Prevent III score is a validated prediction model for amputation-free survival among patients with CLTI 22. The score predicts 1-year amputation-free survival using 5 clinical variables: dialysis-dependence, tissue loss, coronary artery disease, age ≥75 years and hematocrit <30% with higher scores predicting lower amputation-free survival. Revascularizations were classified as ENDO, OPEN or, if a combination of OPEN and ENDO techniques was used, as “HYBRID”. If prior revascularization on the limb occurred, amputations were considered secondary and primary if not.
Main and Secondary Objectives:
The main objective was to determine 5-year incidence trends for major and minor amputations, and OPEN, ENDO and HYBRID revascularizations between 1990 and 2009. The secondary objectives were to determine 5-year incidence trends for revascularizations for claudication vs CLTI and to compare outcomes for OPEN and ENDO/HYBRID (HYBRID was combined with ENDO due to the utilization of endovascular technology and the relatively small number of HYBRID procedures). Specifically, major amputation, major adverse limb events (MALE; major revascularization or major amputation), amputation-free survival and MALE+ (any MALE or minor revascularization) 23 following initial revascularization on a limb were compared between OPEN and ENDO/HYBRID.
Statistical Analysis:
Procedures were analyzed as “limb-procedures” where each limb intervened on during a single procedure was counted as a separate “limb-procedure” 19. Complete demographic characteristics and clinical data were available for all unique patients. This data was compared by calendar year of first limb-procedure and by type of first limb-procedure using linear regression for continuous variables and logistic regression for categorical variables. Rationales for performing major amputations instead of revascularization were ranked from most to least severe (Supplemental Figure 1). Changes in rationale by calendar year were assessed for all major amputations using cumulative logistic models with generalized estimating equations (independent correlation structure) to account for patients with multiple amputations.
Incidence rates were calculated per 100,000 person-years, where incident limb-procedures were defined as Olmsted County, Minnesota residents on the procedure date. Annual age-specific and gender-specific population counts for 1990 through 2009 were also obtained from the REP Census of Olmsted County. Using this denominator, incidence rates were calculated for 1990 through 2009, both overall and for 5-year periods (bins). These rates were adjusted to the gender and 5-year interval age distribution of whites in the United States for 2000 using the direct method. The 95% confidence intervals for the incidence estimates were calculated assuming the cases follow a Poisson distribution. Poisson regression models adjusted for age and sex were used to test secular trends in 5-year incidence rates.
Outcomes of major amputation, MALE, MALE+ and amputation-free survival were determined 23 by following patients from their earliest OPEN or ENDO/HYBRID procedure date on each limb separately until the earliest of the outcomes of interest, or censored at last follow-up (or death for major amputation, MALE and MALE+). Survival estimates for time to each outcome were calculated using the Kaplan Meier method and compared using log-rank tests. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria), and P<.05 was considered statistically significant.
Results:
Study Population Demographics and Procedures:
The electronic search identified 2325 patients who underwent 3972 procedures. Of these, 773 patients met inclusion criteria for the cohort and underwent a total of 1906 limb-procedures. Twenty-five procedures were concomitant revascularization and minor amputation, which were counted as separate limb-procedures and categorized as a revascularization if it was their first limb-procedure (N=11). Etiologies for excluded non-PAD procedures/patients are listed in Supplemental Table 1.
The cohort had more males than females (58.5% vs 41.5%) and was predominantly Caucasian (96.5%; 2.3% African American, 0.6% Hispanic, 0.5% other). The 1906 limb-procedures consisted of 689 OPEN, 611 ENDO, 74 HYBRID, 220 major amputations and 312 minor amputations. Trends in cohort demographics at the time of the patient’s first limb-procedure over the 20-year study period are summarized in Table 1. Body mass index (BMI) increased over time, as did prior coronary artery interventions and diagnoses of dyslipidemia and hypertension. Prevent III score decreased over time and fewer patients had a history of transient ischemic attack (TIA)/stroke/amaurosis. There were fewer “never smokers” and more current smokers in more recent years.
Table 1.
Cohort Characteristics at the Time of First Limb-Procedure Over the Study Period (1990–2009)
| 1990–94 (n=176) |
1995–99 (n=192) |
2000–04 (n=194) |
2005–09 (n=211) |
Trend P | |
|---|---|---|---|---|---|
| Age, mean (SD), years | 70.0 (13.1) | 69.6 (13.0) | 67.4 (13.8) | 67.8 (12.4) | .08 |
| Male gender, no. (%)a | 101 (57.4) | 114 (59.4) | 109 (56.2) | 128 (60.7) | .60 |
| Non-Caucasian race/ethnicity, no. (%)a | 2 (1.1) | 5 (2.6) | 8 (4.1) | 12 (5.7) | .005 |
| Height, mean (SD), cm | 166.9 (9.6) | 167.1 (9.9) | 169.0 (10.7) | 169.4 (10.2) | .002 |
| Weight, mean (SD), kg | 74.1 (18.8) | 74.8 (19.3) | 77.7 (19.6) | 82.1 (21.4) | <.001 |
| BMI, mean (SD), kg/m2 | 26.5 (5.7) | 26.6 (5.8) | 27.0 (5.8) | 28.4 (6.2) | <.001 |
| Smoking, no. (%)a | .04 | ||||
| Never | 43 (24.4) | 44 (22.9) | 40 (20.6) | 31 (14.7) | |
| Former | 76 (43.2) | 81 (42.2) | 83 (42.8) | 98 (46.4) | |
| Current | 57 (32.4) | 67 (34.9) | 71 (36.6) | 82 (38.9) | |
| Creatinine, mean (SD), mg/dLb | 1.3 (0.8) | 1.6 (1.4) | 1.5 (1.1) | 1.3 (1.2) | .73 |
| Hematocrit <30%, no. (%)a | 20 (11.4) | 29 (15.1) | 17 (8.8) | 21 (10.0) | .35 |
| Prevent III score, mean (SD) | 3.2 (2.3) | 3.4 (2.4) | 3.0 (2.5) | 2.6 (2.3) | .009 |
| Prior coronary intervention, no. (%)a | 29 (16.5) | 43 (22.4) | 54 (27.8) | 62 (29.4) | <.001 |
| Functioning kidney transplant, no. (%)a | 4 (2.3) | 3 (1.6) | 9 (4.6) | 4 (1.9) | .69 |
| Comorbidities, no. (%)a | |||||
| Dyslipidemia | 56 (31.8) | 85 (44.3) | 128 (66.0) | 166 (78.7) | <.001 |
| Hypertension | 112 (63.6) | 144 (75.0) | 159 (82.0) | 183 (86.7) | <.001 |
| Diabetes mellitus | 84 (47.7) | 91 (47.4) | 76 (39.2) | 84 (39.8) | .16 |
| Coronary artery disease | 76 (43.2) | 95 (49.5) | 94 (48.5) | 104 (49.3) | .18 |
| Congestive heart failure | 33 (18.8) | 39 (20.3) | 26 (13.4) | 32 (15.2) | .27 |
| Stroke/TIA/amaurosis | 46 (26.1) | 54 (28.1) | 38 (19.6) | 41 (19.4) | .04 |
| COPD | 33 (18.8) | 46 (24.0) | 41 (21.1) | 42 (19.9) | .69 |
| End-stage renal disease | 4 (2.3) | 11 (5.7) | 9 (4.6) | 10 (4.7) | .42 |
BMI = body mass index; COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack.
Percentages have been rounded and may not total 100.
To convert serum creatinine to μmol/L, multiply values by 88.4.
Similar sub-cohort analysis of demographic trends by type of first limb-procedure (OPEN, ENDO/HYBRID, major amputation and minor amputation) showed no noteworthy differences from the cohort-level trends (Supplemental Table 2). As shown in Table 2, when first-procedure OPEN and ENDO/HYBRID subcohorts were compared, ENDO/HYBRID patients had higher BMI and more prior coronary interventions and dyslipidemia. There were no differences, however, in comorbidities such as age, Prevent III score, creatinine, congestive heart failure, end-stage renal disease or COPD. Compared to patients undergoing revascularization (OPEN, ENDO or HYBRID) as a first procedure, patients undergoing major amputation were older, had higher Prevent III score and more hematocrit ≤30%, diabetes mellitus, congestive heart failure, prior stroke/TIA/amaurosis and end-stage renal disease.
Table 2.
Sub-Cohort Comparisons of Characteristics by Type of First Limb Procedure
| OPEN (n=285) |
ENDO/HYBRID (n=309) |
P | Any Revasc. (n=594) |
Major Amputation (n=74) |
P | |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 67.7 (12.7) | 68.1 (12.3) | .77 | 67.9 (12.5) | 75.0 (12.9) | <.001 |
| Male gender, no. (%)a | 169 (59.3) | 176 (57.0) | .56 | 345 (58.1) | 42 (56.8) | .83 |
| Non-Caucasian race/ethnicity, no. (%)a | 5 (1.8) | 14 (4.5) | .06 | 19 (3.2) | 1 (1.4) | .39 |
| Height, mean (SD), cm | 168.0 (9.7) | 167.9 (9.9) | .85 | 167.9 (9.8) | 167.9 (11.0) | .97 |
| Weight, mean (SD), kg | 75.8 (18.0) | 78.9 (20.0) | .054 | 77.4 (19.1) | 70.9 (19.5) | .006 |
| BMI, mean (SD), kg/m2 | 26.7 (5.3) | 27.8 (5.8) | .02 | 27.3 (5.6) | 25.1 (6.0) | .002 |
| Smoking, no. (%)a | .15 | <.001 | ||||
| Never | 40 (14.0) | 48 (15.5) | 88 (14.8) | 28 (37.8) | ||
| Former | 118 (41.4) | 143 (46.3) | 261 (43.9) | 32 (43.2) | ||
| Current | 127 (44.6) | 118 (38.2) | 245 (41.2) | 14 (18.9) | ||
| Creatinine, mean (SD), mg/dLb | 1.3 (0.9) | 1.4 (1.3) | .82 | 1.3 (1.1) | 1.6 (1.0) | .11 |
| Hematocrit <30%, no. (%)a | 20 (7.0) | 19 (6.1) | .67 | 39 (6.6) | 22 (29.7) | <.001 |
| Prevent III score, mean (SD) | 2.5 (2.2) | 2.3 (2.2) | .21 | 2.4 (2.2) | 5.6 (1.7) | <.001 |
| Prior coronary intervention, no. (%)a | 58 (20.4) | 92 (29.8) | .009 | 150 (25.3) | 15 (20.3) | .35 |
| Functioning kidney transplant, no. (%)a | 5 (1.8) | 6 (1.9) | .87 | 11 (1.9) | 4 (5.4) | .06 |
| Comorbidities, no. (%)a | .02 | |||||
| Dyslipidemia | 159 (55.8) | 201 (65.0) | 360 (60.6) | 30 (40.5) | .001 | |
| Hypertension | 214 (75.1) | 248 (80.3) | .13 | 462 (77.8) | 51 (68.9) | .09 |
| Diabetes mellitus | 94 (33.0) | 119 (38.5) | .16 | 213 (35.9) | 41 (55.4) | .001 |
| Coronary artery disease | 130 (45.6) | 146 (47.2) | .69 | 276 (46.5) | 43 (58.1) | .06 |
| Congestive heart failure | 33 (11.6) | 44 (14.2) | .34 | 77 (13.0) | 31 (41.9) | <.001 |
| Prior stroke/TIA/amaurosis | 67 (23.5) | 60 (19.4) | .22 | 127 (21.4) | 34 (45.9) | <.001 |
| COPD | 69 (24.2) | 61 (19.7) | .19 | 130 (21.9) | 19 (25.7) | .46 |
| End-stage renal disease | 9 (3.2) | 10 (3.2) | .96 | 19 (3.2) | 6 (8.1) | .04 |
BMI = body mass index; COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack.
Percentages have been rounded and may not total 100.
To convert serum creatinine to μmol/L, multiply values by 88.4.
For patients undergoing major amputation, the primary rationale for why this was done as opposed to revascularization is illustrated in Supplemental Figure 1. Of the 220 major amputations, the most common indications were septic foot or other tissue loss beyond salvage in 26.4% and no reasonable options for revascularization in 23.6%. There were no changes over time in rationales by calendar year (OR per year 1.01, 95% CI 0.97 to 1.06, P=.57).
Type and level of revascularization for OPEN, ENDO and HYBRID are listed in Supplemental Table 3. Overall, 82% of 689 OPEN procedures involved a bypass, most commonly suprainguinal, though 39% were infrageniculate. Stenting accounted for almost half of 611 ENDO procedures and was primarily suprainguinal (81%), with less than 1% of stents involving infrageniculate vessels. Balloon angioplasty (without stenting) accounted for 42% of ENDO and was most commonly infrainguinal but suprageniculate. Infrageniculate angioplasties accounted for 36% of balloon angioplasties and 60% involved concomitant more proximal angioplasty. HYBRID (N=74) was most commonly infrainguinal but suprageniculate and 82% of these were multilevel (eg, common femoral endarterectomy plus iliac stenting).
Main Objectives: 5-Year Incidence Rate Trends:
Between 1990 and 2009, the rate of total revascularizations increased by 34% (P<.001) (Table 3). As shown in Figure 1, this was primarily due to a 325% increase in ENDO (P<.001), as there was a 53% decrease in OPEN over this period (P<.001). HYBRID increased by 182% (P<.001) but made up only 5% of total revascularizations. There was no change in minor amputations but a 37% decrease in major amputations occurred (P=.002). This decrease remained significant when only primary amputations and only initial major amputation (eg, excluded above knee amputation performed after below knee amputation on same limb) were included in the analysis. Major amputation rates among non-PAD patients (eg, trauma, malignancy) excluded from the cohort did not change between 1990 and 2009 (Supplemental Figure 2).
Table 3.
Five-Year Age- and Sex-Adjusted Incidence Trends
| Procedure Type | Overall | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Rate (95% CI) | No. | Rate (95% CI) | No. | Rate (95% CI) | No. | Rate (95% CI) | No. | Rate (95% CI) | ||
| Any amputation | 532 | 39.0 (35.6–42.3) | 120 | 44.9 (36.7–53.0) | 138 | 45.0 (37.5–52.6) | 149 | 40.8 (34.2–47.4) | 125 | 29.4 (24.2–34.6) | <.001 |
| Major amputation | 220 | 16.3 (14.1–18.5) | 56 | 20.6 (15.1–26.1) | 62 | 20.4 (15.3–25.5) | 48 | 13.5 (9.7–17.4) | 54 | 12.9 (9.4–16.4) | .002 |
| First per leg | 210 | 15.6 (13.4–17.7) | 55 | 20.3 (14.9–25.7) | 59 | 19.4 (14.4–24.4) | 46 | 13.0 (9.2–16.7) | 50 | 12.0 (8.6–15.3) | <.001 |
| Primary | 120 | 8.9 (7.3–10.5) | 35 | 12.8 (8.5–17.1) | 39 | 13.0 (8.9–17.0) | 26 | 7.4 (4.5–10.2) | 20 | 4.8 (2.7–7.0) | <.001 |
| Secondary | 100 | 7.4 (5.9–8.8) | 21 | 7.8 (4.4–11.1) | 23 | 7.5 (4.4–10.5) | 22 | 6.1 (3.6–8.7) | 34 | 8.1 (5.3–10.8) | >.99 |
| Minor amputation | 312 | 22.7 (20.1–25.2) | 64 | 24.3 (18.3–30.3) | 76 | 24.6 (19.0–30.2) | 101 | 27.2 (21.9–32.6) | 71 | 16.5 (12.6–20.4) | .08 |
| Any revascularizationa | 1,374 | 101.1 (95.7–106.5) | 228 | 86.8 (75.5–98.2) | 297 | 96.3 (85.3–107.3) | 365 | 99.7 (89.4–110.0) | 484 | 116.3 (105.8–126.7) | <.001 |
| Claudication | 718 | 52.5 (48.6–56.4) | 101 | 38.3 (30.8–45.8) | 130 | 41.9 (34.7–49.1) | 190 | 51.6 (44.2–59.0) | 297 | 70.6 (62.5–78.7) | <.001 |
| CLTI | 646 | 47.9 (44.2–51.7) | 127 | 48.6 (40.0–57.1) | 164 | 53.4 (45.2–61.6) | 170 | 46.9 (39.8–54.0) | 185 | 45.3 (38.7–51.8) | .33 |
| Open | 689 | 50.3 (46.5–54.0) | 172 | 65.6 (55.8–75.5) | 189 | 61.4 (52.6–70.2) | 196 | 52.5 (45.1–59.9) | 132 | 30.8 (25.5–36.1) | <.001 |
| Claudication | 309 | 22.2 (19.7–24.7) | 75 | 28.2 (21.7–34.6) | 83 | 26.9 (21.1–32.7) | 85 | 22.4 (17.6–27.2) | 66 | 14.8 (11.2–18.5) | <.001 |
| CLTI | 372 | 27.5 (24.7–30.4) | 97 | 37.5 (30.0–45.0) | 103 | 33.5 (27.0–40.0) | 107 | 29.2 (23.6–34.8) | 65 | 15.8 (11.9–19.6) | <.001 |
| Endovascular | 611 | 45.5 (41.9–49.1) | 47 | 17.9 (12.7–23.1) | 97 | 31.5 (25.2–37.8) | 155 | 43.2 (36.4–50.1) | 312 | 76.1 (67.6–84.7) | <.001 |
| Hybrid | 74 | 5.4 (4.1–6.6) | 9 | 3.3 (1.1–5.4) | 11 | 3.4 (1.4–5.4) | 14 | 3.9 (1.9–6.0) | 40 | 9.3 (6.4–12.2) | <.001 |
| Endovascular / hybrid | 685 | 50.9 (47.0–54.7) | 56 | 21.2 (15.6–26.8) | 108 | 34.9 (28.3–41.6) | 169 | 47.2 (40.0–54.3) | 352 | 85.4 (76.4–94.5) | <.001 |
| Claudication | 409 | 30.3 (27.4–33.3) | 26 | 10.1 (6.2–14.1) | 47 | 15.0 (10.7–19.4) | 105 | 29.2 (23.6–34.8) | 231 | 55.7 (48.5–63.0) | <.001 |
| CLTI | 274 | 20.4 (18.0–22.8) | 30 | 11.1 (7.1–15.1) | 61 | 19.9 (14.9–24.9) | 63 | 17.7 (13.3–22.1) | 120 | 29.5 (24.2–34.8) | <.001 |
| Revascularization by arterial level | |||||||||||
| Openb | |||||||||||
| Suprainguinal | 282 | 20.0 (17.6–22.3) | 77 | 28.7 (22.2–35.1) | 81 | 26.3 (20.5–32.0) | 79 | 20.4 (15.9–25.0) | 45 | 9.8 (6.9–12.7) | <.001 |
| Infrainguinal/suprageniculate | 144 | 10.7 (8.9–12.4) | 28 | 10.8 (6.8–14.9) | 41 | 13.4 (9.3–17.5) | 33 | 8.7 (5.7–11.7) | 42 | 10.1 (7.0–13.2) | .52 |
| Infrageniculate | 254 | 18.9 (16.6–21.3) | 60 | 23.4 (17.4–29.4) | 65 | 21.1 (16.0–26.3) | 84 | 23.4 (18.4–28.4) | 45 | 10.9 (7.7–14.1) | <.001 |
| Sympathectomyb | 9 | - | 7 | - | 2 | - | 0 | - | 0 | - | - |
| Endovascular / hybrid | |||||||||||
| Suprainguinal | 303 | 22.5 (19.9–25.0) | 14 | 5.6 (2.6–8.6) | 38 | 12.3 (8.4–16.2) | 97 | 26.8 (21.4–32.2) | 154 | 37.2 (31.3–43.1) | <.001 |
| Infrainguinal/suprageniculate | 224 | 16.7 (14.5, 18.9) | 20 | 7.5 (4.2, 10.8) | 36 | 11.8 (7.9, 15.7) | 39 | 10.8 (7.4, 14.2) | 129 | 32.0 (26.4, 37.5) | <.001 |
| Infrageniculate | 158 | 11.7 (9.8, 13.5) | 22 | 8.1 (4.7, 11.6) | 34 | 10.9 (7.2, 14.5) | 33 | 9.5 (6.3, 12.8) | 69 | 16.3 (12.4, 20.2) | .004 |
CLTI = chronic limb-threatening ischemia.
Ten revascularization procedures (8 open and 2 endovascular) had unknown indication, so are not included in the claudication or CLTI subgroups.
Too few sympathectomy procedures were available for incidence calculations. The most recent sympathectomy was in 1997.
Figure 1 -. Five-year age- and sex-adjusted incidence trends.
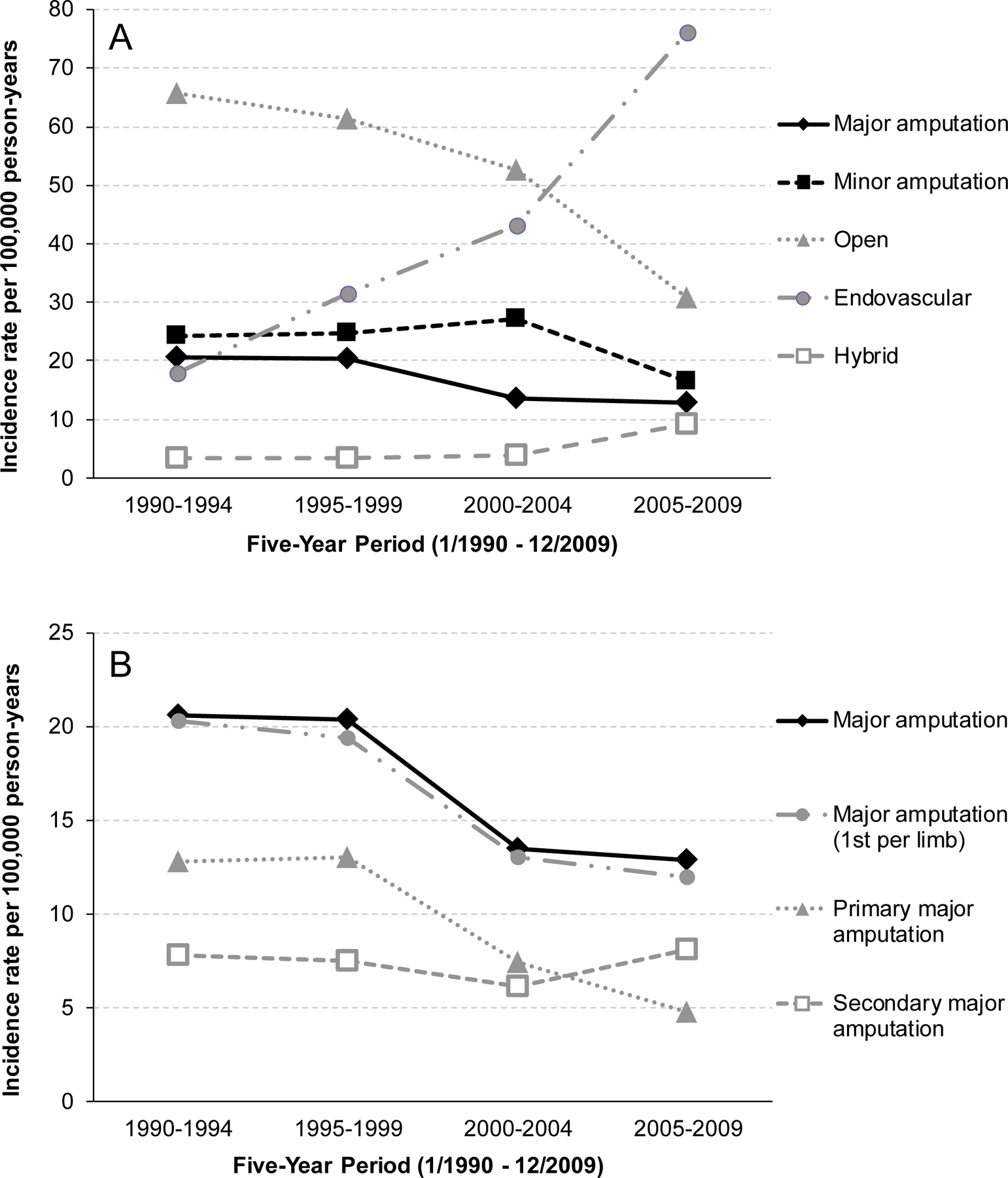
Figure 1a – Five-year incidence trends for amputations and revascularizations between 1990 and 2009. Figure 1b – Incidence trends for major amputations, further subdivided into primary, secondary and excluding revisions to a higher level (1st per limb).
Trends by Indication and Level of Revascularization:
Between 1990 and 2009, there was an 84% increase in any (ENDO, OPEN or HYBRID) revascularization for claudication (P<.001) but no change in revascularizations for CLTI (Table 3). OPEN saw decreases in both claudication and CLTI whereas ENDO/HYBRID saw increases in both. By level of revascularization, there were fewer OPEN revascularizations at the suprainguinal and infrageniculate level but no change in infrainguinal/suprageniculate revascularizations over the study period. ENDO/HYBRID revascularizations increased at all three levels (Table 3).
ENDO/HYBRID vs OPEN Outcomes:
Given the observed decrease in major amputations but stable incidence of revascularizations for CLTI over the study period, outcomes following initial OPEN vs ENDO/HYBRID were compared (median 5.4 years follow-up [interquartile range 2.8–9.0]). Kaplan-Meier analyses of outcomes following ENDO/HYBRID vs OPEN as the initial mode of revascularization for each limb are shown in Figure 2 and Supplemental Figure 3. When all indications were considered together, more limbs with initial ENDO/HYBRID underwent subsequent MALE+ (Supplemental Figure 3). MALE was not significantly different, thus the difference in MALE+ primarily indicated a greater need for minor revascularizations.
Figure 2 – Outcomes following revascularization by initial mode and by indication.
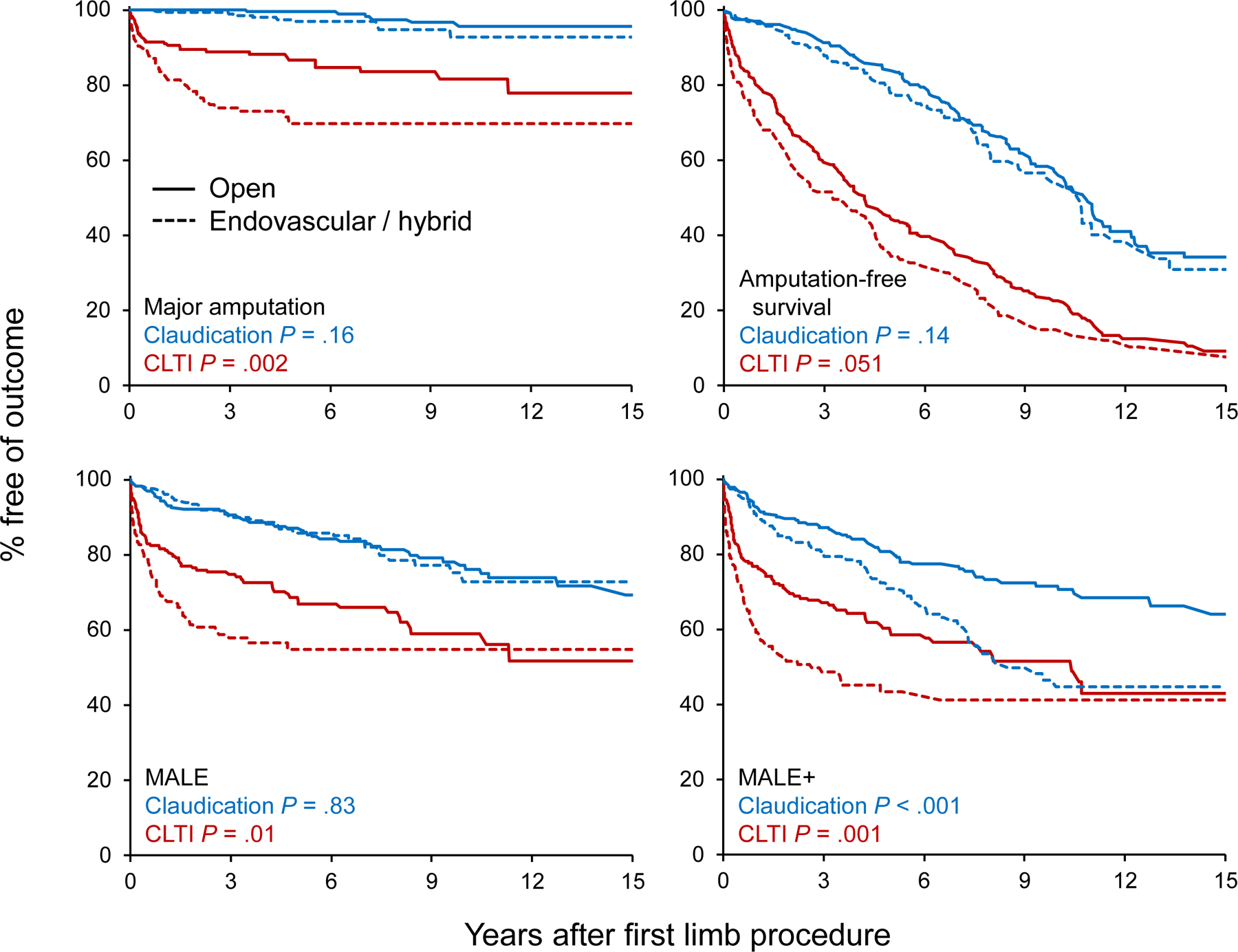
Kaplan-Meier estimates for survival free of specific outcomes comparing patients who initially underwent open vs. endovascular / hybrid revascularization. P values comparing the mode of revascularization were calculated using log-rank tests. Amputation-free survival was a composite outcome defined as the earliest of major amputation on same limb or death due to any cause.
CLTI = chronic limb-threatening ischemia; MALE = major adverse limb events (major revascularization or major amputation); MALE+ = any revascularization or major amputation
When outcomes were analyzed by indication, however, ENDO/HYBRID fared worse than OPEN among CLTI limb-procedures in terms of major amputations, MALE and MALE+ with no difference in amputation-free survival (P=.051; Figure 2). MALE+ was also worse among ENDO/HYBRID claudicant limbs, though MALE, major amputation and amputation-free survival were similar.
Discussion:
Our data show increasing incidence of endovascular and hybrid revascularizations between 1990 and 2009, and decreasing incidence of open surgical revascularizations and major amputations. However, we demonstrated no increase in the incidence of revascularizations for CLTI over this period to coincide with the declining incidence of major amputations. Furthermore, ENDO/HYBRID revascularizations, which supplanted OPEN as the primary mode of revascularization, had a higher incidence of major amputations and MALE compared to OPEN for patients with CLTI. Taken together, these data suggest that the observed decrease in major amputations over this 20-year period was not due to the increased use of endovascular technology per se.
Our data does not suggest, however, that endovascular techniques are without merit. For example, our study did not examine factors such as length of hospital stay or complications (eg, heart attack, wound infection), where ENDO is typically more favorable. That such a large proportion of PAD patients in this population were treated with ENDO/HYBRID instead of OPEN without an increase in major amputations is noteworthy. Finally, endovascular technology continues to improve and better outcomes would be expected to follow.
There are several alternative explanations for the observed decline in major amputations. Major amputation rates have been shown to decrease with revascularization, multidisciplinary care, intermittent pneumatic compression, statin therapy and improved access to care 19,24–28. Practice patterns with respect to threshold for major amputation as opposed to wound care and/or minor amputation have also changed 29,30, favoring these less morbid measures and with reasonable success among PAD patients. Indeed, our data showed a significant decrease in primary amputations over time in the absence of a concomitant increase in total revascularizations for CLTI. Thus, any aforementioned interventions – operative or nonoperative – other than primary major amputation could contribute to a lower major amputation rate on a population level, which should be the subject of further study as to the relative efficacy of such interventions.
The Department of Veterans Affairs’ preventive foot care initiative is credited with an almost 50% decrease in amputation rates 31. Among Medicare patients, increased blood glucose testing and diabetic foot examinations were associated with a decreased rate of major amputations 16. Taylor et al showed that, while ENDO vs OPEN was not predictive of post-revascularization failure of limb salvage, factors such as presence of diabetes, end-stage renal disease and impaired ambulation were 32. Thus, there may be too much focus on the relative limb salvage potential of ENDO vs OPEN and not enough on preventive care, nonoperative measures and clinical decision-making.
Our incidence trends for ENDO, OPEN and major amputations are similar to other epidemiologic studies using Medicare and National Inpatient Sample (NIS) databases over a similar time period 9,13,15. Furthermore, trends for indications for revascularization in our study were comparable to those of Medicare and NIS studies, though our interpretation was somewhat different due to the additional analyses we were able to perform with our study design. Specifically, we were able to gather patient and limb-specific data directly from the medical record, which allowed us to assess outcomes such as amputation-free survival and MALE as well as obtain more reliable clinical data without only using administrative codes. Furthermore, our cohort includes patients of all ages and all amputation/revascularization procedures regardless of whether they were performed as an inpatient or outpatient or insured/uninsured.
Our analysis and conclusions with respect to ENDO vs OPEN outcomes were similar to BASIL 5. Importantly, BASIL was a randomized trial and ours was a population-based cohort study, where same-level revascularizations were not directly compared. Furthermore, BASIL only included patients with CLTI and only compared infrainguinal revascularizations, for which patients had to be candidates for both ENDO and OPEN. Future studies with our cohort could focus on the CLTI subcohort and use multivariate analysis or propensity matching to further study outcomes of the OPEN first vs ENDO first approach. Nationwide studies are currently ongoing to provide more current, level 1 evidence as to the comparability of ENDO and OPEN, such as the ROBUST and BEST-CLI trials 33,34.
There will always be some baseline level of major amputations performed. Our data showed that the majority of amputations (80%) were performed due to nonambulatory status, lack of reasonable options for revascularization, tissue loss beyond salvage, progressive tissue loss despite a patent revascularization or patient preference. While evolving endovascular techniques such as tibial/pedal artery recanalization may create some “reasonable options” beyond our study period, many amputations among PAD patients in our cohort were either not amenable to or not appropriate for revascularization. Preventive measures and earlier intervention for tissue loss may be key to reducing amputation rates in our population further, but this may not be the case in other populations. Moxey et al showed significant variation in amputation rates globally 35. Thus, in many populations, improving access to arterial revascularization of any sort may help lower amputation rates.
Our study has limitations. Compared to the total U.S. population, Olmsted County has a higher proportion of non-Hispanic whites, higher median income and more education, all of which are associated with lower amputation rates and can affect generalizability 15,36. The largest healthcare provider in the county is a tertiary referral center, which is uncommon. Selection criteria for performing ENDO vs OPEN vs amputation are “real world” and not systematic. Data was collected retrospectively. It was often challenging to determine if diabetic patients undergoing minor/major amputation had PAD with the available data. If sufficient data was not available to exclude such a patient, they were included in the cohort. Fontaine stages were used to categorize indications, where a more comprehensive system for CLTI such as WIFi would be more informative 37,38. Finally, our cohort did not include all PAD patients in Olmsted County – only those undergoing amputation/revascularization. To determine trends among all patients with PAD, one would need to broaden the cohort to include patients managed nonoperatively.
Conclusions:
Our study showed an increase in the incidence of ENDO and HYBRID revascularizations for atherosclerotic PAD in a well-defined, stable population between 1990 and 2009. There was a concomitant decrease in OPEN revascularizations and major amputations, though the incidence of total revascularizations for CLTI did not change over the study period. Furthermore, initial ENDO/HYBRID limb-procedures for CLTI had worse major amputation rates, MALE and MALE+ compared to initial OPEN. Taken together, these data suggest that the observed decrease in major amputations was not due to the increase in utilization of endovascular technology per se. Additional emphasis should be placed on improving limb salvage efforts beyond just mode of revascularization among patients with PAD.
Supplementary Material
Acknowledgements:
Funding/Support:
This research was supported by funds from the Gonda Vascular Center and Department of Surgery/Division of Vascular Surgery at the Mayo Clinic in Rochester, MN. These funds were used for statistical analyses. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) (CTSA, REDCap). The REDCap software was used for data entry and distribution. Dr. Nienaber obtained a CTSA Certificate while working on this study. Data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources were not involved in the interpretation of data or preparation/review/approval of the manuscript nor in the decision to submit the manuscript for publication.
Abbreviations:
- CLTI
chronic limb-threatening ischemia
- ENDO
endovascular revascularization
- HYBRID
revascularization using both endovascular and open techniques
- MALE
major adverse limb event
- MALE+
MALE or minor revascularization
- OPEN
open surgical revascularization
- PAD
peripheral arterial disease
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflcts of Interest Disclosures: None reported
Previous Presentation: Portions of this data were presented at the Association of VA Surgeons Annual Meeting May 4, 2015 in Miami Beach, FL and at the Society for Vascular Surgery Annual Meeting May 31, 2013 in San Francisco, CA.
References
- 1.Olin JW, Allie DE, Belkin M, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). J Am Coll Cardiol 2010;56(25):2147–2181. [DOI] [PubMed] [Google Scholar]
- 2.Allie DE, Hebert CJ, Ingraldi A, Patlola RR, Walker CM. 24-carat gold, 14-carat gold, or platinum standards in the treatment of critical limb ischemia: bypass surgery or endovascular intervention? J Endovasc Ther 2009;16 Suppl 1:I134–146. [DOI] [PubMed] [Google Scholar]
- 3.Karam J, Shepard A, Rubinfeld I. Predictors of operative mortality following major lower extremity amputations using the National Surgical Quality Improvement Program public use data. Journal of vascular surgery 2013;58(5):1276–1282. [DOI] [PubMed] [Google Scholar]
- 4.Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis 2011;8(6):A141. [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. Journal of vascular surgery 2010;51(5 Suppl):18S–31S. [DOI] [PubMed] [Google Scholar]
- 6.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. Journal of vascular surgery 2011;54(5):e32–58. [DOI] [PubMed] [Google Scholar]
- 7.Jones WS, Schmit KM, Vemulapalli S, et al. Treatment Strategies for Patients With Peripheral Artery Disease Rockville (MD)2013. [PubMed] [Google Scholar]
- 8.Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J 2014;167(4):489–498 e487. [DOI] [PubMed] [Google Scholar]
- 9.Egorova NN, Guillerme S, Gelijns A, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. Journal of vascular surgery 2010;51(4):878–885, 885 e871. [DOI] [PubMed] [Google Scholar]
- 10.Al-Omran M, Tu JV, Johnston KW, Mamdani MM, Kucey DS. Use of interventional procedures for peripheral arterial occlusive disease in Ontario between 1991 and 1998: a population-based study. Journal of vascular surgery 2003;38(2):289–295. [DOI] [PubMed] [Google Scholar]
- 11.Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. Journal of vascular surgery 2009;49(4):910–917. [DOI] [PubMed] [Google Scholar]
- 12.Cull DL, Langan EM, Gray BH, Johnson B, Taylor SM. Open versus endovascular intervention for critical limb ischemia: a population-based study. J Am Coll Surg 2010;210(5):555–561, 561–553. [DOI] [PubMed] [Google Scholar]
- 13.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. Journal of vascular surgery 2009;50(1):54–60. [DOI] [PubMed] [Google Scholar]
- 14.Skrepnek GH, Armstrong DG, Mills JL. Open bypass and endovascular procedures among diabetic foot ulcer cases in the United States from 2001 to 2010. Journal of vascular surgery 2014;60(5):1255–1264. [DOI] [PubMed] [Google Scholar]
- 15.Hong MS, Beck AW, Nelson PR. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Ann Vasc Surg 2011;25(1):44–54. [DOI] [PubMed] [Google Scholar]
- 16.Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among medicare patients. JAMA Surg 2015;150(1):84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg 2010;97(9):1348–1353. [DOI] [PubMed] [Google Scholar]
- 18.Wendt K, Kristiansen R, Krohg-Sorensen K, Gregersen FA, Fosse E. Norwegian trends in numbers of lower extremity revascularisations and amputations including regional trends in endovascular treatments for peripheral arterial disease: a retrospective cross-sectional registry study from 2001 to 2014. BMJ Open 2017;7(11):e016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallett JW Jr., Byrne J, Gayari MM, Ilstrup DM, Jacobsen SJ, Gray DT. Impact of arterial surgery and balloon angioplasty on amputation: a population-based study of 1155 procedures between 1973 and 1992. Journal of vascular surgery 1997;25(1):29–38. [DOI] [PubMed] [Google Scholar]
- 20.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schanzer A, Goodney PP, Li Y, et al. Validation of the PIII CLI risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. Journal of vascular surgery 2009;50(4):769–775; discussion 775. [DOI] [PubMed] [Google Scholar]
- 23.Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. Journal of vascular surgery 2009;50(6):1462–1473 e1461–1463. [DOI] [PubMed] [Google Scholar]
- 24.Chung J, Modrall JG, Ahn C, Lavery LA, Valentine RJ. Multidisciplinary care improves amputation-free survival in patients with chronic critical limb ischemia. Journal of vascular surgery 2015;61(1):162–169. [DOI] [PubMed] [Google Scholar]
- 25.Kavros SJ, Delis KT, Turner NS, et al. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. Journal of vascular surgery 2008;47(3):543–549. [DOI] [PubMed] [Google Scholar]
- 26.Kumbhani DJ, Steg PG, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J 2014;35(41):2864–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindholt JS, Bovling S, Fasting H, Henneberg EW. Vascular surgery reduces the frequency of lower limb major amputations. Eur J Vasc Surg 1994;8(1):31–35. [DOI] [PubMed] [Google Scholar]
- 28.Williams DT, Majeed MU, Shingler G, Akbar MJ, Adamson DG, Whitaker CJ. A diabetic foot service established by a department of vascular surgery: an observational study. Ann Vasc Surg 2012;26(5):700–706. [DOI] [PubMed] [Google Scholar]
- 29.Chiriano J, Bianchi C, Teruya TH, Mills B, Bishop V, Abou-Zamzam AM Jr. Management of lower extremity wounds in patients with peripheral arterial disease: a stratified conservative approach. Ann Vasc Surg 2010;24(8):1110–1116. [DOI] [PubMed] [Google Scholar]
- 30.Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds 2014;13(3):211–219. [DOI] [PubMed] [Google Scholar]
- 31.Prevention of Amputation in Veterans Everywhere (PAVE) program directive Washington (DC): VHA; 2013. Aug 20. VHA directive 2012–020.: Available from: http://vaww.va.gov/vhapublications/ViewPublication.asp?pub_ID=2778. [Google Scholar]
- 32.Taylor SM, York JW, Cull DL, Kalbaugh CA, Cass AL, Langan EM 3rd. Clinical success using patient-oriented outcome measures after lower extremity bypass and endovascular intervention for ischemic tissue loss. Journal of vascular surgery 2009;50(3):534–541; discussion 541. [DOI] [PubMed] [Google Scholar]
- 33.Malas MB, Qazi U, Glebova N, et al. Design of the Revascularization With Open Bypass vs Angioplasty and Stenting of the Lower Extremity Trial (ROBUST): a randomized clinical trial. JAMA Surg 2014;149(12):1289–1295. [DOI] [PubMed] [Google Scholar]
- 34.Menard MT, Farber A. The BEST-CLI trial: a multidisciplinary effort to assess whether surgical or endovascular therapy is better for patients with critical limb ischemia. Semin Vasc Surg 2014;27(1):82–84. [DOI] [PubMed] [Google Scholar]
- 35.Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations--a review of global variability in incidence. Diabet Med 2011;28(10):1144–1153. [DOI] [PubMed] [Google Scholar]
- 36.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan LX, Branco BC, Armstrong DG, Mills JL Sr. The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing. Journal of vascular surgery 2015;61(4):939–944. [DOI] [PubMed] [Google Scholar]
- 38.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Journal of vascular surgery 2019;69(6S):3S–125S e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


