Abstract
BACKGROUND:
Invasive neuromonitoring is a mainstay of modern management of severe traumatic brain injury (TBI). Complication rates of neuromonitor placement are widely reported, but their effects on long-term outcomes are less studied. We evaluated the association of neuromonitor complications on long-term outcomes in a prospective severe TBI cohort.
METHODS:
We reviewed 599 patients with severe TBI from November 2002 through 2018 for neuromonitor-associated hemorrhage and infection. We compared outcome differences between patients with and without neuromonitoring-associated complications using the Glasgow Outcomes Scale (GOS) at 3, 6, 12, and 24 months post trauma. When analyzing neuromonitoring infections, we removed all patients who expired before discharge as early mortality was associated with reduced infection rates.
RESULTS:
Neuromonitor-associated hemorrhage occurred in 62 out of 534 patients with postplacement imaging (11.6%) and was increased after craniotomy (24% vs. 11%, P = 0.005). Clinical outcomes did not differ in patients with neuromonitor-associated hemorrhage. Neuromonitor-associated infection occurred in 30 of 389 patients (7.7%) who survived to discharge. Infection was associated with worse outcomes at 3 months (P = 0.03), where the proportion of patients with favorable outcomes (P = 0.02) was decreased despite similar mortality (P = 0.24). Patients with an infection recovered by 6 months, at which point there were no differences in total GOS or rates of favorable outcomes then or at later time points (P > 0.26). Neuromonitor-associated infection was associated with increased length of stay (P = 0.01) and depressed skull fractures (P = 0.03) but did not affect rates of shunting (P = 0.99).
CONCLUSIONS:
Complications of neuromonitoring in severe TBI are associated with delayed recovery but not long-term outcomes.
Keywords: External ventricular drain, Glasgow Outcome Scale, Intracranial pressure monitor, Neuromonitor, Traumatic brain injury
INTRODUCTION
Invasive neuromonitoring with either an external ventricular drain (EVD) or intraparenchymal monitors is one of the most commonly performed neurosurgical procedures.1 After severe traumatic brain injury (TBI), intracranial pressure (ICP)-guided therapy is the mainstay of care and recommended in Brain Trauma Foundation guidelines.2 Recent studies suggest therapies that normalize brain tissue oxygenation may also be beneficial.3 Many neurotrauma clinicians believe neuromonitor-guided treatment reduces secondary injury and improves long-term outcomes.3,4
As with any invasive neurosurgical procedure, placement of neuromonitoring carries risks including hemorrhage and infection.5–7 Rates of EVD-associated hemorrhages are well characterized and range from 0% to 42%.1,8–10 While most EVD-associated hemorrhages are small, death after hemorrhage has been reported.8 The incidence of infection after EVD placement is less well described. EVD-associated ventriculitis occurs in upwards of 23% of patients, although 20% of these cases are culture negative.11,12 Intraparenchymal monitor placement complications are likely less frequent, with 1 large trial reporting a 1% hemorrhage rate and no infections.13
Although short-term complications of neuromonitor placement are widely reported, complications of simultaneous EVD and intraparenchymal monitor placement are not clear, as is their association with long-term outcomes. To better assess the risk-benefit ratio of invasive neuromonitoring, we analyzed a prospective database of severe TBI patients. We determined the overall rate of neuromonitor complications, identified risk factors for these complications, and tested the association of neuromonitor-associated complications with long-term outcomes.
METHODS
Study Cohort
This study received approval of the University of Pittsburgh Human Research Protection Office. We performed an analysis of a prospectively collected database of severe TBI patients admitted to a single Level 1 trauma center from November 2002 through December 2018. This database has previously been described14 and includes patients age 16 – 80 with severe TBI (defined as postresuscitation Glasgow Coma Scale [GCS] score of 8 or lower) admitted to UPMC Presbyterian Hospital and excludes patients with imminent brain death (GCS 3 with fixed and dilated pupils on presenting examination), pregnancy, and/or penetrating TBI. We further excluded patients who did not undergo postprocedural head computed tomography (CT) within 24 hours of neuromonitor placement or those for whom screening cerebrospinal fluid (CSF) cultures were never sent. Consent was obtained from a legal representative.
Data Collection
From our prospective registry, we extracted demographic, clinical, and imaging variables including age, gender, race, mechanism of injury, major extracranial injury defined as any non–head injury with an Abbreviated Injury Scale >4, GCS, pupil reactivity, episode of hypoxia or hypotension, glucose, international normalized ratio, alcohol use, and rates of shunting, decompressive craniectomies, and craniotomy. We reviewed each admission CT scan for the Marshall CT score, presence of traumatic subarachnoid hemorrhage, petechial hemorrhage, epidural hemorrhage, subdural hemorrhage, midline shift, obliteration of basal cisterns or third ventricle, presence of nonevacuated hematoma, and skull fractures. Trained neuropsychologists assessed outcomes at 3, 6, 12, and 24 months through a structured interview by using the Glasgow Outcome Scale (GOS): 1 = death; 2 = persistent vegetative state; 3 = severe disability; 4 = moderate disability; 5 = low disability. We also extracted outcomes including total length of stay (LOS), intensive care unit (ICU) LOS, and mortality at discharge.
One author reviewed the medical record for neuromonitor-associated complications. We defined neuromonitoring-associated hemorrhage as any new subdural hemorrhage at the entry point or intraparenchymal hemorrhage along an EVD or intracranial monitor tract on postprocedural imaging. We calculated the volume of each hemorrhage by measuring the maximal distance in the X, Y, and Z planes and using the simplified ABC/2 formula for an ellipsoid. We defined neuromonitor-associated infection as any positive culture from CSF drawn from an EVD unless a physician specializing in infectious diseases documented that the culture was a contaminant.15 We do not typically send the EVD or ICP monitor tips for routine culture. The standard at our institution was to routinely draw CSF daily during the study period 2002 through 2016 and at least twice weekly on Mondays and Thursdays from 2016 through 2018.
Neuromonitor Placement and Indications
The practice at our institution is to place intraparenchymal ICP and brain tissue oxygenation monitors, as well as an EVD, for all severe TBI patients. Both an ICP monitor and an EVD are placed to allow for continuous ICP data from the monitor and continuous CSF drainage from the EVD, which is associated with improved ICP control.2,16–18
All EVDs and monitoring devices were placed in the frontal region using standard technique. Typically, the ICP monitor and EVD were placed on the same side, with a preference for the right side, unless pathology dictated otherwise. Neuromonitoring devices were placed in either the operating room or at the ICU bedside at the discretion of the attending neurosurgeon on call. We typically place both the ICP monitor and EVD at the same time and not in a staged procedure.
Statistical Analysis
We summarized baseline clinical characteristics, neuromonitor complication rates, and patient outcomes using descriptive statistics. To identify baseline characteristics associated with neuromonitor-associated infection or neuromonitor-associated hemorrhage, we used 2-sided t-tests for normally distributed continuous variables and a chi-squared test or Fisher exact test for categorical variables as appropriate. Our a priori plan was to use the same statistical methods as the DECRA and RESCUEicp trials using a mixed-effects ordinal logistic regression to test the independent association of neuromonitor-associated complications with GOS over time.13,19 Similar to the RESCUEicp trial, our results violated the proportional odds assumption and we instead used an unordered chi-squared test to compare GOS across complication categories at each time point.20 To minimize bias when testing the association of neuromonitoring infection with outcomes, we excluded patients who died before discharge because early mortality was associated with reduced infection rates as patients did not survive long enough to get an infection. We specifically chose death at discharge because we did not have reliable documentation for duration of neuromonitoring. To evaluate how the trajectory of patients with and without EVD infections changed with time, we plotted the outcome trajectories for patients who survived to discharge and had a 3-month GOS. We used RStudio version 1.3 (Boston, Massachusetts, USA; 2020) for all analyses.
RESULTS
Patterns of Neuromonitor Hemorrhage or Infection
From November 2002 through December 2018, 599 patients were enrolled in the study (Figure 1). An EVD and ICP monitor were placed in 480 patients, an EVD without ICP monitor in 52 patients, and an ICP monitor only in 6 patients. Among these, 534 had a neuromonitor placed with a postplacement scan within 24 hours and outcomes available for analysis. Of the 519 patients with at least 1 CSF sample drawn for culture, 130 expired before discharge from the hospital, leaving 389 patients included in the analysis of postneuromonitor placement ventriculitis. The overall rate of neuromonitoring hemorrhage was 11.6% (62 patients), and the rate of neuromonitoring-associated infection was 7.7% (30 patients). Table 1 reports the demographic, clinical, radiologic, and laboratory variables collected. The rate of craniotomy before or concurrent with neuromonitoring placement was higher (24%) in the hemorrhage group compared with the no-hemorrhage group (11%; P < 0.005). The distribution of Marshall CT classification was also different between the groups, mainly due to the differences in evacuated mass lesions (P = 0.008). Neuromonitor-associated infections increased the ICU=LOS and overall LOS by 4 days (P = 0.01 and 0.04, respectively), but not the floor LOS (P = 0.60). Patients with infections had higher rates of depressed skull fractures (P = 0.03) but similar rates of shunting (P = 0.12). An infection did not increase the chance for having a haemorrhage or vice versa (P = 0.99).
Figure 1.
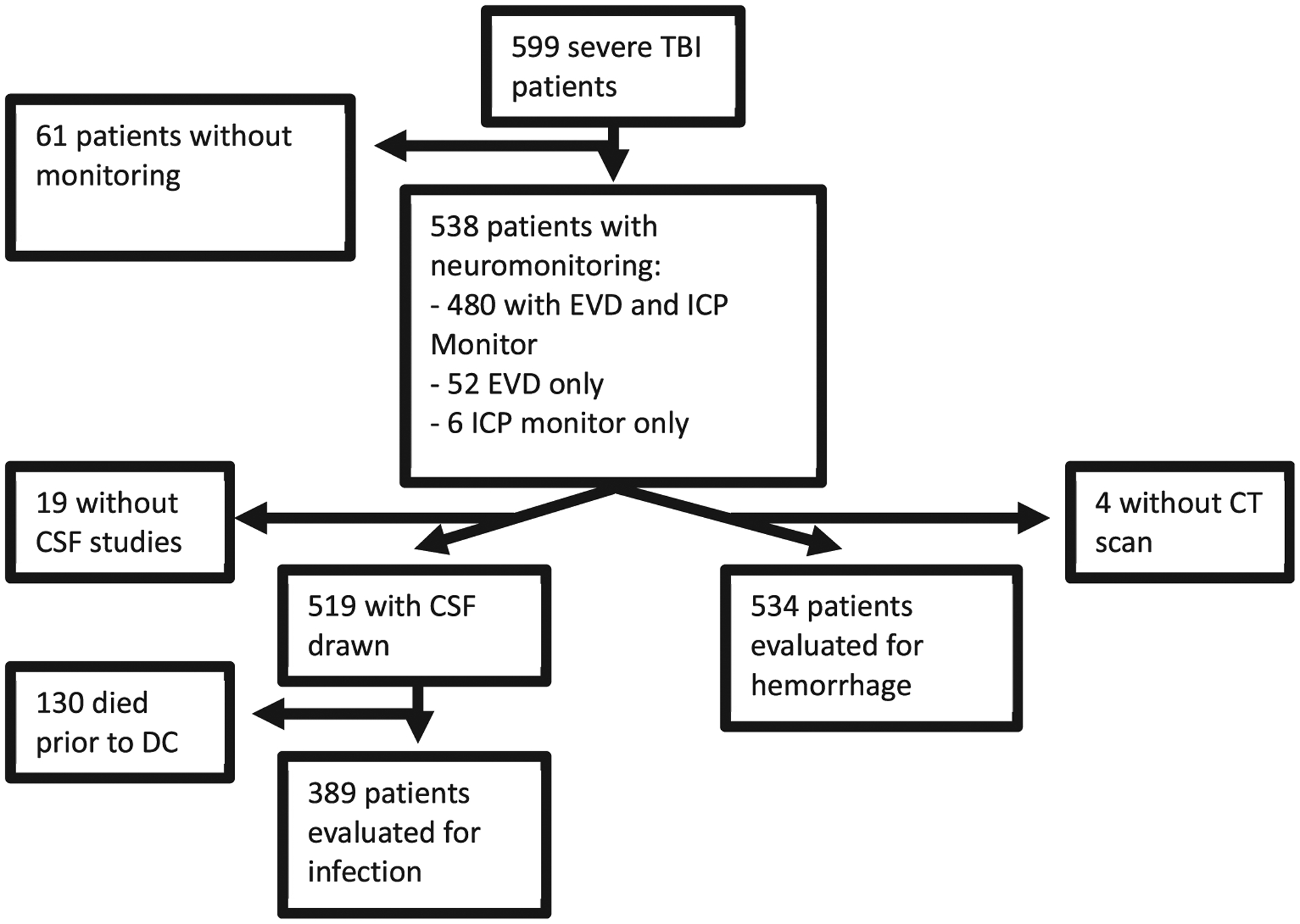
CONSORT diagram for patient selection.
Table 1.
Demographic, Clinical, Radiologic, and Laboratory Variables Associated with Infection and Hemorrhage After Placement of External Ventricular Drain and/or Intracranial Pressure Monitor
| Category | No Infection (n = 359) | Infection (n = 30) | P Value | No Hemorrhage (n = 472) | Hemorrhage (n = 62) | P Value |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age | 32 (23–48) | 34 (21–48) | 0.81 | 37 (23–53) | 42 (26–54) | 0.16 |
| Race | ||||||
| Native Am. | 1% (3/359) | 0% (0/30) | 0.28 | 1% (3/472) | 0% (0/62) | 0.32 |
| Asian | 1% (4/359) | 0% (0/30) | 1% (3/472) | 3% (2/62) | ||
| Black | 7% (25/359) | 17% (5/30) | 8% (37/472) | 8% (5/62) | ||
| White | 91% (327/359) | 83% (25/30) | 90% (428/472) | 89% (55/62) | ||
| Sex (male) | 79% (284/359) | 80% (24/30) | 0.91 | 80% (376/472) | 77% (48/62) | 0.68 |
| Clinical | ||||||
| GCS | ||||||
| 3 | 12% (43/357) | 10% (3/30) | 0.88 | 16% (76/470) | 13% (8/62) | 0.50 |
| 4 | 8% (27/357) | 7% (2/30) | 9% (43/470) | 13% (8/62) | ||
| 5 | 10% (36/357) | 10% (3/30) | 11% (49/470) | 15% (9/62) | ||
| 6 | 20% (71/357) | 17% (5/30) | 19% (89/470) | 11% (7/62) | ||
| 7 | 41% (147/357) | 53% (16/30) | 37% (175/470) | 42% (26/42) | ||
| 8 | 9% (33/357) | 3% (1/30) | 8% (38/470) | 6% (4/62) | ||
| Pupil reactivity | ||||||
| Both | 72% (244/341) | 61% (17/28) | 0.38 | 69% (311/453) | 75% (43/57) | 0.43 |
| One | 9% (32/341) | 14% (4/28) | 10% (45/453) | 11% (6/57) | ||
| None | 19% (65/341) | 25% (7/28) | 21% (97/453) | 14% (8/57) | ||
| Hypoxia | 12% (42/343) | 14% (4/29) | 0.77 | 15% (65/446) | 18% (11/60) | 0.44 |
| Hypotension | 22% (76/341) | 30% (9/30) | 0.34 | 24% (108/444) | 23% (13/57) | 0.80 |
| ICU LOS | 16 (13-22) | 20 (15-32) | 0.01 | 15 (8–20) | 14 (9–19) | 0.48 |
| Floor LOS | 4 (1–9) | 3 (0–9) | 0.60 | 4 (1–9) | 4 (2–9) | 0.77 |
| Overall LOS | 21 (16-30) | 25 (19-35) | 0.04 | 19 (11–28) | 20 (13–26) | 0.84 |
| Craniotomy | 14% (49/359) | 17% (10/30) | 0.59 | 11% (54/472) | 24% (15/62) | 0.005 |
| DHC | 29% (104/359) | 33% (10/30) | 0.65 | 32% (151/472) | 42% (26/62) | 0.12 |
| Shunt | 17% (60/359) | 17% (5/30) | 0.99 | 12% (57/471) | 16% (10/61) | 0.48 |
| EVD hemorrhage | 12% (42/357) | 11% (3/28) | 0.99 | |||
| Radiologic | ||||||
| Marshall CT classification | ||||||
| 1 | 5% (16/346) | 3% (1/29) | 0.21 | 5% (22/446) | 0% (0/61) | 0.008 |
| 2 | 62% (215/346) | 45% (13/29) | 56% (249/446) | 39% (24/61) | ||
| 3 | 7% (23/346) | 17% (5/29) | 10% (46/446) | 11% (7/61) | ||
| 4 | 5% (18/346) | 7% (2/29) | 6% (28/446) | 7% (4/61) | ||
| 5 | 20% (69/346) | 28% (8/29) | 21% (91/446) | 41% (25/61) | ||
| 6 | 1% (5/346) | 0% (0/29) | 2% (10/446) | 2% (1/61) | ||
| SDH | 42% (148/353) | 31% (9/29) | 0.28 | 45% (212/466) | 50% | 0.52 |
| EDH | 11% (35/347) | 17% (5/29) | 0.36 | 9% (42/447) | 8% | 0.76 |
| tSAH | 78% (272/342) | 76% (22/29) | 0.75 | 81% (360/447) | 82% (50/61) | 0.79 |
| Petechial hemorrhage | 68% (220/324) | 70% (19/27) | 0.79 | 67% (286/427) | 69% (10/52) | 0.74 |
| Obliteration of 3rd ventricle | 18% (57/324) | 22% (6/27) | 0.60 | 22% (96/427) | 35% (18/52) | 0.05 |
| Midline shift | 31% (102/324) | 30% (8/27) | 0.84 | 36% (154/427) | 46% (24/52) | 0.16 |
| Midline shift >5 mm | 20% (69/353) | 21% (6/29) | 0.88 | 23% (108/465) | 34% (20/58) | 0.06 |
| Skull fracture | 55% (192/352) | 63% (18/29) | 0.27 | 54% (240/466) | 53% (30/57) | 0.63 |
| Depressed skull fracture | 14% (49/352) | 31% (9/29) | 0.03 | 13% (61/466) | 18% (10/57) | 0.35 |
| Laboratory | ||||||
| Glucose | 139 (117–180) | 152 (110–186) | 0.72 | 145 (121–182) | 133 (108–184) | 0.22 |
| INR | 1.1 (1.1–1.3) | 1.2 (1.0–1.3) | 0.81 | 1.1 (1.1–1.3) | 1.1 (1.0–1.2) | 0.07 |
| EtOH | 49% (142/295) | 58% (15/26) | 0.35 | 47% (176/374) | 46% (23/50) | 0.89 |
Bold signifies statistical significance P < 0.05. The total number of patients or the interquartile range (IQR) is listed in parenthesis.
GCS, Glasgow Coma Scale; ICU, intensive care unit; LOS, length of stay; DHC,; EVD, external ventricular drain; CT, computed tomography; SDH, subdural hemorrhage; EDH,; tSAH, traumatic subarachnoid hemorrhage; INR, international normalized ratio; EtOH.
The vast majority of neuromonitor hemorrhages were small with 24 of the 46 (52%) intraparenchymal hemorrhages being <1 mL (Figure 2). Only 3 of the intraparenchymal hemorrhages were>25 mL. The remaining hemorrhages were subdural hematomas ranging from 3 mm to 2.8 cm in size and 1 solely intraventricular hemorrhage. Three patients had surgery to evacuate a neuromonitor-related hemorrhage or were offered surgery but care was withdrawn as a direct consequence of the hemorrhage.
Figure 2.
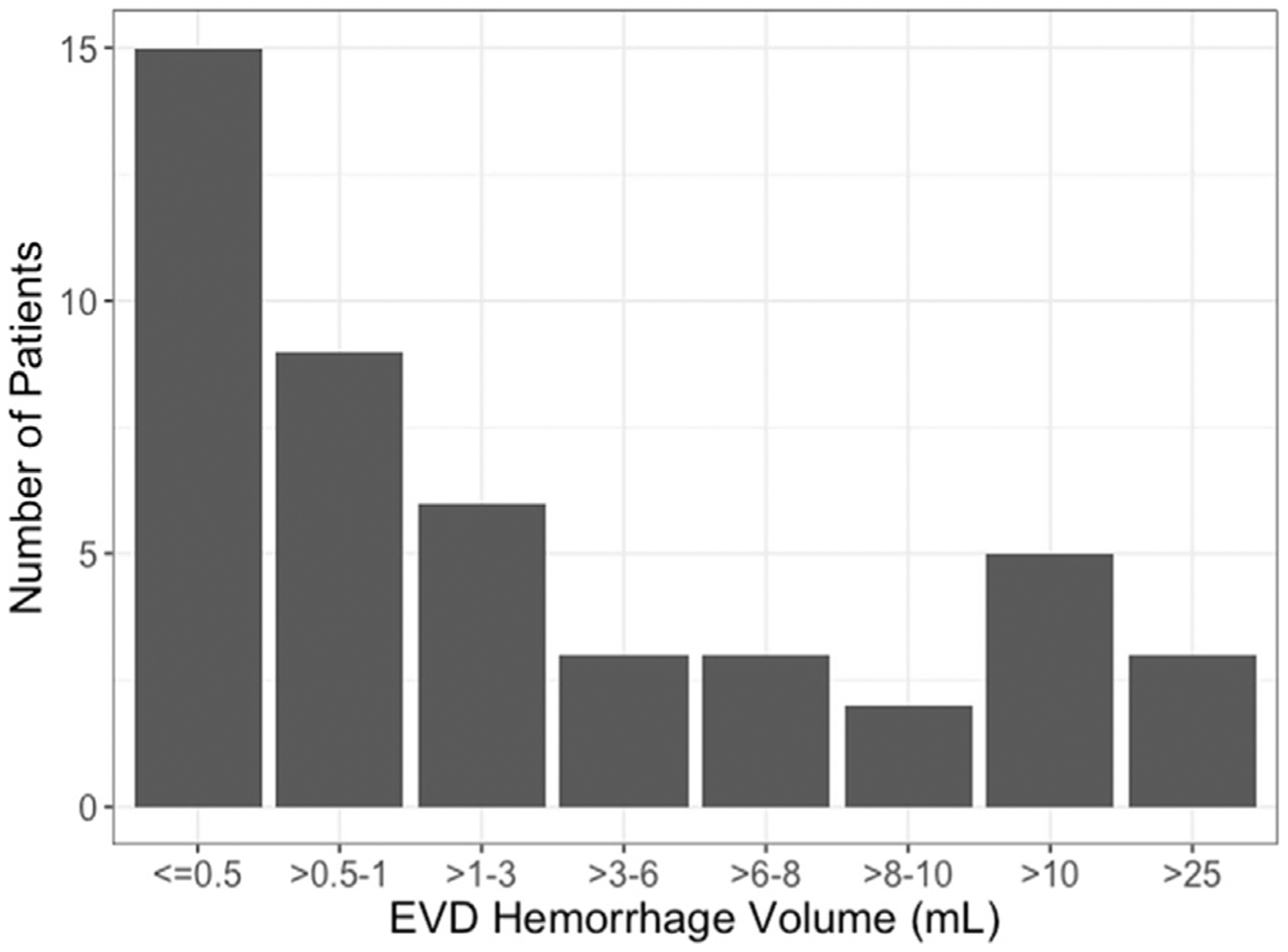
Distribution of the size of external ventricular drain or intracranial pressure monitor–associated hemorrhage
All but 2 of the 30 neuromonitor-associated infections were bacterial, with 1 infection each of Rhodotorula and Cryptococcus. The remaining infectious pathogens included Acinetobacter (3), Enterococcus (4), Escheria (1), Klebsiella (2), Proteus (1), Pseudomonas (3) Serratia (1), and Staphylococcus or Streptococcus (13).
Effects of Neuromonitor Hemorrhages or Infections on Long-Term Outcomes
Neuromonitor-associated hemorrhage did not affect the GOS (P > 0.12), mortality (P > 0.83), or rates of favorable outcomes (P > 0.06) at 3, 6, 12, or 24 months. Tables 2–4 shows the GOS, mortality, and favorable outcome rates, while Figure 3 is the graphical representation.
Table 2.
Glasgow Outcome Scale (GOS) Distributed by Month and External Ventricular Drain or Intracranial Pressure Monitor Hemorrhage
| GOS | 3 Months (n; P = 0.99) | 6 Months (n; P = 0.98) | 12 Months (n; P = 0.12) | 24 Months (n; P= 0.87) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 34% (143) | 32% (18) | 36% (152) | 35% (18) | 40% (157) | 40% (19) | 49% (160) | 49% (20) |
| 2 | 6% (25) | 6% (3) | 2% (9) | 2% (1) | 2% (8) | 0% (0) | 1% (2) | 0% (0) |
| 3 | 39% (167) | 41% (22) | 31% (131) | 35% (18) | 21% (84) | 38% (18) | 17% (56) | 22% (9) |
| 4 | 17% (73) | 17% (9) | 19% (78) | 19% (10) | 19% (75) | 10% (5) | 16% (53) | 17% (7) |
| 5 | 4% (18) | 4% (2) | 12% (51) | 9% (5) | 18% (6) | 12% (6) | 17% (54) | 12% (5) |
| Hemorrhage | No | Yes | No | Yes | No | Yes | No | Yes |
No month was significantly different when stratifying by hemorrhage.
Table 4.
Outcomes by Month for Mortality (Glasgow Outcome Scale 1 vs. 2–5)
| Month | No Hemorrhage | Hemorrhage | P Value |
|---|---|---|---|
| 3 | 34 | 33 | 0.97 |
| 6 | 36 | 35 | 0.83 |
| 12 | 40 | 40 | 0.96 |
| 24 | 49 | 49 | 0.96 |
Figure 3.
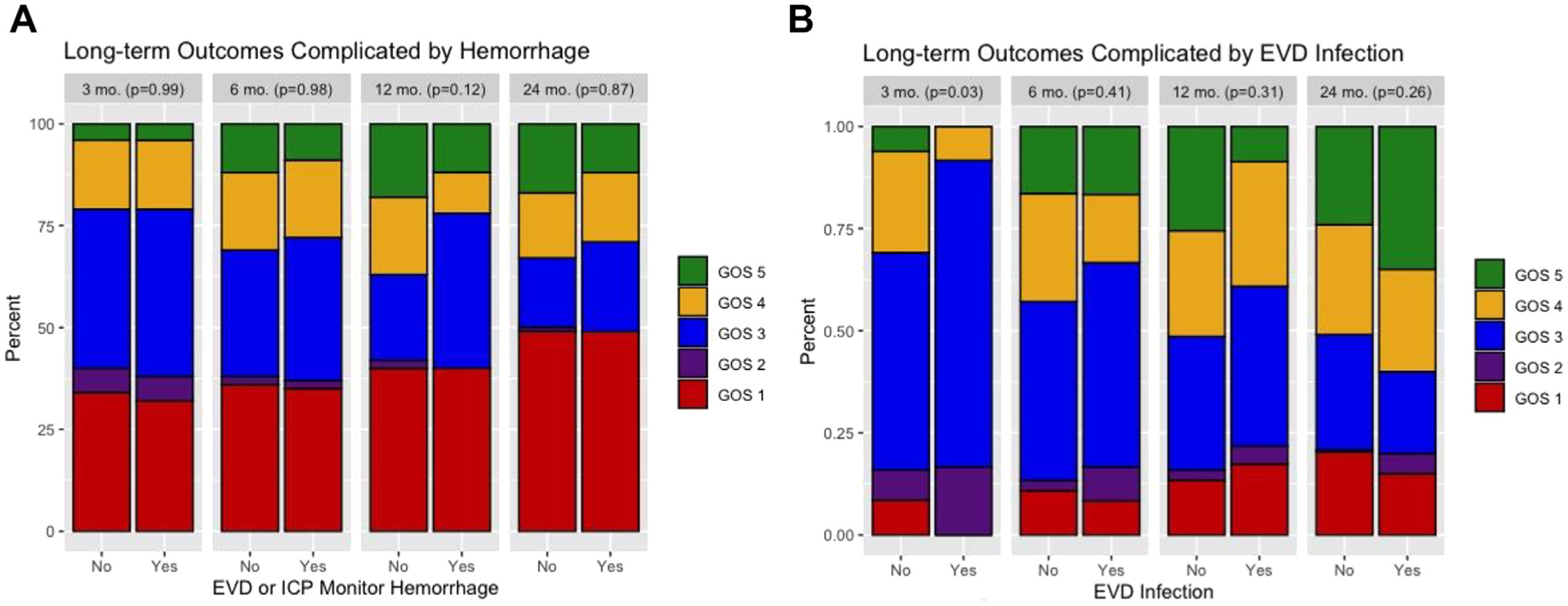
(A) Distribution of outcomes at 3-, 6-, 12-, and 24-month outcomes stratified by external ventricular drain (EVD) or intracranial pressure monitor–associated hemorrhage. (B) Distribution of outcomes at 3-, 6-, 12-, and 24-month outcomes stratified by EVD associated infection.
Among patients who survived to discharge, infection was associated with worse GOS at 3 months (P = 0.03), where the proportion of patients with favorable outcomes (P = 0.02) was decreased despite similar mortality (P = 0.24; Tables5–7). Patients with a neuromonitor-related infection recovered by 6 months, at which point there were no differences in total GOS score or rates of favorable outcomes then or at later time points (P > 0.26). Patients with neuromonitor-associated infection trended toward improved outcomes over time with no patients with a GOS 3 or higher at 3 months falling below GOS 3 at a later point (Figure 4).
Table 5.
Glasgow Outcome Scale (GOS) Distributed by Month and External Ventricular Drain (EVD) Infection
| GOS | 3 Months (n; P = 0.03) | 6 Months (n; P = 0.41) | 12 Months (n; P = 0.31) | 24 Months (n; P = 0.26) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 9% (28) | 0% (0) | 11% (35) | 8% (2) | 13% (39) | 17% (4) | 20% (44) | 15% (3) |
| 2 | 7% (24) | 17% (4) | 3% (8) | 8% (2) | 2% (7) | 4% (1) | 1% (1) | 5% (1) |
| 3 | 53% (174) | 75% (18) | 44% (141) | 50% (12) | 33% (95) | 39% (9) | 28% (61) | 20% (4) |
| 4 | 25% (81) | 8% (2) | 26% (85) | 17% (4) | 26% (75) | 31% (7) | 27% (58) | 25% (5) |
| 5 | 6% (20) | 0% (0) | 16% (53) | 17% (4) | 26% (74) | 9% (2) | 24% (52) | 35% (7) |
| No | Yes | No | Yes | No | Yes | No | Yes | |
Only 3-month outcomes (P = 0.03) differed by EVD infection, while 6-, 12-, and 24-month outcomes were all similar (P > 0.26). Bold is significant (P < 0.05).
Table 7.
Outcomes by Month for Mortality (Glasgow Outcome Scale 1 vs. 2–5)
| Month | No Infection | Infection | P Value |
|---|---|---|---|
| 3 | 9% | 0% | 0.24 |
| 6 | 11% | 8% | 0.99 |
| 12 | 13% | 17% | 0.54 |
| 24 | 20% | 15% | 0.77 |
Figure 4.
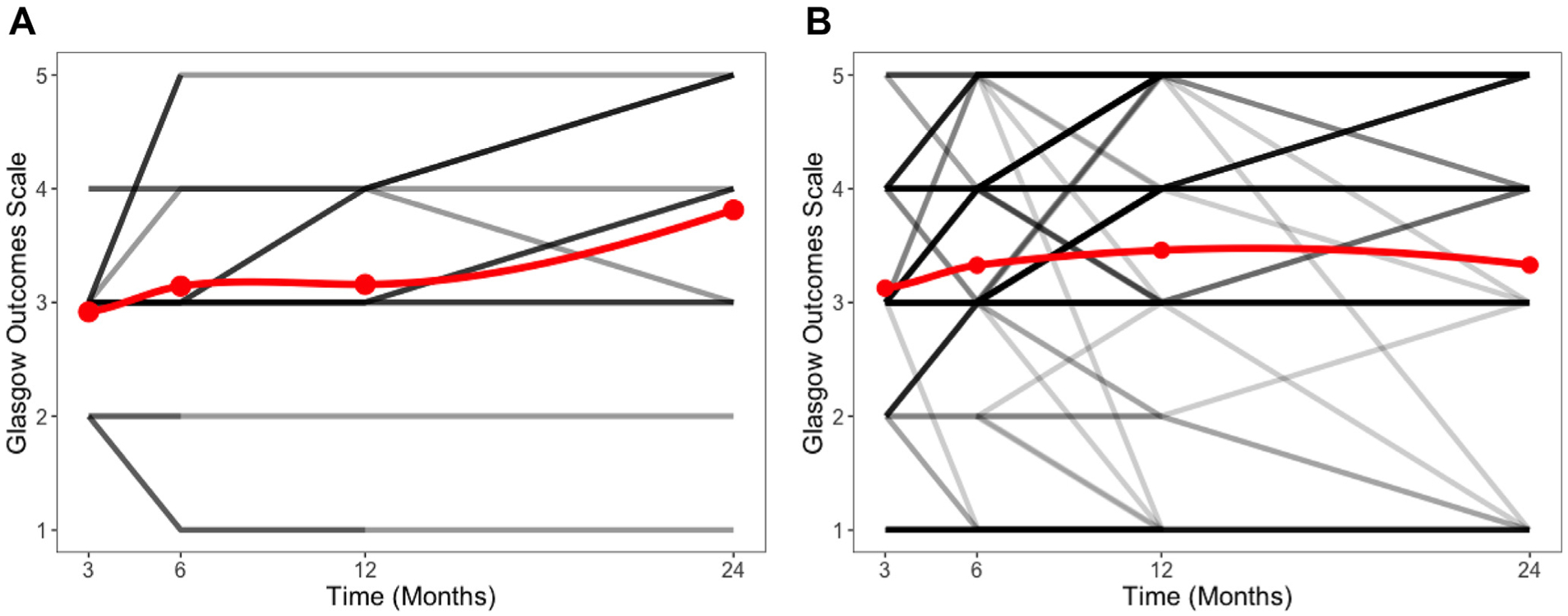
(A) Spaghetti plot evaluating the long-term outcomes among patients with external ventricular drain (EVD) infection who survived to discharge. Note that none of the patients who had a Glasgow Outcome Scale (GOS) of 3 or higher had a decline in GOS to a 1 or 2. Darker lines indicate more patients. The red line reflects the average GOS at each time point. (B) Spaghetti plot evaluating the long-term outcomes among patients without EVD infection who survived to discharge. Darker lines indicate more patients. The red line reflects the average GOS at each time point. Note that the trend line for GOS does not show an improvement. This reflects a small number of patients who had a high GOS passing away despite the overall rate of favorable outcomes increasing from 31% to 51%.
To ensure that our decision to exclude patients who died before discharge did not bias our results, we evaluated the relationship between infection and cause of death in this cohort. Four patients had an infection and passed away before discharge. Among these patients, 1 had an arrest and passed away after. Three others had care withdrawn due to presumed poor neurologic prognosis and failure to regain consciousness.
DISCUSSION
In this prospective cohort of severe TBI patients, hemorrhagic complications from invasive neuromonitoring did not impact long-term outcomes. Infectious complications of invasive neuromonitoring resulted in delayed recovery but did not impact long-term outcomes by 6 months. Despite ventriculostomy or ICP monitor placement being 1 of the most common neurosurgical procedures, the relationship between complications of neuromonitor placement and long-term outcomes is not well studied. This is particularly relevant in TBI, where current guidelines recommend ICP-guided therapies despite the failure of randomized trials proving their effectiveness.2,13 To understand the risk-reward benefit of placing invasive neuromonitoring, we evaluated the effects of complications of neuromonitor placement on long-term outcomes in a prospective cohort of severe TBI patients. We found that although neuromonitor-associated infection delays early favorable functional recovery, patients with neuromonitor hemorrhage or infection ultimately had similar long-term outcomes by 6, 12, and 24 months.
Several studies have previously described EVD or intracranial monitor hemorrhage complications and were similar to both our rate (11.6%) and distribution of sizes of hemorrhages.8,21 A meta-analysis in 2011 of nearly 2500 EVD placements reported a rate of 7.0% for EVD-associated hemorrhage,21 while more recent studies have reported a higher incidence of EVD-associated hemorrhages in up to 22% of patients.8 This large variance may reflect inconsistent definitions of monitor-associated hemorrhage, variability in acquiring postplacement imaging, and different patient populations including stroke and shunt failure patients. Our study involved a defined, single-center prospective cohort of severe TBI patients who had postplacement scans within 24 hours.
Our rate of EVD-associated infections was 7.7% and within the wide range reported in the literature.11,12 A meta-analysis by Dorresteijn et al11 in 2019 found an 11% EVD infection rate in approximately 237 patients with TBI. The rate of neuromonitor infections for TBI patients was much lower than the 23% overall rate, as nearly 60% of the approximately 3,000 patients in the meta-analysis had aneurysmal subarachnoid hemorrhage or hemorrhagic stroke. While their rate was higher than our reported rate, this may reflect that the authors included culture-negative ventriculitis. The Infectious Disease Society of America guidelines for health care–associated ventriculitis notes that “CSF cultures are the most important test to establish the diagnosis of health care–associated ventriculitis” and that CSF pleocytosis or abnormalities in glucose or protein may not be reliable indicators of infection.
Despite the high rate of complications after neuromonitor placement, we did not find that invasive neuromonitor complications altered long-term recovery from severe TBI. Neuromonitor-associated hemorrhage had no effect at 3, 6, 12, or 24 months on outcomes. The rate of severe hemorrhages leading to surgical evacuation or death were rare, occurring in 3 out of 534 patients (0.56%). EVD infections, on the other hand, led to a delay in recovery at 3 months that patients overcame by 6 months. No patients with a CSF infection and a GOS of 3 or greater at 3 months experienced a functional decline to a GOS below 3 at a later time point, highlighting that CSF infections only delay but not limit recovery. This new understanding of the long-term consequences of monitor-associated complications will allow for a better assessment of the risk-benefit ratio for placing invasive neuromonitoring.
Although we analyzed 25 variables for associations with neuromonitor hemorrhage or infection, our predictive findings were limited. Neuromonitor hemorrhage rates increased in patients who underwent a craniotomy. The reason for this is unclear but may reflect that patients with more severe injuries are at higher risk for both craniotomy and coagulopathies. Patients with neuromonitor infections had increased rates of depressed skull fractures. Future studies should evaluate if early treatment of depressed skull fractures or prophylactic antibiotics for open fractures reduces EVD infection rates. Importantly, neuromonitor infections did not result in increased risk for posttraumatic hydrocephalus requiring treatment with shunting.
Our work has several limitations. First, we retrospectively reviewed a prospectively collected database of severe TBI patients. Some patients may have been diagnosed with neuromonitor-associated ventriculitis without a positive culture or did not get a post-EVD placement CSF culture, which may have led to bias. We did not have a reliable method to capture some clinically relevant facets of neuromonitoring including duration of monitor placement, replacement of EVDs or intracranial bolts, number of passes of EVD during placement, or open skull fractures, all of which could influence hemorrhage or infection rate. Second, we do not have clear documentation in the electronic health record of why certain patients received only an EVD or intracranial ICP monitor (not both, in keeping with our institutional standard). While this group constitutes only ~10% of our overall cohort, placing only an EVD or ICP monitor may change the rates of hemorrhage or infection. Third, in 2016, we changed our practice of CSF sampling to twice weekly, which has been shown to decrease rates of ventriculitis and may have introduced bias.22 Exploring how the frequency of CSF sampling affects ventriculitis was outside the scope of this study. In addition, we did not report the effects of antiplatelet medications for neuromonitor hemorrhage, which has previously been reported.9 Patients before 2010 had paper medical records that did not have reliable records of prehospital antiplatelet use. Lastly, we did not explore how antibiotic-impregnated catheters change infection rates. Recent work has suggested that antibiotic impregnated catheters may lower infection rates to <1%. We do not routinely use them at our center in TBI patients.
CONCLUSION
In a review of a prospective cohort of severe TBI patients, the rate of invasive neuromonitor hemorrhage was 11.6% and infection was 7.7%. Neuromonitor-associated infection led to a delay in functional recovery that was overcome by 6 months, while neuromonitor-associated hemorrhage did not affect long-term outcomes. Future studies should evaluate the benefits of neuromonitoring for guiding care in TBI patients.
Table 3.
Outcomes by Month for Percent Favorable Outcome (Glasgow Outcome Scale 4–5 vs. 1–3)
| Month | No Hemorrhage | Hemorrhage | P Value |
|---|---|---|---|
| 3 | 21 | 20 | 0.87 |
| 6 | 31 | 29 | 0.78 |
| 12 | 37 | 23 | 0.06 |
| 24 | 33 | 29 | 0.64 |
Table 6.
Outcomes by Month for Percent Favorable Outcome (Glasgow Outcome Scale 4–5 vs. 1–3)
| Month | No Infection | Infection | P Value |
|---|---|---|---|
| 3 | 31% | 8% | 0.02 |
| 6 | 42% | 34% | 0.36 |
| 12 | 52% | 40% | 0.26 |
| 24 | 51% | 60% | 0.44 |
Bold is significant (P < 0.05).
Conflict of interest statement:
Dr. Elmer’s research time was supported by the NIH through grant 5K23NS097629. This study was supported by grants NIH/NNR and R00 NR13176 from the National Institutes of Health.
Abbreviations and Acronyms
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- EVD
External ventricular drain
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcomes Scale
- ICP
Intracranial pressure
- ICU
Intensive care unit
- LOS
Length of stay
- TBI
Traumatic brain injury
Footnotes
CRediT AUTHORSHIP CONTRIBUTION STATEMENT
Matthew Pease: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Enyinna Nwachuku: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Ezequiel Goldschmidt: Data curation, Writing – original draft, Writing – review & editing. Jonathan Elmer: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. David O. Okonkwo: Conceptualization, Writing – review & editing.
REFERENCES
- 1.Rowe AS, Rinehart DR, Lezatte S, Langdon JR. Intracerebral hemorrhage after external ventricular drain placement: an evaluation of risk factors for post-procedural hemorrhagic complications. BMC Neurol. 2018;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, 4th ed. Neurosurgery. 2017;80:6–15. [DOI] [PubMed] [Google Scholar]
- 3.Okonkwo D, Shutter L, Moore C, et al. Brain tissue oxygen monitoring and management in severe traumatic brain injury (BOOST-II): a phase II randomized trial. Crit Care Med. 2017;45:1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robba C, Graziano F, Rebora P, et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol. 2021;20:548–558. [DOI] [PubMed] [Google Scholar]
- 5.Bauer DF, Razdan SN, Bartolucci AA, et al. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011;69:255–260. [DOI] [PubMed] [Google Scholar]
- 6.Foreman B, Ngwenya LB, Stoddard E, Hinzman JM, Andaluz N, Hartings JA. Safety and reliability of bedside, single burr hole technique for intracranial multimodality monitoring in severe traumatic brain injury. Neurocrit Care. 2018;29: 469–480. [DOI] [PubMed] [Google Scholar]
- 7.Gardner PA, Engh J, Atteberry D, et al. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009;110:1021–1025. [DOI] [PubMed] [Google Scholar]
- 8.Miller C, Tummala RP. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J Neurosurg. 2017;126: 289–297. [DOI] [PubMed] [Google Scholar]
- 9.Woo PYM, Ng BCF, Xiao JX, et al. The importance of aspirin, catheterization accuracy, and catheter design in external ventricular drainage-related hemorrhage: a multicenter study of 1002 procedures. Acta Neurochir (Wien). 2019;161:1623–1632. [DOI] [PubMed] [Google Scholar]
- 10.Majmundar N, Sarris C, Shastri D, Doran J, Gandhi C, Assina R. Hemorrhagic complications of external ventriculostomy in the aspirin and P2Y12 response assay era. World Neurosurg. 2019; 122:e961–e968. [DOI] [PubMed] [Google Scholar]
- 11.Dorresteijn KRIS, Jellema K, Van De Beek D, Brouwer MC. Factors and measures predicting external CSF drain-associated ventriculitis: a review and meta-analysis. Neurology. 2019;93: 964–972. [DOI] [PubMed] [Google Scholar]
- 12.Lang SS, Zhang B, Yver H, et al. Reduction of ventriculostomy-associated CSF infection with antibiotic-impregnated catheters in pediatric patients: a single-institution study. Neurosurg Focus. 2019;47:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschmidt E, Deng H, Puccio AM, Okonkwo DO. Post-traumatic hydrocephalus following decompressive hemicraniectomy: incidence and risk factors in a prospective cohort of severe TBI patients. J Clin Neurosci. 2020;73:85–88. [DOI] [PubMed] [Google Scholar]
- 15.Tunkel AR, Hasbun R, Bhimraj A, et al. Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64:e34–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Wang W, Cheng F, et al. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. 2015;83:794–800. [DOI] [PubMed] [Google Scholar]
- 17.Nwachuku EL, Puccio AM, Fetzick A, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocrit Care. 2014;20:49–53. [DOI] [PubMed] [Google Scholar]
- 18.Shore PM, Thomas NJ, Clark RSB, et al. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21:1113–1122. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt B, Roberts RS, Davis P, et al. NFL.pdf. N Engl J Med. 2003:1695–1702.14585937 [Google Scholar]
- 20.Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375: 1119–1130. [DOI] [PubMed] [Google Scholar]
- 21.Binz DD, Toussaint LG III, Friedman JA. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurocrit Care. 2009;10:253–256. [DOI] [PubMed] [Google Scholar]
- 22.Williams TA, Leslie GD, Dobb GJ, Roberts B, Van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains: clinical article. J Neurosurg. 2011;115:1040–1046. [DOI] [PubMed] [Google Scholar]


