Abstract
One approach to control dengue virus transmission is the symbiont Wolbachia, which limits viral infection in mosquitoes. Despite plans for its widespread use in Aedes aegypti, Wolbachia's mode of action remains poorly understood. Many studies suggest that the mechanism is likely multifaceted, involving aspects of immunity, cellular stress and nutritional competition. A previous study from our group used artificial selection to identify a new mosquito candidate gene related to viral blocking; alpha‐mannosidase‐2a (alpha‐Mann‐2a) with a predicted role in protein glycosylation. Protein glycosylation pathways tend to be involved in complex host–viral interactions; however, the function of alpha‐mannosidases has not been described in mosquito–virus interactions. We examined alpha‐Mann‐2a expression in response to virus and Wolbachia infections and whether reduced gene expression, caused by RNA interference, affected viral loads. We show that dengue virus (DENV) infection affects the expression of alpha‐Mann‐2a in a tissue‐ and time‐dependent manner, whereas Wolbachia infection had no effect. In the midgut, DENV prevalence increased following knockdown of alpha‐Mann‐2a expression in Wolbachia‐free mosquitoes, suggesting that alpha‐Mann‐2a interferes with infection. Expression knockdown had the same effect on the togavirus chikungunya virus, indicating that alpha‐Mann‐2a may have broad antivirus effects in the midgut. Interestingly, we were unable to knockdown the expression in Wolbachia‐infected mosquitoes. We also provide evidence that alpha‐Mann‐2a may affect the transcriptional level of another gene predicted to be involved in viral blocking and cell adhesion; cadherin87a. These data support the hypothesis that glycosylation and adhesion pathways may broadly be involved in viral infection in Ae. aegypti.
Keywords: alpha‐mannosidase, chikungunya virus, cadherin, dengue infection, Wolbachia‐mediated viral blocking
The expression of an Aedes aegypti candidate Wolbachia‐mediated pathogen blocking gene, alpha‐Mann‐2a, is affected by dengue virus (DENV) in a tissue‐ and time‐dependent manner, and Wolbachia infection had no effect. Following knockdown of alpha‐Mann‐2a expression in the midgut, DENV infection prevalence increased in Wolbachia‐free mosquitoes, and the same effect was observed during chikungunya virus infection. Knockdown of alpha‐Mann‐2a expression also decreases another candidate Wolbachia‐mediated pathogen blocking gene, cadherin87a, suggesting that both genes contribute to infection outcomes.
INTRODUCTION
The mosquito Aedes aegypti plays an extensive role in transmitting the following viruses: dengue (DENV), Zika (ZIKV), chikungunya (CHIKV) and yellow fever, which have serious health consequences in humans globally (Gould et al., 2017). Without effective, licensed vaccines, prevention of arboviral diseases is focused on mosquito control via habitat reduction and the use of insecticides. Such interventions are difficult to implement in regions of impoverishment, urbanization and poor sanitation (Fauci & Morens, 2016). Furthermore, insecticide resistance in mosquitoes has hampered these efforts and assisted in the increase in dengue cases (Dusfour et al., 2019). It is estimated that yearly 390 million become infected with dengue, with 96 million experiencing pathological symptoms, and efforts to create a vaccine have faced difficulties due to the circulation of four different dengue serotypes across the world (Izmirly et al., 2020).
One alternative means of disease control is through the use of the bacterium Wolbachia, which is being released globally to decrease virus transmission to humans through population ‘suppression’ and population ‘replacement’ (Ferreira et al., 2020). Wolbachia are endosymbiotic bacteria that naturally infect arthropods and are maternally transmitted to offspring (Zug & Hammerstein, 2012). Wolbachia species native to a range of other insects have been introduced into the naturally Wolbachia‐free Ae. aegypti where they have formed stably inherited infections (McMeniman et al., 2009; Xi et al., 2005). ‘Population suppression’, involving the release of Wolbachia‐infected males whose offspring are not viable when mated with wild females, has led to reductions in mosquito populations where trialled in the United States and China (Crawford et al., 2020; Zheng et al., 2019). ‘Population replacement’ is another control approach and is achieved by releasing female Ae. aegypti that pass on Wolbachia to their offspring, which then spreads through the population. Female mosquitoes infected with Wolbachia harbour reduced dengue viral loads, exhibit longer extrinsic incubation periods or fail to transmit virus in the saliva all together (Moreira et al., 2009; Ye et al., 2015). Field trials of replacement strategies have shown some efficacy in reducing the incidence of dengue fever in humans (Carrington et al., 2018; Nazni et al., 2019; Utarini et al., 2021). Wolbachia also limits the replication of other arboviruses in mosquitoes, including CHIKV, ZIKV, yellow fever and West Nile (WNV), suggesting a fundamental shared mechanism that could be useful for many diseases (Joubert & O'Neill, 2017; Kambris et al., 2009; Moreira et al., 2009; van den Hurk et al., 2012).
The potential use of Wolbachia population replacement for biocontrol by pathogen blocking is immense. While the mechanisms of blocking are still poorly understood, there is a growing consensus that it is multifaceted (Lindsey et al., 2018). Understanding how mosquito–virus–Wolbachia genetic interactions dictate pathogen blocking is necessary to assure the efficacy of the biocontrol agent across a global landscape of mosquito and viral genetic diversity. In addition, understanding the mechanism will be key to developing strategies to counter the evolution of resistance against Wolbachia or its effects (Joubert et al., 2016), either in the mosquito or the virus. Our own work has shown that we can rapidly artificially select for differences in DENV blocking strength in mosquitoes collected from a single field release site (Ford et al., 2019), demonstrating the importance of mosquito genetic diversity in the trait's expression. Genome‐wide association (GWA) in selected lines exhibiting stronger and weaker blocking revealed a set of candidate mosquito genes that underpin blocking phenotypes. One of the top candidate genes was alpha‐mannosidase‐2a (alpha‐Mann‐2a). Alpha‐mannosidase proteins cleave mannose residues on glycoproteins as they move through the secretory pathway in the endoplasmic reticulum (ER) and Golgi, creating the substrate for the next step in a complex process of N‐linked glycosylation (Stanley, 2011). In the ER, alpha‐mannosidases also recognize mis‐folded proteins and facilitate degradation through the ER‐associated degradation pathway (Hosokawa et al., 2001). The predicted ortholog to alpha‐Mann‐2a in Drosophila, alpha‐Man‐II‐b, has also been demonstrated to have deglycosylation activity, though its role is unknown, and its increased variation compared with other Drosophila alpha‐mannosidases suggests a deviation in its function (Rosenbaum et al., 2014).
Alpha‐mannosidases have not directly been associated with DENV infection; however, the expression of an alpha‐mannosidase‐1 positively correlated with Hepatitis B virus during infection (Jiang et al., 2016), and a hantavirus protein colocalizes with mammalian alpha‐mannosidase‐2 protein during infection (Ravkov & Compans, 2001). Another possibility is that alpha‐Mann‐2a may affect the glycosylation of viral proteins rather than those of host origin. Glycoproteins are a major component of viral surface proteins and are important for CHIKV and DENV attachment to cells, and DENV secretes non‐structural glycoproteins (Banerjee & Mukhopadhyay, 2016). With no functional evidence of the role of this gene in mosquitoes, further investigation during infection is required. Since the key single nucleotide polymorphisms (SNPs) in alpha‐Mann‐2a identified in our GWA were located in the non‐coding regions (Ford et al., 2019), we sought to manipulate the expression of this gene to see the most effect. We therefore used RNA interference (RNAi) to assess whether reduced expression of alpha‐Mann‐2a had any impact on the DENV and CHIKV infection loads in mosquitoes. This gene may offer a potential target for genetic modification in the effort to create virus refractory mosquitoes.
RESULTS
The effect of DENV and Wolbachia infection on alpha‐Mann‐2a expression
We hypothesized that infection may regulate the expression of alpha‐Mann‐2a and so we measured the expression in both Wolbachia‐free (W−) and wAlbB mosquitoes (W+) that consumed either DENV‐infected (D+) or DENV‐free blood (D−). The midgut, salivary gland and remaining tissues (remainder) were collected at 3, 7 and 14 days post‐feeding for each treatment group. Expression of the candidate gene was normalized to the housekeeping gene Rps6. The expression levels of alpha‐Mann‐2a were regulated by tissue, time and DENV infection status, but Wolbachia had no effect (mixed effects model: tissue, time and infection p < 0.0001, Wolbachia p = 0.44, Table S1). Overall expression was lowest in the midgut and highest in the salivary glands, where on average expression levels at 7‐ and 14 days post‐feeding on blood without DENV were at least 100‐fold higher than in the other tissues (Figure 1). In the midgut, the impact of DENV was limited to 14 days post‐feeding, where expression in both wild‐type and Wolbachia‐infected mosquitoes decreased more than four‐fold in the presence of DENV when compared with uninfected blood fed mosquitoes (post hoc Tukey time × infection: p < 0.0001). Salivary gland gene expression trends were similar to that of the midgut, with DENV‐infected mosquito lines having 2.5‐ to 3‐fold lower alpha‐Mann‐2a expression at 7 and 14 days post‐feeding, compared with uninfected blood fed mosquitoes (post hoc Tukey time × infection: p = 0.001 and p < 0.0001, respectively). In contrast, in the remainder tissue, alpha‐Mann‐2a expression increased in the presence of DENV at 7 days post‐feeding and remained elevated over 12‐fold at 14 days post‐feeding for both W− and W+ mosquitoes (post hoc Tukey time × infection: p = 0.008 and p = 0.007, respectively). Overall, Wolbachia infection itself had no impact on alpha‐Mann‐2a expression and DENV impacts were tissue‐ and time‐specific. DENV infection induced differential expression of alpha‐Mann‐2a later in infection, decreasing expression in the midgut and salivary glands and increasing expression in the remainder tissue.
FIGURE 1.
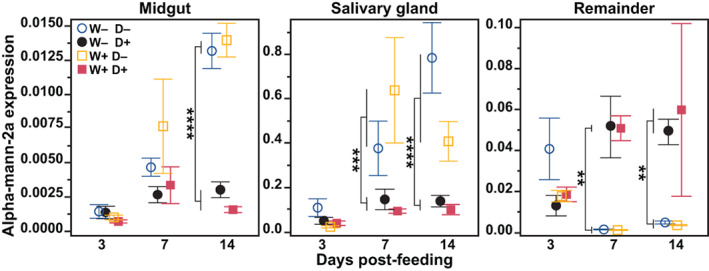
Effect of Wolbachia and dengue virus (DENV) infection on alpha‐Mann‐2a expression. At 3, 7 and 14 days post‐feeding, the midguts, salivary glands and remaining tissues (remainder) were dissected from Wolbachia‐free (W−) and wAlbB (W+) mosquitoes that fed on blood containing DENV (D+) or blood without DENV (D−). The mRNA levels of alpha‐Mann‐2a are expressed relative to RpS6. Symbols represent the mean and bars the standard error of the mean. Tukey post hoc multiple comparisons: **p < 0.01, ***p < 0.001, ****p < 0.0001. N = 7–8
alpha‐Mann‐2a knockdown during DENV infection
We hypothesized that alpha‐Mann‐2a affects DENV infection and dissemination from the midgut to the body in mosquito lines with and without Wolbachia. To investigate the role of alpha‐Mann‐2a during DENV infection and Wolbachia‐mediated pathogen blocking, mosquitoes were injected with double‐stranded RNA (dsRNA) complimentary to alpha‐Mann‐2a to decrease its mRNA expression through the RNAi pathway. On day 3 following dsRNA injection, mosquitoes were fed on infectious blood and unfed mosquitoes were discarded. Midguts and the remaining carcasses containing the salivary glands were sampled at 7 days post‐infection (dpi) and again at 10 dpi, a time point when salivary glands are infected in the majority of mosquitoes (Salazar et al., 2007). The Wolbachia‐mediated blocking significantly reduced DENV‐infected individuals as expected (Figure S1); however, knockdown was not effective in any of the tissues of Wolbachia‐infected mosquitoes (Figure 2a–c). We also checked the Wolbachia densities across treatment groups and found that RNAi had no effect on wAlbB densities (Figure S2). We did see effective knockdown in the midgut of wild‐type mosquitoes infected with DENV 7 dpi, with 90% of the control levels of expression suppressed (Figure 2e). In the carcass the transcript levels surprisingly increased compared to controls (Figure 2f).
FIGURE 2.
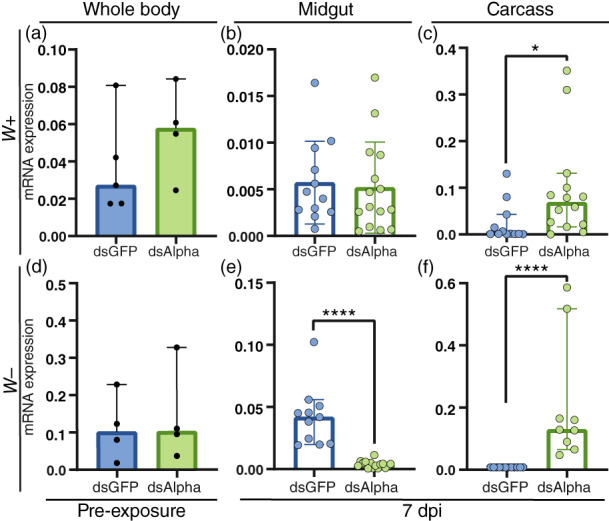
Expression of alpha‐Mann‐2a following RNA interference (RNAi). The expression of alpha‐Mann‐2a in control mosquitoes (dsGFP) and during alpha‐Mann‐2a RNAi (dsAlpha). (a–c) wAlbB mosquitoes (W+). (d–f) Wolbachia‐free mosquitoes (W−). (a, d) The levels of alpha‐Mann‐2a mRNA in whole‐body mosquitoes prior to dengue virus exposure through blood feeding (pre‐exposure) at 3 days post‐RNAi. Black circles represent individual whole‐body samples. N = 4–5. (b, c, e, f) Expression of alpha‐Mann‐2a at 7 dpi in the midgut and carcass. Blue and green circles represent individual tissues. Graphs display the relative expression to RpS6, bars represent the median and whiskers depict the 95% confidence intervals. Mann–Whitney: *p < 0.05, ****p < 0.0001. N = 9–15
Knockdown of alpha‐Mann‐2a significantly increased the intensity (viral load) of DENV infection in the midgut at 7 dpi (Figure 3a, Mann–Whitney test p < 0.01). Of those mosquitoes, the DENV prevalence (percent infected) was 60% higher in alpha‐Mann‐2a knockdown midguts, and prevalence also increased by 33% at 10 dpi in the midgut (Figure 3b, likelihood ratio test, treatment p < 0.0001). Overall, alpha‐Mann‐2a knockdown was associated with increased DENV prevalence, suggesting that alpha‐Mann‐2a interferes with DENV infection in the midgut. When DENV prevalence increased at 7 dpi in the midgut, transcript levels of alpha‐Mann‐2a were reduced by more than 90%, confirming that silencing by RNAi was occurring (Figure 2e, Mann–Whitney p < 0.0001). The lack of the effect in the carcass for either intensity (Figure 3a) or prevalence (Figure 3b) is not surprising given a lack of gene expression knockdown (Figure 2f). Additionally, this could indicate differential roles of this enzyme in the midgut and carcass, which would be supported by our expression data (Figure 1). Overall, a single dose of dsRNA targeting alpha‐Mann‐2a (dsAlpha) prior to infection can increase DENV prevalence in the midgut at 7 and 10 dpi. This is important for potential downstream effects on DENV dissemination and invasion of the salivary glands.
FIGURE 3.
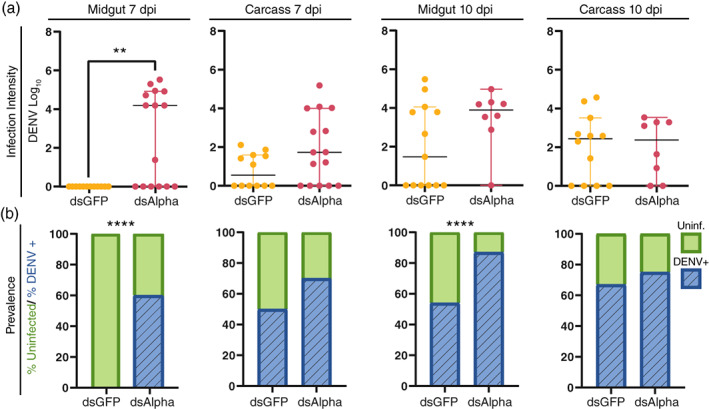
Dengue virus (DENV) infection during RNA interference knockdown of alpha‐Mann‐2a. DENV intensity and prevalence were detected by absolute qRT‐PCR in midgut and carcass samples at 7 and 10 dpi. (a) Infection intensity (genome copy number) over time. Lines mark the median, circles represent DENV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann–Whitney test, **p < 0.01. (b) Prevalence (presence or absence) of DENV. Bars contain the percentage of mosquitoes uninfected (green) and DENV infected (blue). Binary logistical regression for Midgut: p < 0.0001, likelihood ratio test for treatment, ****p < 0.0001. Binary logistical regression for carcass: p = 0.48. N = 8–15
alpha‐Mann‐2a knockdown during CHIKV infection
Next, we were interested in comparing the potential functional role of alpha‐Mann‐2a in the presence of an alphavirus vs. a flavivirus. We used the same dsRNA for knockdown as above and infected mosquitoes with CHIKV. Knowing that CHIKV infection proceeds faster, we took time points at what would be biologically comparable in terms of viral dissemination.
In wild‐type mosquitoes at 10 dpi, the intensity of CHIKV infection was significantly higher in the alpha‐Mann‐2a knockdown mosquito midguts (Figure 4a). This was largely driven by the increase in the number of infected mosquitoes in this group (Figure 4b), and similar to the effects observed during DENV infection (Figure 3). By 10 dpi in control midguts, the prevalence of CHIKV was 81%, and alpha‐Mann‐2a knockdown increased CHIKV prevalence to 100% (likelihood ratio test, p = 0.03). Though similar to the effects observed during DENV infection, comparatively the trend was not as strong. The overall prevalence and intensity of CHIKV infection were higher than in DENV control mosquitoes and likely due to the higher titre that was harvested for the bloodmeal. The expression of alpha‐Mann‐2a in dsAlpha mosquitoes was reduced by 75% prior to infection; however, by 5 dpi expression was not significantly lower (Figure S3). This recovery of expression could be another reason the effects are not as strong. As with DENV infection, no effects were observed in the carcass (Figure S4). Overall, the data supports that alpha‐Mann‐2a limits viral prevalence in the midgut during both DENV and CHIKV infections.
FIGURE 4.
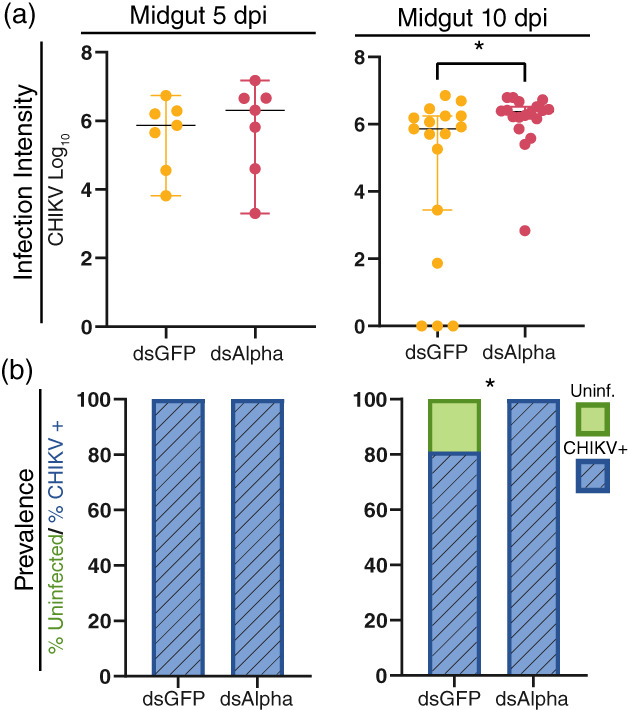
Chikungunya virus (CHIKV) infection during RNA interference knockdown of alpha‐Mann‐2a in the midgut. CHIKV intensity and prevalence were detected by absolute qRT‐PCR in midgut samples at 5‐ and 10 dpi. (a) Infection intensity (genome copy number) over time. Lines mark the median, circles represent CHIKV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann–Whitney test, *p < 0.05. (b) Prevalence (presence or absence) of CHIKV. Bars contain the percentage of mosquitoes uninfected (green) and CHIKV infected (blue). Binary logistical regression for Midgut: p = 0.03, likelihood ratio test for treatment, *p = 0.03. At 5 dpi N = 7 and at 10 dpi N = 17–18
alpha‐Mann‐2a and cadherin87a interactions
Since the conventional role of alpha‐mannosidase‐2 is to help convert a high mannose glycosylated structure to a complex mannose structure, we hypothesized that the role of alpha‐Mann‐2a may function through its enzymatic activity on another protein. For multiple reasons we looked into cadherin87a (AAEL023845), the next top candidate gene from our previous study (Ford et al., 2019). In addition to both genes containing genetic variation that associated with the strength of DENV blocking, some cadherins require glycans in order to mediate cell adhesion (Lommel et al., 2013). Therefore, it is plausible there could be functional interactions with alpha‐Mann‐2a. Second, there are data from mammalian systems showing that decreasing the expression of an alpha‐mannosidase gene also changed the expression of an E‐cadherin (Tian et al., 2008). Finally, these genes are close in proximity to each other, just under 1.5 Mb apart. Cadherins are a large and diverse family of proteins that all have extracellular cadherin repeat domains and many roles including cell–cell adhesion, cell polarity and intracellular signalling (Halbleib & Nelson, 2006). Classical cadherins form adherens junctions with nectin proteins, and nectin is bound by poliovirus, human herpes simplex virus and the measles virus in order to cross the epithelium (Mateo et al., 2015). Though a cadherin protein is yet to be identified as a viral receptor in any organism, multiple mosquito cadherins have been identified that bind DENV proteins (Colpitts et al., 2011; Muñoz et al., 2013). We were interested to see if knockdown of alpha‐Mann‐2a affected expression of cadherin87a.
We checked the levels of cadherin87a in our dsAlpha treated mosquitoes. When alpha‐Mann‐2a expression decreased by more than 90% at 7 dpi in the midguts of dsAlpha mosquitoes (Figure 2e), cadherin87a expression also decreased by 77% (Figure 5). These results suggest that the increases in infection upon alpha‐Mann‐2a knockdown may result from a decrease of both alpha‐Mann‐2a and cadherin87a in the midgut. These results where surprising since we suspected these genes to have opposing functions during infection. The decrease in cadherin87a expression upon alpha‐Mann‐2a KD suggests that there could be direct or indirect interactions in the functions of these genes during infection. For example, the functional activity of alpha‐Mann‐2a may affect cadherin87a protein turnover, and therefore expression, or decreases in cadherin87a expression may be indirect effects from increasing DENV during alpha‐Mann‐2a knockdown.
FIGURE 5.
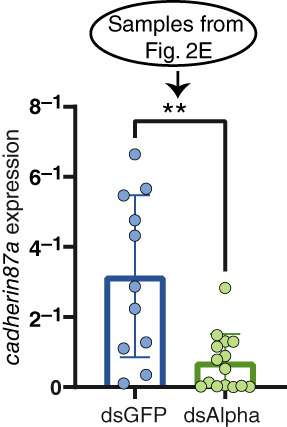
Expression of cadherin87a during alpha‐mann‐2a RNA interference. Transcript levels of cadherin87a following dengue virus infection at 7 dpi in the midgut of control (dsGFP) and dsAlpha injected mosquitoes. Graphs display the relative expression compared with RpS6. Bars represent the median and whiskers depict the 95% confidence intervals. Circles represent individual midguts. Mann–Whitney: **p = 0.003, N = 11–15. The graph of alpha‐mann‐2a transcription in these same samples is found in Figure 2e
We then went back to our initial expression experiment and checked cadherin expression in these same tissues. Cadherin87a expression was impacted by tissue, time, infection and Wolbachia (mixed effects model Wolbachia, time, and infection: p < 0.0001, tissue p = 0.02). Overall expression levels did not vary highly across tissues (Figure 6). In the midgut DENV infection had no impact on cadherin expression (mixed effects model time × infection × Wolbachia: DENV p = 0.37), and Wolbachia decreased expression at 3 days post‐bloodmeal by 2.5‐fold (post hoc Tukey: time × Wolbachia p < 0.009). In the salivary glands of DENV‐free mosquitoes, Wolbachia also reduced expression post‐bloodmeal across all time points (post hoc Tukey: Wolbachia p = 0.004). At 14 dpi in the remainder tissue, Wolbachia reduced cadherin87a by 3‐fold, and DENV also reduced expression in both W− and W+ mosquitoes by 60‐fold or more (post hoc Tukey infection × Wolbachia: Wolbachia and infection p < 0.0001). In summary, the presence of Wolbachia reduced cadherin87a expression in the midgut and DENV reduced expression in the remainder. In the midgut at 14 dpi, cadherin87a was lower in the DENV‐infected samples and trending in the same direction that alpha‐Mann‐2a does in response to DENV in the midgut, though not significantly different. There are also significant differential expression patterns between alpha‐Mann‐2a and cadherin87a. In the remainder, at 14 dpi, DENV had opposite effects, increasing alpha‐Mann‐2a and decreasing cadherin87a expression (Figures 1 and 6).
FIGURE 6.
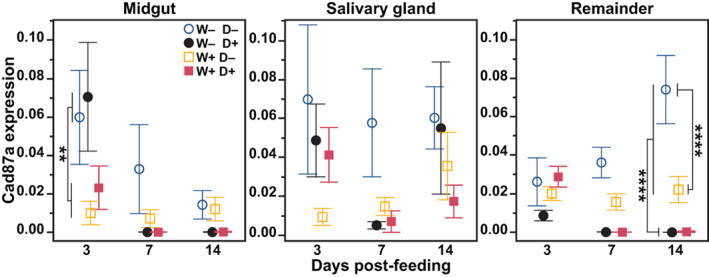
Effect of Wolbachia and dengue virus (DENV) infection on cadherin87a expression. At 3, 7, and 14 days post‐feeding, the midguts, salivary glands and remaining tissues (remainder) were dissected from Wolbachia‐free (W−) and wAlbB (W+) mosquitoes that fed on blood containing DENV (D+) or blood without DENV (D−). The mRNA levels of cadherin87a are expressed relative to RpS6. Symbols represent the mean and bars the standard error of the mean. Tukey post hoc multiple comparisons: **p < 0.01, ****p < 0.0001. N = 7–8
DISCUSSION
Current control approaches releasing Wolbachia‐mosquitoes aim to decrease mosquito susceptibility to viral infection or to reduce the number of wild mosquitoes (Ferreira et al., 2020). Both strategies require large‐scale mosquito rearing facilities for establishment and long‐term monitoring for success (Ritchie & Staunton, 2019). These high investment approaches are proving to have high returns with respect to effectively crashing mosquito populations and for reducing the incidence of dengue fever in humans via pathogen blocking (Carrington et al., 2018; Nazni et al., 2019; Zheng et al., 2019). Insights into how pathogen blocking operates at the molecular level may provide strategies and solutions for maintaining the success of Wolbachia in the face of evolutionary or ecological hinderances. Additionally, independently targeting the mosquito pathways acted upon by Wolbachia could lead to GM technology that is more accessible and easier to deploy in mosquitoes than symbiont‐based methods.
A range of mechanisms has been proposed to explain Wolbachia‐mediated virus blocking. The growing consensus is that the effect is likely multifaceted (Lindsey et al., 2018). There is some evidence that mosquito immunity (Pan et al., 2012; Terradas et al., 2017), cellular stress (Wong et al., 2015) and competition for space or nutritional resources (Caragata et al., 2013, 2016) as well as other factors could play a role. We recently sought to identify the basis of viral blocking using an unbiased approach by artificially selecting upon the natural variation observed in this phenotype (Ford et al., 2019). By identifying SNPs that differentiated low‐ and high‐blocking selected lines, we identified a suite of new candidate loci associated with these phenotypes. Few of the genes we identified containing key SNPs recapitulated previously proposed hypotheses, although there was some evidence of selection driving variation in oxidative stress genes (Ford et al., 2019). The two top candidate genes, surprisingly, pointed to potential impacts on protein glycosylation and or cell adhesion.
In this study, we sought to obtain functional data on the role of alpha‐Mann‐2a, in virus replication in the mosquito by using RNAi. We took a practical and conservative approach in our design of gene knockdown, by using a single dose of dsRNA prior to infection and assessing infection at later time points when the virus has disseminated. A caveat to this design is that we could have missed earlier effects on infection or any larger differences that may have waned over time. We chose this approach first, since the gene being studied was identified through experimental infection via the body cavity, therefore initially bypassing the midgut barrier, and second to see if a single dose of dsRNA could induce RNAi and have lasting effects throughout an infection. With oral delivery systems being developed for larval and adult stage mosquitoes (Wiltshire & Duman‐Scheel, 2020), RNAi‐based strategies have the potential to be deployed for mosquito control if their impacts can last beyond several days. Furthermore, different methods of RNAi induction and delivery, such as microbial‐based delivery, could be pursued to enhance the efficiency of knockdown. Our goal was to initially assess if alpha‐Mann‐2a is a good target for adult stage manipulation of infection.
Although we have no evidence of what alpha‐Mann‐2a and cadherin87a are doing in the mosquito, there is information in the broader literature within these gene families about localization, activity and function, which can inform on how they may affect infection. Both, alpha‐Mann‐2a and cadherin87a, are members of large gene families that are further divided into subfamilies based on sequence domains, localization and protein activity (Gonzalez & Jordan, 2000; Oda & Takeichi, 2011). Individual genes in these families may have novel, distinct functions or provide redundancies in functionality with other genes. In mosquitoes, alpha‐mannosidases have not been studied, and a search for ‘alpha‐mannosidase’ in the Ae. aegypti genome (AaegL5.0) results in 11 alpha‐mannosidase genes, including some that are specifically predicted to be lysosomal or ER degradation enhancing. The exact role of the alpha‐Mann‐2a ortholog in Drosophila melanogaster is unknown, but it is required for proper maturation of a rhodopsin protein in the fly eye (Rosenbaum et al., 2014). A search for ‘cadherin’ in the Ae. aegypti genome results in more than 20 genes and cadherin87a is 1 of 7 that are categorized as protocadherins or neural cadherins. Another cadherin gene in Ae. aegypti has been identified as a receptor for toxins released by the bacterium Bacillus thuringiensis. It is expressed in the digestive tract and essential for development. The strongest localization of this protein was on the apical side of the epithelia and varied in tissue location across developmental stages, with adult females showing highest localization in the foregut (Chen et al., 2020). This gene's distinct expression dynamics and localization underline the diverse function of genes in this family. Further work into the spatial and temporal dynamics of alpha‐Mann‐2a and cadherin87a will inform on their roles during infection. In particular, the DENV‐induced increase in alpha‐Mann‐2a in the tissues of the body, which are opposite of the decreases in expression observed in the midgut and salivary glands, could be reflective of tissue differences or differences in pathogen interactions. Furthermore, these differences may have contributed to the inability to capture an observed knockdown in the carcass. The carcass also includes hemocytes, fat body, the heart, aorta, muscles and additional tissues in which alpha‐Mann‐2a may be expressed, and the time course of knockdown could proceed at a different rate.
In keeping with our data on alpha‐Mann‐2a, we propose two potential modes of action for its involvement in viral infection. The first hypothesis is that alpha‐Mann‐2a enzymatic activity acts upon viral proteins. The second is that alpha‐Mann‐2a enzymatic activity acts upon mosquito proteins that alter infection. In support of the first hypothesis, the presence of N‐linked glycosylation on flavivirus proteins directly impacts binding to factors on the cell's surface (Carbaugh & Lazear, 2020). N‐linked glycosylation can be further classified as high mannose, which is the first step in the stepwise process to further becoming classified as complex mannose (Yap et al., 2017). Alpha‐mannosidase‐2 is required in the first step to convert a high mannose glycosylated structure to a complex mannose structure. Insects and mammals have different glycosylation pathways, and therefore glycosylation patterns are different in these two hosts (Hacker et al., 2009). Analyses of N‐linked glycans on DENV detect high mannose glycans as a majority component when propagated in mosquito cells, and DENV derived from mammalian cells have more complex glycans (Hacker et al., 2009; Lei et al., 2015). This would suggest an absence of alpha‐mannosidase activity on DENV propagated in insect cells. Potentially the addition of complex N‐linked glycans facilitated by alpha‐Mann‐2a could lead to decreased infectivity through changes in protein binding, folding, localization or secretion. The presence of N‐linked glycosylation at a specific position on the WNV Envelope protein reduced viral infectivity in Aedes albopictus C6/36 mosquito cell lines, and this effect was stronger in mosquito cells compared with mammalian (Hanna et al., 2005). In human cells, WNV interactions with the C‐type lectin viral attachment factor DC‐SIGN have been shown to be dependent on the presence of high mannose residues on envelope proteins (Carbaugh & Lazear, 2020). Though this receptor is not present in insects, other C‐type lectins promote DENV, Japanese encephalitis virus and WNV infection (Caragata et al., 2019). The studies mentioned above have compared non‐glycosylated DENV to glycosylated or comparatively studied mosquito versus mammalian derived virus; however, it is unknown if altering a high mannose glycan to a complex mannose, as would be facilitated by alpha‐mannosidase, would change infectivity in mosquitoes. Lastly, it is also possible that high mannose glycosylation of viral proteins results in an evasion of immune responses as has been shown for both flaviviruses and alphaviruses in mammalian cells (Rogers & Heise, 2009), and therefore alpha‐Mann‐2a activity could result in increasing virus vulnerability to immune responses.
Our second hypothesis is that alpha‐Mann‐2a activity may alter the glycosylation of mosquito proteins. This could occur through altering the glycosylation of proteins involved in the viral response or that facilitate infection. Furthermore, the enzymatic activity of alpha‐Mann‐2a could lead to protein turnover, as inhibitors of alpha‐mannosidase lead to decreased degradation of glycoproteins (Segal & Winkler, 1984). In summary, alpha‐Mann‐2a may destabilize the activity or recycling of a protein that facilitates viral infection.
The enzymatic activity of alpha‐Mann‐2a may even act on cadherin87a, as the expression of cadherin87a also decreased during knockdown of alpha‐Mann‐2a. It is not clear how decreasing alpha‐Mann‐2a would also decrease cadherin87a expression, and this would be unexpected based on our hypotheses of opposing function. However, our expression data show opposite directional expression changes in the remainder in the presence of DENV, however this does not occur in the midgut tissue (Figures 1 and 6).One possibility of the observed effects in our KD is that the alpha‐Mann‐2a protein may destabilize the activity or recycling of the cadherin protein. This puts forth the hypothesis that when cadherin87a is in a high mannose glycosylation state (in the absence of alpha‐Mann‐2a) it is more stabilized or recycled, and there is a decreased need to express more cadherin87a, meaning that the cadherin87a expression data may not be reflective of protein levels or activity. In support, classical cadherin activity requires high mannose glycosylation for proper cadherin‐based adhesion in cell junctions (Lommel et al., 2013; Vester‐Christensen et al., 2013). Last, cadherins are trafficked, sorted and recycled to the plasma membrane through endocytosis (Brüser & Bogdan, 2017), and glycosylation could play a role in these processes. Another possibility is that indirectly the effects of alpha‐Mann‐2a KD on DENV then affect cadherin87a expression.
We showed that wAlbB decreased expression of cadherin87a in the midgut, supporting that lower expression could decrease infection; however, DENV also decreased expression in the tissues of the body at later time point in infection. Altogether, cadherin87a may have different roles across tissues and time, and a closer look at protein localization is needed to further assess potential interactions. Interestingly, the cadherin87a gene is 869,996 bp with 27 exons and 5 predicted transcripts (AaegL5.0), so there could also be transcript specificity. Previous studies have identified multiple mosquito cadherins that bind DENV proteins (Colpitts et al., 2011; Muñoz et al., 2013), and one cadherin (AAEL001196) facilitated DENV infection in the Ae. aegypti Aag‐2 cell line. However, wAlbB actually increased the expression of this cadherin gene, and knockdown did not affect pathogen blocking of DENV (Lu et al., 2020). In human cells, an E‐cadherin indirectly facilitates the entry of Hepatitis C virus as it is required for proper localization of receptor proteins in the nearby tight junctions on the cell surface (Li et al., 2016). Interestingly, later in infection, the expression of this E‐cadherin decreased and correlated with increased markers for disease progression in infected cells, likely due to decreased epithelial integrity. This suggests that a single cadherin could have a multifaceted role during an infection, initially facilitating cell entry and later being targeted to disrupt cell to cell adhesions to increase permeability for dissemination. Changes in mosquito cell integrity during infection have not been described; however, in humans haemorrhagic fever during DENV infection associates with decreases in vascular cadherins involved in membrane permeability (Dewi et al., 2008; Kanlaya et al., 2009).
Interestingly, alpha‐Mann‐2a gene knockdown by RNAi only occurred in the absence of Wolbachia. This is intriguing since we hypothesize that alpha‐Mann‐2a decreases viral infection. We show that DENV changes alpha‐Mann‐2a expression but the presence of Wolbachia had little effect. This is not completely surprising since the variation that correlates with pathogen blocking in this gene occurs within introns (Ford et al., 2019). This suggests that Wolbachia may affect splicing and not expression. Recent work in D. melanogaster identified alternative splicing of genes in the presence of Wolbachia and Sindbis virus (Bhattacharya et al., 2017). So further assessment of the role of these genes in pathogen blocking will be done in combination with transcriptomics looking at splicing variation. Wolbachia could also potentially alter the rate of protein turnover, which would indirectly affect transcription and the ability to knockdown a transcript. This is supported by the possibility of overlap in physical space, as alpha‐mannosidase proteins associate with the ER and Golgi, and Wolbachia physically interacts with the ER and alters its subcellular organization (Fattouh et al., 2019). Furthermore, these potential interactions could facilitate the pathogen blocking phenotype by Wolbachia through increasing or stabilizing alpha‐Mann‐2a activity.
Both alpha‐Mann‐2a and cadherin87a remain as interesting candidates in Aedes, Wolbachia and arbovirus tripartite interactions. The gene alpha‐Mann‐2a impacts viral infection and has the potential to regulate or participate in cellular processes that could be crucial for both Wolbachia and arboviruses. Further analysis on the cellular level in combination with enzyme inhibitors and protein markers will distinguish if these genes have roles atypical of other proteins in these super families. Finally, our data indicate that adhesion and glycosylation pathways may broadly be involved in viral infection in Ae. aegypti.
EXPERIMENTAL PROCEDURES
Mosquito rearing
Ae. aegypti mosquitoes previously infected with the wAlbB strain of Wolbachia were backcrossed for seven generations into a wild line, AFM, isolated from Merida Mexico in 2017. All mosquitoes were reared and maintained at 27°C, 60% relative humidity and a 12 h:12 h light/dark photoperiod. Larvae were reared in deionized water and fed fish food (TetraMin) ad libitum. Prior to eclosion, the pupal stage was transferred in small cups of water to a cage, and emerging adults were fed 10% sucrose.
Virus cultivation and mosquito blood feeding
The dengue virus serotype 2 (DENV‐2) strain JAM 1409 (Bennett et al., 2002) was propagated in A. albopictus C6/36 cells, as previously described (Terradas et al., 2017). Briefly, C6/36 cells were maintained in RPMI medium 1640 (Life Technologies) supplemented with 10% heat‐inactivated fetal bovine serum (FBS), (Life Technologies), 20 mM HEPES buffer (Sigma‐Aldrich) and 1% Penicillin–Streptomycin (Life Technologies). On the day of infection, FBS was reduced to 2% in the medium and a frozen aliquot of DENV‐2 was introduced at a multiplicity of infection (MOI) of 0.002 virus particles per cell to a T75 flask at 80% confluency. Flasks were incubated at 27°C for 7 days, and the supernatant was harvested at a titre of 1.0 × 105 focus forming units per ml (FFU protocol below) and mixed with human blood in a ratio of 1:1. The CHIKV strain 20,235‐St. Martin 2013 (BEI Resources) was inoculated into flasks of C6/36 cells at 80% confluency at an MOI of 0.067 virus particles per cell and followed the protocol described for DENV infection. At 2 dpi, the supernatant was harvested at a titre of 8.7 × 105 FFU/ml and mixed 1:1 with human blood. Female mosquitoes of both lines were aged to 7 days and deprived of sugar for 24 h prior to being offered blood. Mosquitoes were fed the virus laden blood meal of either DENV or CHIKV using glass bell feeders covered with a sausage casing, warmed to 37°C with water from a water bath. After 2 h, the mosquitoes were anaesthetized on ice and unfed or partially fed mosquitoes were removed. All CHIKV work was carried out in the Pennsylvania State Pell BSL‐3 Laboratory.
Dissections
Prior to dissection, mosquitoes were anaesthetized with triethylamine (Sigma‐Aldrich) via exposure to a moistened 47 mm circular filter paper. The head was removed with tweezers, followed by removal of the salivary glands from the thorax, and the midgut from the abdomen using insect pins in phosphate‐buffered saline (PBS). The remaining thorax and abdominal tissues are referred to as the remainder, and in cases where the salivary glands were left intact, we refer to as the carcass.
RNA and DNA extractions
Individual tissues from single mosquitoes were placed in 300 μl of TriReagent (Sigma‐Aldrich), or 200 μl for salivary glands, and homogenized on a Bead Ruptor Elite (Omni International, USA) using a 2.8 mm ceramic bead. Total RNA was extracted with the Direct‐zol RNA 96 Magbead Zymo kit (Zymo Research) according to the manufacture's protocol on a MagMAX Express 96 system (Applied biosystems), and RNA was eluted in 50 μl RNase free water. RNA was treated with 5 units of DNase I (Sigma‐aldrich) at room temperature (RT) for 15 min, followed by inactivation with 50 mM EDTA at 70°C for 10 min. To measure Wolbachia DNA, RNA and DNA combination extractions were performed using the column‐based Direct‐zol DNA/RNA Miniprep kit. RNA was eluted in 50 μl RNase free water, followed by DNA elution in 50 μl of Direct‐zol DNA Elution Buffer, and RNA was treated with DNase I (as above).
Gene and Wolbachia quantification
All quantitative polymerase chain reactions (qPCR) were carried out on a LightCycler 480 Real‐Time machine (Roche). For gene quantification, mRNA was used in a one‐step reverse transcriptase‐qPCR (qRT‐PCR) reaction. Using qScript One‐step SYBR Green qRT‐PCR (Quantabio) according to the manufacturer's protocol, the synthesis of cDNA occurred at 50°C for 5 min followed by Taq inactivation at 95°C for 2 min. Amplification and quantification cycled 45 times at 95°C for 3 s and 60°C for 30 s, and the final cycle was followed by a melting curve analysis. Gene‐specific primers were used at a final concentration of 0.2 μM for amplification of target genes (Table S2), and the insect gene 40S Ribosomal protein S6 (Rps6) was used as a housekeeping gene (Pan et al., 2012). For wAlBb quantification by qPCR, DNA samples were amplified using PerfeCTa SYBR Green SuperMix (Quantabio) according to the manufacturer's protocol and genomic primers specific to wAlBb and the mosquito 40S Ribosomal protein S17, and thermocycling conditions as previously published (Dutra et al., 2021). Gene expression and Wolbachia levels were calculated by the 2−ΔCt method (Livak & Schmittgen, 2001).
Virus quantification
DENV was quantified using TaqMan Fast Virus 1‐step Master Mix (Thermo Fisher Scientific) for qRT‐PCR in 10 μl reaction volumes with DENV specific primers and probes (Table S2) (Ye et al., 2014). The following protocol was used: reverse transcription at 50°C for 5 min, followed by 95°C for 20 s, and amplification cycling at 95°C for 3 s and 60°C for 30 s. A standard reference curve of known quantities of a DENV‐2 genomic fragment was used for absolute qRT‐PCR. The DENV‐2 genomic fragment was inserted into a plasmid and transformed into Escherichia coli as previously described (Ye et al., 2014). The linearized and purified fragment was serially diluted ranging from 107, 106, 105, 104, 103, 102, and 10 copies and were used to create a standard curve of DENV amplification. The standard curve was run in duplicate on each 96‐well plate, and the limits of detection were set at 102 copies. CHIKV was quantified using the same reagents and protocols as DENV above, with CHIKV specific primers and probes (Table S2) (Rückert et al., 2017). A 140 bp fragment was amplified and cloned into the PGEM‐T vector system (Promega, Madison, WI), and the plasmid was transformed into E. coli. Plasmid was extracted by phenol‐chloroform, linearized by restriction enzyme digest with PstI (NEB, Ipswich, MA) and a standard curve of 108, 107, 106, 105, 104, 103, 102, 10, 5, 1 was serial diluted based on the copy number obtained using a Qubit fluorometer (Thermo Fisher Scientific). The limits of detection of CHIKV were set at 10 copies.
Focus forming unit assay
Viral titres were quantified by FFU in Vero cells (Brustolin et al., 2018; Payne et al., 2006). Cells were plated and grown for 24 h to confluency in 96‐well plates at 37°C with 5% CO2. The next day, cells were prepared by washing in RPMI medium (described above) without FBS. Viral supernatant or homogenized mosquito bodies were serially diluted 10‐fold in medium at concentrations from 10−1 to 10−6 and added to cells in 30 μl volumes. Plates were incubated for 2 h at 27°C. After incubation, cells were washed with medium and overlaid with 100 μl of 1% methylcellulose in RPMI with 5% FBS and further incubated for 24 h (CHIKV) or 48 h (DENV). Finally, cells were fixed in 4% paraformaldehyde for 10 min, washed in PBS, blocked and permeabilized in 2% bovine serum albumin with 0.1% triton‐X for 30 min at RT, and washed again with PBS. Virus was labelled for 2 h at RT with monoclonal anti‐DENV type 2 envelope protein Clone 3H5‐1 (BEI Resources, Manassas, VA), followed by three washes in PBS. The secondary antibody, Alexa‐488 goat anti‐mouse IgG (Invitrogen, Life Science), was added at a dilution of 1:500, and plates were incubated in the dark for 1 h. Fluorescent foci were visualized and counted on an Olympus BX41 microscope equipped with an UPlanFI ×4 objective and a FITC filter and were used to calculate FFU/ml.
RNA‐interference gene silencing
The Ae. aegypti gene alpha‐Mannosidase‐2a (AAEL004389) was targeted and silenced by RNAi. We designed many primer sets to target different regions of alpha‐Mann‐2a within exons 4–9. Knowing that this gene likely has a high amount of variation, and experiencing multiple primer sets that did not amplify cDNA, we designed the primers in areas where there were low numbers of SNPs based on genomic sequencing (Ford et al., 2019). Additionally appending the T7 promoter sequence for dsRNA synthesis requires a specific nucleotide sequence. To achieve the best binding, we aligned alpha‐Mann‐2a with the predicted ortholog in Anopheles gambiae (AGAP0004032) and chose conserved GGG sequences between these two species for the forward primer T7 binding location and then used the same approach to design the reverse primers. We also designed our dsRNAs to be over 400 bp in length and therefore would be processed into many siRNAs. Gene‐specific dsRNA was synthesized using the MEGAscript T7 transcription kit (Invitrogen) as previously described (Sigle & Hillyer, 2018). Briefly, templates were amplified from mosquito cDNA using PCR and primers appended with the T7 promoter sequence (Table S2). PCR products were gel purified using the PureLink Quick Gel Extraction kit (Invitrogen). Approximately 1 μg of template was used to synthesize dsRNA following the manufacturer's protocol. The dsRNA was purified by phenol/chloroform precipitation and resuspended in sterile PBS. Adult mosquitoes at 4 days post‐eclosion were anaesthetized on ice and 69 nl of dsRNA (300 ng final concentration) in PBS was injected into the hemocoel through the thoracic anepisternal cleft. Only two out of five designed dsRNAs made it through our stringent requirements and both targeted exon 9. We proceeded with the dsAlpha used in this study based on preliminary KD efficiencies being the greatest. As a control, dsGFP was injected in the same cohort of mosquitoes (Pan et al., 2012). At 3 days post‐RNAi, mosquitoes were offered an infectious bloodmeal as described above. Following infection, the midgut was separated from the carcass and each tissue was individually placed in TriReagent as above and stored at −80°C until extracted.
Statistical analysis
For gene expression experiments with 4‐factors, a mixed effects model was run (Wolb +/−, DENV +/−, tissue and time), followed by a tissue‐specific 3‐factor model (Wolb +/−, DENV +/−, and time) with post hoc Tukey comparisons of the identified significant factors. Data were tested for normality using the Kolmogorov–Smirnov test. All significant differences in gene expression are two‐fold or greater changes in expression. Infection prevalence was analysed for each tissue using a binary logistical regression model with the presence or absence of infection as the response and with the variables of treatment (RNAi: dsGFP vs. dsAlpha) and time (dpi) as predicting effects. The significance of the effects of time and treatment on prevalence were compared using a likelihood ratio test. The Mann–Whitney test was used for t‐test comparisons of non‐parametric data. Analyses were performed using prism 6 Software (GraphPad, La Jolla, CA), with the exception that General linear models and logistical regression were performed in jmp pro 15 (SAS, Cary, NC). Effects were deemed significantly different at p < 0.05.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Leah T. Sigle: Conceptualization, formal analysis, investigation, methodology, project administration, validation, visualization, writing‐original draft, writing‐review and editing. Matthew Jones: Investigation, methodology, validation, writing—review and editing. Mario Novelo: Investigation, validation, writing—review and editing. Suzanne A. Ford: Investigation, writing—review and editing. Nadya Urakova: Methodology, resources, writing—review and editing. Konstantinos Lymperopoulos: Conceptualization, funding aquisition. Richard T. Sayre: Conceptualization, funding aquisition. Zhiyong Xi: Conceptualization, formal analysis, funding aquisition, methodology, project administration, resources, supervision, validation, writing—review and editing. Jason L. Rasgon: Conceptualization, formal analysis, funding aquisition, methodology, project administration, resources, supervision, validation, writing—review and editing. Elizabeth A. McGraw: Conceptualization, formal analysis, funding aquisition, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
Supporting information
Figure S1. Wolbachia mediated DENV‐blocking during alpha‐Mann‐2a RNAi. DENV intensity and prevalence were detected by absolute qRT‐PCR in midgut and carcass samples at 10 and 14 dpi. A. Infection intensity (genome copy number) overtime. Lines mark the median, circles represent DENV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann‐Whitney test, not significant. B. Prevalence (presence or absence) of DENV. Bars contain the percentage of mosquitoes uninfected (green) and DENV infected (blue). Binary logistical regression for Midgut: P = 0.09. Binary logistical regression for Carcass: P = 0.07. N = 20‐25.
Figure S2. Wolbachia levels 3 days post‐RNAi. The levels of wAlbB in whole body mosquitoes was similar across treatment groups at 3 days post‐dsRNA injection and prior to DENV infection. Mann‐Whitney test, dsAlpha P = 0.7. Bars represent the median and whiskers depict the 95% confidence intervals. N = 3.
Figure S3. Expression of alpha‐Mann‐2a following RNAi and infection with CHIKV. A. Levels of alpha‐Mann‐2a expression in mosquitoes at 3 days post‐RNAi (pre‐exposure) compared to control mosquitoes (dsGFP injected). Circles represent individual whole‐body samples. N = 4‐5. B‐C. Expression of alpha‐Mann‐2a at 5 dpi in the midgut and carcass. N = 6‐17. Graphs display the relative expression compared to RpS6. Bars represent the median and whiskers depict the 95% confidence intervals. Mann‐Whitney test: * P < 0.05.
Figure S4. CHIKV infection during RNAi knockdown of alpha‐Mann‐2a in the carcass. CHIKV intensity and prevalence were detected by absolute qRT‐PCR in carcass samples at 5‐ and 10 dpi. A. Infection intensity (genome copy number) overtime. Lines mark the median, circles represent CHIKV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann‐Whitney test, P > 0.07 B. Prevalence (presence or absence) of CHIKV. Bars contain the percentage of mosquitoes uninfected (green) and CHIKV infected (blue). Binary logistical regression for carcass: P = 0.48. At 5 dpi N = 7 and at 10 dpi N = 17‐18.
Table S1. Statistical analyses for Figures 1 and 6.
Table S2. Primers.
ACKNOWLEDGEMENTS
We are grateful to the McGraw lab members for helpful discussions and comments. This work was funded by the National Institutes of Health R01 grants AI143758 to Elizabeth A. McGraw and AI116636 to Jason L. Rasgon, and by a grant from Pebble Labs, Inc. to Elizabeth A. McGraw, Jason L. Rasgon and Zhiyong Xi. The graphical figure was created with BioRender.com.
Sigle, L.T. , Jones, M. , Novelo, M. , Ford, S.A. , Urakova, N. , Lymperopoulos, K. et al. (2022) Assessing Aedes aegypti candidate genes during viral infection and Wolbachia‐mediated pathogen blocking. Insect Molecular Biology, 31(3), 356–368. Available from: 10.1111/imb.12764
Funding information National Institute of Allergy and Infectious Diseases, Grant/Award Numbers: AI116636, AI143758; Pebble Labs
Contributor Information
Leah T. Sigle, Email: leahtc@gmail.com.
Elizabeth A. McGraw, Email: eam7@psu.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in figshare at 10.6084/m9.figshare.13726000.
REFERENCE
- Banerjee, N. & Mukhopadhyay, S. (2016) Viral glycoproteins: biological role and application in diagnosis. VirusDisease, 27(1), 1–11. 10.1007/s13337-015-0293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, K.E. , Olson, K.E. , Muñoz, M.D. , Fernández Salas, I. , Farfán Ale, J.A. , Higgs, S. et al. (2002) Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. The American Journal of Tropical Medicine and Hygiene, 67(1), 85–92. 10.4269/ajtmh.2002.67.85 [DOI] [PubMed] [Google Scholar]
- Bhattacharya, T. , Newton, I.L.G. & Hardy, R.W. (2017) Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection. PLoS Pathogens, 13(6), e1006427. 10.1371/journal.ppat.1006427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüser, L. & Bogdan, S. (2017) Adherens junctions on the move—membrane trafficking of E‐cadherin. Cold Spring Harbor Perspectives in Biology, 9(3), a029140. 10.1101/cshperspect.a029140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustolin, M. , Pujhari, S. , Henderson, C.A. & Rasgon, J.L. (2018) Anopheles mosquitoes may drive invasion and transmission of Mayaro virus across geographically diverse regions. PLoS Neglected Tropical Diseases, 12(11), e0006895. 10.1371/journal.pntd.0006895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata, E.P. , Rancès, E. , Hedges, L.M. , Gofton, A.W. , Johnson, K.N. , O'Neill, S.L. et al. (2013) Dietary cholesterol modulates pathogen blocking by Wolbachia . PLoS Pathogens, 9(6), e1003459. 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata, E.P. , Rezende, F.O. , Simões, T.C. & Moreira, L.A. (2016) Diet‐induced nutritional stress and pathogen interference in Wolbachia‐infected Aedes aegypti . PLoS Neglected Tropical Diseases, 10(11), e0005158. 10.1371/journal.pntd.0005158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata, E.P. , Tikhe, C.V. & Dimopoulos, G. (2019) Curious entanglements: interactions between mosquitoes, their microbiota, and arboviruses. Current Opinion in Virology, 37, 26–36. 10.1016/j.coviro.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbaugh, D.L. & Lazear, H.M. (2020) Flavivirus envelope protein glycosylation: impacts on viral infection and pathogenesis. Journal of Virology, 94(11), e00104–e00120. 10.1128/JVI.00104-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington, L.B. , Tran, B.C.N. , Le, N.T.H. , Luong, T.T.H. , Nguyen, T.T. , Nguyen, P.T. et al. (2018) Field‐ and clinically derived estimates of Wolbachia‐mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences of the USA, 115(2), 361–366. 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Aimanova, K.G. & Gill, S.S. (2020) Aedes cadherin receptor that mediates Bacillus thuringiensis Cry11A toxicity is essential for mosquito development. PLoS Neglected Tropical Diseases, 14(2), e0007948. 10.1371/journal.pntd.0007948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts, T.M. , Cox, J. , Nguyen, A. , Feitosa, F. , Krishnan, M.N. & Fikrig, E. (2011) Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector. Virology, 417(1), 179–187. 10.1016/j.virol.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, J.E. , Clarke, D.W. , Criswell, V. , Desnoyer, M. , Cornel, D. , Deegan, B. et al. (2020) Efficient production of male Wolbachia‐infected Aedes aegypti mosquitoes enables large‐scale suppression of wild populations. Nature Biotechnology, 38(4), 482–492. 10.1038/s41587-020-0471-x [DOI] [PubMed] [Google Scholar]
- Dewi, B.E. , Takasaki, T. & Kurane, I. (2008) Peripheral blood mononuclear cells increase the permeability of dengue virus‐infected endothelial cells in association with downregulation of vascular endothelial cadherin. The Journal of General Virology, 89(3), 642–652. 10.1099/vir.0.83356-0 [DOI] [PubMed] [Google Scholar]
- Dusfour, I. , Vontas, J. , David, J.P. , Weetman, D. , Fonseca, D.M. , Corbel, V. et al. (2019) Management of insecticide resistance in the major Aedes vectors of arboviruses: advances and challenges. PLoS Neglected Tropical Diseases, 13(10), e0007615. 10.1371/journal.pntd.0007615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, H.L.C. , Ford, S.A. , Allen, S.L. , Bordenstein, S.R. , Chenoweth, S.F. , Bordenstein, S.R. et al. (2021) The impact of artificial selection for Wolbachia‐mediated dengue virus blocking on phage WO. PLoS Neglected Tropical Diseases, 15(7), e0009637. 10.1371/journal.pntd.0009637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattouh, N. , Cazevieille, C. , & Landmann, F. (2019) Wolbachia endosymbionts subvert the endoplasmic reticulum to acquire host membranes without triggering ER stress. PLoS Neglected Tropical Diseases, 13(3), e0007218. 10.1371/journal.pntd.0007218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci, A.S. & Morens, D.M. (2016) Zika virus in the Americas—yet another arbovirus threat. The New England Journal of Medicine, 374(7), 601–604. 10.1056/nejmp1600297 [DOI] [PubMed] [Google Scholar]
- Ferreira, A.G. , Fairlie, S. & Moreira, L.A. (2020) Insect vectors endosymbionts as solutions against diseases. Current Opinion in Insect Science, 40, 56–61. 10.1016/j.cois.2020.05.014 [DOI] [PubMed] [Google Scholar]
- Ford, S.A. , Allen, S.L. , Ohm, J.R. , Sigle, L.T. , Sebastian, A. , Albert, I. et al. (2019) Selection on Aedes aegypti alters Wolbachia‐mediated dengue virus blocking and fitness. Nature Microbiology, 4(11), 1832–1839. 10.1038/s41564-019-0533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, D.S. & Jordan, I.K. (2000) The α‐Mannosidases: phylogeny and adaptive diversification. Molecular Biology and Evolution, 17(2), 292–300. 10.1093/oxfordjournals.molbev.a026309 [DOI] [PubMed] [Google Scholar]
- Gould, E. , Pettersson, J. , Higgs, S. , Charrel, R. & de Lamballerie, X. (2017) Emerging arboviruses: why today? One health, 4, 1–13. 10.1016/j.onehlt.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker, K. , White, L. & de Silva, A.M. (2009) N‐linked glycans on dengue viruses grown in mammalian and insect cells. The Journal of General Virology, 90(9), 2097–2106. 10.1099/vir.0.012120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib, J.M. & Nelson, W.J. (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes & Development, 20(23), 3199–3214. 10.1101/gad.1486806 [DOI] [PubMed] [Google Scholar]
- Hanna, S.L. , Pierson, T.C. , Sanchez, M.D. , Ahmed, A.A. , Murtadha, M.M. & Doms, R.W. (2005) N‐linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. Journal of Virology, 79(21), 13262–13274. 10.1128/jvi.79.21.13262-13274.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, N. , Wada, I. , Hasegawa, K. , Yorihuzi, T. , Tremblay, L.O. , Herscovics, A. et al. (2001) A novel ER α‐mannosidase‐like protein accelerates ER‐associated degradation. EMBO Reports, 2(5), 415–422. 10.1093/embo-reports/kve084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly, A.M. , Alturki, S.O. , Alturki, S.O. , Connors, J. & Haddad, E.K. (2020) Challenges in dengue vaccines development: pre‐existing infections and cross‐reactivity. Frontiers in Immunology, 16(11), 1055. 10.3389/fimmu.2020.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Hu, S. , Tian, D. , Zou, X. & He, M. (2016) α‐Mannosidase I protein expression in peripheral blood mononuclear cells is upregulated during hepatitis B virus infection. Viral Immunology, 29(1), 33–39. 10.1089/vim.2015.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D.A. & O'Neill, S.L. (2017) Comparison of stable and transient Wolbachia infection models in Aedes aegypti to block dengue and West Nile viruses. PLoS Neglected Tropical Diseases, 11(1), e0005275. 10.1371/journal.pntd.0005275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D.A. , Walker, T. , Carrington, L.B. , De Bruyne, J.T. , Kien, D.H. , Hoang, N.L. et al. (2016) Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathogens, 12(2), e1005434. 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P.E. , Phuc, H.K. & Sinkins, S.P. (2009) Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326(5949), 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanlaya, R. , Pattanakitsakul, S.N. , Sinchaikul, S. , Chen, S.T. & Thongboonkerd, V. (2009) Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. Journal of Proteome Research, 8(5), 2551–2562. 10.1021/pr900060g [DOI] [PubMed] [Google Scholar]
- Lei, Y. , Yu, H. , Dong, Y. , Yang, J. , Ye, W. , Wang, Y. et al. (2015) Characterization of N‐glycan structures on the surface of mature dengue 2 virus derived from insect cells. PLoS One, 10(7), e0132122. 10.1371/journal.pone.0132122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Sodroski, C. , Lowey, B. , Schweitzer, C.J. , Cha, H. , Zhang, F. et al. (2016) Hepatitis C virus depends on E‐cadherin as an entry factor and regulates its expression in epithelial‐to‐mesenchymal transition. Proceedings of the National Academy of Sciences of the USA, 113(27), 7620–7625. 10.1073/pnas.1602701113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey, A.R.I. , Bhattacharya, T. , Newton, I.L.G. & Hardy, R.W. (2018) Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia‐mediated pathogen‐blocking. Viruses, 10(4), 141. 10.3390/v10040141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. & Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408. 10.1006/METH.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lommel, M. , Winterhalter, P.R. , Willer, T. , Dahlhoff, M. , Schneider, M.R. , Bartels, M.F. et al. (2013) Protein O‐mannosylation is crucial for E‐cadherin‐mediated cell adhesion. Proceedings of the National Academy of Sciences of the USA, 110(52), 21024–21029. 10.1073/pnas.1316753110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P. , Sun, Q. , Fu, P. , Li, K. , Liang, X. & Xi, Z. (2020) Wolbachia inhibits binding of dengue and Zika viruses to mosquito cells. Frontiers in Microbiology, 11, 1750. 10.3389/fmicb.2020.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo, M. , Generous, A. , Sinn, P.L. & Cattaneo, R. (2015) Connections matter ‐ how viruses use cell‐cell adhesion components. Journal of Cell Science, 128(3), 431–439. 10.1242/jcs.159400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman, C.J. , Lane, R.V. , Cass, B.N. , Fong, A.W. , Sidhu, M. , Wang, Y.F. et al. (2009) Stable introduction of a life‐shortening Wolbachia infection into the mosquito Aedes aegypti . Science, 323(5910), 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- Moreira, L.A. , Iturbe‐Ormaetxe, I. , Jeffery, J.A. , Lu, G. , Pyke, A.T. , Hedges, L.M. et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium . Cell, 139(7), 1268–1278. 10.1016/J.CELL.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Muñoz, M.D. , Limón‐Camacho, G. , Tovar, R. , Diaz‐Badillo, A. , Mendoza‐Hernández, G. & Black, W.C. (2013) Proteomic identification of dengue virus binding proteins in Aedes aegypti mosquitoes and Aedes albopictus cells. BioMed Research International, 2013, 875958. 10.1155/2013/875958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazni, W.A. , Hoffmann, A.A. , NoorAfizah, A. , Cheong, Y.L. , Mancini, M.V. , Golding, N. et al. (2019) Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Current Biology, 29(24), 4241–48.e5. 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda, H. & Takeichi, M. (2011) Structural and functional diversity of Cadherin at the adherens junction. The Journal of Cell Biology, 193(7), 1137–1146. 10.1083/jcb.201008173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X. , Zhou, G. , Wu, J. , Bian, G. , Lu, P. , Raikhel, A.S. et al. (2012) Wolbachia induces reactive oxygen species (ROS)‐dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti . Proceedings of the National Academy of Sciences of the USA, 109(1), E23–E31. 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, A.F. , Binduga‐Gajewska, I. , Kauffman, E.B. & Kramer, L.D. (2006) Quantitation of flaviviruses by fluorescent focus assay. Journal of Virological Methods, 134, 183–189. 10.1016/j.jviromet.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Ravkov, E.V. & Compans, R.W. (2001) Hantavirus nucleocapsid protein is expressed as a membrane‐associated protein in the perinuclear region. Journal of Virology, 75(4), 1808–1815. 10.1128/JVI.75.4.1808-1815.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S.A. & Staunton, K.M. (2019) Reflections from an old Queenslander: can rear and release strategies be the next great era of vector control? Proceedings of the Biological Sciences, 286(1905), 20190973. 10.1098/rspb.2019.0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, K.M. & Heise, M. (2009) Modulation of cellular tropism and innate antiviral response by viral glycans. Journal of Innate Immunity, 1(5), 405–412. 10.1159/000226422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, E.E. , Vasiljevic, E. , Brehm, K.S. & Colley, N.J. (2014) Mutations in four glycosyl hydrolases reveal a highly coordinated pathway for rhodopsin biosynthesis and N‐glycan trimming in Drosophila melanogaster . PLoS Genetics, 10(5), e1004349. 10.1371/journal.pgen.1004349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert, C. , Weger‐Lucarelli, J. , Garcia‐Luna, S.M. , Young, M.C. , Byas, A.D. , Murrieta, R.A. et al. (2017) Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nature Communications, 8, 15412. 10.1038/ncomms15412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, M.I. , Richardson, J.H. , Sánchez‐Vargas, I. , Olson, K.E. & Beaty, B.J. (2007) Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiology, 7(1), 1–13. 10.1186/1471-2180-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal, H.L. & Winkler, J.R. (1984) Mechanism and regulation of protein turnover: effect of the alpha‐mannosidase inhibitor, swainsonine, on glycoprotein degradation. Current Topics in Cellular Regulation, 24, 229–249. 10.1016/b978-0-12-152824-9.50029-0 [DOI] [PubMed] [Google Scholar]
- Sigle, L.T. & Hillyer, J.F. (2018) Eater and draper are involved in the periostial haemocyte immune response in the mosquito Anopheles gambiae . Insect Molecular Biology, 27(4), 429–438. 10.1111/imb.12383 [DOI] [PubMed] [Google Scholar]
- Stanley, P. (2011) Golgi glycosylation. Cold Spring Harbor Perspectives in Biology, 3(4), 1–13. 10.1101/cshperspect.a005199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas, G. , Joubert, D.A. & McGraw, E.A. (2017) The RNAi pathway plays a small part in Wolbachia‐mediated blocking of dengue virus in mosquito cells. Scientific Reports, 7, 43847. 10.1038/srep43847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y. , Ju, J.Y. , Zhou, Y.Q. , Liu, Y. & Zhu, L.P. (2008) Inhibition of α‐mannosidase Man2c1 gene expression suppresses growth of esophageal carcinoma cells through mitotic arrest and apoptosis. Cancer Science, 99(12), 2428–2434. 10.1111/j.1349-7006.2008.01019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utarini, A. , Indriani, C. , Ahmad, R.A. , Tantowijoyo, W. , Arguni, E. , Ansari, M.R. et al. (2021) Efficacy of Wolbachia‐infected mosquito deployments for the control of dengue. The New England Journal of Medicine, 384(23), 2177–2186. 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk, A.F. , Hall‐Mendelin, S. , Pyke, A.T. , Frentiu, F.D. , McElroy, K. , Day, A. et al. (2012) Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti . PLoS Neglected Tropical Diseases, 6(11), e1892. 10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester‐Christensen, M.B. , Halim, A. , Joshi, H.J. , Steentoft, C. , Bennett, E.P. , Levery, S.B. et al. (2013) Mining the O‐mannose glycoproteome reveals cadherins as major O‐mannosylated glycoproteins. Proceedings of the National Academy of Sciences of the USA, 110(52), 21018–21023. 10.1073/pnas.1313446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire, R.M. & Duman‐Scheel, M. (2020) Advances in oral RNAi for disease vector mosquito research and control. Current Opinion in Insect Science, 40, 18–23. 10.1016/j.cois.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, Z.S. , Brownlie, J.C. & Johnson, K.N. (2015) Oxidative stress correlates with Wolbachia‐mediated antiviral protection in Wolbachia‐Drosophila associations. Applied and Environmental Microbiology, 81(9), 3001–3005. 10.1128/AEM.03847-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Z. , Khoo, C.C.H. & Dobson, S.L. (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science, 310(5746), 326–328. 10.1126/science.1117607 [DOI] [PubMed] [Google Scholar]
- Yap, S.S.L. , Nguyen‐Khuong, T. , Rudd, P.M. & Alonso, S. (2017) Dengue virus glycosylation: what do we know? Frontiers in Microbiology, 8, 1415. 10.3389/fmicb.2017.01415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y.H. , Carrasco, A.M. , Frentiu, F.D. , Chenoweth, S.F. , Beebe, N.W. , van den Hurk, A.F. et al. (2015) Wolbachia reduces the transmission potential of dengue‐infected Aedes aegypti . PLoS Neglected Tropical Diseases, 9(6), e0003894. 10.1371/journal.pntd.0003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y.H. , Ng, T.S. , Frentiu, F.D. , Walker, T. , van den Hurk, A.F. , O'Neill, S.L. et al. (2014) Comparative susceptibility of mosquito populations in North Queensland, Australia to oral infection with dengue virus. American Journal of Tropical Medicine and Hygiene, 90(3), 422–430. 10.4269/ajtmh.13-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Zhang, D. , Li, Y. , Yang, C. , Wu, Y. , Liang, X. et al. (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature, 572(7767), 56–61. 10.1038/s41586-019-1407-9 [DOI] [PubMed] [Google Scholar]
- Zug, R. & Hammerstein, P. (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One, 7(6), e38544. 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Wolbachia mediated DENV‐blocking during alpha‐Mann‐2a RNAi. DENV intensity and prevalence were detected by absolute qRT‐PCR in midgut and carcass samples at 10 and 14 dpi. A. Infection intensity (genome copy number) overtime. Lines mark the median, circles represent DENV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann‐Whitney test, not significant. B. Prevalence (presence or absence) of DENV. Bars contain the percentage of mosquitoes uninfected (green) and DENV infected (blue). Binary logistical regression for Midgut: P = 0.09. Binary logistical regression for Carcass: P = 0.07. N = 20‐25.
Figure S2. Wolbachia levels 3 days post‐RNAi. The levels of wAlbB in whole body mosquitoes was similar across treatment groups at 3 days post‐dsRNA injection and prior to DENV infection. Mann‐Whitney test, dsAlpha P = 0.7. Bars represent the median and whiskers depict the 95% confidence intervals. N = 3.
Figure S3. Expression of alpha‐Mann‐2a following RNAi and infection with CHIKV. A. Levels of alpha‐Mann‐2a expression in mosquitoes at 3 days post‐RNAi (pre‐exposure) compared to control mosquitoes (dsGFP injected). Circles represent individual whole‐body samples. N = 4‐5. B‐C. Expression of alpha‐Mann‐2a at 5 dpi in the midgut and carcass. N = 6‐17. Graphs display the relative expression compared to RpS6. Bars represent the median and whiskers depict the 95% confidence intervals. Mann‐Whitney test: * P < 0.05.
Figure S4. CHIKV infection during RNAi knockdown of alpha‐Mann‐2a in the carcass. CHIKV intensity and prevalence were detected by absolute qRT‐PCR in carcass samples at 5‐ and 10 dpi. A. Infection intensity (genome copy number) overtime. Lines mark the median, circles represent CHIKV quantities in individual dsGFP (yellow) or dsAlpha (magenta) tissue samples, and whiskers depict the 95% confidence intervals. Mann‐Whitney test, P > 0.07 B. Prevalence (presence or absence) of CHIKV. Bars contain the percentage of mosquitoes uninfected (green) and CHIKV infected (blue). Binary logistical regression for carcass: P = 0.48. At 5 dpi N = 7 and at 10 dpi N = 17‐18.
Table S1. Statistical analyses for Figures 1 and 6.
Table S2. Primers.
Data Availability Statement
The data that support the findings of this study are openly available in figshare at 10.6084/m9.figshare.13726000.


