Abstract
OBJECTIVE:
To examine whether laws limiting opioid prescribing have been associated with reductions in the incidence of persistent postoperative opioid use
SUMMARY BACKGROUND DATA:
In an effort to address the opioid epidemic, 26 states (as of 2018) have passed laws limiting opioid prescribing for acute pain. However, it is unknown whether these laws have achieved their reduced the risk of persistent postoperative opioid use.
METHODS:
We identified 957,639 privately insured patients undergoing one of 10 procedures between January 1, 2004 and September 30, 2018. We then estimated the association between persistent postoperative opioid use, defined as having filled ≥10 prescriptions or ≥120 days supply of opioids during postoperative days 91–365, and whether opioid prescribing limits were in effect on the day of surgery. States were classified as having: no limits, a limit of ≤7 days supply, or a limit of >7 days supply. The regression models adjusted for observable confounders such as patient comorbidities and also utilized a difference-in-differences approach, which relied on variation in state laws over time, to further minimize confounding.
RESULTS:
The adjusted incidence of persistent postoperative opioid use was 3.5% (95%CI 3.3%−3.7%) for patients facing a limit of ≤7 days supply, compared with 3.3% (95%CI 3.3%−3.3%) for patients facing no prescribing limits (p=0.13 for difference compared to no prescribing limits) and 3.4%, (95%CI 3.2%−3.6%) for patients facing a limit of >7 days supply (p=0.43 for difference compared to no prescribing limits).
CONCLUSIONS:
Laws limiting opioid prescriptions were not associated with subsequent reductions in persistent postoperative opioid use.
MINI-ABSTRACT
Many states have enacted laws limiting opioid prescribing for acute pain. This study examined whether these laws are associated with reduced risk of persistent postoperative opioid use (PPOU) among adults undergoing several common surgeries. Overall, the study found no association between these laws and the risk of PPOU.
INTRODUCTION
The opioid epidemic remains a pressing concern in the United States, with over 50,000 deaths from opioid overdose in 2019.1 Researchers and policymakers have started to focus on the role that surgery plays in the opioid epidemic, with numerous studies2,3 showing that surgical patients are at an increased risk for long-term postoperative opioid use, a phenomenon known as persistent postoperative opioid use (PPOU).4,5
To address this issue, states have begun instituting opioid prescribing limits for acute pain. By the end of 2018, 26 states had passed limits on opioid prescribing laws for initial opioid prescriptions, with the intended goal of preventing patients who fill an initial opioid prescription from transitioning to persistent opioid use.6 A plurality (9/26) of these states were located in the northeast United States, followed by 8 states in the southern United States, 5 midwestern states, and 4 states in the western United States. Only 4 states (Illinois, Missouri, South Carolina, and Tennessee) enacted opioid prescribing limits before 2016.
However, there are many reasons why limits on opioid prescribing may fail to achieve the intended aim of reducing persistent opioid use. First, the limits may not be effective if prescribers were already prescribing below the mandated limits, or if the penalties for exceeding the limit are small or unlikely to be enforced. Second, since many of the laws limit the number of days supply of opioid, as opposed to limiting the number of pills or the overall potency of the prescription, prescribers may avoid these limits by redefining what constitutes a day’s supply. Finally, even if the laws reduce initial opioid prescribing, it is unclear whether these reductions would lower the risk of persistent opioid use in the longer-term, since exposure to even a reduced amount of opioid could still place some patients at risk for long-term opioid use.2,7 Understanding whether state prescribing limits reduce the risk that patients transition to persistent opioid use has important policy implications. In particular, there is growing interest in developing procedure-specific opioid prescribing guidelines,8 and to the extent that laws limiting opioid prescribing are effective, additional legislation could be used to more broadly implement these guidelines.
In this analysis, we used administrative claims data to examine whether state opioid prescribing limits are associated with reductions in PPOU among patients undergoing several common surgeries. To minimize confounding, our analysis included a difference-in-differences approach that relied on state-level variation in the timing and nature of laws limiting opioid prescribing. Previous studies suggest that state opioid prescribing limits have not been associated with reductions in short-term opioid prescribing, defined as either the initial opioid prescription,9,10 or all prescriptions within 90 days of the initial prescription.6 Our study builds on this work in two ways. First, previous work has largely focused on all acute pain indications, while our analysis focuses on the care of surgical patients. State prescribing laws may be particularly salient for this population, in light of a recent study finding that for some surgeries, the average prescription is for over 9 day’s supply, which would be in excess of the 7-day maximum enacted by many state laws.11 Second, previous analyses have generally focused on the effect of state opioid prescribing limits on short-term opioid use and have not examined whether the laws achieved the intended goal of reductions in long-term, persistent opioid use.
METHODS
Data.
The data consisted of administrative health claims provided by Optum’s Clinformatics® Data Mart Database (CDM), a de-identified database of administrative health claims for beneficiaries of commercial and Medicare Advantage health plans. For medical claims, the data contained details such as diagnosis codes, procedure codes, and dates of service. For pharmacy claims, the data contained details such as dates of prescription fill, number of days supplied, drug generic name and dosage, and number of tablets or capsules supplied. Many studies have used these data to analyze topics related to health policy and health outcomes research.12,13 This study was included in the umbrella Institutional Review Board protocol for de-identified data managed by the Center for Population Heath Sciences at Stanford University, which included a waiver of consent.
Sample.
The initial sample consisted of 2,750,731 surgical procedures between January 1, 2004 and September 30, 2018 for patients 18 to 90 years old undergoing one of the following: total knee arthroplasty, total hip arthroplasty, laparoscopic cholecystectomy, open cholecystectomy, laparoscopic appendectomy, open appendectomy, cesarean section, functional endoscopic sinus surgery, transurethral resection of the prostate, or simple mastectomy. We chose these procedures because they are commonly performed and encompass a wide variety in terms of projected pain intensity. For patients who underwent more than one surgery, we only included the first procedure, leaving an initial sample of 2,612,187 patients. In addition, we excluded patients who underwent any other procedure in the year before or after the surgery of interest (N=212,514), except for postoperative days 0–30 to allow for the possibility of postoperative complications and their subsequent impact on opioid use. We then excluded patients who did not have continuous enrollment in their insurance plan during this 2-year perioperative window (N=1,369,987) and patients who did not have an identifiable ZIP code on the date of their procedure (N=56,049) or whose zip code of residence was located outside the 50 states and the District of Columbia (i.e., a US territory; N=7,998). Finally, we excluded the top 1% of opioid users (N=8,000, >83 oral morphine milligram equivalents (MME) per day). The final sample consisted of 957,639 patients (see Online Supplement for a flow diagram describing sample construction).
Outcomes.
The primary outcome was whether a patient transitioned to PPOU. Using the pharmacy claims data, we identified prescriptions for the following opioids: codeine, fentanyl patch, hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, and tramadol. Each prescription was converted to an average daily dose in oral morphine milligram equivalents based on the dosing and quantity provided in the data.14 Following previous work, we defined PPOU as filling 10 or more prescriptions or 120 or more day’s supply during postoperative days 91–365.2
Secondary outcomes consisted of opioid fills in the immediate perioperative period, based on outpatient opioid prescriptions filled 7 days before surgery to 7 days after surgery. We chose this time window because most patients who fill their initial postoperative opioid prescription do so during this time.15 We identified the average daily MME during this period.
Exposure.
Our independent variable of interest was whether a law restricting opioid prescribing was in effect at the time of the patient’s surgery.6 Since many—but not all—states with restrictions typically imposed a restriction of 7 days supply, we categorized states into three categories: those with no restriction, those with a restriction of 7 days supply or less, and those with restrictions of more than 7 days supply.
Variables.
Additional variables were included to adjust for potential confounding. Age, sex, and type and year of surgery were directly extracted from the claims data. We used previously described methods16 to identify and create variables for medical comorbidities included in the Elixhauser index. Using pharmacy claims data, we also measured opioid use in the year before surgery.
Statistical Analysis.
A simple cross-sectional comparison of outcomes between states with and without opioid prescribing limits is potentially vulnerable to confounding, since patients residing in states that passed laws limiting opioid prescribing could differ from patients living in states that did not. Therefore, we used a difference-in-differences approach to minimize confounding. This approach is often used to evaluate outcomes associated with various state-level policies that are adopted across states in a staggered fashion over time.17–19 Under this approach, state specific controls (i.e., indicator variables for every state) were used to adjust for unobservable state-level factors. Therefore, rather than comparing differences in PPOU incidence across states, the difference-in-differences approach identifies the effect of “opioid prescribing laws by estimating within state changes in PPOU incidence over time among states that enacted opioid prescribing limits. However, this “before-after” comparison can still be confounded by secular time trends, such as general changes in surgical practice over time. Therefore, the second step of a standard difference-in-differences approach involves the use of year effects (i.e., indicator variables for each year of surgery) in order to control for secular time trends at the national level.
We implemented our difference-in-differences analysis by using multivariable linear regression analyses in which the dependent variable was the outcome being studied. For example, in our primary analysis, the outcome was an indicator variable for whether the patient met the criteria for PPOU. The independent variable of interest was whether a law restricting opioid prescribing was in effect on the day of the patient’s surgery. The analyses then implemented the differences-in-differences approach by including fixed effects for the year and state of the surgery.
In addition to these variables, the multivariable linear regression analyses also included adjustments for type of surgery (surgery fixed effects; one indicator variable for each type of surgery studied) and the patient characteristics listed in the supplemental content (eTable 1), including age (modeled as five-year age groups), sex, and patient comorbidities. The multivariable linear regression models also adjusted for preoperative opioid use using three variables based on opioid fills in the year before surgery: an indicator variable for whether the patient experienced chronic opioid use (having filled ≥10 prescriptions or ≥120 days supply), an indicator variable for whether the patient was opioid-naïve (having filled no opioid prescriptions), and the average daily MME filled. Finally, the models also adjusted for the presence of a law requiring practitioners to consult a Prescription Drug Monitoring Program before prescribing opioids.6
In the case of the secondary outcome (average daily opioid fills in the immediate perioperative period), nearly 30 percent of patients did not fill any opioid during this time period. When there are many observations for which the value of the dependent variable is zero, a simple linear regression analysis will tend to be downward-biased.20 We utilized a two-step model to address this issue.21,22 In the first step, we performed a multivariable linear regression in which the dependent variable was an indicator variable equaling one if the patient used any opioid at all and zero otherwise. In the second step, we performed a multivariable linear regression in which the dependent variable was opioid utilization, measured in average daily MME, during the perioperative period. This analysis was restricted to patients who used some MME (i.e., a non-zero average daily MME). Thus, the first step estimated the association between prescribing limits and whether the patient utilized any opioid at all, while the second step estimated the association between prescribing limits and the amount of opioid utilized among the subset of patients who filled at least one prescription for an opioid.
In a multivariable linear regression, our approach estimated a linear probability model in the case of discrete outcomes such as PPOU. We chose a linear probability model over alternatives such as a logistic regression in order to overcome the issue of complete or quasi-complete separation, which can occur when there are many fixed effects in a logistic regression.23,24 An analysis plan outlining the details of the statistical analysis was written prior to analyzing the data. All analyses were performed using STATA 14.0 (College Station, TX). Standard errors for the regression models were clustered at the state level25 and 2-sided p-values were used to assess statistical significance, with a threshold of ≤0.05.
Subgroup Analyses.
Three sets of pre-specified subgroup analyses were performed. First, we performed a subgroup analysis for each individual surgery. Second, we performed a subgroup analysis among patients at high risk for PPOU, hypothesizing that prescribing limits might be most effective for these patients. We identified high risk patients by first performing a regression in which the dependent variable was PPOU and the independent variables consisted of patient characteristics (age, sex, and comorbidities) as well as fixed effects for each surgery. The results of this regression were used to calculate the predicted probability of transitioning to PPOU, and high-risk patients were defined as those in the upper quartile of predicted probability. Finally, subgroup analysis was performed on opioid naïve patients, defined as patients who did not fill an opioid prescription in the year before surgery, as well as non-opioid naïve patients, defined as patient who filled any opioid prescription in the year before surgery. Because of the large number of subgroups, we used the Bonferroni correction to set p=0.0036 (=0.05/14) as the threshold for statistical significance.26
Sensitivity Analyses.
We performed a sensitivity analysis in which, rather than PPOU, we used the average daily MME during post-operative days 91–365 as the outcome. In addition, we performed sensitivity analyses in which the independent variable of interest was whether the patient was whether a law limiting opioid prescribing was enacted within 7 or 30 days after surgery, to allow for the possibility that even if the law was not in effect on the day of surgery, subsequent enactment could affect prescribing behaviors.
RESULTS
In the final sample of 957,639 patients, the average was 52.6 years (s.e. 0.02 years), with 658,935 females (68.8%). Overall, 601,416 (62.8%) patients resided in states with no limits on opioid prescribing, while 249,303 (26.0%) resided in states that limited opioid prescriptions to 7 days supply or less, and 106,920 (11.2%) resided in states with limits of more than 7 days supply. Because of the large sample sizes, differences in patient characteristics between these three groups were generally statistically significant at the p<0.001 level (see Table 1 for a select list of characteristics; the full list of characteristics is presented in the online supplemental content; eTable 1). However, using Hedge’s g as a measure of effect size, the actual magnitude of these differences was small for all of the characteristics we examined (Hedge’s g<0.1 for all characteristics).
Table 1.
Characteristics of the study population
| States without Prescribing Limits (N=601,416, 26 states) | States with limit of 7 days supply or less (N=249,303 patients, 18 states) | States with limit greater than 7 days supply (N=106,920, 7 states) | |||
|---|---|---|---|---|---|
| Demographics | p/g | p/g | |||
| Age, avg (s.e) | 52.6 (0.02) | 52.4 (0.04) | 0.001/0.01 | 53.4 (0.06) | */0.04 |
| Female, % (s.e.) | 68.7 (0.06) | 69.2 (0.09) | */0.01 | 68.4 (0.14) | 0.06/0.01 |
| Procedures, N (%) | |||||
| Total Knee Arthroplasty | 103,972 (17.3) | 43,223 (17.3) | 0.58/0.00 | 19,869 (18.6) | */0.03 |
| Total Hip Arthroplasty | 55,898 (9.3) | 24,631 (9.9) | */0.02 | 10,394 (9.7) | */0.01 |
| Laparoscopic Cholecystectomy | 151,645 (25.2) | 60,108 (24.1) | */0.03 | 28,431 (26.6) | */0.03 |
| Open Cholecystectomy | 7,445 (1.2) | 2,966 (1.2) | 0.07/0.00 | 1,262 (1.2) | 0.12/0.01 |
| Laparoscopic Appendectomy | 41,547 (6.9) | 16,157 (6.5) | */0.02 | 7,031 (6.6) | */0.01 |
| Open Appendectomy | 7,662 (1.3) | 3,425 (1.4) | */0.01 | 1,419 (1.3) | 0.15/0.00 |
| Cesarean Section | 127,729 (21.2) | 54,853 (22.0) | */0.02 | 20,235 (18.9) | */0.06 |
| Functional Endoscopic Sinus Surgery | 55,213 (9.2) | 21,946 (8.8) | */0.01 | 9,337 (8.7) | */0.02 |
| Transurethral Prostate Resection | 16,647 (2.8) | 7,201 (2.9) | 0.002/0.01 | 3,023 (2.8) | 0.28/0.00 |
| Mastectomy | 33,658 (5.6) | 14,793 (5.9) | */0.01 | 5,919 (5.5) | 0.43/0.00 |
| Comorbidities, % (s.e.) | |||||
| Congestive Heart Failure | 6.0 (0.03) | 6.4 (0.05) | */0.01 | 6.6 (0.08) | */0.02 |
| Hypertension, Uncomplicated | 48.1 (0.06) | 49.1 (0.1) | */−0.02 | 51.1 (0.15) | */0.06 |
| Hypertension, Complicated | 8.8 (0.04) | 8.6 (0.06) | 0.004/0.01 | 8.9 (0.09) | 0.90/0.00 |
| Preoperative Opioid Prescription | |||||
| Persistent Opioid Use, % (s.e.) | 4.0 (0.03) | 4.0 (0.04) | 0.31/0.00 | 4.5 (0.06) | */0.03 |
| Opioid-Naïve, % (s.e.) | 64.4 (0.06) | 66.4 (0.09) | */0.04 | 63.8 (0.15) | */0.01 |
| Average Daily MME (s.e.) | 1.8 (0.01) | 1.7 (0.01) | */0.01 | 1.9 (0.02) | */0.01 |
Table 1 presents selected summary, stratified by states with no opioid prescribing limits, states that passed limits of 7 days supply or less, and states with limits of more than 7 days supply. “p” refers to the statistical significance of the difference between the given group and states with no prescribing limits, while “g” refers to the effect size, which was measured using Hedge’s g. Pre-operative opioid prescriptions were measured based on prescriptions filled 8 to 365 days before surgery. “Persistent Opioid Use” was defined as filling ≥10 prescriptions or ≥120 days supply during this timeframe. “Opioid-Naïve” was defined as filling no prescriptions during this timeframe.
=p<0.001; MME=oral morphine milligram equivalents. A full list of summary statistics can be found in eTable 1 in the supplemental content.
Among states that instituted opioid prescribing limits, Figure 1 plots the incidence of PPOU and the average daily MME in the perioperative period (7 days before surgery to 7 days after surgery) for each of the twelve months before and after the limit was instituted. The figure suggests mild declines in perioperative opioid use among states that limited opioid prescribing to 7 days supply or less. In these states, the average daily MME in the perioperative period, fell from 16.9 MME (95% CI 15.9–18.0) in the month before limits were enacted to 14.3 MME (95% CI 13.4–15.2; p<0.001 for the difference) one year after the limits were enacted. While this provides some qualitative evidence that the laws were associated with mild declines in opioid prescribing, figure 1 does not account for secular changes in opioid prescribing or patient characteristics over time, issues that will be addressed in our formal difference-in-differences analysis. Declines in opioid prescribing were more modest and not statistically significant in states with limits of more than 7 days supply, with the average daily MME falling from 17.6 MME (95% CI 15.2–19.9) in the month before limits were enacted to 16.2 MME (95% CI 14.2–18.2; p=0.39 for the difference) one year after the limits were enacted.
Figure 1. Trends in Persistent Postoperative Opioid Use and Perioperative Opioid Use.
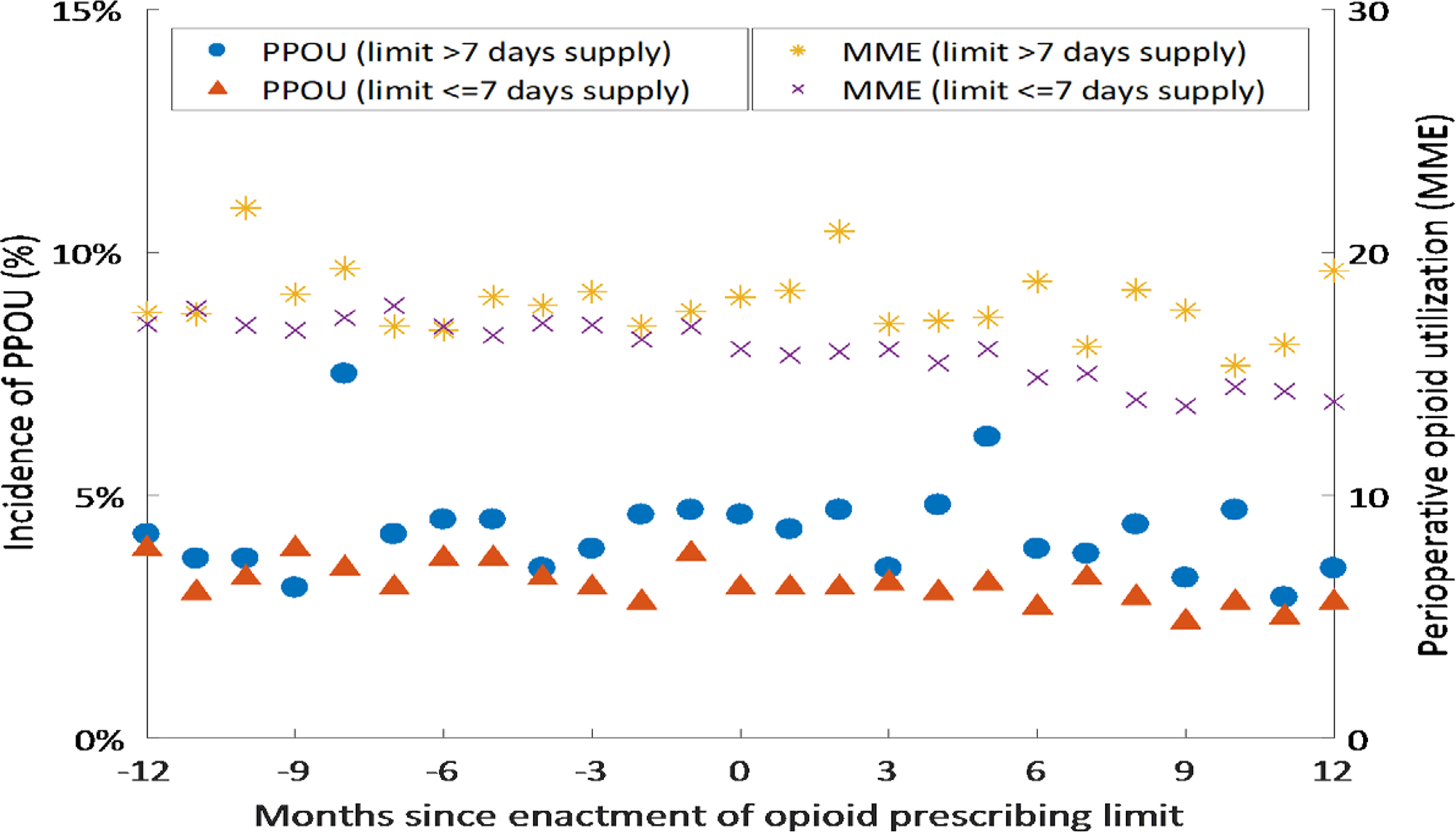
Figure 1 shows the monthly incidence of Persistent Postoperative Opioid Use (left axis) and the monthly average perioperative opioid use (right axis) in states that enacted limits on opioid prescribing, during the 12 months before and after the limit was enacted. Persistent Postoperative Opioid Use was defined as filling 10 or more prescriptions or more than 120 days supply during postoperative days 91–365. Perioperative opioid use is the daily amount of opioid utilized between 7 days before surgery to 7 days after surgery, measured in oral morphine milligram equivalents (MME). The figure shows trends for two groups of states: those that limited opioid prescribing to seven days supply or less (<=7), and those states which enacted limits of more than seven days supply (>7; i.e., a limit of 14 days supply).
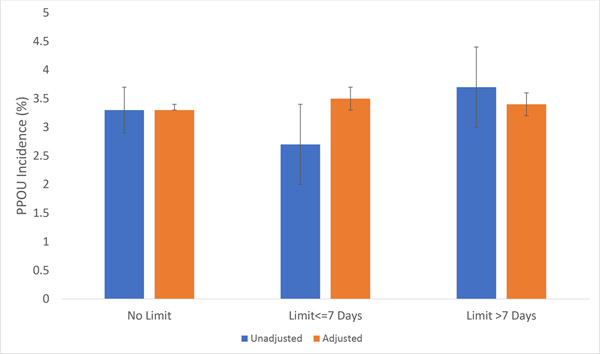
The unadjusted incidence of PPOU was 3.3% (95% CI 2.9%−3.7%) for patients facing no limits on opioid prescribing, 2.7% (95% CI 2.0%−3.4%; p=0.07 for the difference compared to no limit) for patients who faced a limit of 7 days supply or less, and 3.7% (95% CI 3.0%−4.4%; p=0.34 for the difference compared to no limit) for patients who faced a limit of more than 7 days supply (Figure 2). However, these unadjusted differences do not take into account potential confounding from observable and unobservable differences in characteristics between patients in states with no prescribing limits compared to patients in states with a prescribing limit. After utilizing a difference-in-differences approach to adjust for these potential confounders, the adjusted incidence of PPOU was 3.5% (95% CI 3.3%−3.7%) for patients facing a limit of 7 days supply or less, compared with 3.3% for patients in states without a limit (95% CI 3.3%−3.3%; p=0.13 for difference; see eTable 2 for the regression results). Similarly, there was no statistically significant difference in PPOU for patients facing a limit of 7 days supply or more (incidence 3.4%, 95% CI 3.2–3.6, p=0.43 for difference compared to states with no limit). In addition, while not the focus of the study, we also found no significant association between PPOU and the presence of a law requiring practitioners to consult a Prescription Drug Monitoring Program before prescribing opioids (adjusted incidence 3.3% (95% CI 3.3%−3.3% ) for states without a law vs 3.3% (95% CI 3.2%−3.5%) for states with a law (p=0.96 for the difference).
Figure 2. Incidence of Persistent Postoperative Opioid Use and Opioid Prescribing Limits.
Figure 2 shows the unadjusted and adjusted incidence of Persistent Postoperative Opioid Use for patients who, based on the date of surgery and the state where the surgery occurred, faced either no limit on opioid prescribing, a law limiting prescribing to 7 days supply or less, or laws with limits of more than 7 days supply. 95% confidence intervals, shown in brackets, were calculated using standard errors clustered at the state level.
70.5% (95% CI 70.1%−71.0%) of patients who faced no prescribing limit filled a prescription for an opioid in the perioperative period, compared to 69.7% (95% CI 65.7%−73.7%, p=0.69 for the difference compared to no limit) of patients who faced a limit on 7 days supply or less and 75.9% (95% CI 70.8%−81.1%; p=0.06 for the difference compared to no limit) of patients who faced a limit of more than 7 days supply (Table 2). Among patients who did fill an opioid, the average daily MME was 24.0 (95% CI 23.7–24.3) for patients who faced no prescribing limit, 21.0 (95% CI 18.8%−23.2%; p=0.020 for the difference compared to no limit) for patients facing a limit of 7 days supply or less, and 23.3% (95% CI 21.2%−25.4%; p=0.40 for the difference compared to no limit) for patients facing a limit of more than 7 days supply.
Table 2.
Opioid Prescribing Limits and Perioperative Opioid Prescriptions
| Filled an Opioid Prescription (%) | Amount of Opioid (MME) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| No Prescribing Limits (26 states) | 71.0 (68.0, 74.0) | 70.5 (70.1, 71.0) | 23.9 (23.0, 24.9) | 24.0 (23.7, 24.3) |
| Limit <=7 days (18 states) | 63.4 (57.2, 69.6) p=0.014 |
69.7 (65.7, 73.7) p=0.69 |
22.1 (21.4, 22.8) p=0.003 |
21.0 (18.8, 23.2) p=0.020 |
| Limit >7 days (7 states) | 73.5 (72.3, 74.8) p=0.11 |
75.9 (70.8, 81.1) p=0.06 |
23.0 (20.6, 25.4) p=0.49 |
23.3 (21.2, 25.4) p=0.40 |
Table 2 shows the percentage of patients who filled at least one opioid prescription during the perioperative period as well as the average daily amount of opioid filled during this period, measured in oral morphine milligram equivalents (MME). The perioperative period was defined as the period spanning from 7 days before surgery up to 7 days after surgery. Patients were divided into three groups based on the date of surgery and the state where the surgery occurred: patients who faced no opioid prescribing limit, patients who faced a limit of 7 days supply or less, and patients who faced a limit of more than 7 days supply. “Adjusted” refers to analyses which used a multivariable linear regression which adjusted for potential confounders by including state and year fixed effects, surgery fixed effects, and controls for patient comorbidities, patient age, and patient sex. 95% confidence intervals, shown in parentheses, were calculated using standard errors clustered at the state level. p-values refer to the statistical significance of the difference between the given group compared to patients who faced no prescribing limits.
Subgroup analyses generally found no significant association between opioid prescribing laws and PPOU incidence (Table 3) by procedure type, especially after adjusting statistical significance thresholds for multiple hypothesis testing.26 One exception was function endoscopic sinus surgery, where the adjusted incidence of PPOU was 4.0% (95% CI 3.4%−4.5%) for patients facing a limit of more than 7 days supply, compared to 2.8% (95% CI 2.7%−2.8%) for patients facing no limit (p<0.001 for the difference). Similarly, for transurethral prostate resection, the incidence of PPOU was 4.2% (3.4%−5.0%) among patients facing a limit of more than 7 days supply, compared to 2.8% (95% CI 2.8%−2.9%) for patients facing no prescribing limits (p=0.002 for the difference). Additional subgroup analyses also found no significant association between prescribing limits and PPOU incidence for opioid-naïve patients, non-opioid-naïve patients, and for patients predicted to be at high risk for PPOU (Table 3). Finally, sensitivity analyses found no association between opioid prescribing laws and long-term opioid use ( measured in MMEs), and also found no significant association between PPOU incidence and subsequent enactment of prescribing limits within 7 or 30 days after surgery (Online Supplement; eTable 3).
Table 3.
Association Between Opioid Prescribing Limits and Incidence of Persistent Postoperative Opioid Use, Subgroup Analyses
| Subgroup | Unadjusted PPOU Incidence, % (95% CI) | Adjusted PPOU Incidence, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| Procedures | No Limits | Limit<=7 Days | Limit>7 days | No Limits | Limit<=7 Days | Limit>7 days |
| TKA (n=167,064) | 6.7 (5.7, 7.6) | 4.1 (3.0, 5.3) p<0.001 |
6.5 (4.7, 8.3) p=0.88 |
6.5 (6.4, 6.6) | 7.0 (6.3, 7.6) p=0.17 |
6.3 (4.9, 7.6) p=0.75 |
| THA (n=90,923) | 5.2 (4.4, 6.0) | 3.1 (2.1, 4.1) p<0.001 | 5.3 (4.6, 6.1) p=0.76 |
5.1 (5.0, 5.1) | 5.6 (5.1, 6.1) p=0.045 | 4.8 (4.3, 5.2) p=0.30 |
| Laparoscopic Cholecystectomy (n=240,184) | 3.5 (3.1, 3.9) | 3.0 (2.2, 3.8) p =0.24 |
3.6 (2.6, 4.6) p =0.80 |
3.4 (3.4, 3.5) | 3.5 (3.1, 4.0) p =0.67 |
3.5 (3.3, 3.7) p =0.69 |
| Open Cholecystectomy (n=11,673) | 5.0 (4.2, 5.8) | 4.2 (2.5, 5.9) p =0.36 |
3.8 (1.9, 5.8) p =0.25 |
5.1 (4.9, 5.2) | 3.7 (2.0, 5.3) p=0.11 |
3.8 (2.5, 5.1) p=0.07 |
| Laparoscopic Appendectomy (n=64,735) | 1.8 (1.5, 2.0) | 1.6 (0.8, 2.5) p=0.81 |
2.3 (1.6, 2.9) p=0.14 |
1.8 (1.7, 1.8) | 1.7 (1.2, 2.2) p=0.72 |
2.0 (1.7, 2.2) p=0.20 |
| Open Appendectomy (n=12,506) | 1.9 (1.6, 2.2) | 2.0 (0.3, 3.7) p =0.91 |
1.9 (1.6, 2.3) p =0.85 |
2.0 (1.9, 2.1) | 2.0 (0.6, 3.3) p =0.97 |
0.4 (0.0, 2.5) p =0.16 |
| Cesarean Section (n=202,817) | 0.5 (0.5, 0.6) | 0.2 (0.0, 0.4) p <0.001 |
0.5 (0.4, 0.6) p =0.66 |
0.5 (0.5, 0.5) | 0.5 (0.3, 0.6) p =0.46 |
0.5 (0.4, 0.6) p =0.65 |
| FESS (n=86,496) | 2.9 (2.5, 3.2) | 2.2 (1.4, 3.1) p =0.10 |
3.0 (2.3, 3.8) p =0.64 |
2.8 (2.7, 2.8) | 3.0 (2.5, 3.4) p =0.38 |
4.0 (3.4, 4.5) p <0.001 |
| TURP (n=26,871) | 3.0 (2.6, 3.3) | 2.5 (1.6, 3.4) p =0.25 |
3.2 (2.2, 4.1) p =0.66 |
2.8 (2.8, 2.9) | 2.9 (2.4, 3.3) p =0.87 |
4.2 (3.4, 5.0) p =0.002 |
| Mastectomy (n=54,370) | 3.1 (2.7, 3.5) | 1.9 (1.1, 2.8) p =0.005 |
3.0 (2.0, 4.0) p =0.90 |
3.0 (2.9, 3.1) | 3.1 (2.6, 3.6) p =0.69 |
3.4 (2.4, 4.4) p =0.49 |
| High-Risk Patients (n=239,410) | 12.0 (11.4, 12.6) | 16.5 (14.9, 18.1) p <0.001 |
12.9 (11.1, 14.7) p =0.34 |
12.1 (12.1, 12.2) | 12.6 (11.5, 13.7) p =0.39 |
12.5 (12.0, 13.0) p =0.19 |
| Non High-Risk Patients (n=718,229) | 0.4 (0.3, 0.4) | 0.2 (0.1, 0.2) p<0.001 |
0.4 (0.3, 0.4) p=0.98 |
0.3 (0.3, 0.4) | 0.4 (0.3, 0.4) p =0.41 |
0.4 (0.4, 0.5) p =0.02 |
| Opioid-Naïve Patients (n=621,229) | 0.3 (0.2, 0.3) | 0.1 (0.0, 0.2) p<0.001 |
0.3 (0.2, 0.4) p=0.93 |
0.3 (0.3, 0.3) | 0.3 (0.2, 0.3) p=0.57 |
0.3 (0.2, 0.4) p=0.12 |
| Non-Opioid-Naïve Patients (n=336,410) | 8.8 (8.1, 9.5) | 9.7 (8.7, 10.8) p=0.06 |
9.7 (8.1, 11.2) p=0.30 |
8.9 (8.8, 8.9) | 9.3 (8.6, 9.9) p=0.29 |
9.2 (8.7, 9.6) p=0.24 |
Table 3 shows the unadjusted and adjusted incidence of Persistent Postoperative Opioid Use (PPOU) for patients who, based on the date of surgery and the state where the surgery occurred, faced either no limit on opioid prescribing, a law limiting prescribing to 7 days supply or less, or laws with limits of more than 7 days supply. The table shows the results for the following subgroups: each surgery in our sample, patients at high predicted PPOU risk, patients not at high predicted risk for PPOU, opioid naïve patients, and non-opioid naïve patients (defined as having filled at least one opioid prescription in the year before surgery). “Adjusted” refers to analyses which used a multivariable linear regression which adjusted for potential confounders by including state and year fixed effects, surgery fixed effects, and controls for patient comorbidities, patient age, and patient sex. 95% confidence intervals, shown in parentheses, were calculated using standard errors clustered at the state level. p-values refer to the statistical significance of the difference between the given group compared to patients who faced no prescribing limits. TKA=Total Knee Arthroplasty; THA=Total Hip Arthroplasty; FESS=Functional Endoscopic Sinus Surgery; TURP=Transurethral Prostate Resection
DISCUSSION
The ongoing opioid epidemic has led many states to impose limits on opioid prescribing for acute pain with the goal of directly limiting opioid prescribing, and indirectly reducing opioid use and the transition to chronic use, misuse, and opioid use disorders. In this study, we examined whether laws restricting opioid prescribing limits were associated with decrease persistent postoperative opioid use incidence among patients undergoing several common surgical procedures, utilizing a difference-in-differences approach that relied on the timing of implementation of state laws that varied in the limits set for prescribed opioids. Overall, our results suggest no significant association between these laws and persistent postoperative opioid use incidence. While we did find statistically significant associations for some groups, (a) these association generally would not meet the threshold for statistical significance after adjusting for multiple hypothesis testing and (b) were generally of limited clinical significance.
Previous studies generally focused on prescribing behaviors for acute pain episodes, or on prescribing behaviors outside the operating room. Whether these laws would specifically impact surgical patients is unclear. On one hand, for some for some surgeries, one study suggested that the average prescription included over 9 days supply of opioids, which would be in excess of the 7-day maximum enacted by many state laws.11 However, this average is across all procedures, and there may be specific procedures whose average days supply is lower. Furthermore, because of existing guidelines, prescribers may feel they have less discretion over opioid prescribing for surgical patients, compared to other acute pain indications such as musculoskeletal pain. On the whole, our results suggest that limits on opioid prescribing have done little to reduce opioid utilization among surgical patients in either the immediate perioperative period or on a longer-term basis.
One reason why these laws may have had little effect on persistent postoperative opioid use is because, consistent with previous work, 6,9,10 we found no significant association between these laws and opioid prescribing in the immediate perioperative period (7 days before to 7 days after surgery). A large literature suggesting that higher initial opioid prescribing is associated with an increased risk for persistent opioid use,27 so if opioid prescribing laws have little effect on prescribing in the immediate perioperative period they would be unlikely to be associated with increases in longer-term opioid prescribing. In this vein, another important issue is that prescribing behaviors are likely to be dependent on the maximum number of days supply proscribed by state laws. As previously noted, the 7 day limit may not have been binding for some procedures. Moreover, some studies have found that particularly stringent laws or clinical guidelines, such as a limit of 3 days supply or a limit based on the quantity of pills prescribed, are associated with reduced prescribing in the immediate perioperative period.28,29 Therefore, there may be a benefit to more stringent and procedure-specific limits on opioid prescribing. Ultimately, understanding why laws limiting opioid prescribing have had little impact on PPOU has important policy implications. For example, if the laws have been ineffective because they are not binding, then this could argue for more stringent limits on prescribing.
Our study should be viewed in light of its limitations. First, we restricted our analysis to a subset of surgeries. Second, we restricted our analysis to privately insured patients covered by a single insurer. Third, given our use of administrative claims data, we did not measure opioid use that occurred outside of the insurance setting, such as the use of opioids not prescribed to the patient or prescriptions that patients may simply have paid for out of pocket. Along these lines, we were only able to observe opioid prescriptions that were filled, and the findings do not reflect opioid prescriptions that were provided but not filled nor actual opioid use. Fourth, private insurers may have separately instituted their own limits on opioid prescribing, although it is unclear to extent to which these initiatives would be correlated with state laws. Finally, we cannot exclude the possibility that our results were impacted by unobserved confounding. However, it is important to note that in general, there were few differences in observable characteristics between patients who faced no prescribing limits compared to patients who did, making the possibility of differences in unobservable factors less likely. In addition, we utilized a difference-in-differences approach to minimize confounding.
In conclusion, we found no significant association between laws limiting opioid prescribing and persistent postoperative opioid use incidence in a large sample of privately insured patients undergoing a diverse set of surgical procedures. Further studies should examine the impact of these laws on other outcomes such as patient pain scores, which may be particularly relevant if the laws have a limited effect on persistent postoperative opioid use. Further studies could also extend our analysis to other vulnerable populations, particularly those likely to have reduced access to pain management, such as low-income patients and the uninsured.
Supplementary Material
Acknowledgments
Funding: Support was provided by the National Institute on Drug Abuse (Dr. Sun, K08DA042314) and the National Institute on Aging (Dr. Jena, R56AG059620), and the National Institute of General Medical Sciences (Dr. Rishel, T32GM089626). Dr. Brummett receives funding from the National Institutes of Health (NIDA R01 DA042859, Common Fund UM1 NS118922), Michigan Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, and the Centers for Disease Control and Prevention. Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from Internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, MDHHS, or SAMHSA. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures and Conflicts of Interest: Dr. Sun is on the advisory board of Lucid Lane, LLC, and has received consulting fees unrelated to this work from Analysis Group. Dr. Jena reports receiving consulting fees unrelated to this work from Pfizer, Bioverativ, Bristol Myers Squibb, Merck/Sharp/Dohme, Janssen, Edwards Life Sciences, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Precision Health Economics, and Analysis Group. Dr. Jena also reports being retained as an expert witness in lawsuits against opioid manufacturers and distributors. Dr. Brummet serves as a consultant for Heron Therapeutics, Vertex Pharmaceuticals, and Alosa Health, and provides expert testimony.
REFERENCES
- 1.Provisional Drug Overdose Death Counts. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed.
- 2.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent ML, Hurley RW, Oderda GM, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative-4 Joint Consensus Statement on Persistent Postoperative Opioid Use: Definition, Incidence, Risk Factors, and Health Care System Initiatives. Anesth Analg. 2019;129(2):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jivraj NK, Raghavji F, Bethell J, et al. Persistent Postoperative Opioid Use: A Systematic Literature Search of Definitions and Population-based Cohort Study. Anesthesiology. 2020;132(6):1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacks DW, Hollingsworth A, Nguyen TD, Simon KI. CAN POLICY AFFECT INITIATION OF ADDICTIVE SUBSTANCE USE? EVIDENCE FROM OPIOID PRESCRIBING. NBER Working Paper No 25974. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy N, Mills P, Rockett M. Post-surgical pain management: time for a paradigm shift. Br J Anaesth. 2019;123(2):e182–e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence. Washington, D.C.: National Academies of Science, Engineering, and Medicine;2020. [PubMed] [Google Scholar]
- 9.Dave CV, Patorno E, Franklin JM, et al. Impact of State Laws Restricting Opioid Duration on Characteristics of New Opioid Prescriptions. J Gen Intern Med. 2019;34(11):2339–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S, Bryan JD, Hu HM, et al. Association of State Opioid Duration Limits With Postoperative Opioid Prescribing. JAMA Netw Open. 2019;2(12):e1918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikosz CA. Indication-Specific Opioid Prescribing for US Patients with Medicaid or Private Insurance, 2017. https://www.cdc.gov/injury/pdfs/bsc/Mikosz_Opioid-Prescribing-Estimates-Project_NCIPC-BSC-meeting_7.22.2020-508.pdf. Published 2020. Accessed. [DOI] [PMC free article] [PubMed]
- 12.Zhao X, Bhattacharjee S, Innes KE, LeMasters TJ, Dwibedi N, Sambamoorthi U. The impact of telemental health use on healthcare costs among commercially insured adults with mental health conditions. Curr Med Res Opin. 2020;36(9):1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston SS, Morton JM, Kalsekar I, Ammann EM, Hsiao CW, Reps J. Using Machine Learning Applied to Real-World Healthcare Data for Predictive Analytics: An Applied Example in Bariatric Surgery. Value Health. 2019;22(5):580–586. [DOI] [PubMed] [Google Scholar]
- 14.Analyzing Opioid Prescription Data and Oral Morphine Milligram Equivalents (MME). Centers for Disease Control and Prevention. https://www.cdc.gov/drugoverdose/resources/data.html. Published 2018. Accessed.
- 15.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surgery. 2017;152(6):e170504–e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 17.Buckley J, Shang Y. Estimating policy and program effects with observational data: the “differences-in-differences” estimator. Practical Assessment, Research & Evaluation. 2003;8(24):Retrieved April 16, 2015 from http://PAREonline.net/getvn.asp?v=2018&n=2012. [Google Scholar]
- 18.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. [DOI] [PubMed] [Google Scholar]
- 19.Vetter TR, Mascha EJ, Kilgore ML. Physician Supervision of Nurse Anesthetists: To Opt In or To Opt Out? Anesth Analg. 2016;122(6):1766–1768. [DOI] [PubMed] [Google Scholar]
- 20.Amemiya T Regression analysis when the dependent variable is truncated normal. Econometrica. 1973;41(6):997–1016. [Google Scholar]
- 21.Goldman DP, Jena AB, Lakdawalla DN, Malin JL, Malkin JD, Sun E. The value of specialty oncology drugs. Health services research. 2010;45(1):115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell O, Doorslaer Ev, Wagstaff A, Lindelow M Nonlinear Models for Health and Expenditure Data. In: Analyzing Health Equity Data Using Household Survey Data: A Guide to Techniques and Their Implementation. Washington, D.C: The World Bank; 2008:131–145. [Google Scholar]
- 23.Hellevik O Linear versus logistic regression when the dependent variable is a dichotomy. Quality and Safety. 2009;43:59–74. [Google Scholar]
- 24.Wooldridge JM. Econometric Analysis of Cross Section and Panel Data. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 25.Bertrand M, Duflo E, Mullainathan S. How Much Should We Trust Differences-In-Differences Estimates? Quarterly Journal of Economics. 2004;119(1):249–275. [Google Scholar]
- 26.Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–508. [DOI] [PubMed] [Google Scholar]
- 27.Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet. 2019;393(10180):1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hincapie-Castillo JM, Goodin A, Possinger MC, Usmani SA, Vouri SM. Changes in Opioid Use After Florida’s Restriction Law for Acute Pain Prescriptions. JAMA Netw Open. 2020;3(2):e200234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CS, Vu JV, Howard RA, et al. Assessment of a quality improvement intervention to decrease opioid prescribing in a regional health system. BMJ Qual Saf. 2021;30(3):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.