Abstract
Objective:
The long-term impact of hypertensive disorders of pregnancy (HDP) exposure on offspring health is an emerging research area. The objective of this study was to evaluate the association between a maternal diagnosis of HDP (gestational hypertension and preeclampsia) and adverse neurodevelopmental outcomes in the offspring.
Study Design:
This was a secondary analysis of two parallel multicenter clinical trials of thyroxine therapy for subclinical hypothyroid disorders in pregnancy. Women with singleton non-anomalous gestations diagnosed with subclinical hypothyroidism or hypothyroxinemia were randomized to thyroxine therapy or placebo. The primary outcome was child intelligence quotient (IQ) at 5 years of age. Secondary outcomes included several neurodevelopmental measures, including Bayley-III cognitive, motor, and language scores at 12- and 24- months, DAS-II scores at 36 months, the Conners’ Rating Scales-Revised at 48 months, and scores from the Child Behavior Checklist at 36 and 60 months. Thyroxine therapy did not influence neurodevelopment in either of the primary studies. Associations between neurodevelopment outcomes and maternal HDP were examined using univariable and multivariable analyses.
Results:
A total of 112 woman-child dyads with HDP were compared with 1067 woman-child dyads without HDP. In univariable analysis, mean maternal age (26.7±5.9 vs. 27.8±5.7 years, p=0.032) and the frequency of nulliparity (45.5% vs. 31.0%, p=0.002) differed significantly between the two groups. Maternal socioeconomic characteristics did not differ between the groups. After adjusting for potential confounders, there were no significant differences in any primary or secondary neurodevelopment outcome between offspring exposed to HDP and those unexposed. However, when dichotomized as low or high scores, we found higher rates of language delay (language scores <85: −1 standard deviation) at two years of age among offspring exposed to HDP compared with those unexposed (46.5% versus 30.5%, adjusted odds ratio 2.22, 95% CI 1.44 - 3.42).
Conclusions:
In this cohort of pregnant women, HDP diagnosis was associated with language delay at 2 years of age. However, other long-term neurodevelopmental outcomes in offspring were not associated with HDP.
Introduction
Hypertensive disorders of pregnancy (HDP) affect about 7%-10% of pregnancies worldwide.1 Pregnancies complicated by HDP are at increased risk for maternal and/or fetal mortality or serious morbidity.2-4 Lifelong consequences of HDP affect the maternal-child dyad with increased risk of developing cardiovascular disease.3, 5-8 Moreover, new evidence is emerging that in utero exposure to HDP may be an independent risk factor for adverse neurologic outcomes in the offspring.9-13
Recent studies investigating long-term effects of HDP on offspring health suggest that maternal HDP may be associated with lower cognitive ability of the offspring.10-14 Several studies have suggested that HDP may adversely affect neurodevelopment during childhood and into early adulthood, including intelligence quotient (IQ) scores.14-16 Mechanisms proposed to explain this association include genetic components that may predispose to HDP, as well as cardiovascular and neurodevelopment diseases in later life, which may be inherited by the offspring, as well as inflammatory components that are present in preeclampsia and may disrupt placental signaling and/or cross to the fetal circulation to alter fetal neurodevelopment trajectories. Other studies, however, did not confirm the association between HDP and offspring neurodevelopment.17-19
In 2017, two parallel multicenter randomized placebo-controlled trials were published examining the effect of treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy on cognitive function in offspring.20 The study followed 1,179 children up to 5 years of age and reported several neurodevelopmental outcome measures including IQ. Therefore, we proposed to use this unique long-term data base to examine the association between maternal diagnosis of HDP and offspring neurodevelopment. The objective of this study was to compare full-scale intelligence quotient assessed with the use of the Wechsler Preschool and Primary Scale of Intelligence III (WPPSI-III) at 5 years of age, or with the overall score from the Differential Ability Scales–II at 3 years of age between offspring of women with and without diagnosis of HDP. Our hypothesis was that offspring of women with diagnosis of HDP will have lower full-scale intelligence quotient at 5 years of age or lower overall score from Differential Ability Scales–II at 3 years of age.
Methods
This was a secondary analysis of two multicenter randomized placebo-controlled trials of thyroxine therapy for hypothyroid disorders in pregnancy.20 The trials were conducted in 15 centers within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Women with a singleton pregnancy who were diagnosed with subclinical hypothyroidism or hypothyroxinemia at a gestational age between 8 weeks 0 days and 20 weeks 6 days were included in separate but parallel trials . These women were randomized to receive thyroxine therapy or placebo. Apart from the underlying condition of the participants (subclinical hypothyroidism versus hypothyroxinemia) and dosing for the active and placebo groups, the trials were identical. For the purposes of this analysis they are combined and designated as the parent study. The primary outcome in the original trial was the full-scale intelligence quotient (IQ) assessed with the use of the Wechsler Preschool and Primary Scale of Intelligence III (WPPSI-III) at 5 years of age, or with the overall (General Conceptual Ability) score from the Differential Ability Scales–II (DAS) at 3 years of age if the WPPSI-III score was not available, or death before 3 years of age (because it was a competing event for IQ score). The primary outcome for this analysis was child intelligence quotient (IQ) at 5 years of age. We did not include deaths in this analysis since the primary outcome for this analysis was looking at IQ at five years of age. The number of deaths were low and occurred mainly before hospital discharge. Secondary outcomes included several neurodevelopmental measures, including Bayley-III cognitive, motor, and language scores at 12- and 24-months, DAS-II scores at 36 months, the Conners’ Rating Scales-Revised at 48 months, and scores from the Child Behavior Checklist at 36 and 60 months. Composite scores are derived for cognitive, language, and motor development and scaled to a metric, with a mean of 100, standard deviation of 15, and range of 40 to 160.21 There was no specific language assessment done at 5 years, nor the WPPSI-III receptive or expressive tests. For the Child intelligence quotient at the age of 5, if there was an impairment, they were assigned a score less than 50 (N=5). The score of 50 was the lower limit of the test score and it was given instead of having a missing score. The impairment could have been a visual, hearing, or motor impairment severe enough that the test could not be administered. For other assessments, only valid non-missing values were included. Of note, in the parent study thyroxine therapy did not influence neurodevelopment.
We applied the following exclusion criteria to this analysis: miscarriage, stillbirth or neonatal death. Maternal diagnosis of HDP was abstracted in the parent study from the electronic health records and included gestational hypertension and preeclampsia. In the original study, preeclampsia was defined as hypertension (defined as a systolic blood pressure of ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg on 2 occasions 4 hours apart) plus proteinuria (either ≥300 mg per 24 hours or 2+ or more by dipstick on 2 or more occasions 4 hours apart). Women with chronic hypertension were not included in the diagnosis of HDP. The primary and secondary neurodevelopmental and behavior outcomes from the parent study were compared between women with and without diagnosis of HDP.
Maternal baseline characteristics including demographic characteristics (maternal age, marital status, education, body mass index (BMI), insurance type), clinical characteristics (parity, smoking during pregnancy, alcohol use during pregnancy, thyroid status (subclinical hypothyroidism vs. hypothyroxinemia), treatment group (levothyroxine vs. placebo), and neonatal sex were compared between those with and without HDP. Maternal baseline characteristics were compared using the Wilcoxon rank sum, chi-square, or Fisher exact test, as appropriate. Differences between HDP groups for the primary and secondary outcomes were analyzed using Wilcoxon rank sum test, Fisher exact, or chi-square tests, as appropriate. Multivariable quantile regression was used to assess differences in scores adjusting for potential confounders. Multivariable logistic regression was used to evaluate dichotomized outcomes. Baseline characteristics that were found to be different between HDP groups at a level p<.0.05 in univariable analysis were included in the models. In addition, the original trial treatment group assignment and maternal baseline thyroid status were included in the models. .Adjusted beta coefficients (demonstrating differences in scores) and adjusted odds ratios with 95% confidence intervals were reported. Additional analyses stratifying by preterm (less than 37 weeks) and term (greater than or equal to 37 weeks) deliveries were performed. The rationale behind stratifying by preterm delivery is the inverse relationship between gestational age and risk for developmental impairment as preterm delivery can predispose the offspring to neurodevelopmental delays.22 Since gestational age at birth could be in the causal pathway for the association between HDP and offspring’s neurodevelopment we did not adjust for it in the regression model but examined term and preterm groups, separately. A p-value <0.05 was considered statistically significant. No imputation for missing data was performed. Statistical analysis was performed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC). The parent study was approved by the Institutional Review Board at each center prior to trial initiation. All STROBE checklist items were followed when planning, conducting and reporting the original RCT which this secondary analysis is based on.
Results
A total of 1,179 women-children dyads were included in the analysis (Figure 1). Of these, 112 (9.5%) were exposed to HDP. Table 1 describes baseline demographic and clinical characteristics of the cohort. Women who developed HDP were younger, more likely to be nulliparous, have higher BMI and higher rates of preterm delivery. Marital status, insurance type, substance abuse, maternal education and receipt of levothyroxine treatment did not differ between women with and without a diagnosis of HDP.
Figure 1.
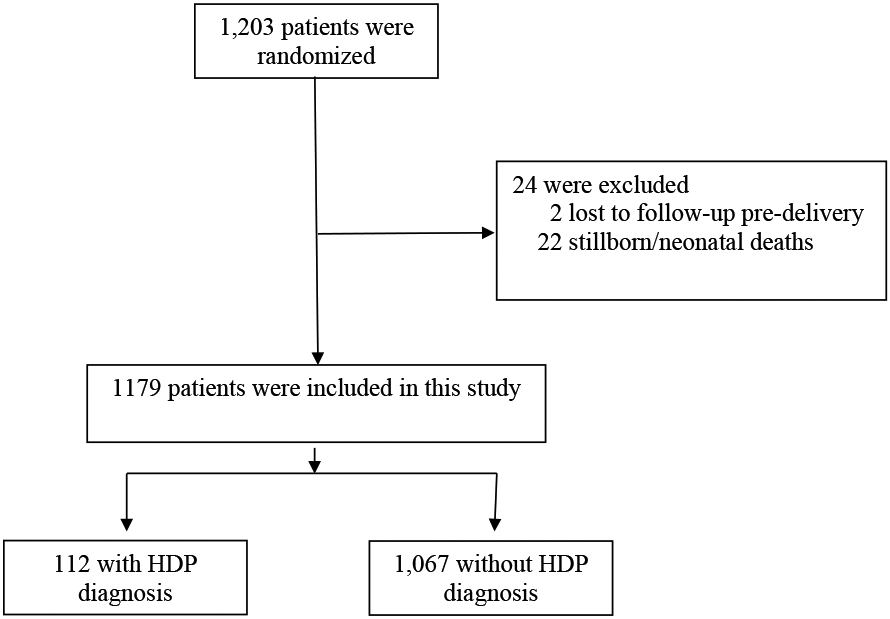
Study flowchart.
Table 1.
Comparison of neurodevelopment outcomes among offspring by maternal diagnosis of hypertensive disorders of pregnancy.
| Outcome | HDP diagnosis (N=112) |
No HDP diagnosis (N=1067) |
P-value |
|---|---|---|---|
| Maternal age (years) | 26.7 ± 5.9 | 27.8 ± 5.7 | 0.03 |
| Marital status | 0.27 | ||
| Married / living with partner | 76 (67.9) | 795 (74.5) | |
| Divorced / widowed / separated | 6 (5.4) | 41 (3.8) | |
| Never married | 30 (26.8) | 231 (21.6) | |
| BMI kg/m2 | 31.8 ± 7.8 | 28.8 ±6.4 | <0.001 |
| Preterm delivery < 37 weeks | 25 (22.3) | 76 (7.1) | <0.001 |
| Smoking during pregnancy | 8 (7.1) | 83 (7.8) | 0.81 |
| Alcohol during pregnancy | 4 (3.6) | 61 (5.7) | 0.34 |
| Insurance type | 0.22 | ||
| Government assisted | 73 (65.2) | 610 (57.2) | |
| Private | 28 (25.0) | 304 (28.5) | |
| Self-pay | 11 (9.8) | 153 (14.3) | |
| Maternal education | 0.52 | ||
| Less than High School graduate | 51 (45.5) | 478 (44.8) | |
| High school graduate | 42 (37.5) | 362 (33.9) | |
| College graduate and higher | 19 (17.0) | 227 (21.3) | |
| Parity | 0.002 | ||
| Nulliparous | 51 (45.5) | 331 (31.0) | |
| Multiparous | 61 (54.5) | 736 (69.0) | |
| Levothyroxine treatment group | 53 (47.3) | 542 (50.8) | 0.48 |
| Thyroid status | 0.24 | ||
| Subclinical hypothyroidism | 69 (61.6) | 595 (55.8) | |
| Subclinical hypothyroxinemia | 43 (38.4) | 472 (44.2) | |
| Infant male sex | 64 (57.1) | 545 (51.1) | 0.22 |
Data displayed as mean ± SD or n (%).
Table 2 describes neurodevelopmental outcomes among offspring by diagnosis of HDP. The full-scale IQ assessed with the use of the WPPSI-III at 5 years of age, both as continuous outcome and as IQ< 85 (one standard deviation below the mean), did not differ between children exposed to HDP and those unexposed. Median scores were 94 [interquartile range: (IQR): 85 – 101.5] and 95 [IQR: 84-104] for those exposed to HDP versus unexposed to HDP, respectively (p=0.75). Similarly, there were no significant differences between the two groups, for a low WPPSI-III score, 22.2% of HDP group and 25.3% of the non-HDP group had an IQ < 85 (p=0.48). Among secondary outcomes, both groups were similar with the exception of the Bayley-III language assessment at two years of age. Children exposed to HDP had higher rates of language score <85 at two years of age, indicating language delay (46.5% versus 30.5%, p=0.001).
Table 2.
Comparison of neurodevelopmental outcomes among offspring by maternal diagnosis of hypertensive disorders of pregnancy.
| Outcome | HDP diagnosis (N=112) |
No HDP diagnosis (N=1067) |
Adjusted Difference in Medians or Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| WPPSI-III IQ at 5y | 94 [85–101.5] | 95 [84–104] | −0.82 (−4.31 to 2.67) |
| WPPSI-III IQ<85 (n, %) | 24 (22.2) | 254 (25.3) | 0.80 (0.50 to 1.31) |
| Bayley-III assessment at 1y: | |||
| Cognitive | 100 [95-110] | 100 [90-110] | 0 (−2.58 to 2.58) |
| Cognitive <85 (n, %) | 7 (6.6) | 90 (9.0) | 0.76 (0.34 to 1.70) |
| Motor | 97 [91-107] | 97 [91-103] | 0 (−1.56 to 1.56) |
| Motor <85 (n, %) | 8 (7.5) | 98 (9.8) | 0.79 (0.37 to 1.70) |
| Language | 95.5 [89-103] | 94 [86-103] | 1.84 (−0.90 to 4.58) |
| Language<85 (n, %) (indicating language delay) | 17 (16.0) | 181 (18.1) | 0.93 (0.54 to 1.62) |
| Bayley-III assessment at 2y: | |||
| Cognitive | 90 [85-95] | 90 [85-100] | −0.39 (−3.03 to 2.25) |
| Cognitive <85 (n, %) | 19 (18.8) | 235 (24.0) | 0.73 (0.43 to 1.25) |
| Motor | 97 [91-103] | 97 [91-103] | 0.47 (−2.23 to 3.16) |
| Motor <85 (n, %) | 8 (7.9) | 96 (9.9) | 0.74 (0.34 to 1.60) |
| Language | 86 [79-97] | 91 [83-97] | −4.63 (−9.09 to −0.17) |
| Language<85 (n, %) (indicating language delay) | 47 (46.5) | 292 (30.5) | 2.22 (1.44 to 3.42) |
| DAS-II General Conceptual Ability score (GCA)at 3y | 89 [79-97] | 90 [81-100] | −1.04 (−6.01 to 3.93) |
| DAS-II (GCA) at 3y <85 | 39 (37.9) | 348 (35.2) | 1.11 (0.72 to 1.72) |
| CBCL T score at 3y | 46.5 [41-57] | 46.5 [40-54] | 0.21 (−3.21 to 3.63) |
| CBCL T score at 3y >60 | 12 (11.5) | 97 (9.8) | 1.32 (0.68 to 2.53) |
| Conners’ Rating Scale-Revised ADHD score at 4y | 48.5 [44-57.5] | 49 [44-57] | −0.48 (−3.55 to 2.59) |
| Conners’ Rating Scale-Revised ADHD T score > 60 | 17 (16.3) | 160 (16.5) | 0.92 (0.53 to 1.61) |
| CBCL T score at 5y | 44.5 [37-53] | 44.5 [37-53] | 0.02 (−3.05 to 3.10) |
| CBCL T score at 5y >60 | 10 (9.3) | 105 (10.4) | 0.87 (0.43 to 1.75) |
Data displayed as median and interquartile range unless otherwise indicated.
WPPSI-III, Wechsler Preschool and Primary Scale of Intelligence-III; DAS-II, Differential Ability Scales-II; Bayley, Bayley Scales of Infant Development-III; Conners, Conners’ Rating Scales-Revised, for assessment of attention; CBCL, Child Behavior Checklist, for behavioral and social competency
Number of participants in each outcome: WPPSI-III: 1111; 12 month Bayley cognitive: 1111; 12 month Bayley motor: 1108; 12 month Bayley language: 1104; 24 month Bayley cognitive: 1080; 24 month Bayley motor: 1069; 24 month Bayley language: 1057; DAS II: 1091; 36 month CBCL: 1096; Conners: 1071; 60 month CBCL: 1114.
Quantile regression model adjusters included parity, maternal age, maternal BMI, treatment group, and baseline thyroid status.
Multivariable regression analyses were performed and showed similar findings. Regression models were adjusted for baseline maternal age, parity and BMI, since these were significantly different between the two groups, and for the original trial treatment group assignment and baseline thyroid status. The adjusted difference in medians between the two groups for the WPPSI-III IQ was −0.82, 95% CI: −4.31 to 2.67. The adjusted odds ratio for the HDP group compared with the non-HDP group for low WPPSI-III score was 0.80, 95% CI: 0.50 to 1.31. The adjusted odds ratio for the HDP group compared with the non-HDP group for low Bayley-III language score at two years was significant (aOR 2.22, 95%CI 1.44 to 3.42). In addition, median language scores were lower among children exposed to HDP compared with the non-HDP group. No other secondary outcomes were significantly different in the adjusted analyses.
Analysis stratified by preterm delivery (<37 weeks) was conducted and described in Tables 3 and 4. The results confirmed the finding of higher rates of language score <85, both in preterm and term groups. In addition, median language scores were lower in the preterm group among children exposed to HDP compared with the non-HDP group.
Table 3.
Comparison of neurodevelopmental outcomes among offspring stratified by maternal diagnosis of hypertensive disorders of pregnancy for women who delivered preterm (<37 weeks).
| Outcome | HDP diagnosis (N=25) |
No HDP diagnosis (N=76) |
Adjusted Difference in Medians or Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| WPPSI-III IQ at 5y | 89 [81 – 103] | 90.5 [80 -102] | 1.13 (−11.33 to 13.59) |
| WPPSI-III IQ<85 (n, %) | 7 (29.2) | 24 (34.3) | 0.63 (0.21 to 1.94) |
| Bayley-III assessment at 1y | |||
| Cognitive | 100 [95-110] | 100[95-110] | 2.89 (−4.93 to 10.70) |
| Cognitive <85 (n, %) | 1 (4.5) | 3 (4.3) | 0.69 (0.06 to 8.57) |
| Motor | 97 [91-100] | 100 [91-110] | −3.81 (−9.82 to 2.21) |
| Motor < 85 (n, %) | 4 (18.2) | 6 (8.7) | 2.03 (0.48 to 8.64) |
| Language | 97 [89-109] | 94 [89-106] | −3.05 (−13.64 to 7.53) |
| Language <85 (n, %) | 4 (18.2) | 13 (18.8) | 0.81 (0.21 to 3.11) |
| Bayley-III assessment at 2y | |||
| Cognitive | 90 [85-95] | 90 [80-100] | 1.22 (−6.03 to 8.47) |
| Cognitive <85 (n, %) | 3 (13.6) | 17 (25.8) | 0.54 (0.13 to 2.29) |
| Motor | 94 [91-107] | 97 [91-107] | 0.00 (−7.39 to 7.39) |
| Motor < 85 (n, %) | 2 (9.1) | 5 (7.7) | 1.17 (0.16 to 8.41) |
| Language | 83 [77-94] | 91 [83-97] | −6.52 (−14.53 to −1.50) |
| Language <85 (n, %) | 12 (54.5) | 17 (26.6) | 3.32 (1.12 to 9.84) |
| DAS-II General Conceptual Ability score at 3y | 82 [78-95] | 87 [77-96] | −1.32 (−13.43 to 10.79) |
| DAS II (GCA) at 3y < 85 | 12 (54.5) | 30 (43.5) | 1.73 (0.60 to 4.96) |
| CBCL T score at 3y | 48 [41-59] | 47 [39-58] | 3.14 (−3.01 to 9.30) |
| CBCL T score at 3y >60 | 4 (18.2) | 13 (8.8) | 0.62 (0.14 to 2.83) |
| Conners’ Rating Scale-Revised ADHD score at 4y | 50 [44-58]) | 49.5 [44-57] | −0.34 (−7.00 to 6.32) |
| Conners’ Rating Scale-Revised ADHD T score >60 | 5 (22.7) | 12 (19.4) | 2.61 (0.57 to 12.02) |
| CBCL T score at 5y | 51 [45-55.5] | 46 [39-54] | 5.55 (−0.74 to 11.85) |
| CBCL T score at 5y >60 | 3 (12.5) | 10 (14.3) | 0.55 (0.12 to 2.67) |
Data displayed as median and interquartile range.
WPPS III, Wechsler Preschool and Primary Scale of Intelligence-III; DAS II, Differential Ability Scales-II; Bayley, Bayley Scales of Infant Development; Conners, Conners’ Rating Scales-Revised, for assessment of attention; CBCL, Child Behavior Checklist, for behavioral and social competency
Number of participants in each outcome: WPPS III: 94; 12 month Bayley cognitive: 92; 12 month Bayley motor: 91; 12 month Bayley language: 91; 24 month Bayley cognitive: 88; 24 month Bayley motor: 87; 24 month Bayley language: 86; DAS II: 91; 36 month CBCL: 91; Conners: 84; 60 month CBCL: 94.
Quantile regression models included parity, maternal age, maternal BMI, treatment group, and baseline thyroid status.
Table 4.
Comparison of neurodevelopmental outcomes among offspring stratified by diagnosis of HDP for women who delivered at term (>=37 weeks)
| Outcome | HDP diagnosis (N=87) |
No HDP diagnosis (N=991) |
Adjusted Difference in Medians or Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| Primary outcome | |||
| WPPSI-III IQ at 5y | 94.5 [86-101] | 95 [85 -104] | −1.12 (−4.70 to 2.46) |
| WPPSI-III IQ<85 (n, %) | 17 (20.2) | 230 (24.7) | 0.73 (0.42 to 1.30) |
| Secondary outcomes | |||
| Bayley-III assessment at 1y: | |||
| Cognitive | 100 [92.5-110] | 100 [90-110] | 0.87 (−1.97 to 3.71) |
| Cognitive <85 (n, %) | 6 (7.1) | 87 (9.3) | 0.81 (0.34 to 1.94) |
| Motor | 97 [92.5-110] | 97 [91-103] | 0.00 (−2.78 to 2.78) |
| Motor <85 (n, %) | 4 [4.8) | 92 (9.9) | 0.50 (0.18 to 1.40) |
| Language | 94 [89-103] | 94 [86-103] | 1.82 (−1.07 to 4.71) |
| Language<85 (n, %) (indicating language delay) | 13 (15.5) | 168 (18.1) | 0.92 (0.49 to 1.71) |
| Bayley-III assessment at 2y: | |||
| Cognitive | 90 [85-95] | 90 [85-95] | −0.30 (−3.65 to 3.05) |
| Cognitive <85 (n, %) | 16 (20.3) | 218 (23.9) | 0.79 (0.44 to 1.42) |
| Motor | 97 [91-103] | 97 [91-103] | 0.56 (−1.68 to 2.80) |
| Motor <85 (n, %) | 6 (7.6) | 91 (10.1) | 0.70 (0.29 to 1.68) |
| Language median | 86 [79-100] | 90 [83-98.5] | −2.91 (−7.40 to 1.58) |
| Language<85 (n, %) (indicating language delay) | 35 (44.3) | 275 (30.8) | 2.01 (1.24 to 3.25) |
| DAS-II General Conceptual Ability score (GCA) at 3y | 89 [80-97] | 90 [81-100] | −0.62 (−5.40 to 4.15) |
| DAS-II (GCA)<85 | 27 (33.3) | 318 (34.6) | 0.92 (0.56 to 1.52) |
| CBCL T score at 3y | 46 [41-56] | 46 [40-54] | −0.61 (−4.71 to 3.48) |
| CBCL T score at 3y >60 | 8 (9.8) | 84 (9.1) | 1.24 (0.57 to 2.70) |
| Conners’ Rating Scale-Revised ADHD score at 4y | 47.5 [44-57]) | 49 [44-56] | −0.30 (−3.90 to 3.30) |
| Conners’ Rating Scale-R >60 ADHD T score > 60 | 12 (14.6) | 148 (16.4) | 0.80 (0.42 to 1.52) |
| CBCL T score at 5y | 42 [37-51] | 44.0 [37-53] | −1.57 (−4.58 to 1.45) |
| CBCL T score at 5y >60 | 7 (8.3) | 95 (10.1) | 0.82 (0.36 to 1.86) |
Data displayed as median and interquartile range.
WPPS III, Wechsler Preschool and Primary Scale of Intelligence-III; DAS II, Differential Ability Scales-II; Bayley, Bayley Scales of Infant Development; Conners, Conners’ Rating Scales-Revised, for assessment of attention; CBCL, Child Behavior Checklist, for behavioral and social competency
Number of participants in each outcome: WPPS III: 1017; 12 month Bayley cognitive: 1019; 12 month Bayley motor: 1017; 12 month Bayley language: 1013; 24 month Bayley cognitive: 982; 24 month Bayley motor: 982; 24 month Bayley language: 971; DAS II: 1000; 36 month CBCL: 1005; Conners: 987; 60 month CBCL: 1114.
Quantile regression model adjusters included parity, maternal age, maternal BMI, treatment group, and baseline thyroid status.
Discussion
In this study, we found that maternal diagnosis of HDP was associated with higher rates of language delay in the offspring at the age of 2 but did not have an effect on any other neurodevelopmental measures at the age of 2 or at the age of 5. The results did not differ when analysis was stratified by preterm birth.
Our findings of language delay were similar to the study by Whitehouse et al that found lower verbal ability in offspring exposed to HDP.16 Whitehouse et al examined verbal ability in 1,389 children at age 10 using the “Peabody Picture Vocabulary Test – Revised” and found that exposure to preeclampsia or gestational hypertension was associated with 1.83 (95% Confidence Interval −3.48 - −0.17) points lower score.16 This study controlled for birthweight and gestational age which suggested that reduced verbal ability cannot be attributed solely to immaturity or intrauterine growth restriction. In contrast, Sverrisson et al analyzed nationwide data from the Icelandic registries and reported no difference in language art scores among children age 9 to 15 years old.18 This study, however, lacked information about maternal smoking status, maternal BMI and maternal medical co-morbidities, such as diabetes, all of which have been associated with child cognitive development.22-24
In regard to the rest of the neurodevelopmental outcomes, including child IQ assessed at the age of 5, our study did not find an association between offspring exposure to HDP and long-term neurodevelopmental concerns. This conflicts with prior studies reporting positive association.10, 11, 13 Spinillo et al found that infants delivering preterm secondary to HDP compared to spontaneous preterm birth, had higher rates of minor neurodevelopmental impairment.13 Cheng et al followed infants born at <32 weeks with and without an exposure to preeclampsia up to 2 years of age and found that preeclampsia was associated with a significantly lower score on the mental development index of the Bayley Scales of Infant Development.10 Other studies that included full term deliveries found an association between exposure to HDP and childhood behavioral issues and mild lower cognitive ability in the offspring.14, 15, 19, 25 A recent systematic review showed that exposure to HDP was associated with a 35% increase in the odds of Autism Spectrum Disorder (ASD) when compared to those unexposed to HDP.11 Another recent study from Norway using medical birth registry of term deliveries found that offspring from preeclampsia had higher odds ratio of attention-deficit/hyperactivity disorder, ASD, epilepsy and intellectual disability.26 The limitation of that study included lack of information on maternal smoking status and overall, as this was a registry ascertainment of exposures and outcomes may have been limited to more severe cases.26 It is important to note, that other studies that found a positive association between preeclampsia and neurodevelopmental outcomes in the offspring did not control for certain key variables such as maternal age, socio-economic status, ethnic origin and maternal depression, calling into question the validity of findings.11,19 Other studies focused on preterm population,10,13 a factor that by itself can predispose the offspring to neurodevelopmental delays.27 Finally, other long cohort studies did not support the association between exposure to HDP and impaired neurodevelopment in the offspring.17, 18, 25
Given the epidemiologic evidence for an association between exposure to HDP and offspring’s language delay, confirmed by our study, the question arise what could be the mechanisms that mediate this association. These include a genetic component that may predispose to preeclampsia, cardiovascular and neurodevelopment diseases in later life and may be inherited by offspring.1 Another mechanism is inflammation. Inflammation is recognized as a core clinical feature in preeclampsia.2 The decrease in placental perfusion in preeclampsia leads to an increased production of pro-inflammatory cytokines such as IL-6 and TNF-α. These cytokines may disrupt placenta signaling and/or cross to the fetal circulation to alter fetal neurodevelopment trajectories, which may increase the risk of suboptimal neurodevelopmental outcomes, including verbal ability, in offspring exposed to pre-eclampsia. The potential effect of these mechanisms on neurologic pathways related to language and verbal ability is intriguing and deserves further elucidation.
The limitations of this study should be noted. First, this analysis is limited to the population included in the parent study and sample size used for our analysis was fixed. Moreover, all women in the parent study had a diagnosis of subclinical thyroid disease. There has been a controversy surrounding the contribution of untreated maternal subclinical thyroid disease in pregnancy towards offspring long-term cognitive development.20, 28-34 However recent large randomized controlled trials, including the parent trial20 and the Controlled Antenatal Thyroid Screening study30 with a follow-up up to 9.5 years of age35 did not show that levothyroxine in pregnancy improves child cognitive scores. The IQ scores were similar across the groups and within the general population range. Furthermore, studies investigating maternal thyroid dysfunction did not find an association between thyroid function tests and offspring language ability.32, 33 This supports that our findings are not related to maternal diagnosis of subclinical thyroid disease. Second limitation included lack of information whether women with HDP required antihypertensive medication, and if so, what type of medication. Therefore, we are unable to control our results for antihypertensive medication exposure or the severity of maternal HDP disease. Third, we analyzed both gestational hypertension and preeclampsia in the same group of HDP as a separate analysis for each of these disease stages was not feasible due to limited sample size. Fourth, it should be emphasized that the language delay was detected at the age of 2. There was no specific language assessment done at 5 years; therefore, we are unable to assess whether the language delay persisted. It is possible that the language difference was a type 1 error due to comparison of multiple neurodevelopment measures in this analysis, Furthermore, we did not account for an important confounder when assessing offspring language, and that is the primary language of the parents. Finally, it is important to note that while missing data for the primary outcome of this analysis, WPPSI-III, was missing in only 5.8% of participants, some of the data for secondary outcomes was missing in up to 10%.
In contrast, our study has several strengths. First, our analysis stands apart from other studies as it is based on the only prospective cohort of women-children dyads assessing long-term neurodevelopmental outcomes of the offspring and collecting a detailed maternal information on prenatal clinical, social and demographic variables. Second, the association between offspring language delay and HDP exposure was significant both in the entire cohort as well as in the sub-group analysis limited to term deliveries. Therefore, this is less likely to represent a random association.
In conclusion, in this secondary analysis, diagnosis of maternal HDP was associated with language score <85 at 2 years of age but not with any other long-term neurodevelopmental outcomes in offspring.
Acknowledgements:
We thank Lisa Moseley, R.N., B.S.N., and Gail Mallett, R.N., B.S.N., C.C.R.C., for protocol development and coordination between clinical research centers; Barbara Jones-Binns, J.D., M.P.H., for protocol and data management, overall coordination, and quality control; and Elizabeth A. Thom, Ph.D., Alan M. Peaceman, M.D., and Catherine Y. Spong, M.D. for protocol development and oversight.
Funding:
Supported by grants (HD34116, HD40512, HD27917, HD34208, HD40485, HD40560, HD53097, HD27869, HD40500, HD40545, HD27915, HD40544, HD53118, HD21410, and U10HD36801) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
The University of Utah Health Sciences Center, Salt Lake City, UT – K. Hill, A. Sowles, S. Timothy, P. Reed (Intermountain Healthcare), S. Esplin (Intermountain Healthcare)
University of Texas Southwestern Medical Center, Dallas, TX – L. Moseley, J. Price, C. Melton, M. Garcia, J. Gerald, M. Santillan
University of Pittsburgh, Pittsburgh, PA – M. Cotroneo, D. DeAngelis, M. Luce, R. Kennedy, D. Nowinski
University of Alabama at Birmingham, Birmingham, AL – S. Harris, F. Biasini, M. Parks, J. Grant, C. Lee, A. Todd, K. Domnanovich, W. Andrews
Wayne State University, Detroit, MI – N. Hauff, L. Goldston, D. Driscoll
The Ohio State University, Columbus, OH – F. Johnson, J. Iams, S. Wylie, R. Devlin, B. Selegue, C. Latimer, J. Bauer
Brown University, Providence, Rhode Island – D. Allard, T. Leach, V. Watson, B. Hughes
Columbia University, New York, NY – S. Bousleiman, V. Carmona, A. Zygmunt, Y. Williams (Drexel University), M. Grant (Drexel University), C. Kitto (Christiana Care Health Systems), B. Higley (Christiana Care Health Systems), M. Falk (St. Peter's University Hospital); L. Padovano (St. Peter's University Hospital)
University of Texas Medical Branch, Galveston, TX – A. Salazar, A. Acosta, K. Smith, G. Hankins, S. Jain, M. Munn, L. Pacheco
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH – C. Milluzzi, B. Nielsen, W. Dalton, H. Cozart, E. Chien
The University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX – F. Ortiz, S. Blackwell, B. Rech, M. Hutchinson, P. Givens
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Clark, S. Timlin, K. Dorman, E. Campos, H. Byers, S. Brody (WakeMed Health & Hospitals)
Northwestern University, Chicago, IL – G. Mallett, M. Ramos-Brinson, M. Weissbourd (Lurie Children's Hospital), M. Dinsmoor (NorthShore University HealthSystem), K. Paychek (NorthShore University HealthSystem), P. Campbell
Oregon Health & Science University, Portland, OR – M. Rincon, L. Pereira, P. Blasco, S. Saxton, K. Beach, J. Snyder
George Washington University Biostatistics Center, Washington, DC – E.A. Thom. B. Jones-Binns, L. Mele
National Institute of Neurological Disorders and Stroke, Bethesda, MD – D.G. Hirtz
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
MFMU Network Steering Committee Chair (Medical University of South Carolina, Charleston, SC) – J. P. VanDorsten, M.D.
Footnotes
Disclosures: The author reports no conflict of interest.
Presented in poster format at the 40th annual meeting of the Society for Maternal-Fetal Medicine, Grapevine, Texas, Feb 3-8, 2020.
Contributor Information
Anna Palatnik, Departments of Obstetrics and Gynecology of Northwestern University, Chicago, IL.
Lisa Mele, George Washington University Biostatistics Center, Washington, DC.
Brian M Casey, University of Texas - Southwestern, Dallas, TX.
Michael W Varner, University of Utah Health Sciences Center, Salt Lake City, UT.
Yoram Sorokin, Wayne State University, Detroit, MI.
Uma M Reddy, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Ronald J Wapner, Columbia University, New York, NY.
John M Thorp, Jr, University of North Carolina, Chapel Hill, NC.
George R Saade, University of Texas Medical Branch, Galveston, TX.
Alan TN Tita, University of Alabama at Birmingham, Birmingham, AL.
Dwight J Rouse, Brown University, Providence, RI.
Baha Sibai, University of Texas – Houston, Houston, TX.
Maged M Costantine, The Ohio State University, Columbus, OH.
Brian M Mercer, Case Western Reserve University, Cleveland, OH.
Jorge E. Tolosa, Oregon Health Sciences University, Portland, OR.
Steve N Caritis, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European journal of obstetrics, gynecology, and reproductive biology. 2013;170(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Duley L The global impact of pre-eclampsia and eclampsia. Seminars in perinatology. 2009;33(3):130–7. [DOI] [PubMed] [Google Scholar]
- 3.Pauli JM, Repke JT. Preeclampsia: Short-term and Long-term Implications. Obstetrics and gynecology clinics of North America. 2015;42(2):299–313. [DOI] [PubMed] [Google Scholar]
- 4.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet (London, England). 2010;376(9741):631–44. [DOI] [PubMed] [Google Scholar]
- 5.Goffin SM, Derraik JGB, Groom KM, Cutfield WS. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy hypertension. 2018;12:11–5. [DOI] [PubMed] [Google Scholar]
- 6.Seely EW, Rich-Edwards J, Lui J, Nicklas JM, Saxena A, Tsigas E, et al. Risk of future cardiovascular disease in women with prior preeclampsia: a focus group study. BMC pregnancy and childbirth. 2013;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by Women's Health Care Providers of Long-Term Cardiovascular Disease Risk After Preeclampsia. Obstetrics and gynecology. 2015;125(6):1287–92. [DOI] [PubMed] [Google Scholar]
- 8.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. American heart journal. 2008;156(5):918–30. [DOI] [PubMed] [Google Scholar]
- 9.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Seminars in perinatology. 2014;38(3):151–8. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SW, Chou HC, Tsou KI, Fang LJ, Tsao PN. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early human development. 2004;76(1):39–46. [DOI] [PubMed] [Google Scholar]
- 11.Maher GM, McCarthy FP, McCarthy CM, Kenny LC, Kearney PM, Khashan AS, et al. A perspective on pre-eclampsia and neurodevelopmental outcomes in the offspring: Does maternal inflammation play a role? International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2019;77:69–76. [DOI] [PubMed] [Google Scholar]
- 12.Many A, Fattal A, Leitner Y, Kupferminc MJ, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertension in pregnancy. 2003;22(1):25–9. [DOI] [PubMed] [Google Scholar]
- 13.Spinillo A, Iasci A, Capuzzo E, Egbe TO, Colonna L, Fazzi E. Two-year infant neurodevelopmental outcome after expectant management and indicated preterm delivery in hypertensive pregnancies. Acta obstetricia et gynecologica Scandinavica. 1994;73(8):625–9. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenstein V, Rothman KJ, Pedersen L, Hatch EE, Sorensen HT. Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. American journal of epidemiology. 2009;170(8):1025–31. [DOI] [PubMed] [Google Scholar]
- 15.Heikura U, Hartikainen AL, Nordstrom T, Pouta A, Taanila A, Jarvelin MR. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatric and perinatal epidemiology. 2013;27(2):188–98. [DOI] [PubMed] [Google Scholar]
- 16.Whitehouse AJ, Robinson M, Newnham JP, Pennell CE. Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatric and perinatal epidemiology. 2012;26(2):101–8. [DOI] [PubMed] [Google Scholar]
- 17.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. British journal of obstetrics and gynaecology. 1991;98(10):1009–14. [DOI] [PubMed] [Google Scholar]
- 18.Sverrisson FA, Bateman BT, Aspelund T, Skulason S, Zoega H. Preeclampsia and academic performance in children: A nationwide study from Iceland. PloS one. 2018;13(11):e0207884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuovinen S, Eriksson JG, Kajantie E, Raikkonen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. Journal of the American Society of Hypertension : JASH. 2014;8(11):832–47.e1. [DOI] [PubMed] [Google Scholar]
- 20.Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, et al. Treatment of Subclinical Hypothyroidism or Hypothyroxinemia in Pregnancy. The New England journal of medicine. 2017;376(9):815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayley N Bayley Scales of Infant and Toddler Development. San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- 22.Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, Lind JN. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics. 2015;135(5):e1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthopolos R, Edwards SE, Miranda ML. Effects of maternal prenatal smoking and birth outcomes extending into the normal range on academic performance in fourth grade in North Carolina, USA. Paediatr Perinat Epidemiol. 2013;27(6):564–74 [DOI] [PubMed] [Google Scholar]
- 24.Camprubi Robles M, Campoy C, Garcia Fernandez L, Lopez-Pedrosa JM, Rueda R, Martin MJ. Maternal Diabetes and Cognitive Performance in the Offspring: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(11):e0142583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson M, Mattes E, Oddy WH, de Klerk NH, Li J, McLean NJ, et al. Hypertensive diseases of pregnancy and the development of behavioral problems in childhood and adolescence: the Western Australian Pregnancy Cohort Study. The Journal of pediatrics. 2009;154(2):218–24. [DOI] [PubMed] [Google Scholar]
- 26.Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring. JAMA Psychiatry. 2020;77(8):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–37. [DOI] [PubMed] [Google Scholar]
- 28.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. The New England journal of medicine. 1999;341(8):549–55. [DOI] [PubMed] [Google Scholar]
- 29.Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. The lancet Diabetes & endocrinology. 2016;4(1):35–43. [DOI] [PubMed] [Google Scholar]
- 30.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. The New England journal of medicine. 2012;366(6):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levie D, Korevaar TIM, Bath SC, Dalmau-Bueno A, Murcia M, Espada M, et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. The Journal of clinical endocrinology and metabolism. 2018;103(8):2967–79. [DOI] [PubMed] [Google Scholar]
- 32.Nelson SM, Haig C, McConnachie A, Sattar N, Ring SM, Smith GD, et al. Maternal thyroid function and child educational attainment: prospective cohort study. BMJ (Clinical research ed). 2018;360:k452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noten AM, Loomans EM, Vrijkotte TG, van de Ven PM, van Trotsenburg AS, Rotteveel J, et al. Maternal hypothyroxinaemia in early pregnancy and school performance in 5-year-old offspring. European journal of endocrinology. 2015;173(5):563–71. [DOI] [PubMed] [Google Scholar]
- 34.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. The Journal of clinical endocrinology and metabolism. 2011;96(10):3234–41. [DOI] [PubMed] [Google Scholar]
- 35.Hales C, Taylor PN, Channon S, Paradice R, McEwan K, Zhang L, et al. Controlled Antenatal Thyroid Screening II: Effect of Treating Maternal Suboptimal Thyroid Function on Child Cognition. The Journal of clinical endocrinology and metabolism. 2018;103(4):1583–91. [DOI] [PubMed] [Google Scholar]


