Abstract
Background:
An association of eosinophilic esophagitis (EoE) with esophageal dysmotility has been described, however the related mechanism remains unclear. We aimed to evaluate clinical and physiologic characteristics, including esophageal distensibility, associated with secondary peristalsis in patients with EoE.
Methods:
199 consecutive adult patients with EoE (ages 18–78, 32% female) that completed 16-cm functional luminal imaging probe (FLIP) during endoscopy were evaluated in a cross-sectional study. FLIP Panometry contractile response (CR) patterns were classified as normal CR or borderline CR if antegrade contractions were present, while “abnormal CRs” included impaired/disordered CR, absent CR, or spastic-reactive CR. The distensibility plateau (DP) of the esophageal body and esophagogastric junction distensibility was measured with FLIP.
Results:
FLIP CR patterns included 68 (34%) normal CR, 65 (33%) borderline CR, 44 (22%) impaired/disordered CR, 16 (8%) absent CR, and 6 (3%) spastic-reactive CR. Compared with normal CRs, abnormal CRs more frequently had reduced esophageal distensibility (DP<17mm in 56% vs 32%), greater total EREFS scores (median (interquartile range, IQR) 5 (3–6) vs 4 (3–5) with more severe ring scores, and a greater duration of symptoms (10 (4–23) vs 7 (3–15) years). Mucosal eosinophil density, however, was similar between abnormal CRs and normal CRs: 34 (14–60) vs 25 (5–50) eosinophils/hpf.
Discussion:
While normal secondary peristalsis was frequently observed in this EoE cohort, abnormal esophageal CRs were related to EoE disease severity, especially features of fibrostenosis. This study evaluating secondary peristalsis in EoE suggests that esophageal wall remodeling, rather than eosinophilic inflammatory intensity, was associated with esophageal dysmotility in EoE.
Keywords: dysphagia, achalasia, esophageal spasm, manometry, impedance
Introduction
A relationship between esophageal eosinophilia and esophageal motor disorders, including achalasia, has been reported.1–5 This has prompted speculation that eosinophils and associated products may cause esophageal motility disorders though neurotoxic, neuroactive, or myoactive effects. Eosinophilic esophagitis (EoE) is the paradigm eosinophilic esophageal disorder and is characterized by its typical presentation of obstructive esophageal symptoms, histopathologic detection of mucosal eosinophils, and fibrostenotic remodeling of the esophageal wall.6 However, studies evaluating esophageal motility using manometry in patients with EoE have shown that normal esophageal motility is the most common finding while a motor pattern consistent with achalasia is infrequently observed.4, 7 Additionally, mechanical esophageal obstruction is recognized to cause reactive esophageal contractile responses and thus, fibrostenosis in EoE is suspected to impact esophageal motility.8, 9 Ultimately, the pathophysiologic role of esophageal eosinophilia on esophageal motility is incompletely understood.
Functional luminal imaging probe (FLIP) Panometry provides a unique method to evaluate the esophageal response to distension and can simultaneously assess both esophageal biomechanics and esophageal motility.10, 11 Reduced distensibility of the esophageal body was reported in EoE with an associated risk for food impaction and need for endoscopic dilation.10 The esophageal contractile response (CR) to distension (i.e. secondary peristalsis) can also be classified using FLIP Panometry as a pathophysiologic progression from normal to abnormal esophageal peristaltic function.12 This FLIP Panometry CR classification was recently shown to largely parallel primary peristalsis on high resolution manometry (HRM) in patients undergoing evaluation for esophageal motility disorders.11 Thus, evaluation of FLIP Panometry in patients with EoE may provide insight on the impact of esophageal wall mechanics and fibrostenosis on esophageal dysmotility in EoE. We hypothesize that esophageal dysmotility is associated with obstructive mechanics related to EoE pathogenesis and thus this study aimed to evaluate secondary peristalsis in patients with EoE and the association with measures of EoE disease activity.
Methods
Subjects
Adult patients (age ≥18 years) presenting to the Esophageal Center of Northwestern for evaluation of esophageal symptoms between January 2015 and December 2020 who completed FLIP during upper endoscopy were prospectively evaluated and data were maintained in an esophageal motility registry. Consecutive patients that completed FLIP during sedated endoscopy and with a diagnosis of EoE were included and evaluated via a cross-sectional study design. Patients with technically limited FLIP, with previous foregut surgery, or without EoE were excluded. FLIP was typically performed for patients completing endoscopy for a primary complaint of dysphagia and EoE was suspected based on the presence of endoscopic features of EoE (i.e. longitudinal furrows, rings, or strictures). Patients were diagnosed with EoE per consensus guidelines with ≥ 15 eosinophils/hpf on esophageal biopsies (during previous endoscopy, or endoscopy with FLIP included in this study) in the absence of identified, secondary causes of esophageal eosinophilia.6 Some patients completed a validated dysphagia symptom severity score, the Brief Esophageal Dysphagia Questionnaire (BEDQ), on the day of the FLIP test (supplementary material); greater scores indicate greater symptom severity.13 Additional clinical features were obtained from patients’ electronic medical records including EoE-related treatment at the time of endoscopy with FLIP (proton pump inhibitor (PPI) and/or topical steroid or elimination diet), history of previous endoscopic dilation, and duration of esophageal symptoms (a likely correlate with EoE disease-duration). HRM, when recommended by treating physician and completed within one month of endoscopy with FLIP, was performed and analyzed per the Chicago Classification v4.0.14 The study protocol was approved by the Northwestern University Institutional Review Board.
Endoscopic and histologic assessment
Subjects underwent upper endoscopy in the left lateral decubitus position. Endoscopy was generally performed using conscious sedation with midazolam and fentanyl, but other medications, e.g., propofol, were also used in some cases with monitored anesthesia care at the discretion of the performing endoscopist. Although these medications used for endoscopic sedation can alter esophageal motility, the patterns of motility during the FLIP protocol are reproducible and have been shown to correlate with motility patterns during standard manometry performed without these medications.15–17 During endoscopy, four esophageal mucosal biopsies were obtained at 5 and 15-cm above the squamocolumnar junction. Histologic evaluation of biopsy specimens was performed by expert gastrointestinal pathologists. The peak number of eosinophils per hpf (0.196 mm2) was recorded for each patient. Histologic remission was defined by an eosinophil count <15/hpf, as opposed to active eosinophilia when eosinophil count was ≥15/hpf.
Endoscopic features of EoE (edema, rings, exudate, furrows, and stricture) were graded during the upper endoscopy (performed by experienced esophageal endoscopists: DAC, NG, IK, PJK, and JEP) according to a validated endoscopic assessment instrument, Endoscopic EoE Reference Score (EREFS).18 Edema (score 0–1), furrows (score 0–1), and exudates (score 0–2) were considered inflammatory endoscopic changes and their scores were summed to generate an inflammatory endoscopic score. Rings (score 0–3) and stricture (score 0–1) were considered fibrotic/remodeling endoscopic changes and were summed to generate a fibrotic endoscopic score.
FLIP Study Protocol
The FLIP study using 16-cm FLIP (EndoFLIP® EF-322N; Medtronic, Inc, Shoreview, MN) was performed during sedated endoscopy as previously described.12, 16, 17 With the endoscope withdrawn and after calibration to atmospheric pressure, the FLIP was placed transorally and positioned within the esophagus with 1–3 impedance sensors beyond the EGJ, maintaining this positioning throughout the FLIP study. Stepwise 10-ml FLIP distensions beginning with 40 ml and increasing to a target volume of 70 ml were then performed; each stepwise distension volume was maintained for 30–60 seconds. In some studies, excessive pressures (typically >60 mmHg, though no formal threshold was applied) limited the extent of FLIP distension to a maximum fill volume <70ml. Also in some studies, after completion of the initial distension ramp, the FLIP was partially emptied and repositioned in the proximal esophagus immediately distal to the upper esophageal sphincter (UES), using the FLIP narrowing at the UES as the landmark. Stepwise FLIP filling was then again performed to facilitate distensibility measurement of the proximal esophagus.
FLIP Panometry Analysis
FLIP data were exported to a customized program (available open source at http://www.wklytics.com/nmgi) to generate FLIP Panometry plots for analysis as previously described.11, 12 FLIP Panometry analysis was performed blinded to clinical characteristics.
Esophageal body contractility was identified by transient decreases in the luminal diameter spanning at least 3 cm in axial length; distinct antegrade contractions spanned at least 6cm in axial length. Studies were reviewed for specific features and patterns of contractility that were then applied to assign a contractile response (CR) pattern (Figure 1; Table 1).11, 12 Repetitive antegrade contractions (RACs) or repetitive retrograde contractions (RRCs) involved contractions of similar directionality that occurred consecutively at a consistent time interval and meeting rate criteria (Table 1).11, 12
Figure 1. FLIP Panometry contractile response patterns in eosinophilic esophagitis.
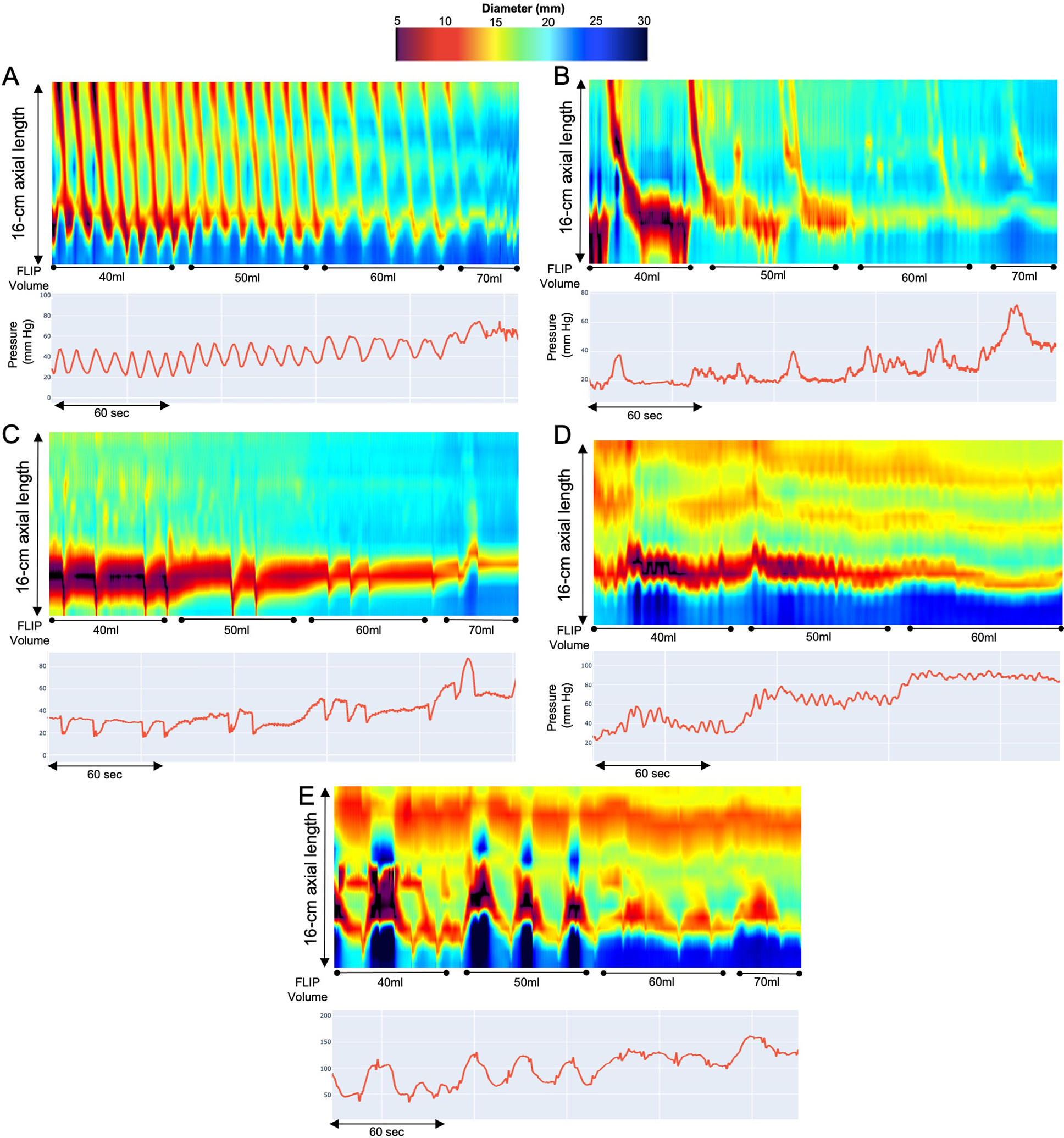
FLIP Panometry contractile response (CR) patterns. FLIP Panometry output from four patients (A-E) is displayed as length (16-cm) × time × color-coded diameter FLIP topography (top panels) with corresponding FLIP pressure (bottom panel). A) Normal CR, B) Borderline CR, C) Impaired/disordered CR, D) Absent CR, E) Spastic-reactive CR. The distensibility plateaus (DPs) were measured at the areas indicated by “*” and were A) 19mm, B) 19mm, C) 19mm, D) 14mm, and E) 11mm. Figure used with permission from the Esophageal Center of Northwestern.
Table 1. Contractile response pattern evaluation with FLIP Panometry.
The contractile response to distension, e.g. presence of secondary peristalsis, was based on evaluation of the FLIP study protocol including from the 40ml to 70ml fill volume.
| FLIP Panometry Contractile Response Patterns | Definition |
|---|---|
|
Normal Contractile Response
NCR |
Repetitive Antegrade Contraction (RAC) - Rule of 6s (Ro6s)
|
|
Borderline Contractile Response
BCR |
|
|
Impaired/Disordered Contractile Response
IDCR |
|
|
Absent Contractile Response
ACR |
|
|
Spastic-Reactive Contractile Response
SRCR |
|
Analysis of esophageal distensibility included measurement of the distensibility plateau (DP) of the esophageal body and measurement of EGJ-distensibility index (DI) and maximum EGJ diameter. For measurement of each of these metrics, areas of the FLIP Panometry output (body and EGJ) that were affected by dry catheter artifact (i.e. artifact that impacts diameter measurement when occlusion of the FLIP balloon disrupts the electrical current utilized for the impedance planimetry technology) were omitted from analysis.12
The DP was measured as the narrowest, fixed diameter (after excluding esophageal contractions) that was observed in response to increasing FLIP volume and pressure. When both the distal and proximal esophageal body were evaluated (n=115), the lower of the two DPs was applied for analysis. A DP threshold of <17mm was applied based on its previously demonstrated association with risk for food impaction; DP <13mm was utilized as a marker of severely reduced distensibility.10
The EGJ-DI was preferentially measured at the 60ml FLIP fill volume and the maximum EGJ diameter during the 60ml or 70ml fill volume when these fill volumes were completed (as previously described).12 If the study protocol was limited to FLIP fill volumes <60ml, the EGJ-DI and maximum EGJ diameter were measured at the greatest fill volume. The EGJ-DI (calculated as EGJ-midline cross-sectional area divided by pressure) was measured at the peaks of EGJ-opening (greatest diameters) that occurred in response to antegrade contractions, and/or to avoid times of lower esophageal sphincter (LES) contraction or crural contractions; the median of three EGJ-DI values was applied for analysis. ‘Normal’ EGJ opening was previously defined as an EGJ-DI >2.0mm2/mmHg and a maximum EGJ-diameter >16mm.12
Statistical Analysis
Summary statistics were reported as mean (standard deviation; SD), or median (interquartile range; IQR) depending on data distribution. Bivariate correlation was assessed using Spearman’s rho. Groups were compared using Chi-square test for categorical variables and ANOVA/t-tests or Kruskal-Wallis/Mann-Whitney U for continuous variables, depending on data distribution. Binary logistic regression was performed utilizing an abnormal CR as the dependent variable. Additionally, stratified analysis was performed related to mucosal eosinophilia and treatment status (i.e., on treatment considered as on PPI, topical steroid, or elimination diet). Statistical tests were two-tailed and significance set at 5%. Post-hoc comparison testing, as appropriate, was completed using a Bonferroni correction.
Results
Patient Characteristics
199 EoE patients (mean (SD) age 38 (12) years; 32% female) were included (Table 2). The median (IQR) symptom (disease) duration was 8 (4–17) years. 155 (78%) patients were on treatment during the time of endoscopy with FLIP: 105 treated only with PPI, 22 on topical steroid (11 also on PPI), and 28 on elimination diet (13 also on PPI). The mean (SD) eosinophil density was 36 (32) eos/hpf and 60 (30%) of patients were in histologic remission (eosinophil density <15 eos/hpf). 74 (37%) of patients had a previous endoscopic dilation with the median (IQR) time between dilation and FLIP being 29 (9–51) months.
Table 2.
Comparison of EoE characteristics between normal and abnormal contractile responses.
| ‘Normal’ CRs | Abnormal CRs | ||
|---|---|---|---|
| n (%) | 133 (67) | 66 (33) | |
| Age, years, mean (SD) | 37 (12) | 41 (12)* | |
| Female, n (%) | 52 (39) | 12 (18)* | |
| Symptom duration, years | 7 (3–15) | 10 (4–23)* | |
| [n (%) completed BEDQ] | [55 (41)] | [30 (45)] | |
| Off treatment, n (%) | 27 (20) | 17 (26) | |
| On PPI, n (%) | 86 (65) | 43 (65) | |
| On topical steroid, n (%) | 15 (11) | 7 (11) | |
| On elimination diet, n (%) | 24 (18) | 6 (9) | |
| Previous dilation, n (%) | 43 (32) | 31 (47)* | |
| Eosinophil count (eos/hpf) | 25 (5–50) | 34 (14–60) | |
| Eosinophil count <15/hpf, n (%) | 44 (33) | 15 (23) | |
| Endoscopic score (EREFS) | |||
| 1 | 87 (65) | 50 (76) | |
| 3 | 1 (1) | 7 (11)* | |
| 2 | 15 (11) | 8 (12) | |
| 1 | 98 (74) | 48 (73) | |
| 1 | 102 (77) | 54 (82) | |
| Total EREFS score | 4 (3–5) | 5 (3–6)* | |
| Total EREFS-Fibrotic score | 2 (1–2) | 3 (2–3)* | |
| Total EREFS-Inflammatory score | 2 (1–3) | 2 (1–3) | |
| FLIP metrics | |||
| Distensibility plateau (mm) | 18 (15–19) | 16 (12–19)* | |
| Distensibility plateau <17mm, n (%) | 43 (32) | 37 (56)* | |
| Distensibility plateau <13mm, n (%) | 7 (5) | 21 (32)* | |
| Pressure, maximum FLIP fill volume (mmHg) | 54 (46–67) | 57 (49–70) | |
| Maximum EGJ diameter (mm) | 16 (14–18) | 13 (12–14)* | |
| ≥16mm | 72 (54) | 10 (15)* | |
| EGJ-Distensiblity Index (DI) (mm2/mmHg) | 3.3 (2.4–4.4) | 1.7 (1.2–2.9)* | |
| ≥2.0 mm2/mmHg | 108 (81) | 27 (41)* | |
Values reported as median (IQR) unless otherwise noted.
P<0.05 on comparison with ‘Normal’ i.e. normal or borderline, contractile response (CR). Abnormal CRs included impaired/disordered (CR), absent CR, or spastic-reactive CR. Brief Esophageal Dysphagia Questionnaire (BEDQ) score completed on day of endoscopy with FLIP.
Secondary peristalsis among the EoE cohort
Antegrade contractions (i.e. secondary peristalsis) were observed on FLIP Panometry in 133 (67%) patients: 68 (34%) had normal CR and 65 (33%) had borderline CR (Table 2; Figure 1). Thus, abnormal contractile response patterns were observed in 66 (33%) patients: 44 (22%) impaired/disordered CR, 16 (8%) absent CR, and 6 (3%) spastic-reactive CR.
HRM was completed in 9 patients: 8 had normal primary peristalsis and CRs among these included 3 (38%) normal CR, 2 (25%) borderline CR, and 3 (38%) impaired-disordered CR. One patient had IEM on HRM and absent CR on FLIP Panometry.
Association of esophageal distensibility with contractile response patterns
Patients with abnormal CRs had lower DPs, lower EGJ-DI, and lower maximum EGJ diameter than patients with normal CRs (normal CR or borderline CR); Table 2. DP differed between the five CR patterns (P=0.002; Figure 2). Reduced DP and severely reduced DP (i.e., DP <17mm and DP<13mm, respectively) were observed least frequently in normal CR (19% and 3%), compared with 69% and 38% of absent CR, and 67% and 50% of spastic-reactive CR (Table S1). Maximum EGJ diameter and EGJ-DI also differed between the CR patterns (Table S1). Normal EGJ opening was observed in 69% of normal CR, 34% borderline BCR, 16% impaired/disordered CR, 0% absent CR, and 17% of spastic-reactive CR (P<0.001); Figure S1.
Figure 2. Distensibility plateau (A) and mucosal eosinophil density (B) related to FLIP Panometry contractile response pattern.
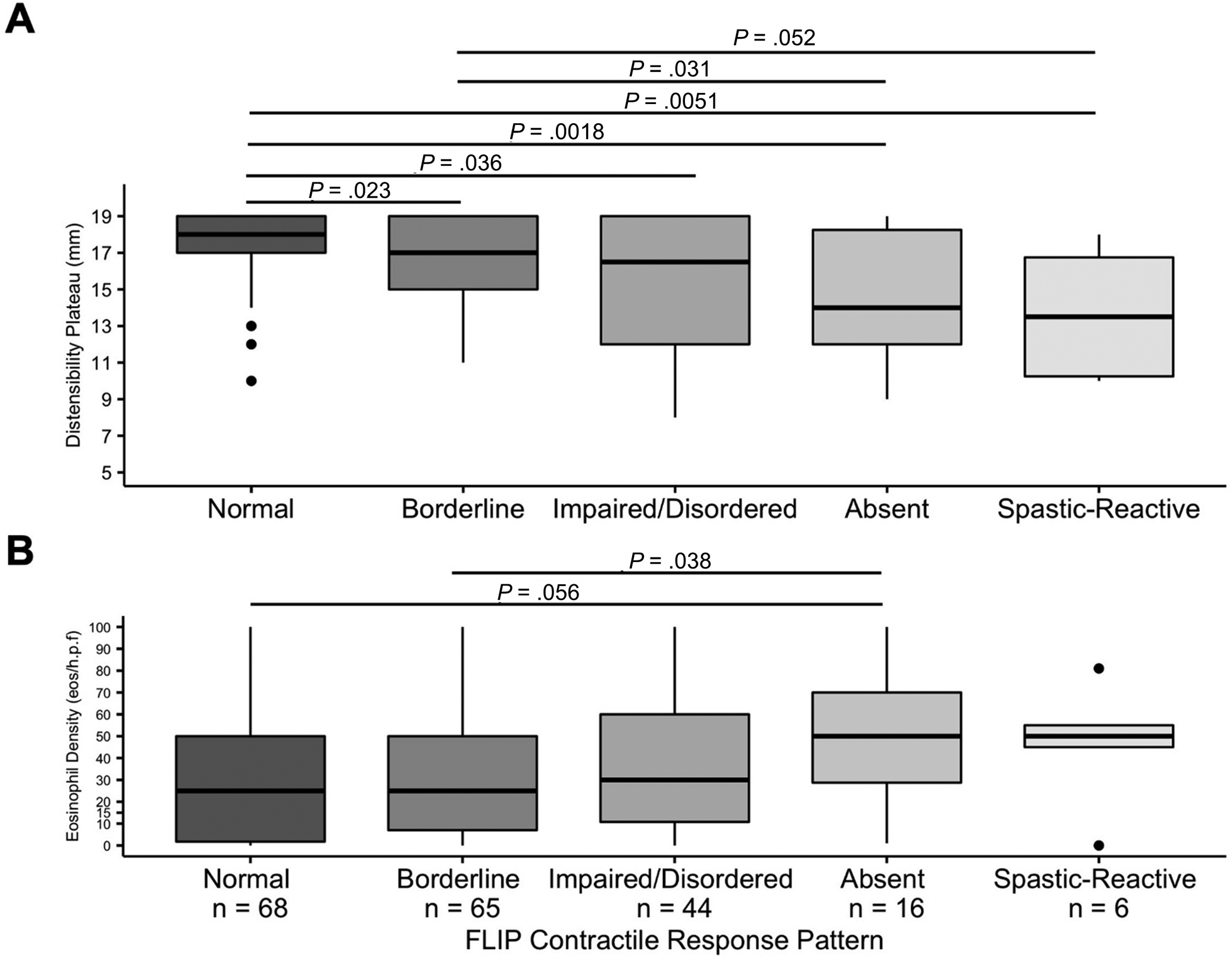
Outlier values are represented by “●”. Unadjusted pairwise comparison results are reported when the unadjusted P<0.1; a Bonferroni correction applied P<0.007 for statistical significance.
DP, EGJ-DI, and maximum EGJ diameter were significantly correlated with each other (rho values 0.385–0.715), as well as with symptom duration, mucosal eosinophil density, and EREFS scores (Table S2).
Association of EoE disease features with contractile response patterns
Compared to patients with normal or borderline CR, EoE patients with abnormal CRs were older, more frequently male, had longer symptom duration, and were more likely to have previously had endoscopic dilation (Table 2). Dysphagia symptom scores (BEDQ) were similar between normal/borderline CR and abnormal CRs. Treatment status (i.e. on versus off PPI, topical steroid, and/or elimination diet) was otherwise similar, as was mucosal eosinophil density and frequency of histologic remission. Endoscopic scores differed with more severe ring scores, greater total EREFS scores, and greater total fibrotic scores occurring in patients with abnormal CRs. However, inflammatory features (edema, exudates, furrows) and total inflammatory score were similar.
Comparisons across the five CR patterns demonstrated differences in age (normal CR younger than impaired/disordered CR) and sex (smaller proportion of males with normal CR); Table S1. There was a trend toward difference in symptom duration (P=0.099) with shorter disease duration in normal CR than impaired/disordered CR (P=0.007). Treatment status did not exhibit statistically significant differences between CR patterns. Absolute mucosal eosinophil density did not statistically differ across five CR patterns, however there were trends toward greater eosinophil density (and less frequent eosinophil density <15 eos/hpf) in absent CR than normal CR or borderline CR (Figure 2). There were also differences in endoscopic ring score, total EREFS score, and total EREFS-fibrotic score (greater scores in absent ACR than normal CR and borderline CR groups). Other endoscopic scores (edema, furrows, exudate, and stricture) and total inflammatory score did not differ among the five CRs.
Univariable binary logistic regression analysis for prediction of an abnormal CR demonstrated significant associations with age, sex, symptom duration, previous dilation, EREFS-total score, EREFS-fibrotic score, and FLIP parameters of DP, EGJ-DI, and maximum EGJ diameter (Table 3). Mucosal eosinophil density (applying absolute values on a continuum or using a 15 eos/hpf threshold) was not significantly associated with abnormal CR, nor was EREFS-inflammatory score.
Table 3. Associations with abnormal contractile response.
The strength of association among each individual parameter with an abnormal contractile response (CR) was evaluated using univariable binary logistic regression; i.e. abnormal CR (impaired/disordered, absent, or spastic-reactive) was utilized as the dependent variable. hpf – high-powered field. Ref = reference category.
| Univariable | B (SE) | OR (95% CI) | P |
|---|---|---|---|
| Age | 0.03 (0.01) | 1.0 (1.0–1.1) | 0.015 |
| Sex (male) | 1.1 (0.4) | 2.9 (1.4–5.9) | 0.004 |
| Symptom duration | 0.05 (0.02) | 1.1 (1.0–1.1) | 0.002 |
| On treatment | −0.3 (0.4) | 0.7 (0.4–1.5) | 0.383 |
| Previous dilation (Yes) | 0.6 (0.3) | 1.9 (1.0–3.5) | 0.038 |
| Eosinophil count | 0.01 (0.01) | 1.0 (1.0–1.0) | 0.208 |
| Eosinophil count <15/hpf | −0.44 (0.34) | 0.6 (0.3–1.3) | 0.202 |
| EREFS total score | 0.2 (0.1) | 1.3 (1.1–1.5) | 0.005 |
| EREFS Fibrotic score | 0.6 (0.2) | 1.9 (1.3–2.7) | <0.001 |
| EREFS Inflammatory score | 0.2 (0.1) | 1.2 (0.9–1.5) | 0.162 |
| Stricture (Yes) | 0.3 (0.4) | 1.4 (0.7–2.9) | 0.409 |
| Distensibility plateau | −0.2 (0.5) | 0.8 (0.7–0.9) | <0.001 |
| Distensibility plateau <17mm | 1.0 (0.3) | 2.7 (1.5–4.9) | 0.001 |
| Distensibility plateau <13mm | 2.1 (0.5) | 8.4 (3.3–21.1) | <0.001 |
| EGJ-distensibility index (DI) | −0.5 (0.1) | 0.6 (0.5–0.7) | <0.001 |
| EGJ-DI <2.0 mm 2 /mmHg | 1.8 (0.3) | 6.2 (3.2–12.0) | <0.001 |
| Maximum EGJ diameter (FLIP) | −0.4 (0.1) | 0.7 (0.6–0.8) | <0.001 |
| >=16mm | ref | ref | |
| FLIP Pressure (greatest fill volume) | 0.013 (0.01) | 1.0 (1.0–1.10) | 0.127 |
Finally, stratified analysis was performed to assess impact of DP on CR relative to mucosal eosinophilia or treatment status (Table 4). This demonstrated that abnormal DP (i.e. DP <17mm) remained more frequent among abnormal CRs than with normal CRs in patients with active eosinophilia and in patients off of treatment. Numeric trends were instead observed among patients in histologic remission and on treatment.
Table 4. Stratified analysis of esophageal body distensibility and contractile response (CR) to distension.
Frequency of abnormal distensibility plateau (DP) was compared between ‘normal’ CRs (i.e. normal CR or borderline CR) and abnormal CRs (impaired/disordered, absent, or spastic-reactive) after stratifying by eosinophil (eos) count or treatment status. hpf – high-powered field.
| DP | ‘Normal’ CRs | Abnormal CRs | P-value | |
|---|---|---|---|---|
| Eosinophilia, mucosal | ||||
| <15 eos/hpf; n=60; n (%) | DP <17mm | 9 (15) | 7 (12) | 0.100 |
| DP ≥17mm | 35 (58) | 9 (15) | ||
| >15 eos/hpf; n=139; n (%) | DP <17mm | 34 (25) | 30 (22) | 0.021 |
| DP ≥17mm | 55 (40) | 20 (14) | ||
| Treatment status | ||||
| On Treatment; n=155; n (%) | DP <17mm | 35 (23) | 24 (16) | 0.075 |
| DP ≥17mm | 71 (46) | 25 (16) | ||
| Not on treatment; n=44; n (%) | DP <17mm | 8 (18) | 13 (30) | 0.005 |
| DP ≥17mm | 19 (43) | 4 (9) |
Discussion
The main findings of this study was that while the majority of patients with EoE had evidence of normal or borderline secondary peristalsis, abnormal CRs (seen in 33% of this EoE cohort) were related to features of fibrostenotic remodeling, such as reduced esophageal distensibility and endoscopically evident rings. Among the 66 patients with abnormal CRs in this cohort, 56% had DPs < 17mm and 52% had endoscopic rings scores (per EREFS) of 2 or 3, compared with 32% and 23% (respectively) of those with normal or borderline CR. However, inflammatory features of EoE such as mucosal eosinophil density or inflammatory endoscopic findings (furrows, exudates), were not directly associated with abnormal CRs. This suggests that esophageal dysmotility in EoE is primarily associated with esophageal remodeling, as opposed to eosinophilic inflammation.
While this study is the first to evaluate secondary peristalsis in EoE, previous studies have evaluated primary peristalsis and swallow associated esophageal motility using manometry in EoE. In a previous study describing HRM diagnoses in 48 patients with EoE, 96% had a diagnoses equivalent to normal motility or IEM (extrapolating to updated HRM criteria from Chicago Classification v4.0), while none of these patients had achalasia.7, 14 However, in another study of 109 patients with EoE, while 84% had normal motility or IEM, they also reported achalasia (subtypes I, II, and III) in 8 patients (7%), 50% of whom were ultimately treated with achalasia-type therapies.4 Although only a small portion of the patients in the present study completed HRM (as our typical practice is typically only to obtain HRM if symptoms persist despite EoE treatment), none had major motility disorders on HRM. However, it can also be noted that the distribution of contractile response patterns in this EoE cohort was similar to what we recently described among a cohort of 164 symptomatic patients (none with EoE) with normal esophageal motility on HRM: 43% had normal CR, 34% borderline CR, 12% impaired/disordered CR, 3% absent CR, and 8% spastic-reactive CR.11 Another consideration is that secondary peristalsis and primary peristalsis may differ among patients, as was demonstrated in previous studies using manometry and eliciting secondary peristalsis with focal balloon distension.19
This study demonstrated that various factors associated with EoE disease severity were related to abnormal secondary peristalsis. Principal among these were EoE physiomarkers indicative of fibrostenotic change, such as fibrotic EREFS features and reduced esophageal distensibility.18 Symptom duration (which likely parallels EoE disease duration) and previous treatment with dilation also reflect greater EoE disease severity associated with fibrostenotic remodeling; male sex also may be related to more severe fibrostenotic disease.20, 21 The regression analysis demonstrated that metrics of EGJ distensibility exhibited the strongest relationship with abnormal CR. This is consistent with previous physiologic data the demonstrated abnormal peristalsis with experimental models of EGJ outflow obstruction.9 Additionally, we also previously showed that patients with absent or impaired CRs may have diminished EGJ opening dimensions related to lower pressures generated in the FLIP.11, 12 Further, although esophageal eosinophilia was not an independent predictor of abnormal secondary peristalsis, the stratified analysis demonstrated a potential interaction of eosinophilia (and treatments that target reduction in mucosal eosinophilia) with the relationship between abnormal CRs and reduced esophageal distensibility. That is, the impact of reduced DP on abnormal CR appeared mitigated among patients in eosinophilic remission or on treatment, thus suggesting that eosinophilic activity may serve to modulate effects related to fibrostenosis. These findings are not intended to question the basic treatment principles of EoE, but instead to provide insight into potential mechanisms associated with dysmotility in EoE. Ultimately, however, future study evaluating longitudinal treatment responses is essential and is a focus of ongoing efforts.
The clinical impact of this study relates to the application of esophageal motility evaluation in patients with EoE, a patient cohort with high potential for mechanical obstruction. Mechanical obstruction of the esophagus is recognized as a potential cause for reactive motor responses, recognized as spastic or hypercontractile features, and even the potential for development of pseudoachalasia involving absent peristalsis.8, 9, 22 Impaired esophageal motor function may also worsen dysphagia in patients with esophageal strictures. However, while mucosal eosinophilia can be observed in achalasia, it is not common and when present, is typically at low intensity.23 This suggests that mucosal eosinophilia in achalasia may be an epiphenomenon or reactive response, rather than a common causal mechanism.23 That said, achalasia treatments have not been shown to improve the low-level mucosal eosinophilia in achalasia as might be expected if it were a response to esophageal stasis.23 Ultimately, as esophageal motility disorders may exhibit esophageal eosinophilia, motility disorders should be considered if EoE-focused treatments are ineffective. However, the concept that mechanical obstruction can result in reactive and abnormal esophageal motility patterns is fundamental to diagnosis of esophageal motility disorders and is essential to consider in clinical practice. This is particularly relevant before considering irreversible achalasia-type treatments, such as myotomy.
While this study carries strengths related to its comprehensive evaluation of a large cohort of patients with EoE, there are several limitations as well. Even within this sizable EoE cohort, the sample sizes of the most severely abnormal CRs (i.e. absent and spastic-reactive CR) were small, which somewhat limits specific comparisons of these groups. This, however, highlights the infrequency of these motor abnormalities in EoE (which again occurred at similar proportions as observed in patients with normal motility on HRM).12 Also, histologic eosinophilia was defined here with the standard approach using mucosal biopsies, though eosinophilic involvement in muscle may be more impactful in the pathology of esophageal motility disorders (however, muscularis eosinophilia remains relatively rare in muscle samples in achalasia).1, 24 Finally, while treatment status and response were evaluated in this analysis (with heterogeneity of these factors noted among the cohort), the study does represent a cross-sectional assessment of EoE disease status and motor function. Hence, additional study to evaluate longitudinal impact of treatment and clinical outcomes remains essential.
In conclusion, the results of this study evaluating secondary peristalsis in EoE suggest that esophageal dysmotility in EoE is associated with fibrostenotic remodeling and evidence of esophageal obstruction, rather than indices of eosinophilic inflammation. The concept that eosinophilic activity (or associated pathology, e.g. mast cell degranulation) may have direct neuromyogenic effects on esophageal motility remains intriguing and warrants additional study. However, the impact of abnormal esophageal contractility seen with fibrostenotic features in EoE is important to consider in the clinical setting, recognizing that the primary role of manometry is to assess non-obstructive dysphagia.1, 4, 5 Ultimately, additional investigation into the role of eosinophilia on the pathophysiology of achalasia, as well as the pathophysiologic effects of EoE on esophageal motor function, remains necessary.
Supplementary Material
Grant support:
This work was supported by P01 DK117824 (JEP) from the Public Health service and American College of Gastroenterology Junior Faculty Development Award (DAC).
Disclosures:
JEP, PJK, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic Inc.
DAC: Medtronic (Speaking, Consulting); Phathom Pharmaceuticals (Consulting)
NG: Allakos (consulting), Astra-Zenca (consulting), Takeda (speakers bureau), Abbvie (consulting), Sanofi-Regeneron (consulting), Nutricia (consulting), Knopp Pharma (consulting)
IH: Adare/Ellodi (consulting, clinical trial support), Allakos (consulting, clinical trial support), AstraZeneca (consulting, clinical trial support), Celgene/Receptos/Bristol Meyers Squibb (consulting, clinical trial support), Sanofi/Regeneron (consulting, clinical trial support, speaking), and Shire/Takeda (consulting, clinical trial support, speaking); Amgen (consulting), Arena (consulting, clinical trial support), Eli Lilly (consulting), EsoCap (consulting), Gossamer Bio (consulting), Parexel/Calyx (consulting)
PJK: Ironwood (Consulting); Reckitt Benckiser (Consulting), Johnson & Johnson (consulting)
JEP: Sandhill Scientific/Diversatek (Consulting, Speaking, Grant), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking, Consulting, Patent, License), Torax (Speaking, Consulting), Ironwood (Consulting)
CS, SP, JP, DAF, WK: none
References
- 1.Spechler SJ, Konda V, Souza R. Can Eosinophilic Esophagitis Cause Achalasia and Other Esophageal Motility Disorders? Am J Gastroenterol. 2018;113(11):1594–9. [DOI] [PubMed] [Google Scholar]
- 2.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18(4):327–37. [PubMed] [Google Scholar]
- 3.Nakajima N, Sato H, Takahashi K, et al. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil. 2017;29(3). [DOI] [PubMed] [Google Scholar]
- 4.Ghisa M, Laserra G, Marabotto E, et al. Achalasia and Obstructive Motor Disorders Are Not Uncommon in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Nelson M, Zhang X, Genta RM, et al. Lower esophageal sphincter muscle of patients with achalasia exhibits profound mast cell degranulation. Neurogastroenterol Motil. 2021;33(5):e14055. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155(4):1022–33 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23(3):208–14, e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton PR, Brown W, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction: analysis using high-resolution video manometry. Obes Surg. 2009;19(7):905–14. [DOI] [PubMed] [Google Scholar]
- 9.Little AG, Correnti FS, Calleja IJ, et al. Effect of incomplete obstruction on feline esophageal function with a clinical correlation. Surgery. 1986;100(2):430–6. [PubMed] [Google Scholar]
- 10.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11(9):1101–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Baumann AJ, Prescott JE, et al. Validation of secondary peristalsis classification using FLIP panometry in 741 subjects undergoing manometry. Neurogastroenterol Motil. 2021:e14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson DA, Baumann AJ, Donnan EN, Krause A, Kou W, Pandolfino JE. Evaluating esophageal motility beyond primary peristalsis: Assessing esophagogastric junction opening mechanics and secondary peristalsis in patients with normal manometry. Neurogastroenterol Motil. 2021:e14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil. 2016;28(12):1854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil. 2021;33(1):e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31(5):601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol. 2019;17(4):674–81 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol. 2016;111(12):1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–95. [DOI] [PubMed] [Google Scholar]
- 19.Schoeman MN, Holloway RH. Secondary oesophageal peristalsis in patients with non-obstructive dysphagia. Gut. 1994;35(11):1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch KL, Dhalla S, Chedid V, et al. Gender is a determinative factor in the initial clinical presentation of eosinophilic esophagitis. Dis Esophagus. 2016;29(2):174–8. [DOI] [PubMed] [Google Scholar]
- 21.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–6 e1–2. [DOI] [PubMed] [Google Scholar]
- 22.Cruiziat C, Roman S, Robert M, et al. High resolution esophageal manometry evaluation in symptomatic patients after gastric banding for morbid obesity. Dig Liver Dis. 2011;43(2):116–20. [DOI] [PubMed] [Google Scholar]
- 23.Cools-Lartigue J, Chang SY, McKendy K, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2013;26(8):766–75. [DOI] [PubMed] [Google Scholar]
- 24.Sodikoff JB, Lo AA, Shetuni BB, Kahrilas PJ, Yang GY, Pandolfino JE. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28(1):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


