Abstract
Combining blood flow restriction (BFR) with exercise is considered a relevant, helpful method in load-compromised individuals and a viable replacement for traditional heavy-load strength training. BFR exercise may be particularly useful for those unable to withstand high mechanical stresses on joints resulting in skeletal muscle dysfunction, such as patients with chronic kidney disease (CKD). Current literature suggests that BFR training displays similar positive health benefits to exercise training alone for CKD patients, including maintenance of muscle strength, glomerular filtration rate maintenance, uremic parameters, inflammatory profile, redox status, glucose homeostasis, blood pressure adjustments, and low adverse reports. In this review of nine studies in CKD patients, we clarify the potential safety and health effects of exercise training with BFR compared to exercise training alone and recommend insights for future research and practical use. Furthermore, we introduce relevant gaps in this emerging field, providing substantial guidance, critical discussion, and valuable preliminary conclusions in this demographic of patients. However, based on the limited studies in this area, more research is necessary to determine the optimal BFR exercise programming.
Keywords: Chronic kidney disease, Blood flow restriction, Inflammation, Renal function, exercise
INTRODUCTION
Exercise training with blood flow restriction (BFR) is considered a potentially useful method for clinical musculoskeletal rehabilitation as a replacement for traditional heavy load strength training for populations who are unable to withstand high mechanical stressors (Hughes et al., 2017). BFR exercise involves applying external pressure around the muscle using a tourniquet-inflated cuff to the most proximal region of the upper or lower limbs (Patterson et al., 2019). During BFR, there is gradual mechanical compression of the vasculature underneath the cuff, resulting in partial restriction of arterial blood flow to structures distal to the cuff, and diminished venous outflow from under the cuff that is proposed to impede venous return (Patterson et al., 2019). This method can be used during resistance and aerobic exercise as well as applied passively without exercise (Patterson et al., 2019). Longitudinal results indicate that low-load exercise training with BFR can produce greater muscular strength responses than low-load exercise without BFR with comparable gains in muscle mass compared to heavy load exercise (Hughes et al., 2017; Lixandrao et al., 2018).
Those positive musculoskeletal benefits observed in both clinical and healthy populations highlight the need for nephrologists, physiotherapists, and physical education professionals to understand the possible use of this modality on treating functional impairments and the disablement process of patients with chronic kidney disease (CKD) (Centner et al., 2019; Hughes et al., 2017; Lixandrao et al., 2018; Roshanravan et al., 2017). CKD consists of kidney damage and progressive and irreversible loss of kidney function (glomerular, tubular, and endocrine) and for didactic and conceptual purposes, CKD is divided into six functional stages according to the patient’s degree of renal function, with the last stage requiring some renal replacement therapy (KDIGO, 2021). Patients with CKD display considerable strength and muscle mass loss contributing to a high prevalence of frailty, mobility disability, and increased mortality risk, especially in the more advanced stages of the disease (Moorthi and Avin, 2017; Roshanravan et al., 2017).
However, exercise training can ameliorate aerobic capacity, muscle strength, and physical function, health-related quality of life, decrease blood pressure, improve inflammation and oxidative stress biomarkers, and maintain kidney function (Cheema et al., 2014; Heiwe and Jacobson, 2011; Howden et al., 2012; Smart et al., 2013). Current clinical practice guidelines suggest that intermittent compression (e.g., a milder form of BFR) of upper arms veins by tourniquet before arteriovenous fistula (AVF) construction may positively influence the forearm vascular access and enhance quality of life of CKD patients who undergo hemodialysis (Rus et al., 2005a; Rus et al., 2005b). Thus, the beneficial effects of BFR training (e.g., a more targeted and restrictive form of intermittent compression) in CKD patients should also be investigated in relevant cardiovascular dimensions (e.g., resting blood pressure), nutritional measures (e.g., muscle mass), physical fitness (e.g., muscle strength), physical functioning and activity (e.g., walking capacity), systemic inflammation (e.g., serum interleukin 6), and arterial and vein diameters to determine if there exists a dose-response relationship with ischemia, compression and/or exercise under restriction.
Although BFR is practically useful for those with physical impairments, there exist concerns regarding safety and adverse events (e.g., numbness, nausea, hypertension, headache, venous thrombus, deterioration of ischemic heart disease, fainting, tingling, excessive pain, central retinal vein occlusion, and rhabdomyolysis) during and following exercise, particularly for those with comorbidities (da Cunha Nascimento et al., 2020; de Queiros et al., 2021; Noto et al., 2017; Ozawa et al., 2015; Patterson and Brandner, 2018; Wong et al., 2021). The concern is primarily related to the skeletal muscle exercise pressor reflex during BFR exercise (Cristina-Oliveira et al., 2020; Spranger et al., 2015). As a result of the ischemia and stimulation of the afferents in the exercising muscle, potentially exaggerated cardiovascular and hemodynamic responses may occur during exercise exceeding the same exercise performed without BFR and approaching heavy load strength training (Domingos and Polito, 2018). In addition, exercise training with BFR might have deleterious effects on vascular health in those with CKD as they display an increased abundance of vascular alpha-1 adrenergic receptors that contributes to abnormal neurocirculatory control (da Cunha Nascimento et al., 2020; Sprick et al., 2019).
Therefore, the beneficial health effects of exercise training with BFR and safety (e.g., possible adverse effects and a risk stratification tool) for CKD patients remain not fully elucidated. As such, the purpose of this review paper is to examine the evidence available on BFR exercise in patients with CKD to make recommendations about BFR exercise prescription and programming and ascertain relevant safety considerations when implementing it into clinical practice. Importantly this review focuses on clinically relevant outcomes associated with CKD and its complications as previously reported (Cheema et al., 2014; Heiwe and Jacobson, 2011; Howden et al., 2012; Rus et al., 2005a; Rus et al., 2005b; Smart et al., 2013). Furthermore, we introduce relevant gaps in this emerging field, provide substantial guidance, critical discussion, and valuable preliminary conclusions in the area.
EFFECTS OF BFR EXERCISE ON PHYSICAL FITNESS, PHYSICAL FUNCTION, AND NUTRITIONAL MEASURES
CKD can lead to frailty, muscle mass wasting, and sarcopenia, presenting a significant challenge to functional mobility and reductions in quality-of-life measures (Dubey et al., 2021). The inflammatory status and redox state inherent to CKD, nutrient loss during dialysis and the harmful changes in the musculoskeletal system related to natural aging can partially explain the increased protein catabolism present in this condition (Chatzipetrou et al., 2022; Dubey et al., 2021; Sabatino et al., 2021; Workeneh et al., 2021; Yu et al., 2021). Markers of muscle mass, strength, and functional capacity are important predictors of outcomes in this patient population subjected to a catabolic protein-energy wasting (PEW) syndrome and consequently, muscle atrophy (Chatzipetrou et al., 2022; Sabatino et al., 2021). Importantly, assessment of muscle functionality may provide additional diagnostic and prognostic information to clinical outcomes, quality of life, and mortality rates (Wilkinson et al., 2021).
Previous randomized clinical trials compared the effects of 6 months of periodized resistance training with and without BFR 3 days a week in male and female patients with stage 2 CKD with hypertension and diabetes (Corrêa et al., 2021a; Corrêa et al., 2021b). The training program included eight exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl. Fifty percent (50%) of limb occlusion pressure (LOP) was applied for the upper and lower limbs in a continuous application method during all exercise training sessions. Results of the studies indicate similar increases in dynamic and isometric strength (hand grip strength) for upper and lower limbs regardless of the application of BFR.
Another randomized clinical trial compared the effects of 8 weeks of exercise training including tennis ball, dumbbell weights, and handgrip exercise 5 times a week (2 being supervised by the physiotherapist and 3 unsupervised) between the BFR group and exercise training alone (Silva et al., 2021). Male and female patients with stage 4/5 CKD under conservative or dialysis treatment participated in this study. All patients were hypertensive, and the majority were diabetic and presented with cardiovascular disease (CVD). Fifty percent (50%) LOP was applied continuously for the upper limbs during all training sessions. Results demonstrated that the BFR group displayed no superior improvement in isometric muscle strength and arm circumference when compared to group without BFR training with no within group improvements after 8 weeks of training.
In addition, the Barbosa et al. (2018) randomized clinical trial evaluated the effect of 8 weeks of exercise training with and without BFR on handgrip strength in male and females with stage 4/5 CKD. All patients were hypertensive, and the majority were diabetic. The protocol consisted of 5 times per week (2 being supervised by the physiotherapist and 3 unsupervised) of training using tennis balls, dumbbells, and handgrip exercise. Pressure was also applied continuously at 50% LOP and only released following completion of the exercises. The results showed that handgrip strength improved in 81.82% of the control group patients while the BFR group did not show demonstrable increases indicating superiority of conventional exercise approaches.
A previous 12-week randomized clinical trial evaluated the effect of intradialytic exercise training with BFR compared to conventional exercise on muscle strength and walking endurance test in male and female end-stage CKD patients (Cardoso et al., 2020). The protocol consisted of aerobic training using cycle ergometry during hemodialysis sessions 3 times a week. Fifty percent of LOP was applied to the lower limbs continuously during all training session. Results of this study demonstrated no differences for functional capacity (walking distance in 6 min) between groups despite a larger improvement in distance walked in the BFR group with no change in muscle strength in either condition.
The contrasting results between studies demonstrate that progressive and periodized exercise training may be an essential issue to consider with BFR prescription for CKD patients. It is worth mentioning that supervised exercise programs are associated with greater benefits than unsupervised exercise for CKD patients (Heiwe and Jacobson, 2011; Smart et al., 2013). In the included studies in our review, two randomized clinical trials adopted some unsupervised sessions (Barbosa et al., 2018; Smart et al., 2013) while only three studies adopted some form of progressive and periodized training with dynamic and isometric strength increments observed post-intervention (Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021). Furthermore, only one study evaluated the effects of exercise on muscle mass.
Increases in dynamic and isometric strength from BFR training (Corrêa et al., 2021a; Corrêa et al., 2021b) represents an important health outcome for CKD patients. Muscle strength independently predicts the composite renal end points (mortality before commencing long-term dialysis or reaching end-stage renal disease, ESRD) across different stages of CKD (stages 1 to 5) in men and women (Chang et al., 2011). Patients reaching renal end points display lower muscle strength than patients that do not reach renal end points (Chang et al., 2011). From a clinical point of view, current literature suggests that it seems essential to start exercise training as early as possible to obtain strength and functional capacity improvements. Considering this, the use of BFR training for muscle strength maintenance in the initial stages of estimated glomerular filtration rate (eGFR) reduction (G2 stage) could be a reliable entry point to foster functional capacity during the progression of CKD.
For muscle mass, arm circumference is considered a readily available criteria for assessing PEW in CKD patients (Fouque et al., 2008), and the lack of change observed in Silva et al. (2021) might demonstrate that CKD patients are at increased risk for PEW. Causes of PEW include endocrine disorders, vitamin D deficiency, diabetes, nutrient loss during dialysis, production of inflammatory cytokines, oxidative stress, and low nutrient intake (Fouque et al., 2008). Although more common in ESRD patients (Fouque et al., 2008), PEW should also be considered in the initial stages of CKD and exercise-based strategies including BFR should be investigated in future studies to determine optimal application parameters to mitigate PEW.
EFFECTS OF BFR EXERCISE ON CARDIOVASCULAR OUTCOMES AND RENAL INTEGRITY AND FUNCTION
In general, patients with CKD exhibit an elevated cardiovascular risk compared to healthy individuals, manifesting hypertension, coronary artery disease, heart failure, and arrhythmias (Jankowski et al., 2021). The kidney can release hormones, enzymes, and cytokines in response to kidney injury or insufficiency, leading to maladaptive changes in the vasculature and autonomic system (Jankowski et al., 2021). In this respect, CKD mimics the accelerated aging process of the cardiovascular system.
Few randomized clinical trials compared the effect of BFR exercise training with exercise training alone on blood pressure and heart rate variability. The first study compared the effects of 6 months of periodized resistance training with and without BFR 3 days a week in male and female patients with stage two CKD disease (Corrêa et al., 2021a). The training included eight exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl at 50% LOP for both upper and lower limbs. Results displayed similar decreases on clinic and 24-hr ambulatory blood pressure monitoring (systolic and diastolic blood pressure) and an attenuated exercise pressor response for both groups, highlighting potential adaptation to the ischemic stimulus. Using the same protocol as previously stated, de Deus et al. (2021) demonstrated that after 6 months of training, both groups demonstrated higher delta (Δ) values of R–R intervals and standard deviation of N–N interval time series. Besides, only BFR training displayed a higher Δ values of root mean square of successive differences in the N–N intervals. Thus, both interventions improved cardiac autonomic function (quantified by Δ heart rate variability analysis).
From a clinical point of view, current literature suggests starting exercise training as early as possible to obtain blood pressure and heart rate variability improvements. However, the changes in response to short term BFR (<3 months of duration) (Heiwe and Jacobson, 2011) and different periodization schemes (e.g., linear or undulatory) remain largely unknown. Additional studies should be carried out to test these relevant factors since they may be key elements that contribute to new insights into patients’ adaptations. The time-course effects of BFR can have specific relevance to these beneficial health effects. In this context, adherence, adhesion, and dropout reasons for an exercise program should be considered as well.
The randomized clinical trial of Clarkson et al. (2020) evaluated the hemodynamic (heart rate, systolic blood pressure, diastolic blood pressure, and mean arterial pressure) and perceived exertional response to an acute exercise session with BFR in end-stage CKD patients. Patients were examined in three conditions using a cycle ergometer with bilateral BFR - non-BFR exercise during dialysis, BFR exercise off dialysis, and BFR exercise during dialysis. Training consisted of 5 min of warm-up followed by two bouts of 10 min of cycling separated by 20 minutes of rest at 50% LOP. No differences in hemodynamics were observed between groups despite rate of perceived exertion (RPE) being lower in the non-BFR condition (Clarkson et al., 2020). This indicates BFR is perceptually more demanding than the same exercise performed without BFR.
Even though higher RPE and reports of fatigue during BFR training appear to occur to a greater degree compared to similar exercise performed without BFR, it does not appear to interfere with adherence (Silva et al., 2021). There may even be a habituation effect with chronic training. Corrêa et al. (2021a) showed that exercise-induced discomfort decreased compared to baseline after 6 months of continuous BFR training. This study reinforces that perceptual demands may attenuate with repeated use of the technique as previously reported (Clarkson et al., 2017). Of note, a higher number of sessions were attended in the BFR training group compared with the no-BFR exercise group (Corrêa et al., 2021b).
Another strong concern when using BFR during intradialytic exercise is that it does not impact hemodialysis treatment (Clarkson et al., 2020). Dias et al. (2020) demonstrated that exercise training with BFR was effective as exercise alone in improving hemodialysis adequacy (e.g., how well blood is being cleansed), displaying a positive result in single-pool Kt/V-urea, equilibrated Kt/V-urea, urea reduction ratio, and urea rebound. Also, during intradialytic BFR training, the acute effect on dialysis adequacy and ultrafiltration rates were not different from the same patient’s usual care hemodialysis sessions (Clarkson et al., 2020). Considering the long-term effect, Corrêa et al. (2021b) reported no changes after 6 months on creatinine concentration between BFR exercise and exercise alone for stage 2 CKD. Therefore, we consider that these protocols should receive special attention as they could be crucial for rehabilitation purposes and open new avenues for therapeutic interventions.
This important concern regarding BFR’s potential effect on hemodialysis is essential because most published studies with CKD kept the cuff inflated throughout the exercise during all sessions. However, considering that every study used 50% LOP, blood flow is relatively unchanged within this percentage of LOP that is independent of cuff widths, as previously reported (Mouser et al., 2017). In addition, only pressures above diastolic pressure will begin squeezing the major arteries (Mouser et al., 2017). Thus, setting the restriction pressure relative to the cuff being used and the individual ensures that no participant reaches complete arterial occlusion due to unnecessarily high pressure (Mouser et al., 2017). Furthermore, it may be more appropriate to use 50% of LOP for participant safety and comfort, although the acute and chronic effects of other applied pressures remain to be elucidated, especially as BFR is shown in most studies to be no worse than exercise alone.
Last, exercise training with BFR displays similar results compared to exercise training alone in attenuating the decrease of eGFR verified by creatinine levels, and cystatin C (Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022). Therefore, it appears that exercise training with BFR is a promising strategy to control blood pressure, autonomic function, decelerate the decline eGFR, improve dialysis adequacy, and provide great adherence to training in CKD patients. However, significantly more research is needed in this area to make firmer conclusions.
EFFECTS OF BFR EXERCISE ON DIAMETER OF ARTERIES AND VEINS
Activities that improve vessel status before AVF construction have greater applicability for adequate vascular access in ESRD during hemodialysis (Rus et al., 2003; Rus et al., 2005a; Rus et al., 2005b). Maximizing the local vascular function near the AVF appears crucial as a large majority of radiocephalic AVFs fail due to inadequate blood supply, insufficient maturation and/or venous thromboembolism formation (Goh et al., 2016). Strategies including exercise and intermittent compression are frequently integrated to promote vascular health, function and maturation of the AVF to increase the chance of long-term patency (Goh et al., 2016) and reduce healthcare burden (Nordyke et al., 2019).
Research has shown local exercise using handgrip training increases the diameter of the forearm arteries and vessels and endothelium-dependent vasodilation (Rus et al., 2003). Furthermore, clinical practice suggests that intermittent compression of upper arms veins by tourniquet before AVF construction may positively influence the forearm vessels (Rus et al., 2005a; Rus et al., 2005b). To determine the potential additive effects of ischemic compression and local forearm exercise, one 8-month study had patients on nondialysis days use an elastic band or rubber ring and perform intermittent compression of the upper arm veins 6 times per day for 1.5 min (Rus et al., 2005a; Rus et al., 2005b). Patients were instructed that the radial artery pulse was palpable during compression, minimizing the risk of complete ischemic compression of the limb (Rus et al., 2005a; Rus et al., 2005b). Results showed increases in the diameter of the arteries, veins, and improved endothelium-dependent vasodilation (Rus et al., 2005a; Rus et al., 2005b). The increase in venous diameter observed probably resulted from the intermittent increase of forearm venous pressure that led to venous distension (Mouser et al., 2019; Rus et al., 2005b). The observed effect of this long-term intervention presents considerable benefits for CKD patients with suboptimal venous diameters before creating AFV.
The practice of elastic band use and providing that radial artery be palpable during compression appears like the BFR method, where the goal is to partially restrict arterial inflow and occlude venous return (Patterson et al., 2019). Previous studies in other populations have demonstrated that BFR exercise improves vascular endothelial function, vascular conductance, and venous compliance (Mouser et al., 2019; Shimizu et al., 2016) all of which could be beneficial to maintain AVF patency. These results may have valuable insights for CKD patients provided similar responses occur. In the only study to date in CKD patients, Barbosa et al. (2018) demonstrated that BFR exercise compared to exercise alone similarly increased radial artery diameter while only exercise without BFR increased cephalic vein diameter. Thus, the use of BFR to increase venous diameters before creating AFV in CKD patients remains to be determined in future studies.
EFFECTS OF BFR EXERCISE ON BIOCHEMICAL AND MOLECULAR OUTCOMES
Due to the advancements in basic research, many mediators and pathways have been reported in the literature regarding CKD (Torino et al., 2021), but several mechanisms underpinning the benefits of BFR exercise are still up for debate (Pearson and Hussain, 2015; Vogel et al., 2020). A better understanding of the common cellular and molecular mechanisms of CKD may be key to the successful identification of new therapeutic targets and effective nonpharmacological strategies to combat the deleterious effects inherent to CKD (Henaut et al., 2019; Ribeiro et al., 2016; Torino et al., 2021). Thus, a key factor in intervention selection for CKD patients is insights on the mechanisms underlying risk factors associated with disease and comorbidities.
Previous randomized controlled trials evaluated the effects of BFR exercise (6 months) compared to exercise alone in stage 2 CKD patients on biochemical and molecular variables related to inflammation, oxidative stress, metabolic and hormonal responses. Corrêa et al. (2021b) displayed similar benefits of BFR exercise and exercise alone on reducing tumor necrosis factor-alpha (TNF-α), and interleukin-18 (IL-18). Improvements on klotho-fibroblast growth factor 23 (FGF23), interleukin-10 (IL-10), and interleukin-15 (IL-15) were also observed, indicating health status improvement.
In same experimental design, Corrêa et al. (2021a) showed similar benefits of BFR exercise and exercise alone on decreasing vasopressin, asymmetric dimethylarginine (ADMA), F2-isoprostanes, increasing angiotensin 1-17 (Ang 1-17), nitric oxide (NO2-), antioxidant defense, and catalase activity. Altering the concentrations of these markers in this way indicates improvements in metabolism, systemic health, and the vascular system via reductions in inflammation and increased endothelial function.
Using the same experimental design previously reported (Corrêa et al., 2021a; Corrêa et al., 2021b), de Deus et al. (2021) displayed similar benefits of exercise training with BFR and exercise alone on reducing delta (Δ) values of myeloperoxidase (MPO) and increasing Δ values of paraoxonase 1 (PON1). Thus, BFR exercise appears to positively alter concentrations of molecules associated with CVD. These molecular findings reveal promising clinical outcomes considering that both molecules potentially participate in the prospective mortality risk in patients who use dialysis (Ndrepepa, 2019). Furthermore, Deus et al. (2022) demonstrated similar benefits of BFR exercise and exercise alone on improving glucose homeostasis by regulation of hormonal mediators of glucose uptake in circulation (e.g., insulin, irisin, adiponectin, and sirtuin 1). In addition, improvements in inflammation and lipid profile were observed within groups with reductions on C-reactive protein and triglycerides levels. These protective effects are important since individuals with diabetes mellitus are at elevated risk of developing kidney disease secondary to metabolic conditions (Kuo et al., 2019).
In summary, inflammation is regulated (e.g., decrements of TNF-α, C-reactive protein, and IL-18), with increments of anti-inflammatory cytokines (IL-10 and IL-15) and improvements on klotho-FGF23, representing an anti-inflammatory effect and attenuation of renal deterioration. For pathways that influence blood pressure, BFR exercise augments Ang 1-17 and NO2-, reduces vasopressin and oxidative stress (decrements of MPO), and improves antioxidant defense demonstrated by decrements on ADMA, F2-isoprostanes, and PON1. Moreover, improvements in glucose homeostasis were also observed. Notably, these molecular mechanisms contribute toward healthier phenotypes and impact cellular longevity.
However, the pleiotropic effects of BFR and the complexity of molecular responses in the current literature suggest that there is no singular pathway mediating beneficial exercise adaptation. Thus, it is important to emphasize that cellular homeostasis is achieved by a delicate balance between multiple pathways and the players required to carry out complex physiological processes (Henaut et al., 2019; Ribeiro et al., 2016; Torino et al., 2021). Moreover, the crosstalk between the tissues, organs and the physiological systems via molecular, cellular, paracrine, endocrine, and neuronal factors are essential in regulating these molecular networks (Henaut et al., 2019; Ribeiro et al., 2016; Torino et al., 2021).
BFR EXERCISE AND SAFETY CONCERNS
It is important to note that for the significant part of studies with BFR and CKD (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; Deus et al., 2022; Silva et al., 2021), patients presented with hypertension, diabetes mellitus, CVD, and metabolic syndrome. Although concerns related to the safety of BFR in medically compromised populations is an undeniable requisite to mitigate risk, there appears to be limited reporting of adverse events in the CKD literature with BFR exercise. One randomized clinical study observed isolated reports of tingling and fatigue in the upper limb of some patients who underwent BFR exercise; however, these complaints were not sufficient for them to withdraw from the study (Silva et al., 2021). Clarkson et al. (2020) reported one case of exercise-related syncope that occurred with BFR exercise during hemodialysis (systolic and diastolic blood pressure of 88 and 68 mmHg, respectively). However, no prolonged effects of the adverse event occurred, and the participant chose to remain enrolled in the study. In the same study, one additional instance of a participant feeling ‘light-headed’ in recovery was reported (systolic and diastolic blood pressure of 85 and 56 mmHg, respectively) during which ultrafiltration was stopped briefly. However, this was self-resolving, and ultrafiltration resumed within 5 minutes. It is important to note that both patients presented with fluid overload and there was no temporal association with BFR condition.
Furthermore, four studies with BFR and CKD stated no adverse events from training in conventional exercise or with BFR (Barbosa et al., 2018; Cardoso et al., 2020; Corrêa et al., 2021a; Dias et al., 2020), while three did not specifically report adverse events (Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022). Thus, BFR exercise appears to be a potentially viable option for the kidney health professional to incorporate in a resistance or aerobic training exercise program. However, special care should be given to screening relevant comorbidities, using recommended strategies to attenuate intra-exercise discomfort and perceptual exertion, and performing adequate supervision to maximize safety during application.
Further, other relevant BFR-related considerations include performing exercise during the first 2 hr of hemodialysis (e.g., when blood pressure control is best), including regular blood pressure and electrocardiogram (ECG) monitoring during training and considering contraindications resulting in electrolyte imbalances (due to their effect on ECG). Further, care (and likely avoidance of BFR) should be given to those patients who have gained >4 kg of weight since their last dialysis appointment/exercise session, appear unstable on dialysis, have a frequently changing medication regimen, pulmonary congestion or peripheral edema (Smart et al., 2013). Given the current state of evidence, if patients pass the screening process, exercise training with BFR may be a safe and reliable option compared to traditional exercise regimens when performed at 50% LOP as the included studies indicate at worst, BFR is no worse than traditional approaches. A summary of intervention characteristics and outcomes are displayed in Table 1.
Table 1.
Exercise intervention characteristics and outcomes of the studies included in this review
| Study | Type of study | Study groups | Clinical status | Sex (F:M) | Age (yr) | Exercise modality | Exercise protocol | Exercise frequency (day/wk) | Intervention (effects) | Vascular occlusion method | Outcomes | Adverse effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | ||||||||||||
| Barbosa et al. (2018) | RCT | EG+BFR EG |
Stage 4 and 5 CKD; EG+ BFR: 100% HTN, 75% diabetic; EG: 64% HTN, 21.4% diabetic | NR for EG+BFR; 66.7% F, and 71.4% M for EG | 61.33; 60.14 | Physiotherapy | Tennis ball: 6 sets of 10 tennis ball squeezes with 1 min RI with 5 squeezes added each week; Dumbbells: 3 sets of 10 reps of elbow flexion with 1 kg during first 2 weeks, followed by 2 kg for the last 2 weeks of the first month, and 3 kg for the last 4 weeks; Handgrip: 3 sets of 20 contractions with 1 min RI with 40% of the 1-RM | 5 | 8 Weeks | 50% of LOP | ↔ Strength for EG+BFR; ↑ strength for EG; ↑ cephalic vein diameter for EG; ↔ cephalic vein diameter for EG+BFR; ↑ Radial artery diameter for both groups | No | |
| Dias et al. (2020) | CD | EG+BFR EG |
NR, but patients were on HD for more than 3 months | 45.4% M and 63.6% M | 46.6–53.9 | AT | Cycling exercise, 20 min during the first 2 hours of HD with RPE of 12–13 Borg scale (moderate intensity) | - | √ | 50% of LOP | ↑ eKt/V for EG+BFR; ↑ eKt/V for EG; ↑ spKt/V for EG+BFR; ↑ spKt/V for EG; ↓ Urea reduction for EG+BFR; ↓ Urea reduction for EG; ↓ Urea rebound for EG+BFR; ↓ Urea rebound for EG | No | |
| Cardoso et al. (2020) | RCT | EG+BFR EG |
ESRD; EG+ BFR: 26% diabetic; EG: 39% diabetic | 10 F and 9 M for EG+BFR; 9 F and 11 M for EG | 49.4; 59.8 | AT | Cycling exercise, 20 min for 4 hr during HD; Fist 6 week: 60%–63% of maximal HR and 10–11 of RPE; Following 6 weeks 64%–76% of maximal HR and 12–13 of RPE | 3 Times during HD | 12 Weeks | 50% of LOP | ↔ strength for EG+BFR; ↔ strength for EG; ↑ 6 MWT for EG+BFR; ↔ 6MWT for EG | No | |
| Clarkson et al. (2017) | CD | EG+BFR EG |
Stage 5 renal disease; 2 patients were diabetic and 4 HTN | 3 F and 7 M | 61 | AT | Cycling exercise, 5 min of warm-up and cool down followed by 10 min bouts of cycling separated by 20 min of RI; Workload for each 10 min bout of 10 W and 30 W equivalent to low-to-moderate RPE | - | √ | 50% of LOP | ↔ Kt/v, and UF between groups; ↔ HR, SBP, DBP and MAP between groups; ↑ RPE for EG+BFR than EG | Yes | |
| Silva et al. (2021) | RCT | EG+BFR EG |
Stage 4 or 5 CKD; EG+ BFR: 100% HTN, 75% diabetic, 50% with CVD; EG: 64% HTN, 21% diabetic, 14% with CVD | NR | 61.33; 60.14 | Physiotherapy | Tennis ball: 6 sets of 10 tennis ball squeezes with 1 min RI with 5 squeezes added each week; Dumbbells: 3 sets of 10 reps of elbow flexion with 1 kg during first 2 weeks, followed by 2 kg for the last 2 weeks of the first month, and 3 kg for the last 4 weeks; Handgrip: 3 sets of 20 contractions with 1 min RI with 40% of the 1-RM. | 5 | 8 Weeks | 50% of LOP | ↔ Strength and arm circumference for EG+BFR; ↔ Strength and arm circumference for EG | Yes | |
| Corrêa et al. (2021a) | RCT | EG+BFR EG |
Stage 2 CKD; Patients in both groups were HTN (100%) | 10 F and 20 M; 12 F and 18 M | 60; 58 | RT | Exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl; first 2 months with 3 sets of 12 reps at 30% of 1-RM; Next 2 months with 3 sets of 10 reps at 40% of 1-RM; Last 2 months, 3 sets of 8 reps at 50% of 1-RM | 3 | 6 Months | 50% of LOP | ↓ Decelerate the decline in eGFR for both groups; ↓ Exercise pressor response for both groups; ↓ Clinic and ambulatory 24 hr blood pressure for both groups; ↓ Vasopressin for both groups; ↑Ang 1–17 for both groups; ↑ NO2-, antioxidant defense, and catalase activity for both groups; ↓ ADMA and F2-isoprostanes for both groups; ↑ Strength for both groups | No | |
| Corrêa et al. (2021b) | RCT | EG+BFR EG |
Stage 2 CKD; Patients in both groups were HTN (100%) and diabetic (100%) | 10 F and 25 M for EG+BFR; 12 F and 23 M for EG | 58; 58 | RT | Exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl; first 2 months with 1–3 sets of 12 reps at 30% of 1-RM; Next 2 months with 2–3 sets of 10 reps at 40% of 1-RM; Last 2 months, 3 sets of 8 reps at 50% of 1-RM | 3 | 6 Months | 50% of LOP | ↓ Decelerate the decline in eGFR for both groups; ↔ Creatinine concentrations for both groups; ↑ Klotho-FGF23 for both groups; ↓TNF-α for both groups; ↑ IL-10 and IL-15 for both groups; ↓ IL-18 for both groups; ↑ Strength for both groups | NR | |
| de Deus et al. (2021) | RCT | EG+BFR EG |
Stage 2 CKD | NR | 58.06; 58.09 | RT | Exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl; first 2 months with 1–3 sets of 12 reps at 30% of 1-RM; Next 2 months with 2–3 sets of 10 reps at 40% of 1-RM; Last 2 months, 3 sets of 8 reps at 50% of 1-RM | 3 | 6 Months | 50% of LOP | ↓ Decelerate the decline in eGFR for both groups; ↓ Δ values of MPO for both groups; ↑ Δ values of PON1 for both groups; ↑ Δ values of RR and SDNN; ↑ Δ values of RMSSD for EG+BFR; ↑ Δ values of VLF for EG | NR | |
| Deus et al. (2022) | RCT | EG+BFR EG |
Stage 2 CKD; Patients in both groups displayed metabolic syndrome | 10 F and 25 M; 12 F and 23 M | 58.06; 58.09 | RT | Exercises: bench press, seated row, shoulder press, triceps pulley, barbell curls, leg press 45°, leg extension, and leg curl; first 2 months with 1–3 sets of 12 reps at 30% of 1-RM; Next 2 months with 2–3 sets of 10 reps at 40% of 1-RM; Last 2 months, 3 sets of 8 reps at 50% of 1-RM | 3 | 6 Months | 50% of LOP | ↓ Decelerate the decline in eGFR for both groups; ↓ Blood glucose, HbAlc and GTT; ↓ HOMA-IR for both groups; ↑ Irisin for both groups; ↔ Adiponectin for EG; ↑ Adiponectin for EG+BFR; ↑ SIRT-1 for both groups; ↓ Leptin for both groups; ↓ Insulin for both groups; ↓ C-reactive protein for both groups; ↓ Triglycerides for both groups | NR | |
RCT, randomized clinical trial; CD, crossover design; EG, experimental group without BFR; EG+BFR, experimental group with BFR; ESRD, end-stage renal disease; BFR, blood flow restriction; CKD, chronic kidney disease; HTN, hypertensive; CVD, cardiovascular disease; Reps, repetitions; RI, rest interval; RM, repetitions maximum; Min, minutes; HR, heart rate; AE, aerobic training; RT, resistance training; NR, not reported; F, female; M, male; HD, hemodialysis; UF, ultrafiltration rate; eKt/V, equilibrated Kt/V-urea; spKt/V, single-poll Kt/V-urea; RPE, rate of perceived exertion; eGFR, estimated glomerular filtration rate; LOP, limb occlusion pressure; TNF-α, tumor necrosis factor-alpha; IL-18, interleukin-18; FGF23, fibroblast growth factor 23; IL-10, interleukin-10; IL-15, interleukin-15; ADMA, asymmetric dimethylarginine; Ang 1-17, angiotensin 1-17; NO2-, nitric oxide; MPO, myeloperoxidase; PON1, paraoxonase 1; Δ, delta; RR, R–R intervals; SDNN, standard deviation of N–N interval time series; RMSSD, root mean square of successive differences in the N–N intervals; VLF, very low frequency; HbAlc, glycated hemoglobin; GTT, glucose tolerance test; ↓, significant decrease in the mean value (P<0.05); ↔, no significant change in the mean value (P> 0.05); ↑, significant increase in the mean value (P<0.05).
RISK STRATIFICATION FOR BFR EXERCISE IN CKD PATIENTS
A previous report stated that CKD is characterized by multiple risk factors that increase CVD risk (Levey et al., 1998; Provenzano et al., 2019). Traditional risk factors in conjunction with the patient characteristics of CKD can help explain the excessive risk for CVD, as advanced age, hypertension, hyperlipidemia, diabetes, physical inactivity, proteinuria, increased extracellular fluid volume, electrolyte imbalance, anemia, albuminuria, and thrombogenic factors all appear in a greater proportion of CKD patients than the general populace (Ballew and Matsushita, 2018; Levey et al., 1998).
For CKD patients, while exercise is beneficial (Heiwe and Jacobson, 2011; Howden et al., 2012; Johansen, 2005), the risk associated with vigorous exercise is increased and assessment of the individual likelihood of CVD risk should be incorporated before BFR. Individuals with severe CKD (G3b stage with eGFR <30 mL/min/1.73 m2) are classified in the very high cardiovascular risk category (Pelliccia et al., 2021). Clinical evaluation for people with high or very high CVD risk who intend to engage in intensive exercise programs should include a maximal exercise test, risk assessment with a functional imaging test, coronary computed tomography angiography, and carotid or femoral artery ultrasound (Pelliccia et al., 2021).
For patients in ESRD, some critical contraindications to exercise should be considered such as electrolyte abnormalities, recent changes in the ECG, excess of interdialytic weight gain >4 kg since the last dialysis or exercise session, unstable on dialysis treatment, changing medication regime, pulmonary congestion, and peripheral edema (Smart et al., 2013). However, a preliminary CVD risk score, built specially on CKD patients, is highly awaited. While there is insufficient data available utilizing BFR training in CKD patients to make definite recommendations about risk stratification and training protocols to optimize beneficial adaptations, the information gathered from the 9 published BFR studies herein can be used to form the basis for some preliminary recommendations. The exclusion criteria adopted by all studies (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Dias et al., 2020; Silva et al., 2021) in conjunction with contraindications proposed by Smart et al. (2013) appears to be a good initial risk stratification for BFR in CKD patients. Also, clinical acute coronavirus disease 2019 infection was considered because of its deleterious effect on many other organ systems including hepatic, thromboembolism, cardiac, endocrine, neurologic, gastrointestinal, and dermatological (Gupta et al., 2020).
Safety considerations should be a priority before initiating an exercise program with BFR due to high number of comorbidities in CKD populations, heightening the potential for adverse responses during- or following BFR exercise. Nonetheless, if kidney health professionals understand safety concerns, perform a comprehensive screening of the medical history along with a sound physical examination and follow published BFR training application recommendations to mitigate risk, exercise training with BFR can be successfully integrated into clinical practice (Patterson et al., 2019; Smart et al., 2013). Thus, before prescribing BFR training, patients should not present any of the following contraindications or clinical conditions cited in Table 2.
Table 2.
Proposed contraindications before exercise training with blood flow restriction exercise for chronic kidney disease patients
| Hemodynamic instability during hemodialysis over the last month (blood pressure of 180/105 mmHg systolic/diastolic, respectively during hemodialysis) |
| Pre-exercise blood pressure above 160/100 mmHg for systolic/diastolic, respectively before exercise |
| Neurodegenerative diseases |
| Autoimmune diseases (i.e., lupus erythematosus) |
| Human immunodeficiency |
| Symptomatic heart failure |
| History of nephrolithiasis |
| Coagulation or presence of signs of thrombophlebitis |
| Clinical acute coronavirus 2019 disease or other virus infection |
| Surgery within the past 3 months |
| Drug or alcohol abuse |
| Previous diagnosis of coronary artery disease or signs of symptomatic cardiovascular or peripheral vascular disease |
| Severe arrhythmia, angina or cerebrovascular disease |
| D-dimer values not in the normal range (220–500 ng/mL) |
| Admission to an intensive care unit |
| Previous surgery or vascular access in the upper limbs |
| Gains above >4 kg of weight since their last dialysis appointment/exercise session |
| Unstable on dialysis |
| Frequently changing medication regimen |
| Pulmonary congestion or peripheral edema |
Adapted from previous studies (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Dias et al., 2020; Gupta et al., 2020; Silva et al., 2021; Smart et al., 2013).
CLINICAL PRACTICE OF BFR EXERCISE IN CKD PATIENTS
Based on studies included in this review, implementing BFR training into clinical setting requires some basic programming considerations. Table 3 includes some general considerations to theoretically maximize safety with BFR training in CKD patients based on the current limited body of research. However, this consideration should be tempered somewhat because it only refers to exercise-induced changes in physical fitness, physical function, cardiovascular dimensions, systemic inflammation, glucose metabolism, and maintenance of eGFR and not to other potential benefits.
Table 3.
Considerations for blood flow restriction exercise in chronic kidney disease patients
| Programming variables to consider | Recommendation | Notes |
|---|---|---|
| Resistance training | First 2 months with 1–3 sets of 12 reps at 30% of 1-RM; Next 2 months with 2–3 sets of 10 reps at 40% of 1-RM; Last 2 months, 3 sets of 8 reps at 50% of 1-RM | Six months of a periodized all body RT displayed to be a proper method to increase muscle strength, regulate inflammation, glucose homeostasis, decelerate the decline in glomerular filtration rate, attenuate renal deterioration, control blood pressure, autonomic function, and antioxidant defense for stage 2 CKD. |
| Aerobic training | Cycling exercise, 20 min during the first 2 hr of HD. During the 3 first weeks use 60%–63% of maximal HR and 10–11 of RPE, progressing to 64%–76% of maximal HR and 12–13 of RPE. | Kidney health professional should use the following criteria to interrupt BFR training for ESRD patients during HD:
|
| % LOP | Arms: 50% Legs: 50% |
BFR training with 50% of LOP was effective as exercise alone in improving hemodialysis adequacy, comfort, and adherence. Thus, use of a validated doppler vascular device is necessary. |
| Frequency | 2 to 3 x/week | All patients should exercise under the individualized supervision of kidney health professionals. |
RM, repetition maximum; RT, resistance training; HD, hemodialysis; HR, heart rate; RPE, rate of perceived exertion; CKD, chronic kidney disease; ESRD, end-stage renal disease; LOP, limb occlusion pressure; BFR, blood flow restriction.
GAPS IN THE LITERATURE AND FUTURE DIRECTIONS
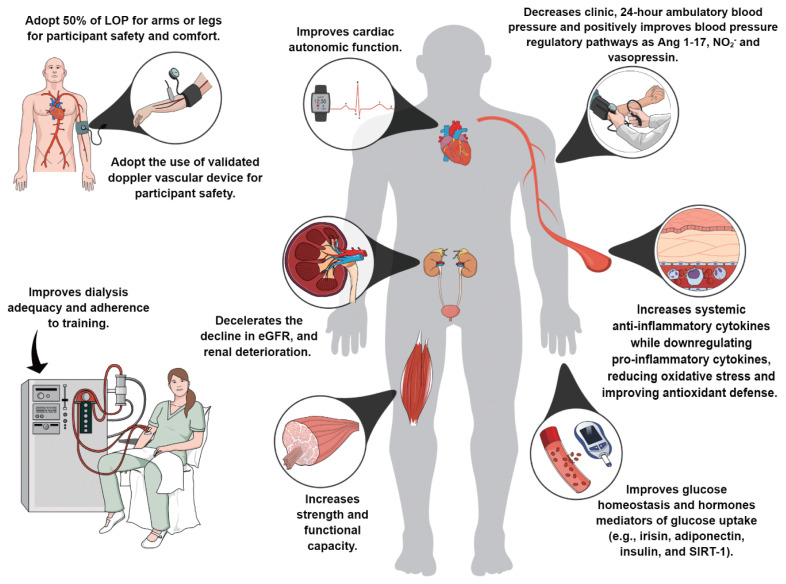
As summarized in Fig. 1, current literature suggests that BFR exercise may improve strength, physical function, blood pressure control, glucose homeostasis, ameliorate autonomic function, decelerate the decline of eGFR, attenuate renal deterioration, improve dialysis adequacy, increase anti-inflammatory cytokines and antioxidant defenses while down-regulating proinflammatory cytokines, oxidative stress (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Dias et al., 2020; Silva et al., 2021).
Fig. 1.
Impact of BFR exercise on health-related outcomes in patients with CKD. The figure was created in the Mind the Graph platform (www.mindthegraph.com). BFR, blood flow restriction; CKD, chronic kidney disease. Adapted from previous studies (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Dias et al., 2020; Silva et al., 2021).
However, considering that few studies till now have evaluated the health benefits of BFR exercise compared with exercise training alone in CKD patients (Barbosa et al., 2018; Cardoso et al., 2020; Clarkson et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Dias et al., 2020), there exists the need for more rigorous randomized controlled trials with reliable outcome measures that will provide better estimations of BFR dosage (intensity, frequency, duration, and modality) for patients with CKD, particularly those with end-stage CKD. However, seven randomized control trials (Barbosa et al., 2018; Cardoso et al., 2020; Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021; Deus et al., 2022; Silva et al., 2021) implemented 8 weeks or longer of regular BFR exercise that induced positive alterations in systemic inflammation, physical fitness, physical functioning, cardiovascular dimensions, glucose metabolism, compliance, and adequate reporting of adverse events. Furthermore, the 6-month experimental design of previous randomized clinical trials provides support for the long-term integration of BFR exercise into an exercise program in those with CKD that could be used in future studies (Corrêa et al., 2021a; Corrêa et al., 2021b; de Deus et al., 2021). In our opinion, an evaluation of clinical outcomes, functional assessments, and concomitant biochemical responses might help to furnish a complete picture of the patient’s adaptation in response to BFR.
There is a need for further research to understand the potential differences between eGFR categories (mL/min/1.73 m2) in response to distinct exercise protocols, the effects of interrupting BFR exercise (e.g., intermittent BFR) or detraining, the effect on muscle mass, muscle quality, AVF, and the impact of lifelong exercise training, not just for a predetermined intervention period. It is also essential to understand whether aerobic or resistance exercise with BFR or a combination of both will provide optimal benefits to CKD patients superior to exercise alone. Most of the available data is derived from studies of patients in stage 2 CKD. More information about the effects of different training programs in other groups of patients with different stages of CKD is needed, including those undergoing hemodialysis or peritoneal dialysis as well as those who are pre-ESRD patients awaiting kidney transplant. Similarly, it is important to know about CKD patient preference regarding BFR exercise so that successful strategies can be implemented, and kidney health professionals can be confident that a reasonable proportion of patients will participate. Finally, the effects of BFR exercise on coagulation, fibrinolysis or hemostasis in CKD patients remains speculative, as studies are not designed to address whether BFR exercise affects coagulation markers (Nascimento et al., 2019).
It has recently been proposed that “exerkines” (peptides, metabolites and RNAs with beneficial effects) released into circulation can be secreted by any organ in response to acute or chronic training protocols (Magliulo et al., 2022). However, to date, studies have evaluated only the systemic adaptations, limiting the understanding of tissue-specific pathways. Since the secretome of exercising skeletal muscle has the power to act through endocrine signaling mediators, spreading specific effects on muscular tissue itself and diverse physiological systems (Magliulo et al., 2022), skeletal muscle biopsy in patients with CKD would be necessary to examine mechanisms at a cellular level. This approach can clarify potential beneficial or adverse effects that are not detectable in the bloodstream. This was previously demonstrated by Nielsen et al. (2020) after muscle tissue sampling.
In response to BFR exercise, clear indications of increases in perivascular membrane properties were observed (Nielsen et al., 2020). Increases in perivascular membrane properties poses a barrier to the diffusion of oxygen and nutritional supply into skeletal muscle fibers (Baum and Bigler, 2016). Notably, perivascular basal membrane thickening has been observed in hypertensive, diabetes mellitus, and chronic inflammation phenotypes (Baum and Bigler, 2016). Of note, none of the included trials in this review implemented a high-frequency exercise protocol (i.e., 1–2 sessions/ day for 3 weeks) similar to Nielsen et al. (2020). A high-frequency resistance exercise protocol may exaggerate vascular stress compared to studies with less frequent training sessions, although that remains speculative. Thus, more in-depth knowledge of the systems-level crosstalk regulation is needed to understand the pathophysiology and complex molecular adaptations in BFR exercise protocols and the magnitude of differences (if any) between exercise alone.
The variability between BFR protocols (duration, intensity, volume, frequency), patient characteristics, and time-points evaluated precluded us from performing a systematic review and meta-analysis, limiting this review to descriptive rather than quantitative comparisons. Future larger, well-designed, and standardized investigations are needed to establish the optimal parameters to modulate beneficial health effects in CKD patients. We believe that this review will pave the way for obtaining critical knowledge in this field. However, given the rapid development of research in this area, annual updates of this review are likely needed to keep pace with the latest findings regarding the relationship between BFR exercise and effectiveness in CKD patients.
CONCLUSIONS
The purpose of this review was to outline the mechanisms through BFR exercise that may improve health outcomes in CKD patients. Discussions in this review may yield clinically useful information and help to effectively design BFR interventions in CKD patients by providing a narrative synthesis of the current state of the BFR literature. However, proposed new exercise modalities for clinical populations need to be safe, tolerable, better, or similarly efficacious than the current alternatives, and BFR exercise appears to not be a limiting mode of exercise for the kidney health professional to utilize in a rehabilitation setting despite current studies indicating it lacks superiority to current management strategies.
ACKNOWLEDGMENTS
The authors received no financial support for this article.
Footnotes
CONFLICT OF INTEREST
NR is the founder of The BFR PROS and teaches BFR training workshops to fitness and rehabilitation practitioners using a variety of BFR training devices around North America and Europe. The other authors declare no potential or actual financial interests that are directly or indirectly related to the work submitted for publication.
REFERENCES
- Ballew SH, Matsushita K. Cardiovascular risk prediction in CKD. Semin Nephrol. 2018;38:208–216. doi: 10.1016/j.semnephrol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Barbosa JB, Maia TO, Alves PS, Bezerra SD, Moura EC, Medeiros AIC, Fuzari LG, Rocha LG, Marinho PE. Does blood flow restriction training increase the diameter of forearm vessels in chronic kidney disease patients? a randomized clinical trial. J Vasc Access. 2018;19:626–633. doi: 10.1177/1129729818768179. [DOI] [PubMed] [Google Scholar]
- Baum O, Bigler M. Pericapillary basement membrane thickening in human skeletal muscles. Am J Physiol Heart Circ Physiol. 2016;311:H654–H666. doi: 10.1152/ajpheart.00048.2016. [DOI] [PubMed] [Google Scholar]
- Cardoso RK, Araujo AM, Del Vechio FB, Bohlke M, Barcellos FC, Oses JP, de Freitas MP, Rombaldi AJ. Intradialytic exercise with blood flow restriction is more effective than conventional exercise in improving walking endurance in hemodialysis patients: a randomized controlled trial. Clin Rehabil. 2020;34:91–98. doi: 10.1177/0269215519880235. [DOI] [PubMed] [Google Scholar]
- Centner C, Wiegel P, Gollhofer A, Konig D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med. 2019;49:95–108. doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YT, Wu HL, Guo HR, Cheng YY, Tseng CC, Wang MC, Lin CY, Sung JM. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26:3588–3595. doi: 10.1093/ndt/gfr013. [DOI] [PubMed] [Google Scholar]
- Chatzipetrou V, Begin MJ, Hars M, Trombetti A. Sarcopenia in chronic kidney disease: a scoping review of prevalence, risk factors, association with outcomes, and treatment. Calcif Tissue Int. 2022;110:1–31. doi: 10.1007/s00223-021-00898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema BS, Chan D, Fahey P, Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med. 2014;44:1125–1138. doi: 10.1007/s40279-014-0176-8. [DOI] [PubMed] [Google Scholar]
- Clarkson MJ, Brumby C, Fraser SF, McMahon LP, Bennett PN, Warmington SA. Hemodynamic and perceptual responses to blood flow-restricted exercise among patients undergoing dialysis. Am J Physiol Renal Physiol. 2020;318:F843–850. doi: 10.1152/ajprenal.00576.2019. [DOI] [PubMed] [Google Scholar]
- Clarkson MJ, Conway L, Warmington SA. Blood flow restriction walking and physical function in older adults: a randomized control trial. J Sci Med Sport. 2017;20:1041–1046. doi: 10.1016/j.jsams.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Corrêa HL, Neves RVP, Deus LA, Maia BCH, Maya AT, Tzanno-Martins C, Souza MK, Silva JAB, Haro AS, Costa F, Moraes MR, Simões HG, Prestes J, Stone W, Rosa TS. Low-load resistance training with blood flow restriction prevent renal function decline: the role of the redox balance, angiotensin 1–7 and vasopressin. Physiol Behav. 2021a;230:113295. doi: 10.1016/j.physbeh.2020.113295. [DOI] [PubMed] [Google Scholar]
- Corrêa HL, Neves RVP, Deus LA, Souza MK, Haro AS, Costa F, Silva VL, Santos CAR, Moraes MR, Simões HG, Navalta JW, Prestes J, Rosa TS. Blood flow restriction training blunts chronic kidney disease progression in humans. Med Sci Sports Exerc. 2021b;53:249–257. doi: 10.1249/MSS.0000000000002465. [DOI] [PubMed] [Google Scholar]
- Cristina-Oliveira M, Meireles K, Spranger MD, O’Leary DS, Roschel H, Pecanha T. Clinical safety of blood flow-restricted training? a comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am J Physiol Heart Circ Physiol. 2020;318:H90–H109. doi: 10.1152/ajpheart.00468.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha Nascimento D, Schoenfeld BJ, Prestes J. Potential implications of blood flow restriction exercise on vascular health: a brief review. Sports Med. 2020;50:73–81. doi: 10.1007/s40279-019-01196-5. [DOI] [PubMed] [Google Scholar]
- de Deus LA, Neves RVP, Correa HL, Reis AL, Honorato FS, Silva VL, de Araujo TB, Souza MK, Sousa CV, Simoes HG, Prestes J, Silva Neto LS, Rodrigues Santos CA, Melo GF, Stone WJ, Rosa TS. Improving the prognosis of renal patients: the effects of blood flow-restricted resistance training on redox balance and cardiac autonomic function. Exp Physiol. 2021;106:1099–1109. doi: 10.1113/EP089341. [DOI] [PubMed] [Google Scholar]
- de Queiros VS, Dantas M, Neto GR, da Silva LF, Assis MG, Almeida-Neto PF, Dantas MS, Cabral B. Application and side effects of blood flow restriction technique: a cross-sectional questionnaire survey of professionals. Medicine (Baltimore) 2021;100:e25794. doi: 10.1097/MD.0000000000025794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus LA, Corrêa HL, Neves RVP, Reis AL, Honorato FS, Araújo TB, Souza MK, Haro AS, Silva VL, Barbosa JMDS, Padula IA, Andrade RV, Simões HG, Prestes J, Stone WJ, Melo GF, Rosa TS. Metabolic and hormonal responses to chronic blood-flow restricted resistance training in chronic kidney disease: a randomized trial. Appl Physiol Nutr Metab. 2022;47:183–194. doi: 10.1139/apnm-2021-0409. [DOI] [PubMed] [Google Scholar]
- Dias EC, Orcy R, Antunes MF, Kohn R, Rombaldi AJ, Ribeiro L, Oses JP, Ferreira GD, Araujo AM, Boff IF, BohIke M. Intradialytic exercise with blood flow restriction: something to add to hemodialysis adequacy? Findings from a crossover study. Hemodial Int. 2020;24:71–78. doi: 10.1111/hdi.12793. [DOI] [PubMed] [Google Scholar]
- Domingos E, Polito MD. Blood pressure response between resistance exercise with and without blood flow restriction: a systematic review and meta-analysis. Life Sci. 2018;209:122–131. doi: 10.1016/j.lfs.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Dubey AK, Sahoo J, Vairappan B, Parameswaran S, Ps P. Prevalence and determinants of sarcopenia in Indian patients with chronic kidney disease stage 3 & 4. Osteoporos Sarcopenia. 2021;7:153–158. doi: 10.1016/j.afos.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- Goh MA, Ali JM, Iype S, Pettigrew GJ. Outcomes of primary arteriovenous fistulas in patients older than 70 years. J Vasc Surg. 2016;63:1333–1340. doi: 10.1016/j.jvs.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon M, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011. p. CD003236. [DOI] [PMC free article] [PubMed]
- Henaut L, Grissi M, Brazier F, Assem M, Poirot-Leclercq S, Lenglet G, Boudot C, Avondo C, Boullier A, Choukroun G, Massy A, Kamel S, Chilon JM. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci Rep. 2019;9:6432. doi: 10.1038/s41598-019-42933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden EJ, Fassett RG, Isbel NM, Coombes JS. Exercise training in chronic kidney disease patients. Sports Med. 2012;42:473–488. doi: 10.2165/11630800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51:1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KL. Exercise and chronic kidney disease: current recommendations. Sports Med. 2005;35:485–499. doi: 10.2165/00007256-200535060-00003. [DOI] [PubMed] [Google Scholar]
- KDIGO . KDIGO guidelines focus on topics related to the prevention or management of individuals with kidney diseases [Internet] KDIGO; p. c2021. [cited 2021 Dec 20]. Available from: https://kdigo.org/guidelines/ [Google Scholar]
- Kuo IC, Wu PH, Lin HY, Niu SW, Huang JC, Hung CC, Chiu YW, Chen HC. The association of adiponectin with metabolic syndrome and clinical outcome in patients with non-diabetic chronic kidney disease. PLoS One. 2019;14:e0220158. doi: 10.1371/journal.pone.0220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PWF, Wright JT. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- Lixandrao ME, Ugrinowitsch C, Berton R, Vechin FC, Conceicao MS, Damas F, Libardi CA, Hoschel H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48:361–378. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- Magliulo L, Bondi D, Pini N, Marramiero L, Di Filippo ES. The wonder exerkines-novel insights: a critical state-of-the-art review. Mol Cell Biochem. 2022;477:105–113. doi: 10.1007/s11010-021-04264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26:219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Buckner SL, Counts BR, Loenneke JP. A tale of three cuffs: the hemodynamics of blood flow restriction. Eur J Appl Physiol. 2017;117:1493–1499. doi: 10.1007/s00421-017-3644-7. [DOI] [PubMed] [Google Scholar]
- Mouser JG, Mattocks KT, Buckner SL, Dankel SJ, Jessee MB, Bell ZW, Abe T, Bentley JP, Loenneke JP. High-pressure blood flow restriction with very low load resistance training results in peripheral vascular adaptations similar to heavy resistance training. Physiol Meas. 2019;40:035003. doi: 10.1088/1361-6579/ab0d2a. [DOI] [PubMed] [Google Scholar]
- Nascimento DDC, Petriz B, Oliveira SDC, Vieira DCL, Funghetto SS, Silva AO, Prestes J. Effects of blood flow restriction exercise on hemostasis: a systematic review of randomized and non-randomized trials. Int J Gen Med. 2019;12:91–100. doi: 10.2147/IJGM.S194883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndrepepa G. Myeloperoxidase - a bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. 2019;493:36–51. doi: 10.1016/j.cca.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Nielsen JL, Frandsen U, Jensen KY, Prokhorova TA, Dalgaard LB, Bech RD, Nygaard T, Suetta C, Aagaard P. Skeletal muscle microvascular changes in response to short-term blood flow restricted training-exercise-induced adaptations and signs of perivascular stress. Front Physiol. 2020;11:556. doi: 10.3389/fphys.2020.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordyke RJ, Reichert H, Bylsma LC, Jackson JJ, Gage SM, Fryzek J, Roy-Chaudhury P, Lithgow T. Costs attributable to arteriovenous fistula and arteriovenous graft placements in hemodialysis patients with medicare coverage. Am J Nephrol. 2019;50:320–328. doi: 10.1159/000502507. [DOI] [PubMed] [Google Scholar]
- Noto T, Hashimoto G, Takagi T, Awaya T, Araki T, Shiba M, Lijima R, Hara H, Moroi M, Nakamura M, Sugi K. Paget-Schroetter syndrome resulting from thoracic outlet syndrome and Kaatsu training. Intern Med. 2017;56:2595–2601. doi: 10.2169/internalmedicine.7937-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Koto T, Shinoda H, Tsubota K. Vision loss by central retinal vein occlusion after Kaatsu training: a case report. Medicine (Baltimore) 2015;94:e1515. doi: 10.1097/MD.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SD, Brandner CR. The role of blood flow restriction training for applied practitioners: a questionnaire-based survey. J Sports Sci. 2018;36:123–130. doi: 10.1080/02640414.2017.1284341. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, Abe T, Nielsen JL, Libardi CA, Laurentino G, Neto GR, Brandner C, Martin-Hernandez J, Loenneke JP. Blood flow restriction exercise: considerations of methodology, application, and safety. Front Physiol. 2019;10:533. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Ross-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M, ESC Scientific Document Group 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- Provenzano M, Coppolino G, Faga T, Garofalo C, Serra R, Andreucci M. Epidemiology of cardiovascular risk in chronic kidney disease patients: the real silent killer. Rev Cardiovasc Med. 2019;20:209–220. doi: 10.31083/j.rcm.2019.04.548. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Garrido P, Fernandes J, Vala H, Rocha-Pereira P, Costa E, Belo L, Reis F, Santos-Silva A. Pathological and molecular mechanisms underlying resistance to recombinant human erythropoietin therapy in the remnant kidney rat model of chronic kidney disease associated anemia. Biochimie. 2016;125:150–162. doi: 10.1016/j.biochi.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Roshanravan B, Gamboa J, Wilund K. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis. 2017;69:837–852. doi: 10.1053/j.ajkd.2017.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus R, Ponikvar R, Kenda RB, Buturovic-Ponikvar J. Effects of handgrip training and intermittent compression of upper arm veins on forearm vessels in patients with end-stage renal failure. Ther Apher Dial. 2005a;9:241–244. doi: 10.1111/j.1774-9987.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- Rus RR, Ponikvar R, Kenda RB, Buturovic-Ponikvar J. Effect of intermittent compression of upper arm veins on forearm vessels in patients with end-stage renal disease. Hemodial Int. 2005b;9:275–280. doi: 10.1111/j.1492-7535.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- Rus RR, Ponikvar R, Kenda RB, Buturovic-Ponikvar J. Effect of local physical training on the forearm arteries and veins in patients with end-stage renal disease. Blood Purif. 2003;21:389–394. doi: 10.1159/000073441. [DOI] [PubMed] [Google Scholar]
- Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34:1347–1372. doi: 10.1007/s40620-020-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Matsuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016;116:749–757. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- Silva IB, Barbosa JBN, Araujo AXP, Marinho PEM. Effect of an exercise program with blood flow restriction on the muscular strength of patients with chronic kidney disease: a randomized clinical trial. J Bodyw Mov Ther. 2021;28:187–192. doi: 10.1016/j.jbmt.2021.06.022. [DOI] [PubMed] [Google Scholar]
- Smart NA, Williams AD, Levinger I, Selig S, Howden E, Coombes JS, Fasset RG. Exercise & sports science Australia (ESSA) position statement on exercise and chronic kidney disease. J Sci Med Sport. 2013;16:406–411. doi: 10.1016/j.jsams.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol. 2015;309:H1440–H1452. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprick JD, Morison DL, Stein CM, Li Y, Paranjape S, Fonkoue IT, Da Costa DR, Park J. Vascular alpha1-adrenergic sensitivity is enhanced in chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2019;317:R485–R490. doi: 10.1152/ajpregu.00090.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torino C, Dounousi E, Abd ElHafeez S, Prohic N. Editorial: molecular mechanisms in chronic kidney disease. Front Cell Dev Biol. 2021;9:712834. doi: 10.3389/fcell.2021.712834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Niederer D, Jung G, Troidl K. Exercise-induced vascular adaptations under artificially versus pathologically reduced blood flow: a focus review with special emphasis on arteriogenesis. Cells. 2020;9:333. doi: 10.3390/cells9020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TJ, Miksza J, Yates T, Lightfoot CJ, Baker LA, Watson EL, Zaccardi F, Smith AC. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: a UK Biobank study. J Cachexia Sarcopenia Muscle. 2021;12:586–598. doi: 10.1002/jcsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Song JS, Bell ZW, Yamada Y, Spitz RW, Abe T, Loenneke JP. Blood flow restriction training on resting blood pressure and heart rate: a meta-analysis of the available literature. J Hum Hypertens. 2021. Jun 17, [Epub]. [DOI] [PubMed]
- Workeneh BT, Kalantar-Zadeh K, Moore LW. Progress in the identification and management of protein-energy wasting and sarcopenia in chronic kidney disease. J Ren Nutr. 2021;31:335–339. doi: 10.1053/j.jrn.2021.04.004. [DOI] [PubMed] [Google Scholar]
- Yu MD, Zhang HZ, Zhang Y, Yang SP, Lin M, Zhang YM, Wu JB, Hong FY, Chen WX. Relationship between chronic kidney disease and sarcopenia. Sci Rep. 2021;11:20523. doi: 10.1038/s41598-021-99592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]