Abstract
Metabolomics is the systematic study of all the metabolites present within a biological system, which consists of a mass of molecules, having a variety of physical and chemical properties and existing over an extensive dynamic range in biological samples. Diverse analytical techniques are needed to achieve higher coverage of metabolites. The application of mass spectrometry (MS) in metabolomics has increased exponentially since the discovery and development of electrospray ionization and matrix-assisted laser desorption ionization techniques. Significant advances have also occurred in separation-based MS techniques (gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry, capillary electrophoresis-mass spectrometry, and ion mobility-mass spectrometry), as well as separation-free MS techniques (direct infusion-mass spectrometry, matrix-assisted laser desorption ionization-mass spectrometry, mass spectrometry imaging, and direct analysis in real time mass spectrometry) in the past decades. This review presents a brief overview of the recent advanced MS techniques and their latest applications in metabolomics. The software/websites for MS result analyses are also reviewed.
Metabolomics is the systematic study of all the metabolites present within a biological system, supply functional information and has received extensive attention in the field of life sciences.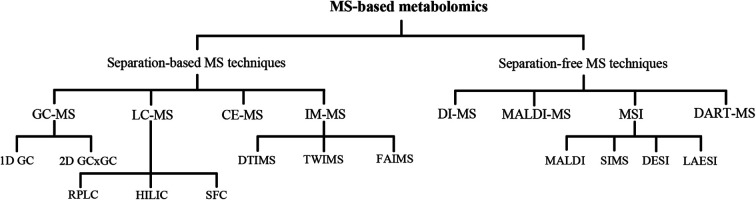
1. Introduction
Metabolomics is a booming omics field arising after genomics, transcriptomics, and proteomics, and it is a vital part of systems biology. Biological fluids (e.g., serum, plasma, urine, saliva, cerebrospinal fluid, bile, amniotic fluid, tears, pancreatic juice, intestinal fluid, and breast milk), human tissues, and cells are the common analysis objects in metabolomics. Metabolites are the end products of complex cellular regulation networks,1 and they can also influence or even alter regulation via feedback loops.2 Through the overall analysis of metabolites, the insightful knowledge of physiological, pathological, and biochemical status can be directly understood, which can then be combined with chemical and informatics methods. Further, endogenous small-molecule metabolic changes can also be determined, and the corresponding biomarkers can be obtained, which can be used to characterize or reveal the functional states for a particular time or environment.3 Thus, metabolomics may provide the “fingerprint” of metabolic systems that can reveal the clinical phenotype in the best possible manner.4 The studies of metabolomics can help us to enhance the understanding of disease mechanisms and drug effects, as well as improve the ability to predict personal disease progression or variation in drug response phenotypes.5–8
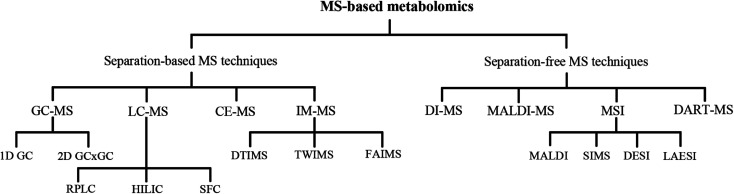
Metabolomics refers to a large number of compounds that belong to multiple categories, such as amino acids, lipids, nucleotides, carbohydrates, and organic acids. The physical and chemical properties of metabolites are diverse and are often distributed over a wide range of concentrations.9 Consequently, metabolomics research is a challenge in analytical chemistry. The most common analytical techniques used in metabolomics are nuclear magnetic resonance spectroscopy and mass spectrometry (MS).10 Due to the evident superiorities of speed, sensitivity, and wide dynamic range over other techniques, MS has provided new dimensionality to medical research. The applications of MS are rapidly broadening, encompassing molecular and cellular biology. MS-based metabolomics technologies (Fig. 1) aid the analysis of in vivo metabolite changes, understanding of the possible pathogenesis and mechanism of diseases, and identification of potential biomarkers that may improve the diagnosis and treatment of diseases.11
Fig. 1. MS-based technologies currently used for metabolomics. CE: capillary electrophoresis; DART: direct analysis in real time; DESI: desorption electrospray ionization; DI: direct infusion; DTIMS: drift-time ion mobility-mass spectrometry; FAIMS: field asymmetric ion mobility-mass spectrometry; GC: gas chromatography; HILIC: hydrophilic interaction chromatography; IM-MS: ion mobility-mass spectrometry; LAESI: laser ablation electrospray ionization; LC: liquid chromatography; MALDI: matrix-assisted laser desorption ionization; MSI: mass spectrometry imaging; RPLC: reversed-phase liquid chromatography; SFC: supercritical fluid chromatography; SIMS: secondary ion mass spectrometry; TWIMS: traveling-wave ion mobility-mass spectrometry.
The large-scale commercialization of two soft ionization techniques, electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI), has made MS the dominant analytical technique in metabolomics research, and has proven to be particularly helpful in large-scale omics studies.12,13 The development of an ESI source extends the applications of MS, as it offers a conventional method by generating gas-phase solute ions from the solution droplets by solution evaporation.14,15 MALDI is widely used primarily because of the rise of proteomics; in the last decade, MALDI has been extensively used in the detection and quantification of small molecular metabolites.16–18 Mass spectrometry imaging (MSI) opened a new chapter in the intuitive presentation of the metabolites distribution in tissues or cells. Herein, we categorically review the MS-based technologies employed in metabolomics and the corresponding analysis using software/website. Table 1 summarizes the advantages and disadvantages of these MS techniques in order to facilitate research applications.
Advantages and disadvantages of MS-based metabolomics.
| Advantages | Disadvantages | |
|---|---|---|
| GC-MS | Suitable for the detection of volatile metabolites | Unsuitable for non-volatile and thermally unstable metabolites |
| Highly repeated retention times | Sample pre-processing process is tedious and often requires derivatization | |
| Universal database facilitates the identification of the structure | ||
| LC-MS | Simple sample preparation | Ion suppression |
| Can be matched with multiple MS detectors | Metabolites can not be detected without form adduct ions | |
| Wide coverage of detectable metabolites | ||
| CE-MS | Low sample volume required for the detection, especially suitable for precious samples | System stability is less stable than LC-MS, GC-MS |
| Affected by salt in the sample | ||
| IM-MS | Isomers can be distinguished | Determination structural information indirectly from CCS values |
| Fast detection speed | ||
| The three-dimensional structure of metabolites can be determined in dynamic motion | ||
| DI-MS | High-throughput detection of samples | Ion suppression |
| The data processing process is relatively simple | Isomers cannot be distinguished | |
| MALDI-MS | Short sample analysis time | Reproducibility is greatly affected by the matrix and sample processing |
| Low sample consumption | Quantitative analysis is difficult to achieve | |
| High salt tolerance | ||
| MSI | Enables in situ detection and presentated in situ metabolites information | Imaging effect is affected by resolution |
| It takes longer time to complete the detection under the high resolution | ||
| DART-MS | Samples can be analyzed directly without the extraction process | Polar compounds are difficult to ionize |
| Low sample consumption | Ion suppression | |
| Sample analysis cycle was sharply shortened |
2. Separation-based MS techniques
2.1. Gas chromatography-mass spectrometry
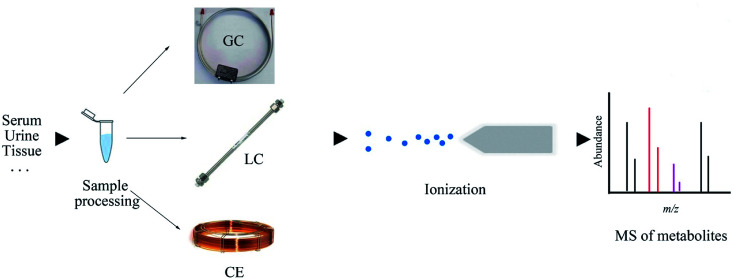
Gas chromatography-mass spectrometry (GC-MS) is the preferred instrument for volatile metabolites analysis, which yields high sensitivity and resolution, prominent reproducibility, and highly repeatable fragmentation. The availability of a spectral library (such as NIST and AMDIS) makes the identification of biomarkers more convenient and helpful for the elucidation of subsequent mechanisms of biology or pathology alterations.19,20 Furthermore, although low cost and ease of operation are its merits, GC-MS usually requires tedious sample processing and derivatization when the analysis targets are nonvolatile constituents. To increase the volatility and thermal stability of the analytes, various derivatizations, such as alkylation, acylation, and silylation, can be employed to “protect” functional groups.1,9 Among these derivatization methods, methoximation and trimethylsilylation are commonly used in large-scale metabolomics studies with GC-MS.21 On account of the difference in the derivatization efficiencies of metabolites, the reproducibility of the overall analysis may be affected.9,22 It should also should be noted that the formation of byproducts and structural transformation in the derivatization process may lead to the misinterpretation of the generated data. The simple scheme of the GC-MS technique is illustrated in Fig. 2.
Fig. 2. Flowchart depicting the basic workflow of GC-MS, LC-MS, and CE-MS techniques.
Zhao et al. developed an automated high-throughput sample derivatization and quantitative method by using the GC-MS platform, and simultaneously measured over one hundred microbial metabolites in human serum, urine, feces, and Escherichia coli cell samples within 15 min for each sample.23 This established method is suitable for future microbiome metabolomics research that require good linearity, reproducibility, and stability. Hadi et al. profiled serum metabolites for the prediction of breast cancer diagnosis, grading, staging, and neoadjuvant status by GC-MS.24 The robust analytical approach highlights the potential of these metabolic features as prognostic factors, thus helping to improve the treatment of breast cancer. Yan et al. performed GC-MS to investigate urinary metabolic alterations related to systemic lupus erythematosus: 70 endogenous metabolites were identified that involved nucleotide metabolism, energy metabolism, oxidative stress, and gut-microbiome-derived metabolism.25 The present study manifested that GC-MS is a promising technique for metabolites analysis and understanding the pathology of diseases.
Since 1991, the use of comprehensive GC (GC × GC) has increased both peak capacity and sensitivity, which aid the identification of metabolites.26–28 In GC × GC, the compounds eluted from the primary column are imported into the secondary column to perform a second independent separation.29 Most of the reported applications of GC × GC are executed using the nonpolar stationary phase in the first dimension of separation and polar stationary phase in the second dimension. This choice is usually made as the elution behavior of the compound class is predictable, and these compounds are subjected to the first-dimension separation according to the different boiling points. Due to the similar boiling points, first-dimension separation fails to isolate the compounds. Therefore, these compounds were separated according to polarity in the second-dimension separation; consequently, the separation power was significantly enhanced.30 Miyazaki et al. examined the temporal changes of serum metabolites in neonatal calves after the first ingestion of colostrum by GC × GC-MS.31 Approximately 1400 calf serum metabolites were detected, the number of which was significantly higher than that of conventional GC-MS. About 180 metabolites were detected in the same serum sample, thus enhancing our understanding of serum metabolites.
2.2. Liquid chromatography-mass spectrometry
Liquid chromatography-mass spectrometry (LC-MS) has become the dominating analytical technique in the field of global metabolite profiling. As compared to GC-MS, the mobile phase changes from gas to liquid, eliminating the need for metabolites to be volatile. Thus, no sample derivatization is required, which indicates that a broader range of analytes can be detected. In 2004, the introduction of commercialized ultra-high-performance LC (UPLC) with sub-2 μm-particles drastically increased the throughput of regular LC methods.32 UPLC usually employs sub-2 μm or 2.6–2.8 μm (fused-core) stationary phase particles at the chromatography column and can withstand high solvent flow rates and high pressures in the range of 6000–19 000 psi, which can reduce the peak width, yield shorter analytical run times, increase the peak capacity, provide better ionization, and reduce mass spectral overlap, leading to improved structural determination and confirmation.33,34 It is reported that the separation of human serum metabolites with UPLC-MS brings about the detection of 20% more metabolites when compared with those detected by HPLC-MS.35 Currently, UPLC-MS has become the mainstay in metabolites separation and identification. The simple scheme of the LC-MS technique is illustrated in Fig. 2.
2.2.1. Reversed-phase liquid chromatography-mass spectrometry
At present, most of the LC-MS-based metabolomics studies have been performed using RPLC column with C18 or C8 materials.36 Reversed-phase liquid chromatography (RPLC) provides an extensive range of selectivity values for the analysis of diverse metabolites, which are highly compatible for the analysis of organisms.37 For RPLC separation, gradient elution is often employed, which starts with highly aqueous and organic contents.38 For the sake of improving the separation efficiency, various buffer modifiers (e.g., formic acid, acetic acid, and ammonium acetate) can be added into the mobile phase to adjust the separation selectivity or detection sensitivity.39
Wang et al. investigated a comprehensive metabolome of jaundice syndrome by RP-UPLC-MS, which established a specific metabolite phenotype and identified 44 potential biomarkers. The disturbance of alanine, aspartate, and glutamate metabolism, as well as the synthesis and degradation of ketone bodies were found in jaundice syndrome patients, which can yield a better understanding of the pathophysiology of the jaundice syndrome, and laid the foundation for the scientific interpretation of the pathology of traditional Chinese medicine syndrome.40 Based on the chinmedomics analysis method, Li et al. analyzed the global constituents of Phellodendri amurensis cortex and serum metabolites by RP-UPLC-MS.41 Herein, 34 different metabolites were found to be related with prostate cancer, and Phellodendri amurensis cortex can observably reverse the abovementioned biomarkers to the normal level, indicating its therapeutic effect. Zhang et al. profiled urine metabolites by RP-UPLC-MS to probe the phenotypic characteristics of nanshi oral liquid, altering the metabolic features of the Kidney-Yang deficiency syndrome.42 The established metabolomics platform characterized the metabolic pathways of the Kidney-Yang deficiency syndrome and evaluated the intervention effect of the nanshi oral liquid against this syndrome, which is conducive to further understanding the pathogenesis of traditional Chinese medicine syndrome, and supplied novel information for studying the pharmacology of traditional Chinese medicine.
However, highly polar and ionic compounds are difficult to retain in RPLC; the analysis of polar compounds is practicable using RPLC by adding ion-pairing agents into the mobile phase by means of ion-pairing liquid chromatography (IPLC).43 The ion-pair reagent is a compound that has an opposite charge when compared with the target metabolites in an aqueous solution.44 As for cation analysis, the ion-pair reagent usually includes hydrochloric acid, perchloric acid, perfluorocarboxylic acids, pentane sulfonic acids, hexane sulfonic acids, heptane sulfonic acids, and octane sulfonic acids, while for anion analysis, it usually includes tetramethylammonium, tetraethylammonium, tetrapropylammonium, tetrabutylammonium, tributylamine, and hexylamine.45 Guo et al. exploited the IP-RP-UHPLC-MS method to separate and analyze biochemical-related metabolites that contain phosphate and carboxylic acid in cellular metabolism.46 Diisopropylethylamine and hexafluoroisopropanol were used as the IP reagents, which enhanced the sensitivity and chromatographic separation, thus assisting in the precise analysis of metabolites in cell center energy metabolism and proving the in-depth knowledge of mechanisms related to metabolic adaptations. Though the usage of an ion-pair reagent is helpful, the remaining unpaired reagent may contaminate the ion source that may influence the sensitivity or reproducibility of LC-MS.44,47
2.2.2. Hydrophilic interaction chromatography-mass spectrometry
Hydrophilic interaction chromatography (HILIC) utilizes a polar stationary phase and a high proportion of organic mobile phase, which usually requires at least 3% water, followed by increasing the percentage of water, as the eluent.9,48 Contrary to RPLC, in HILIC, the more lipophilic compounds are barely attracted to the stationary phase and get rapidly eluted, whereas the more polar compounds are retained and only eluted when the aqueous phase content increases. HILIC-MS is complementary to RPLC-MS since early eluting analytes in RPLC-MS are usually well retained in HILIC-MS. However, HILIC usually generates a wider peak than RPLC, leading to lower peak capacity and higher dependence on the peak resolution of the mass spectrometer.44 Overall, HILIC-MS analysis provides a broader view for metabolites analysis.
It is well known that the simultaneous quantitative measurements of amino acids, neurotransmitters, purines, and pyrimidines exhibit immense difficulties. Chen et al. established a novel method based on HILIC-MS to target the quantification of the abovementioned metabolites in rat brain and serum, which displayed high selectivity, high throughput, and better chromatographic behavior than existing approaches.49 The results demonstrated that HILIC-MS can monitor a wide range of metabolites. Boelaert et al. developed a HILIC-MS-based metabolomics study in searching for novel potential biomarkers of chronic kidney disease.50 Herein, glycoursodeoxycholic acid, 2-hydroxyethane sulfonate, and pregnenolone sulfate were the newly identified metabolites in uremic retention solutes, which extends the range of metabolites of chronic kidney disease, opening a new perspective for future study. Cífková et al. systematically optimized the separation of acidic lipid classes and other lipid classes by HILIC-MS.51 It is a fairly tough task to separate acidic lipid classes due to the broadening or tailing of the chromatographic peaks by them. The established method obtained a higher number of lipids, providing a basis for the future detailed research on lipids.
2.2.3. Supercritical fluid chromatography-mass spectrometry
A substance having its temperature and pressure above a critical point is defined as a supercritical fluid.52 Supercritical fluid chromatography (SFC) uses supercritical fluids, usually CO2, as the primary mobile phase, which possesses a modest supercritical point (31.1 °C and 7.38 MPa). Moreover, the polarity of supercritical CO2 is similar to that of hexane, which can be adjusted by polar solvents (such as methanol). Its nontoxicity and ease of handling makes CO2 to be often used as the mobile phase.53,54 The properties of low viscosity and high diffusivity of supercritical fluids permits higher flow rates and lower pressure drops than that those encountered in traditional HPLC.55 The use of CO2 makes the SFC environmentally friendly, which requires only a small quantity of organic solvent as the auxiliary solvent. However, this was once considered to be the main reason that restricts SFC in the separation of nonpolar and relatively low polar compounds. At present, SFC is recognized as a suitable means for nonpolar as well as polar compound separation.55,56
Lísa et al. developed a novel analytical strategy for high throughput and comprehensive analysis of lipids by SFC-MS, which enables a quick separation of 30 classes of nonpolar and polar lipids within 6 min.57 Taguchi et al. established a rapid method to target profile bile acids in rat serum with SFC-MS.58 Twenty four bile acids were simultaneously quantified without any solid-phase extraction and complex sample preparation within 13 min. These applications confirm that SFC-MS is an alternative to existing analytical methods for metabolomics research.
2.3. Capillary electrophoresis-mass spectrometry
Electrophoresis is defined as the migration of ions under the influence of a spatially uniform electric field.59 Capillary electrophoresis (CE) requires the application of high voltage to produce an electrophoretic flow of different ionic species in a narrow-bore capillary (20–200 μm i.d.).60 CE-MS is a robust technique for polar and charged metabolite analyses, which is a complementary tool to the chromatographic separation technique.61 Capillary zone electrophoresis, the most common pattern of CE, when combined with MS, initially separates metabolites on the basis of their charge-to-size ratios and then on the basis of the mass-to-charge ratios, thus providing an alternative to LC.62,63 CE is considered to be a new technique among metabolomics and till date, it has not been routinely applied in metabolites profiling due to certain drawbacks, such as poor system stability and easy blockage of narrow capillaries by salt. While CE is a valuable analytical tool for those who have mastered the required skills and knowledge,64 it has some distinct advantages over other instrumental techniques employed for metabolomics: low separation volume, which is particularly suitable for the study of biological fluids in small experimental animals, homogeneous separation environment, easy sample preparation process, and capability of concentrating analytes.62 CE-MS exhibits semblable or even better separation efficiency than GC-MS and LC-MS due to the analysis time. The simple scheme of the CE-MS technique is illustrated in Fig. 2.
Significant sensitivity enhancement of modern MS and optimized methods have transformed CE-MS into a potential tool for metabolomics analysis, and the application of CE-MS in metabolomics has drastically increased in the past decade.65 Onjiko et al. developed “microprobe single-cell CE-ESI-MS” for the in situ analysis of metabolites in single cells in a freely developing vertebrate embryo.66 Only <0.02% of the single cell content can allow the detection of ∼230 different molecules in the positive ion mode, which extends the biological toolbox of the system cell biology and opens a new direction toward understanding normal and impaired developments of cell biology.66 Zeng et al. employed CE-MS to profile metabolites in hepatocellular carcinoma patients, which is helpful to cover polar metabolites. Few studies have used CE-MS to evaluate serum polar metabolites in hepatocellular carcinoma, and this study may provide novel insights into the complicated biological processes in hepatocellular carcinoma.67 Fujii et al. applied CE-MS to detect metabolic changes in the postmortem brain of schizophrenia patients, and the results pointed out that glucose metabolism and proteolysis were altered, revealing that several pathways are changed in the brains of schizophrenia patients.68
2.4. Ion mobility-mass spectrometry
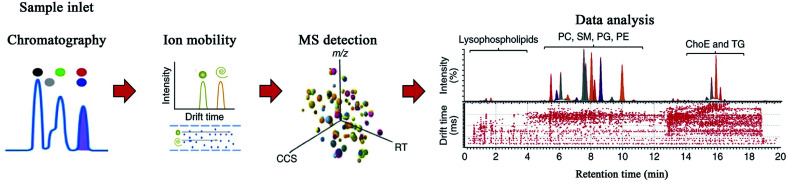
Ion mobility-mass spectrometry (IM-MS), a gas-phase electrophoretic technology, enables the differentiation of ions in the gas phase and electric field on the basis of their size, shape, charge, and mass, and can provide important supplementary information regarding molecular chromatographic separation and ion mass spectrometry separation.69–71 The advantages of IM-MS include distinguishing isobars and isomers, increased signal-to-noise ratio, and determining the conformational state caused by the charge location.72 The separation of IM-MS usually occurs in milliseconds, making it suitable for collaborating with MS, which adds a new dimension in the analysis. The scheme of IM-MS technique is displayed in Fig. 3.
Fig. 3. Scheme of IM-MS technique. Reprinted with permission from ref. 72. Copyright (2017) Nature.
There are three major ion mobility spectrometry (IMS) techniques used in IM-MS: drift-time IMS (DTIMS), traveling-wave IMS (TWIMS), and field asymmetric IMS (FAIMS). DTIMS is the most straightforward method for measuring ion mobility. In the presence of an axial linear electric-field gradient, ions migrate through the buffer gas, thus demonstrating high resolution.73 However, DTIMS suffers from two deficiencies that limit its broad applications: low sensitivity and difficulty in coupling with commercial instruments.74 TWIMS is similar to DTIMS; however, in TWIMS, a sequence of applied voltages engenders a traveling wave that drives the ions through the buffer gas.13 This characteristic facilitates its coupling with the MS platform, greatly enhancing the sensitivity results.75 Since it is difficult to quantify traveling wave voltages, the measurement accuracy of the ion collision cross-section value (CCS: distinct physicochemical property of a compound, with which the compound's molecular structure can be predicted) is the primary disadvantage of TWIMS.76 It is possible for TWIMS to determine CCS accurately, which can be calibrated with the highly accurate CCS obtained from DTIMS.77–79 As the name suggests, FAIMS employs an asymmetric voltage waveform composed of a low- and high-field component to induce ions that can migrate between two fields when they are swept through the flow of gas. Only the stable ions in the applied waveform can pass through FAIMS, thus becoming capable of producing an effective narrow band-pass ion mobility filtering device. When compared with DTIMS and TWIMS, FAIMS operates with high sensitivity when the ions pass through it in a space-dispersive mode by varying the compensation voltage, making it suitable as a commercial technique for several MS instrument platforms.74,80
At present, numerous metabolomics studies using IM-MS have been published. Zhang et al. used ESI IM-MS to study the striatal metabolomics in Parkinson's-like disease for a rat model, and 9 metabolites were selected as potential biomarkers.81 A major discovery of this research was the finding of a dopamine isomer, which has not been reported previously, which was separated using IM-MS. This finding implied that the rangeability of dopamine in Parkinson's disease may be masked by the presence of its isomer. With the maturity of metabolomics, it is found that the function of isomers is not exactly the same, making isomer recognition particularly important. Bowman et al. explored FAIMS-MS to separate lipid isomers (the main differences including sn, chain length, double bond position, and cis/trans), and a success rate of approximately 75% was found in the four main types of glycerolipids and phospholipids.82 This study demonstrates FAIMS-MS as a powerful tool for lipid isomer analysis. Dwivedi et al. profiled human blood by IM-MS with about 1100 metabolites ions detected and 300 isomeric metabolites separated.83 Here, the peaks of low-abundance metabolites that may vanish in MS noise can be distinguished in the mobility space. Furthermore, the peak capacity of IM-MS is enhanced six-fold when compared with individual MS analysis. Thus, IM-MS seems to be a promising analytical technique for assessing metabolomics.
3. Separation-free MS techniques
3.1. Direct infusion-mass spectrometry
Direct infusion-mass spectrometry (DI-MS) is an analysis platform based on the direct injection or infusion of samples into MS, particularly ultra-high-resolution and accurate MS (such as Fourier transform ion cyclotron resonance-MS and Orbitrap-MS), without prior chromatographic or electrophoretic separation, which is clearly advantageous for high-throughput screening.3,84 This feature considerably shortens the total analysis time, thus minimizing the drift of sequence analysis, and improves the repeatability and accuracy among the intersamples.9,85 In addition, subsequent data processing is also simplified because it needs no alignment of the retention time.
Anand et al. detected and confirmed serum lipid biomarkers for preeclampsia using DI-MS, which provides a high throughput method for the analysis of lipid species without a chromatographic separation step.86 It represents a versatile method that requires relatively simple sample preparation and sufficient reproducibility. González-Domínguez et al. analyzed regional metabolic abnormalities in the brains of transgenic APP/PS1 mice with DI-MS; when compared with wild-type control mice, the levels of phospholipids, fatty acids, acylcarnitines, purine and pyrimidine metabolites, sterols, and amino acids in transgenic APP/PS1 mice significantly changed, which is helpful to explore the possible pathogenesis of Alzheimer's disease.87
Although DI-MS can achieve rapid sample analysis, the ion source contamination of sample residue is tedious, and requires a long time to remove. The emergence of chip-based nano-ESI has excellently resolved this problem.88 The nozzle of chip-based nano-ESI is engraved on a silicon board, and the analyzed liquid is passed through the nozzle and sprayed under a relatively gentle pressure.89 Since the requirement of the sample volume is very small and each nozzle can be used only once, the sample residue effect is eliminated. It has been reported that this technique can reduce or even eliminate the differences in the MS response among analytes and adduct ions.90
Zhang et al. employed chip-based direct-infusion nano-electrospray Fourier transform ion cyclotron resonance-mass spectrometry to simultaneously quantify 6 free fatty acids in healthy controls, 5 types of cancer (colorectal, gastric, pancreatic, breast, and lung cancer) patients, and corresponding benign diseases. The results suggest that this platform could provide high sensitivity and high throughput analysis of free fatty acids, and serum unsaturated free fatty acids might have important clinical significance for the early detection of the abovementioned cancers.91–95
Although DI-MS is easy to perform, since it lacks chromatographic or electrophoretic separation capabilities, ion suppression becomes the main obstacle and isomeric compounds cannot be separated.
3.2. Matrix-assisted laser desorption ionization-mass spectrometry
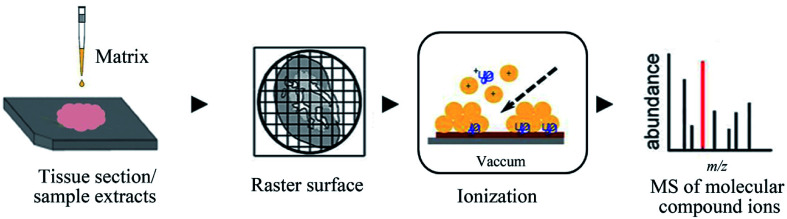
MALDI, another powerful soft ionization source, has become an indispensable analytical tool for complex sample analyses.96–99 This technique provides distinguishing features such as rapid analysis ability, low sample consumption, and relatively high tolerance towards salts.100 The sample analysis time of MALDI-MS is mainly dependent on the amount of laser shots required for the experiment to be suitable for a high-throughput analysis (Fig. 4). In addition, 0.1–1 μL sample or the sample and matrix mixture is deposited on the target plate for MALDI-MS analysis, and only a small fraction of the spotted sample was consumed during the analysis. For LC-MS, usually, 2–20 μL sample volume is infused into the LC, which separates and concentrates the compounds prior to ionization.
Fig. 4. Flowchart depicting the basic workflow of MALDI-MS. Reprinted with permission from ref. 101. Copyright (2018) ACS Publications.
Due to the poor reproducibility of the signal intensities of the analytes, the quantitative analysis of MALDI-MS is not very outstanding; also, the reproducibility of the signal intensities is largely determined by the type of matrices used and sample preparation.102,103 The most commonly used MALDI matrices are α-cyano-4-hydroxycinnamic acid (CHCA) and 2,5-dihydroxybenzoic acid (DHB). The selection of the matrix is also an important aspect that affects the experimental results. MALDI is an essential tool for analyzing macromolecule metabolites, such as proteins, peptides, and nucleic acids. With the discovery of various novel matrices, MALDI can also be used for the analysis of small molecule metabolites in the low mass region. Nanomaterials, such as carbon nanotubes,104 carbon dots,105 graphene,106 and graphene oxide,107 have been extensively developed as effective matrices for the MALDI profiling of smaller molecules.
He et al. adopted MALDI-MS for the in situ determination of the membrane constituents of human mammary epithelial cells and 6 breast cancer cell lines without any extraction and separation steps, thus providing a new rapid method for analyzing membrane lipids.108 Zhang et al. employed an ammonia-treated N-(1-naphthyl)ethylenediamine dihydrochloride (NEDC) as a new matrix for the rapid quantitative and qualitative determination of serum free fatty acids by MALDI-MS. Here, 9 free fatty acids were quantified in 339 serum samples, including healthy controls and non-hyperglycemia and hyperglycemia patients.109 This study indicated that MALDI-MS is not only suitable for metabolomics analysis, but can also quantitatively analyze the contents of metabolites. Ren et al. used graphene oxide as the matrix to rapidly and simultaneously quantify serum non-esterified and esterified fatty acids in 1440 subjects by MALDI-MS.18 Further, 12 non-esterified and esterified fatty acids were quantified within an analysis time of less than 1 min, and the potential biomarker panels presented good diagnostic ability to distinguish benign lung diseases from lung cancer with respect to the clinical uses of carcinoembryonic antigen or cytokeratin 19 fragment antigen 21-1.
3.3. Mass spectrometry imaging
MSI is a powerful tool for determining metabolites in situ, which can simultaneously map multiple molecules, from low-molecular-weight metabolites and drugs to large proteins while simultaneously maintaining the morphological integrity of the analyzed tissue slides.12,17,110 Typically, the spatial information of the biological processes in the tissue was obtained from histology staining, or immunohistochemistry. However, histology staining is non-molecular-specific, and immunohistochemistry requires the knowledge of the target analyte prior to investigation and limited to the simultaneous investigation of only a small number of analytes at any given time.111 Currently, MSI is an emerging technology that can enhance the understanding of the in situ distribution of metabolites or biomarkers directly in tissue sections or cells.
The general workflow of MSI can be briefly summarized as follows: the tissue is cut into thin slices, usually 10–20 μm, and placed on an indium tin oxide-coated slide; then, according to the analysis' requirements, it is selected to either wash the tissue or not and spray an appropriate matrix or not on the basis of the selected ion source; and then, the analysis is performed.112–114 Tissue section is detected according to a predefined raster, which generates mass spectrum at each measurement point; then, the measured information is used to perform image reconstruction, yielding the distribution of molecules in the tissue.115
A variety of ionization methods have been employed for MSI, such as MALDI,17 secondary ion mass spectrometry (SIMS),116,117 desorption electrospray ionization (DESI),118–120 and laser ablation electrospray ionization (LAESI).121,122
3.3.1. MALDI mass spectrometry imaging
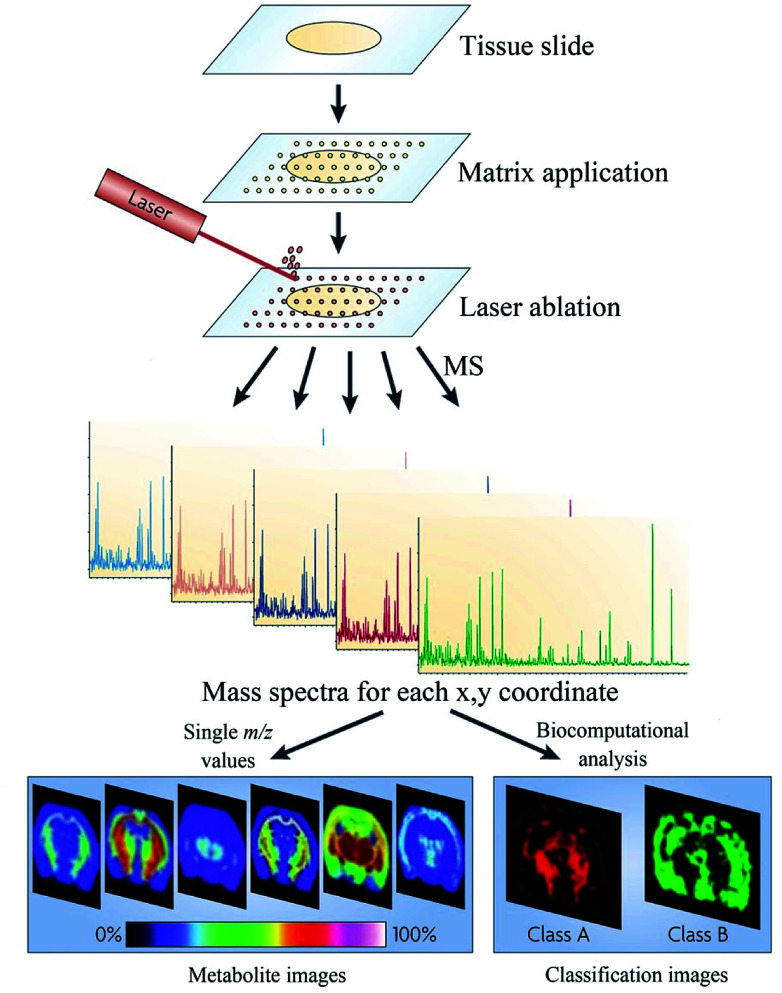
Many techniques are capable of carrying out MSI, among which MALDI is the most commonly used (Fig. 5).112,123,124 MALDI is a so-called “soft” ionization technology, which allows the analysis of metabolites across a wide mass range.126 In MALDI MSI, the laser beam is rastered across the matrix-coated tissue surface to achieve tissue scanning.127 Moreover, the lateral resolution is limited by the diameter of the laser beam, the raster step size, and the diffusion of the biomolecules due to matrix addition.128 One of the most important factors affecting MALDI MSI's performance is the manner in which an appropriate substrate can be added to the tissue sample.129 The selected matrix should form good co-crystals with the tissue biomolecules while maintaining the analytes localization within the tissue, which is critical for good imaging resolution.130
Fig. 5. Flowchart depicting the basic workflow of MALDI MSI. Reprinted with permission from ref. 125. Copyright (2010) Nature.
Guo et al. investigated 134 tissue samples from 6 types of cancer patients by MALDI MSI.17 The analysis of such a large sample size revealed that monosaturated lipids were significantly increased relative to polyunsaturated lipids and the associated enzymes were overexpressed in the tumor microenvironment, which also indicated that the MALDI MSI platform is a powerful tool to study metabolomics and cancer mechanisms. Zhou et al. employed graphene oxide as a matrix to image small molecules in the tissue; here, in the negative ion mode, 212 small molecules were detected and spatially visualized in mouse brain tissues, which reiterated the robust characteristics of MALDI MSI.131
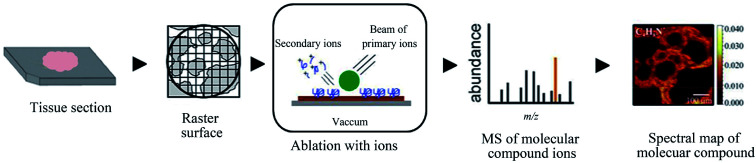
3.3.2. Secondary ion mass spectrometry imaging
Secondary ion mass spectrometry (SIMS), as the “oldest” MSI technique, works by exposing the tissue sample surface to a high-energy primary ion beam (such as Ar+, Ga+, and In+) and then assembling and analyzing the generated secondary ions. This technique currently provides the highest lateral spatial resolution for cellular or subcellular exploration of metabolomics (Fig. 6).12,132 Without applying any matrix, SIMS is capable of acquiring higher spatial resolution than that by MALDI, thus reducing the possibilities of diffusion. However, this usually leads to lower chemical specificity at the expense of molecular fragments.133 Traditionally, SIMS has been the primary method for inorganic surface analysis. However, SIMS has recently undergone a revival in pursuing new chemical information on tissue, single cell, and microbial systems. Phan et al. performed the SIMS of lipids across drosophila brain and obtained a detailed understanding of the biomolecular distribution as well as their chemical structures with high accuracy based on the ion images of its fragments.134 The present study is also useful in studying the relationship between biomolecules and their synthetic precursors or metabolite products.
Fig. 6. Flowchart depicting the basic workflow of SIMS MSI. Reprinted with permission from ref. 101. Copyright (2018) ACS Publications.
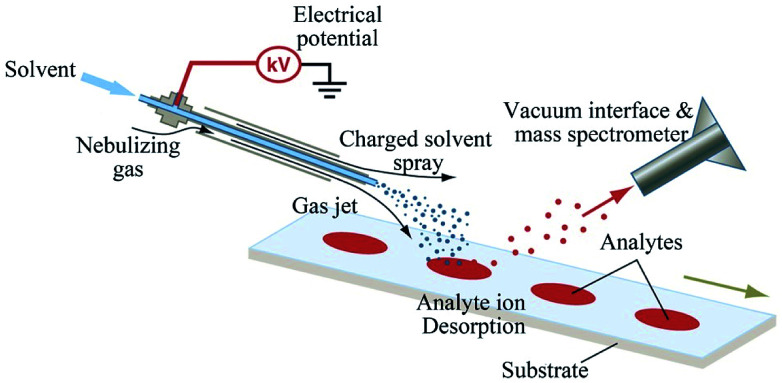
3.3.3. Desorption electrospray ionization mass spectrometry imaging
Desorption electrospray ionization (DESI), an atmospheric pressure ionization technique developed in 2004, is derived from electrospray ionization and desorption ionization. It is performed by conducting electrosprayed charged droplets and solvent ions onto the surface to be analyzed, i.e., DESI relies upon the regional desorption of analyte molecules into the liquid bridge formed between two fused silica capillaries, and the extracted analytes were transferred to spray ionization and mass spectrometer inlet (Fig. 7).121,135 In contrast to MALDI, multicharged ions of molecules (i.e., proteins) may be observed on the spectra of DESI. As a matrix-free technique, DESI is not hampered by matrix-analyte co-crystallization issues and can serve as an alternative platform when compounds cannot be ionized in MALDI.137 With further research, the spatial resolution of DESI has been improved to 10 μm.138 The development of ambient pressure ionization of DESI further expands the applications of MSI.
Fig. 7. Scheme of DESI MSI technique. Reprinted with permission from ref. 136. Copyright (2006) Science.
Jarmusch et al. examined human brain tumors by DESI MSI. A cluster of lower abundance signals were observed, which aided in differentiating brain parenchyma and gliomas.139 This operation can also be performed using tissue smears during intraoperative analyses, which may provide surgeons with near-real-time pathological information and guide the intraoperative resection of tumors whose perimeters are difficult to detect. Lanekoff et al. performed in situ imaging by nano-DESI MSI, obtained efficient separation, and identified difficult-to-isolate isomeric phospholipids in the full scan mode.140 This novel feature opens up exciting opportunities for metabolomics.
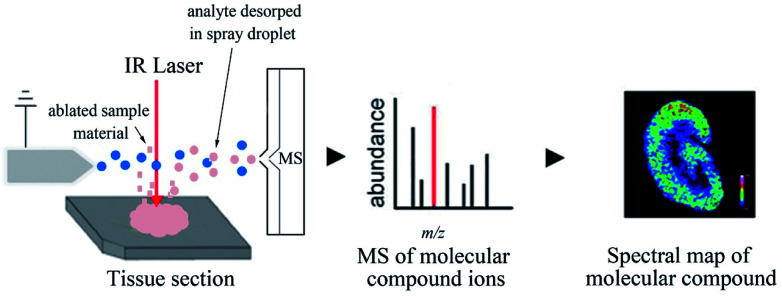
3.3.4. Laser ablation electrospray ionization mass spectrometry imaging
Laser ablation electrospray ionization (LAESI) is a mixed ambient ionization source grounded on mid-infrared laser ablation with charged droplets produced by ESI.141 When rapid micro-ablation is driven by phase explosions, the mixture of molecules and particulate matter is emitted from the samples.142 The molecules are then bonded with the charged droplets generated by the electrospray, and a part of these molecules is transformed into gas-phase ions.143 LAESI is particularly suitable for biological samples with high water content, and it usually employs a focused mid-infrared laser beam of 2940 nm wavelength to excite OH vibrations of water molecules in the sample. Here, the water acts as an “intrinsic matrix” (Fig. 8).144 Li et al. utilized 3D LAESI MSI to characterize the distributions of a wide array of metabolites and lipids in E. coli and B. subtilis model microorganisms interacting with antibiotics in Kirby–Bauer-type experiments. This provided a more in-depth molecular understanding of microbe-antibiotic interactions and improved the quantitative properties of antibiotic susceptibility testing while conspicuously reducing the required incubation time.145
Fig. 8. Flowchart depicting the basic workflow of LAESI MSI. Reprinted with permission from ref. 101. Copyright (2018) ACS Publications.
Nowadays, MSI has evolved from a simple detection technique for different molecules present on various tissue surfaces or cells to a complex, in-depth imaging technique, revealing a mature analytical technology.
3.4. Direct analysis in real time-mass spectrometry
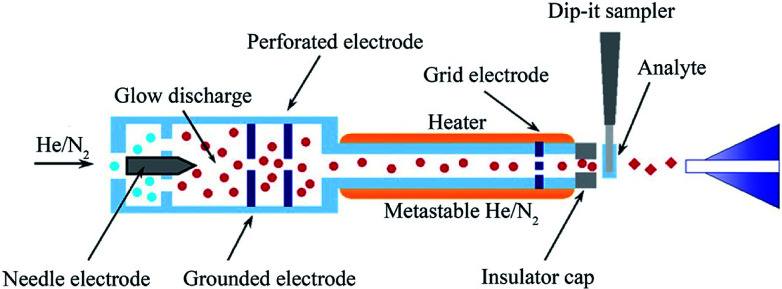
Direct analysis in real time (DART) is another versatile, new ambient ionization technology demonstrated by Cody et al. in 2005, which enables the rapid analyzing of solids, liquids, and gases at atmospheric pressure without sample preparation.146 Helium is conducted via an axial tube and supports a corona discharge that engenders ions, electrons, and excited atoms. Then, helium passes through the other two chambers, where the electrons, cations, and anions are removed, passing into the atmospheric reaction zone that include only electronically excited neutral substances (Fig. 9).147,148 These released atoms in the atmosphere will cause the environmental gas (e.g., atmospheric water or solvent) to undergo gas-phase reaction ionization cascade. Further, these ions serve as chemical ionizing reagents near the surface of the analyzed sample, resulting in analyte ions finally getting transferred to the mass analyzer.147,149 Penning ionization is the most important step in DART ionization, and the flow rate of carrier gas and temperature are the two major factors that affect the DART ionization performance.10 The prominent features of DART include high throughput, minor cross-contamination, and simplicity. In fact, DART is typically used to analyze small molecular compounds with m/z of 50–1200.150
Fig. 9. Scheme of DART MSI technique. Reprinted with permission from ref. 149. Copyright (2011) Elsevier.
From the very beginning, DART was utilized in plant component analysis, vegetable pesticide residue monitoring, forensics, and now gradually used in metabolomics research. Cajka et al. employed DART-MS, where the flow rate of carrier gas and temperature were optimized, as a rapid tool to evaluate the response of dietary supplementation in fish metabolomics.151 Distinguishing carp muscles according to dietary supplementation was practicable when using DART-MS, which represents a powerful analytical technique in metabolomics-based aquaculture research. Zhou et al. reported metabolomics fingerprinting research of human serum by DART-MS.152 In this approach, volatile metabolites were derivatized from protein precipitate, followed by DART-MS analysis. The detection of each sample required only 1.2 min, during which more than 1500 different spectral characteristics were observed. This shows that DART-MS is an invaluable tool for metabolomic fingerprinting. Exploring the changes in human metabolism with DART-MS may provide a better outlook on the amount of biological information.
4. Tools for MS result analysis
There are various open access and commercial software/websites available for MS data processing and analysis (Table 2). These software/websites involve peak alignment, peak extraction, metabolites identification, and metabolic pathway analysis by searching metabolomics databases. The commonly used metabolomics databases include KEGG, HMDB, Metlin, and Massbank. However, there is no currently available standardized software/website for MS data processing and analysis. Using different software, the results will be very different,153–155 which shows that data processing and analysis software/websites have a great influence on data processing. Data processing is a vital part of metabolomics research and each software/website has its own characteristics. Researchers should choose the software/website to process and analyze data according to their own needs. It is believed that with the development of metabolomics methodologies and databases, data processing and analysis software/websites can be continuously enriched and perfected.
MS-based metabolomics data processing and analysis software/websites.
5. Limitations and challenges
MS-based metabolomics technology plays an important role in metabolites profiling due to its high sensitivity, high speed, and high throughput. However, some problems are constantly present in the continuous exploration of this field. First, since the concentration of metabolites in organisms varies greatly and some metabolites with lower concentrations may have important regulatory effects, the detection of trace-level metabolites presents a challenge for MS-based metabolomics. Therefore, the combined use of multiple separation, extraction methods, and MS detectors is imperative. Second, with the continuous improvement of sample processing methods, chromatographic separation capabilities, and MS sensitivity, massive amounts of data can be obtained. Thus, data processing will become another challenge for metabolomics. Third, on account of the different forms of adduct ions, the large amount of data obtained after peak alignment, and the imperfection in the databases and standards, the identification of metabolites is considerably challenging. Fourth, accurate concentration data are roughly relevant to the experimental conditions and data collection methods, which are considered to be comparable among different groups. At present, most MS-based metabolomic studies are limited to the relative quantification of metabolites, which is also regarded as the Achilles heel of metabolomics.156 The constant enrichment of isotopic affinity tags and standards will be conducive to the quantification of metabolites. Last, with the upgrading of the MS instrument, the construction and popularization of the Metabolomics Standards Initiative should be accelerated to achieve data sharing, effective use, and verification of experimental results.157–160
6. Conclusion and future perspective
As a part of systems biology, metabolomics has received extensive attention in the field of life sciences, which represents the final omics level, supplying “functional” information among the omics studies.161–169 The latest advances in novel MS techniques have greatly improved the progression of metabolomics over the past decade (Fig. 10). The high selectivity, flexibility, and lower detection limits of MS along with its compatibility with various separation techniques and the capacity to provide qualitative and quantitative data makes MS an ideal instrument for metabolomics applications. At present, MS not only allows chemical information analysis of the extract content by LC-MS or DI-MS methods, but also can use imaging methods to analyze intact tissue or cells to provide spatial information for the location of given metabolites. In order to achieve better matching with clinical results, the sample processing, data analysis, method validation, and other details need to be standardized. In the future, it is necessary to continually develop new technologies to expand the coverage of metabolites and improve data quality. The measurement sensitivity, spatial resolution, and sample consumption should be improved constantly. Up to now, numerous published studies have demonstrated the promising potential of MS in metabolomics research. As another rapidly growing field of omics, it is expected that there will be more exciting new MS technologies applied in metabolomics in the near future.
Fig. 10. Trends in MS-based metabolomics.
Conflicts of interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
This study was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81430093, 81373930, 81673586, 81302905), National Key Subject of Drug Innovation (Grant No. 2015ZX09101043-005, 2015ZX09101043-011), TCM State Administration Subject of Public Welfare of (Grant No. 2015468004), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118).
References
- Fiehn O. Metabolomics – the link between genotypes and phenotypes. Plant Mol. Biol. 2002;48(1–2):155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- Gruning N. M. Rinnerthaler M. Bluemlein K. et al., Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 2011;14(3):415–427. doi: 10.1016/j.cmet.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. Sun H. Wang X. Urinary metabolic profiling of rat models revealed protective function of scoparone against alcohol induced hepatotoxicity. Sci. Rep. 2014;4:6768. doi: 10.1038/srep06768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Y. Liu J. Cheng X. L. et al., Urinary metabonomics study on biochemical changes in an experimental model of chronic renal failure by adenine based on UPLC Q-TOF/MS. Clin. Chim. Acta. 2012;413(5–6):642–649. doi: 10.1016/j.cca.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Li X. Zhang A. Sun H. et al., Metabolic characterization and pathway analysis of berberine protects against prostate cancer. Oncotarget. 2017;8:65022–65041. doi: 10.18632/oncotarget.17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Zhang A. Sun H. Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma. Hepatology. 2013;57(5):2072–2077. doi: 10.1002/hep.26130. [DOI] [PubMed] [Google Scholar]
- Sun H. Zhang A. Yan G. et al., Metabolomic analysis of key regulatory metabolites in hepatitis C virus-infected tree shrews. Mol. Cell. Proteomics. 2013;12(3):710–719. doi: 10.1074/mcp.M112.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Zhang A. Zhou X. et al., An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Sci. Rep. 2016;6:18997. doi: 10.1038/srep18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. Liu Q. Zhao H. et al., Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Sci. Rep. 2016;6:19333. doi: 10.1038/srep19333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. B. Bailey N. J. Johnson H. E. Measuring the metabolome: current analytical technologies. Analyst. 2005;130(5):606–625. doi: 10.1039/B418288J. [DOI] [PubMed] [Google Scholar]
- Yang H. Lau W. B. Lau B. et al., A mass spectrometric insight into the origins of benign gynecological disorders. Mass Spectrom. Rev. 2017;36(3):450–470. doi: 10.1002/mas.21484. [DOI] [PubMed] [Google Scholar]
- Zhang A. Yan G. Sun H. et al., Deciphering the biological effects of acupuncture treatment modulating multiple metabolism pathways. Sci. Rep. 2016;6:19942. doi: 10.1038/srep19942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Zhang A. Wang L. et al., High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Sci. Rep. 2016;6:38437. doi: 10.1038/srep38437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. Sun H. Wang X. Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spectrom. Rev. 2018;37(3):307–320. doi: 10.1002/mas.21529. [DOI] [PubMed] [Google Scholar]
- Nguyen S. Fenn J. B. Gas-phase ions of solute species from charged droplets of solutions. Proc. Natl. Acad. Sci. U. S. A. 2007;104(4):1111–1117. doi: 10.1073/pnas.0609969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalluge J. J. Matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) Anal. Bioanal. Chem. 2002;372(1):18–19. doi: 10.1007/s00216-001-1151-4. [DOI] [PubMed] [Google Scholar]
- Guo S. Wang Y. Zhou D. et al., Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci. Rep. 2014;4:5959. doi: 10.1038/srep05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. Zhang D. Liu Y. et al., Simultaneous Quantification of Serum Nonesterified and Esterified Fatty Acids as Potential Biomarkers to Differentiate Benign Lung Diseases from Lung Cancer. Sci. Rep. 2016;6:34201. doi: 10.1038/srep34201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridis G. A. Gika H. G. Want E. J. et al., Liquid chromatography-mass spectrometry based global metabolite profiling: a review. Anal. Chim. Acta. 2012;711:7–16. doi: 10.1016/j.aca.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Dagan S. Comparison of gas chromatography-pulsed flame photometric detection-mass spectrometry, automated mass spectral deconvolution and identification system and gas chromatography-tandem mass spectrometry as tools for trace level detection and identification. J. Chromatogr. A. 2000;868(2):229–247. doi: 10.1016/S0021-9673(99)01138-3. [DOI] [PubMed] [Google Scholar]
- Dunn W. B. Broadhurst D. Begley P. et al., Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011;6(7):1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Kanani H. Chrysanthopoulos P. K. Klapa M. I. Standardizing GC-MS metabolomics. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008;871(2):191–201. doi: 10.1016/j.jchromb.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Zhao L. Ni Y. Su M. et al., High Throughput and Quantitative Measurement of Microbial Metabolome by Gas Chromatography/Mass Spectrometry Using Automated Alkyl Chloroformate Derivatization. Anal. Chem. 2017;89(10):5565–5577. doi: 10.1021/acs.analchem.7b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi N. I. Jamal Q. Iqbal A. et al., Serum Metabolomic Profiles for Breast Cancer Diagnosis, Grading and Staging by Gas Chromatography-Mass Spectrometry. Sci. Rep. 2017;7(1):1715. doi: 10.1038/s41598-017-01924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B. Huang J. Dong F. et al., Urinary metabolomic study of systemic lupus erythematosus based on gas chromatography/mass spectrometry. Biomed. Chromatogr. 2016;30(11):1877–1881. doi: 10.1002/bmc.3734. [DOI] [PubMed] [Google Scholar]
- Wong Y. F. Hartmann C. Marriott P. J. Multidimensional gas chromatography methods for bioanalytical research. Bioanalysis. 2014;6(18):2461–2479. doi: 10.4155/bio.14.186. [DOI] [PubMed] [Google Scholar]
- Chin S. T. Marriott P. J. Multidimensional gas chromatography beyond simple volatiles separation. Chem. Commun. 2014;50(64):8819–8833. doi: 10.1039/C4CC02018A. [DOI] [PubMed] [Google Scholar]
- Mostafa A. Edwards M. Gorecki T. Optimization aspects of comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2012;1255:38–55. doi: 10.1016/j.chroma.2012.02.064. [DOI] [PubMed] [Google Scholar]
- Cortes H. J. Winniford B. Luong J. et al., Comprehensive two dimensional gas chromatography review. J. Sep. Sci. 2009;32(5–6):883–904. doi: 10.1002/jssc.200800654. [DOI] [PubMed] [Google Scholar]
- Ryan D. Morrison P. Marriott P. Orthogonality considerations in comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2005;1071(1–2):47–53. doi: 10.1016/j.chroma.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Miyazaki T. Okada K. Yamashita T. et al., Two-dimensional gas chromatography time-of-flight mass spectrometry-based serum metabolic fingerprints of neonatal calves before and after first colostrum ingestion. J. Dairy Sci. 2017;100(6):4354–4364. doi: 10.3168/jds.2017-12557. [DOI] [PubMed] [Google Scholar]
- Wang X. Yang B. Sun H. Zhang A. Pattern recognition approaches and computational systems tools for ultra performance liquid chromatography-mass spectrometry-based comprehensive metabolomic profiling and pathways analysis of biological data sets. Anal. Chem. 2012;84(1):428–439. doi: 10.1021/ac202828r. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Liu P. Li Y. et al., Exploration of metabolite signatures using high-throughput mass spectrometry coupled with multivariate data analysis. RSC Adv. 2017;7(11):6780–6787. doi: 10.1039/C6RA27461G. [DOI] [Google Scholar]
- Zhang A. Sun H. Yan G. et al., Recent developments and emerging trends of mass spectrometry for herbal ingredients analysis. TrAC, Trends Anal. Chem. 2017;94:70–76. doi: 10.1016/j.trac.2017.07.007. [DOI] [Google Scholar]
- Nordstrom A. O'Maille G. Qin C. et al., Nonlinear data alignment for UPLC-MS and HPLC-MS based metabolomics: quantitative analysis of endogenous and exogenous metabolites in human serum. Anal. Chem. 2006;78(10):3289–3295. doi: 10.1021/ac060245f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Li J. Zhang A. H. Urine metabolic phenotypes analysis of extrahepatic cholangiocarcinoma disease using ultra-high performance liquid chromatography-mass spectrometry. RSC Adv. 2016;6(67):63049–63057. doi: 10.1039/C6RA09430A. [DOI] [Google Scholar]
- Liang Q. Liu H. Xing H. et al., UPLC-QTOF/MS based metabolomics reveals metabolic alterations associated with severe sepsis. RSC Adv. 2016;6:43293–43298. doi: 10.1039/C6RA07514B. [DOI] [Google Scholar]
- Want E. J. Wilson I. D. Gika H. et al., Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010;5(6):1005–1018. doi: 10.1038/nprot.2010.50. [DOI] [PubMed] [Google Scholar]
- Liang Q. Liu H. Xing H. et al., Urinary UPLC-MS metabolomics dissecting the underlying mechanisms of huaxian capsule protects against sepsis. RSC Adv. 2016;6:40436–40441. doi: 10.1039/C6RA07987C. [DOI] [Google Scholar]
- Wang X. Zhang A. Han Y. et al., Urine metabolomics analysis for biomarker discovery and detection of jaundice syndrome in patients with liver disease. Mol. Cell. Proteomics. 2012;11(8):370–380. doi: 10.1074/mcp.M111.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. N. Zhang A. Wang M. et al., Screening the active compounds of Phellodendri amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Sci. Rep. 2017;7:46234. doi: 10.1038/srep46234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q. Liu H. Xie L. et al., High-throughput metabolomics enables biomarker discovery in prostate cancer. RSC Adv. 2017;7(5):2587–2593. doi: 10.1039/C6RA25007F. [DOI] [Google Scholar]
- Lu W. Bennett B. D. Rabinowitz J. D. Analytical strategies for LC-MS-based targeted metabolomics. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008;871(2):236–242. doi: 10.1016/j.jchromb.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri S. P. Yamamoto S. Tsugawa H. et al., Current metabolomics: technological advances. J. Biosci. Bioeng. 2013;116(1):9–16. doi: 10.1016/j.jbiosc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Joachim Weiss T. W., Handbook of Ion Chromatography, Wiley-VCH, Weinheim, 3rd edn, 2004 [Google Scholar]
- Guo L. Worth A. J. Mesaros C. et al., Diisopropylethylamine/hexafluoroisopropanol-mediated ion-pairing ultra-high-performance liquid chromatography/mass spectrometry for phosphate and carboxylate metabolite analysis: utility for studying cellular metabolism. Rapid Commun. Mass Spectrom. 2016;30(16):1835–1845. doi: 10.1002/rcm.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher J. M. Czernik D. Ewald J. C. et al., Cross-platform comparison of methods for quantitative metabolomics of primary metabolism. Anal. Chem. 2009;81(6):2135–2143. doi: 10.1021/ac8022857. [DOI] [PubMed] [Google Scholar]
- Tang D. Q. Zou L. Yin X. X. et al., HILIC-MS for metabolomics: an attractive and complementary approach to RPLC-MS. Mass Spectrom. Rev. 2016;35(5):574–600. doi: 10.1002/mas.21445. [DOI] [PubMed] [Google Scholar]
- Chen J. Hou W. Han B. et al., Target-based metabolomics for the quantitative measurement of 37 pathway metabolites in rat brain and serum using hydrophilic interaction ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016;408(10):2527–2542. doi: 10.1007/s00216-016-9352-z. [DOI] [PubMed] [Google Scholar]
- Boelaert J. Lynen F. Glorieux G. et al., Metabolic profiling of human plasma and urine in chronic kidney disease by hydrophilic interaction liquid chromatography coupled with time-of-flight mass spectrometry: a pilot study. Anal. Bioanal. Chem. 2017;409(8):2201–2211. doi: 10.1007/s00216-016-0165-x. [DOI] [PubMed] [Google Scholar]
- Cifkova E. Hajek R. Lisa M. et al., Hydrophilic interaction liquid chromatography-mass spectrometry of (lyso)phosphatidic acids, (lyso)phosphatidylserines and other lipid classes. J. Chromatogr. A. 2016;1439:65–73. doi: 10.1016/j.chroma.2016.01.064. [DOI] [PubMed] [Google Scholar]
- Matsubara A. Fukusaki E. Bamba T. Metabolite analysis by supercritical fluid chromatography. Bioanalysis. 2010;2(1):27–34. doi: 10.4155/bio.09.160. [DOI] [PubMed] [Google Scholar]
- Ikushima Y. S. N. Arai M. Arai K. Solvent polarity parameters of supercritical carbondioxide as measured by infraredspectroscopy. Bull. Chem. Soc. Jpn. 1991;64(7):2224–2229. doi: 10.1246/bcsj.64.2224. [DOI] [Google Scholar]
- Fekete S. Grand-Guillaume Perrenoud A. Guillarme D. Evolution and Current Trends in Liquid and Supercritical Fluid Chromatography. Curr. Chromatogr. 2014;1:15–40. doi: 10.2174/22132406114019990002. [DOI] [Google Scholar]
- Pinkston J. D. Wen D. Morand K. L. et al., Comparison of LC/MS and SFC/MS for screening of a large and diverse library of pharmaceutically relevant compounds. Anal. Chem. 2006;78(21):7467–7472. doi: 10.1021/ac061033l. [DOI] [PubMed] [Google Scholar]
- Taguchi K. Fukusaki E. Bamba T. Supercritical fluid chromatography/mass spectrometry in metabolite analysis. Bioanalysis. 2014;6(12):1679–1689. doi: 10.4155/bio.14.120. [DOI] [PubMed] [Google Scholar]
- Lisa M. Holcapek M. High-Throughput and Comprehensive Lipidomic Analysis Using Ultrahigh-Performance Supercritical Fluid Chromatography-Mass Spectrometry. Anal. Chem. 2015;87(14):7187–7195. doi: 10.1021/acs.analchem.5b01054. [DOI] [PubMed] [Google Scholar]
- Taguchi K. Fukusaki E. Bamba T. Simultaneous and rapid analysis of bile acids including conjugates by supercritical fluid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2013;1299:103–109. doi: 10.1016/j.chroma.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Masuo Y. Imai T. Shibato J. et al., Omic analyses unravels global molecular changes in the brain and liver of a rat model for chronic Sake (Japanese alcoholic beverage) intake. Electrophoresis. 2009;30(8):1259–1275. doi: 10.1002/elps.200900045. [DOI] [PubMed] [Google Scholar]
- Beale D. J. Jones O. A. Karpe A. V. et al., A Review of Analytical Techniques and Their Application in Disease Diagnosis in Breathomics and Salivaomics Research. Int. J. Mol. Sci. 2016;18(1):E24. doi: 10.3390/ijms18010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfender J. L. Marti G. Thomas A. et al., Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A. 2015;1382:136–164. doi: 10.1016/j.chroma.2014.10.091. [DOI] [PubMed] [Google Scholar]
- Kleparnik K. Recent advances in combination of capillary electrophoresis with mass spectrometry: methodology and theory. Electrophoresis. 2015;36(1):159–178. doi: 10.1002/elps.201400392. [DOI] [PubMed] [Google Scholar]
- Zhao S. S. Zhong X. Tie C. et al., Capillary electrophoresis-mass spectrometry for analysis of complex samples. Proteomics. 2012;12(19–20):2991–3012. doi: 10.1002/pmic.201200221. [DOI] [PubMed] [Google Scholar]
- Garcia A. Godzien J. Lopez-Gonzalvez A. et al., Capillary electrophoresis mass spectrometry as a tool for untargeted metabolomics. Bioanalysis. 2017;9(1):99–130. doi: 10.4155/bio-2016-0216. [DOI] [PubMed] [Google Scholar]
- Stalmach A. Albalat A. Mullen W. et al., Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis. 2013;34(11):1452–1464. doi: 10.1002/elps.201200708. [DOI] [PubMed] [Google Scholar]
- Onjiko R. M. Portero E. P. Moody S. A. et al., In Situ Microprobe Single-Cell Capillary Electrophoresis Mass Spectrometry: Metabolic Reorganization in Single Differentiating Cells in the Live Vertebrate (Xenopus laevis) Embryo. Anal. Chem. 2017;89(13):7069–7076. doi: 10.1021/acs.analchem.7b00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J. Yin P. Tan Y. et al., Metabolomics study of hepatocellular carcinoma: discovery and validation of serum potential biomarkers by using capillary electrophoresis-mass spectrometry. J. Proteome Res. 2014;13(7):3420–3431. doi: 10.1021/pr500390y. [DOI] [PubMed] [Google Scholar]
- Fujii T. Hattori K. Miyakawa T. et al., Metabolic profile alterations in the postmortem brains of patients with schizophrenia using capillary electrophoresis-mass spectrometry. Schizophrenia Research. 2017;183:70–74. doi: 10.1016/j.schres.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Kanu A. B. Dwivedi P. Tam M. et al., Ion mobility-mass spectrometry. J. Mass Spectrom. 2008;43(1):1–22. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- Lapthorn C. Pullen F. Chowdhry B. Z. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: separating and assigning structures to ions. Mass Spectrom. Rev. 2013;32(1):43–71. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- Holcapek M. Jirasko R. Lisa M. Recent developments in liquid chromatography-mass spectrometry and related techniques. J. Chromatogr. A. 2012;1259:3–15. doi: 10.1016/j.chroma.2012.08.072. [DOI] [PubMed] [Google Scholar]
- Paglia G. Astarita G. Metabolomics and lipidomics using traveling-wave ion mobility mass spectrometry. Nat. Protoc. 2017;12(4):797–813. doi: 10.1038/nprot.2017.013. [DOI] [PubMed] [Google Scholar]
- Dugourd P. Hudgins R. R. Clemmer D. E. Jarrold M. F. High-resolution ion mobility measurements. Rev. Sci. Instrum. 1997;68(2):1122–1129. doi: 10.1063/1.1147873. [DOI] [Google Scholar]
- Kliman M. May J. C. McLean J. A. Lipid analysis and lipidomics by structurally selective ion mobility-mass spectrometry. Biochim. Biophys. Acta. 2011;1811(11):935–945. doi: 10.1016/j.bbalip.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanucara F. Holman S. W. Gray C. J. et al., The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 2014;6(4):281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- Shvartsburg A. A. Smith R. D. Fundamentals of traveling wave ion mobility spectrometry. Anal. Chem. 2008;80(24):9689–9699. doi: 10.1021/ac8016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotolo B. T. Benesch J. L. Sandercock A. M. et al., Ion mobility-mass spectrometry analysis of large protein complexes. Nat. Protoc. 2008;3(7):1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- Bush M. F. Hall Z. Giles K. et al., Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal. Chem. 2010;82(22):9557–9565. doi: 10.1021/ac1022953. [DOI] [PubMed] [Google Scholar]
- Jurneczko E. Barran P. E. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136(1):20–28. doi: 10.1039/C0AN00373E. [DOI] [PubMed] [Google Scholar]
- Guevremont R. High-field asymmetric waveform ion mobility spectrometry: a new tool for mass spectrometry. J. Chromatogr. A. 2004;1058(1–2):3–19. doi: 10.1016/S0021-9673(04)01478-5. [DOI] [PubMed] [Google Scholar]
- Zhang X. Chiu V. M. Stoica G. et al., Metabolic analysis of striatal tissues from Parkinson's disease-like rats by electrospray ionization ion mobility mass spectrometry. Anal. Chem. 2014;86(6):3075–3083. doi: 10.1021/ac4040967. [DOI] [PubMed] [Google Scholar]
- Bowman A. P. Abzalimov R. R. Shvartsburg A. A. Broad Separation of Isomeric Lipids by High-Resolution Differential Ion Mobility Spectrometry with Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017;28(8):1552–1561. doi: 10.1007/s13361-017-1675-2. [DOI] [PubMed] [Google Scholar]
- Dwivedi P. Schultz A. J. Hill H. H. Metabolic Profiling of Human Blood by High Resolution Ion Mobility Mass Spectrometry (IM-MS) Int. J. Mass Spectrom. 2010;298(1–3):78–90. doi: 10.1016/j.ijms.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dominguez R. Sayago A. Fernandez-Recamales A. Direct infusion mass spectrometry for metabolomic phenotyping of diseases. Bioanalysis. 2017;9(1):131–148. doi: 10.4155/bio-2016-0202. [DOI] [PubMed] [Google Scholar]
- Lin L. Yu Q. Yan X. et al., Direct infusion mass spectrometry or liquid chromatography mass spectrometry for human metabonomics? A serum metabonomic study of kidney cancer. Analyst. 2010;135(11):2970–2978. doi: 10.1039/C0AN00265H. [DOI] [PubMed] [Google Scholar]
- Anand S. Young S. Esplin M. S. et al., Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. J. Lipid Res. 2016;57(4):687–696. doi: 10.1194/jlr.P064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dominguez R. Garcia-Barrera T. Vitorica J. et al., Metabolomic screening of regional brain alterations in the APP/PS1 transgenic model of Alzheimer's disease by direct infusion mass spectrometry. J. Pharm. Biomed. Anal. 2015;102:425–435. doi: 10.1016/j.jpba.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Kapron J. T. Pace E. Van Pelt C. K. et al., Quantitation of midazolam in human plasma by automated chip-based infusion nanoelectrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17(18):2019–2026. doi: 10.1002/rcm.1145. [DOI] [PubMed] [Google Scholar]
- Hop C. E. Chen Y. Yu L. J. Uniformity of ionization response of structurally diverse analytes using a chip-based nanoelectrospray ionization source. Rapid Commun. Mass Spectrom. 2005;19(21):3139–3142. doi: 10.1002/rcm.2182. [DOI] [PubMed] [Google Scholar]
- Valaskovic G. A. Utley L. Lee M. S. et al., Ultra-low flow nanospray for the normalization of conventional liquid chromatography/mass spectrometry through equimolar response: standard-free quantitative estimation of metabolite levels in drug discovery. Rapid Commun. Mass Spectrom. 2006;20(7):1087–1096. doi: 10.1002/rcm.2414. [DOI] [PubMed] [Google Scholar]
- Zhang Y. He C. Qiu L. et al., Serum Unsaturated Free Fatty Acids: A Potential Biomarker Panel for Early-Stage Detection of Colorectal Cancer. J. Cancer. 2016;7(4):477–483. doi: 10.7150/jca.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Qiu L. Wang Y. et al., Unsaturated free fatty acids: a potential biomarker panel for early detection of gastric cancer. Biomarkers. 2014;19(8):667–673. doi: 10.3109/1354750X.2014.977951. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Qiu L. Wang Y. et al., High-throughput and high-sensitivity quantitative analysis of serum unsaturated fatty acids by chip-based nanoelectrospray ionization-Fourier transform ion cyclotron resonance mass spectrometry: early stage diagnostic biomarkers of pancreatic cancer. Analyst. 2014;139(7):1697–1706. doi: 10.1039/C3AN02130K. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Song L. Liu N. et al., Decreased serum levels of free fatty acids are associated with breast cancer. Clin. Chim. Acta. 2014;437:31–37. doi: 10.1016/j.cca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y. He C. Qiu L. et al., Serum unsaturated free fatty acids: potential biomarkers for early detection and disease progression monitoring of non-small cell lung cancer. J. Cancer. 2014;5(8):706–714. doi: 10.7150/jca.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan T. Kirkbride P. Pigou P. E. et al., Surface-assisted laser desorption ionization mass spectrometry techniques for application in forensics. Mass Spectrom. Rev. 2015;34(6):627–640. doi: 10.1002/mas.21431. [DOI] [PubMed] [Google Scholar]
- Lu M. Cai Z. Advances of MALDI-TOF MS in the analysis of traditional Chinese medicines. Top. Curr. Chem. 2013;331:143–164. doi: 10.1007/128_2012_383. [DOI] [PubMed] [Google Scholar]
- Sandrin T. R. Goldstein J. E. Schumaker S. MALDI TOF MS profiling of bacteria at the strain level: a review. Mass Spectrom. Rev. 2013;32(3):188–217. doi: 10.1002/mas.21359. [DOI] [PubMed] [Google Scholar]
- Lu M. Yang X. Yang Y. et al., Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules. Nanomaterials. 2017;7(4):87. doi: 10.3390/nano7040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kampen J. J. Burgers P. C. de Groot R. et al., Biomedical application of MALDI mass spectrometry for small-molecule analysis. Mass Spectrom. Rev. 2011;30(1):101–120. doi: 10.1002/mas.20268. [DOI] [PubMed] [Google Scholar]
- Cleland T. P. Schroeter E. R. A Comparison of Common Mass Spectrometry Approaches for Paleoproteomics. J. Proteome Res. 2018;17(3):936–945. doi: 10.1021/acs.jproteome.7b00703. [DOI] [PubMed] [Google Scholar]
- Sleno L. Volmer D. A. Some fundamental and technical aspects of the quantitative analysis of pharmaceutical drugs by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19(14):1928–1936. doi: 10.1002/rcm.2006. [DOI] [PubMed] [Google Scholar]
- van Kampen J. J. Burgers P. C. Gruters R. A. et al., Quantitative analysis of antiretroviral drugs in lysates of peripheral blood mononuclear cells using MALDI-triple quadrupole mass spectrometry. Anal. Chem. 2008;80(13):4969–4975. doi: 10.1021/ac800218a. [DOI] [PubMed] [Google Scholar]
- Xu S. Li Y. Zou H. et al., Carbon nanotubes as assisted matrix for laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2003;75(22):6191–6195. doi: 10.1021/ac0345695. [DOI] [PubMed] [Google Scholar]
- Baker S. N. Baker G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem., Int. Ed. Engl. 2010;49(38):6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- Novoselov K. S. Geim A. K. Morozov S. V. et al., Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Kim Y. K. Min D. H. Mechanistic study of laser desorption/ionization of small molecules on graphene oxide multilayer films. Langmuir. 2014;30(42):12675–12683. doi: 10.1021/la5027653. [DOI] [PubMed] [Google Scholar]
- He M. Guo S. Li Z. In situ characterizing membrane lipid phenotype of breast cancer cells using mass spectrometry profiling. Sci. Rep. 2015;5:11298. doi: 10.1038/srep11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Wang Y. Guo S. et al., Ammonia-treated N-(1-naphthyl)ethylenediamine dihydrochloride as a novel matrix for rapid quantitative and qualitative determination of serum free fatty acids by matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chim. Acta. 2013;794:82–89. doi: 10.1016/j.aca.2013.07.060. [DOI] [PubMed] [Google Scholar]
- Chughtai K. Heeren R. M. Mass spectrometric imaging for biomedical tissue analysis. Chem. Rev. 2010;110(5):3237–3277. doi: 10.1021/cr100012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P. M. Caprioli R. M. Matrix-assisted laser desorption ionization imaging mass spectrometry: in situ molecular mapping. Biochemistry. 2013;52(22):3818–3828. doi: 10.1021/bi301519p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E. M. Hummon A. B. Imaging mass spectrometry: from tissue sections to cell cultures. Adv. Drug Delivery Rev. 2013;65(8):1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Norris J. L. Caprioli R. M. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem. Rev. 2013;113(4):2309–2342. doi: 10.1021/cr3004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismeh R. Waldon D. J. Teffera Y. et al., Localization and quantification of drugs in animal tissues by use of desorption electrospray ionization mass spectrometry imaging. Anal. Chem. 2012;84(12):5439–5445. doi: 10.1021/ac3011654. [DOI] [PubMed] [Google Scholar]
- Ly A. Buck A. Balluff B. et al., High-mass-resolution MALDI mass spectrometry imaging of metabolites from formalin-fixed paraffin-embedded tissue. Nat. Protoc. 2016;11(8):1428–1443. doi: 10.1038/nprot.2016.081. [DOI] [PubMed] [Google Scholar]
- Kaganman I. SIMS for membranes. Nat. Methods. 2006;3(12):962–963. doi: 10.1038/nmeth1206-962a. [DOI] [PubMed] [Google Scholar]
- Kim Y. P. Oh E. Oh Y. H. et al., Protein kinase assay on peptide-conjugated gold nanoparticles by using secondary-ion mass spectrometric imaging. Angew. Chem., Int. Ed. Engl. 2007;46(36):6816–6819. doi: 10.1002/anie.200701418. [DOI] [PubMed] [Google Scholar]
- Comi T. J. Ryu S. W. Perry R. H. Synchronized Desorption Electrospray Ionization Mass Spectrometry Imaging. Anal. Chem. 2016;88(2):1169–1175. doi: 10.1021/acs.analchem.5b03010. [DOI] [PubMed] [Google Scholar]
- Eberlin L. S. DESI-MS imaging of lipids and metabolites from biological samples. Methods Mol. Biol. 2014;1198:299–311. doi: 10.1007/978-1-4939-1258-2_20. [DOI] [PubMed] [Google Scholar]
- Takats Z. Wiseman J. M. Gologan B. et al., Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- Nemes P. Barton A. A. Li Y. et al., Ambient molecular imaging and depth profiling of live tissue by infrared laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2008;80(12):4575–4582. doi: 10.1021/ac8004082. [DOI] [PubMed] [Google Scholar]
- Nemes P. Barton A. A. Vertes A. Three-dimensional imaging of metabolites in tissues under ambient conditions by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2009;81(16):6668–6675. doi: 10.1021/ac900745e. [DOI] [PubMed] [Google Scholar]
- Groseclose M. R. Massion P. P. Chaurand P. et al., High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics. 2008;8(18):3715–3724. doi: 10.1002/pmic.200800495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino S. Groseclose M. R. Wagner D. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development. Bioanalysis. 2011;3(21):2427–2441. doi: 10.4155/bio.11.232. [DOI] [PubMed] [Google Scholar]
- Schwamborn K. Caprioli R. M. Molecular imaging by mass spectrometry – looking beyond classical histology. Nat. Rev. Cancer. 2010;10:639–646. doi: 10.1038/nrc2917. [DOI] [PubMed] [Google Scholar]
- Franck J. Arafah K. Elayed M. et al., MALDI imaging mass spectrometry: state of the art technology in clinical proteomics. Mol. Cell. Proteomics. 2009;8(9):2023–2033. doi: 10.1074/mcp.R800016-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas M. Kruger R. Ion formation in MALDI: the cluster ionization mechanism. Chem. Rev. 2003;103(2):427–440. doi: 10.1021/cr010376a. [DOI] [PubMed] [Google Scholar]
- Murray K. K. Seneviratne C. A. Ghorai S. High resolution laser mass spectrometry bioimaging. Methods. 2016;104:118–126. doi: 10.1016/j.ymeth.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurand P. Introducing specificity and sensitivity in imaging MS. Bioanalysis. 2015;7(18):2279–2281. doi: 10.4155/bio.15.160. [DOI] [PubMed] [Google Scholar]
- Banstola B. Grodner E. T. Cao F. et al., Systematic assessment of surfactants for matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal. Chim. Acta. 2017;963:76–82. doi: 10.1016/j.aca.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Zhou D. Guo S. Zhang M. et al., Mass spectrometry imaging of small molecules in biological tissues using graphene oxide as a matrix. Anal. Chim. Acta. 2017;962:52–59. doi: 10.1016/j.aca.2017.01.043. [DOI] [PubMed] [Google Scholar]
- Bodzon-Kulakowska A. Suder P. Imaging mass spectrometry: instrumentation, applications, and combination with other visualization techniques. Mass Spectrom. Rev. 2016;35(1):147–169. doi: 10.1002/mas.21468. [DOI] [PubMed] [Google Scholar]
- Kraft M. L. Klitzing H. A. Imaging lipids with secondary ion mass spectrometry. Biochim. Biophys. Acta. 2014;1841(8):1108–1119. doi: 10.1016/j.bbalip.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Phan N. T. N. Munem M. Ewing A. G. et al., MS/MS analysis and imaging of lipids across Drosophila brain using secondary ion mass spectrometry. Anal. Bioanal. Chem. 2017;409(16):3923–3932. doi: 10.1007/s00216-017-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach P. J. Laskin J. Laskin A. Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 2010;135(9):2233–2236. doi: 10.1039/C0AN00312C. [DOI] [PubMed] [Google Scholar]
- Graham Cooks R. Ouyang Z. Takats Z. et al., Ambient Mass Spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- Ifa D. R. Wu C. Ouyang Z. et al., Desorption electrospray ionization and other ambient ionization methods: current progress and preview. Analyst. 2010;135(4):669–681. doi: 10.1039/B925257F. [DOI] [PubMed] [Google Scholar]
- Laskin J. Heath B. S. Roach P. J. et al., Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 2012;84(1):141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmusch A. K. Pirro V. Baird Z. et al., Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc. Natl. Acad. Sci. U. S. A. 2016;113(6):1486–1491. doi: 10.1073/pnas.1523306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanekoff I. Burnum-Johnson K. Thomas M. et al., High-speed tandem mass spectrometric in situ imaging by nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 2013;85(20):9596–9603. doi: 10.1021/ac401760s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes P. Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal. Chem. 2007;79(21):8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- Akos Vertes P. N. Bindesh S. Alexis A. B. et al., Molecular imaging by Mid-IR laser ablation mass spectrometry. Appl. Phys. A: Mater. Sci. Process. 2008;93(4):885–891. doi: 10.1007/s00339-008-4750-5. [DOI] [Google Scholar]
- Nemes P. Woods A. S. Vertes A. Simultaneous imaging of small metabolites and lipids in rat brain tissues at atmospheric pressure by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2010;82(3):982–988. doi: 10.1021/ac902245p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Dill A. L. Eberlin L. S. Cooks R. G. Ifa D. R. et al., Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 2013;32(3):218–243. doi: 10.1002/mas.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Balan P. Vertes A. Molecular Imaging of Growth, Metabolism, and Antibiotic Inhibition in Bacterial Colonies by Laser Ablation Electrospray Ionization Mass Spectrometry. Angew. Chem., Int. Ed. Engl. 2016;55(48):15035–15039. doi: 10.1002/anie.201607751. [DOI] [PubMed] [Google Scholar]
- Cody R. B. Laramee J. A. Durst H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005;77(8):2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- Calvano C. D. van der Werf I. D. Palmisano F. et al., Revealing the composition of organic materials in polychrome works of art: the role of mass spectrometry-based techniques. Anal. Bioanal. Chem. 2016;408(25):6957–6981. doi: 10.1007/s00216-016-9862-8. [DOI] [PubMed] [Google Scholar]
- Pierce C. Y. Barr J. R. Cody R. B. et al., Ambient generation of fatty acid methyl ester ions from bacterial whole cells by direct analysis in real time (DART) mass spectrometry. Chem. Commun. 2007;8:807–809. doi: 10.1039/B613200F. [DOI] [PubMed] [Google Scholar]
- Hajslova J. Cajka T. Vaclavik L. Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC, Trends Anal. Chem. 2011;30(2):204–218. doi: 10.1016/j.trac.2010.11.001. [DOI] [Google Scholar]
- Gross J. H. Direct analysis in real time – a critical review on DART-MS. Anal. Bioanal. Chem. 2014;406(1):63–80. doi: 10.1007/s00216-013-7316-0. [DOI] [PubMed] [Google Scholar]
- Cajka T. Danhelova H. Vavrecka A. et al., Evaluation of direct analysis in real time ionization-mass spectrometry (DART-MS) in fish metabolomics aimed to assess the response to dietary supplementation. Talanta. 2013;115:263–270. doi: 10.1016/j.talanta.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Zhou M. McDonald J. F. Fernandez F. M. Optimization of a direct analysis in real time/time-of-flight mass spectrometry method for rapid serum metabolomic fingerprinting. J. Am. Soc. Mass Spectrom. 2010;21(1):68–75. doi: 10.1016/j.jasms.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gürdeniz G. Kristensen M. Skov T. et al., The Effect of LC-MS Data Preprocessing Methods on the Selection of Plasma Biomarkers in Fed vs. Fasted Rats. Metabolites. 2012;2(1):77–99. doi: 10.3390/metabo2010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble J. B. Fraga C. G. Comparative evaluation of preprocessing freeware on chromatography/mass spectrometry data for signature discovery. J. Chromatogr. A. 2014;1359:155–164. doi: 10.1016/j.chroma.2014.06.100. [DOI] [PubMed] [Google Scholar]
- Chen Y. Xu J. Zhang R. et al., Assessment of data pre-processing methods for LC-MS/MS-based metabolomics of uterine cervix cancer. Analyst. 2013;138(9):2669–2677. doi: 10.1039/C3AN36818A. [DOI] [PubMed] [Google Scholar]
- Scalbert A. Brennan L. Fiehn O. et al., Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5:435–458. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O. Robertson D. Griffin J. et al., The metabolomics standards initiative (MSI) Metabolomics. 2007;3(3):175–178. doi: 10.1007/s11306-007-0070-6. [DOI] [Google Scholar]
- Sumner L. W. Amberg A. Barrett D. et al., Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K. Salek R. M. Conesa P. et al., MetaboLights-an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2012;41:D781–D786. doi: 10.1093/nar/gks1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek R. M. Steinbeck C. Viant M. R. et al., The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience. 2013;2(1):13. doi: 10.1186/2047-217X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S. Zhang A. Zhang T. et al., Dissect new mechanistic insights for geniposide efficacy on the hepatoprotection using multiomics approach. Oncotarget. 2017;8(65):108760–108770. doi: 10.18632/oncotarget.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. Sun H. Wang X. Emerging role and recent applications of metabolomics biomarkers in obesity disease research. RSC Adv. 2017;7(25):14966–14973. doi: 10.1039/C6RA28715H. [DOI] [Google Scholar]
- Zhang A. Fang H. Wang Y. et al., Discovery and verification of the potential targets from bioactive molecules by network pharmacology-based target prediction combined with high-throughput metabolomics. RSC Adv. 2017;7:51069–51078. doi: 10.1039/C7RA09522H. [DOI] [Google Scholar]
- Zhang A. H. Sun H. Han Y. et al., Ultraperformance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets. Anal. Chem. 2013 Aug 6;85(15):7606–7612. doi: 10.1021/ac401793d. [DOI] [PubMed] [Google Scholar]
- Zhang A. Sun H. Yan G. et al., Mass spectrometry-based metabolomics: applications to biomarker and metabolic pathway research. Biomed. Chromatogr. 2016;30(1):7–12. doi: 10.1002/bmc.3453. [DOI] [PubMed] [Google Scholar]
- Nan Y. Zhou X. Liu Q. et al., Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016;1026:217–226. doi: 10.1016/j.jchromb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Liang Q. Liu H. Jiang Y. et al., High-throughput metabolic profiling for discovering metabolic biomarkers of sepsis-induced acute lung injury. RSC Adv. 2016;6:11008–11013. doi: 10.1039/C5RA25961D. [DOI] [Google Scholar]
- Zhang A. Yan G. Sun H. et al., Deciphering the biological effects of acupuncture treatment modulating multiple metabolism pathways. Sci. Rep. 2016;6:19942. doi: 10.1038/srep19942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H. Zhang A. Han Y. et al., Metabolomics approach to explore the effects of Kai-Xin-San on Alzheimer's disease using UPLC/ESI-Q-TOF mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016;1015–1016:50–61. doi: 10.1016/j.jchromb.2016.02.007. [DOI] [PubMed] [Google Scholar]