Abstract
Purpose: Blood loss along with inadequate evacuation after cardiac surgery leads to retained blood syndrome (RBS) in the pleural and/or pericardial cavity. Re-sternotomy is often needed for clot evacuation. Video-assisted thoracoscopic surgery (VATS) evacuation is a less-invasive procedure. However, sufficient evidence on safety and outcomes is lacking.
Methods: Thirty patients who developed hemothorax and/or hemopericardium after cardiac surgery and underwent VATS evacuation between April 2015 and September 2020 were included in this retrospective single-center analysis.
Results: The median patient age was 70 (interquartile range: IQR 62–75) years, body mass index (BMI) was 24.7 (IQR 22.8–29) kg/m2, time between initial cardiac surgery and VATS was 17 (IQR 11–21) days, 30% of the patients were female, 60% resided in the ICU, and 17% were nicotine users. Coronary artery bypass graft was the most frequent initial cardiac procedure. Median operation time was 120 (IQR 90–143) min, 23% of the patients needed an additional VATS, and the median length of hospital stay after VATS was 8 (IQR 5–14) days. All patients survived VATS, and we experienced no mortality related to the VATS procedure.
Conclusion: In our study, VATS for evacuation of RBS after cardiac surgery was a feasible, safe, and efficient alternative approach to re-sternotomy in selected patients.
Keywords: hemothorax, hemopericardium, cardiac surgery, retained blood syndrome, video- assisted thoracoscopic surgery
Introduction
Bleeding after cardiac surgery is a common complication, occurring in 5%–9% cardiac surgeries.1,2) The blood loss leads to a broad spectrum of complications, from acute cardiac tamponade to subacute hemothorax. Boyle et al. described the need for new labels for post-surgical bleeding, including the acute, subacute, and chronic phases of bleeding, and proposed the term retained blood syndrome (RBS).3) The incidence of RBS is 13.8%–22.7% after cardiac surgery.3) In acute RBS, early exploration within the first 48 postoperative hours via an emergency re-sternotomy is the gold standard. In subacute RBS (>48 hr), the remaining clots activate inflammatory mechanisms that cause increased postoperative atrial fibrillation, in-hospital mortality, and longer in-hospital stays.4) In addition, a large number of clots cause mechanical compression of the lung and severe atelectasis. Furthermore, clot contamination can lead to pleural empyema.5) Despite the consequences, proper management of subacute RBS has not been studied. Currently, reopening the sternum is the gold standard for the evacuation of coagulated blood. However, re-sternotomy is an invasive procedure associated with complications. Moreover, the presence of adhesions leads to the risk of graft damage and is associated with increased deep sternal wound infection rates. Therefore, alternative methods are needed to ensure sufficient clot evacuation while avoiding re- sternotomy. Video-assisted thoracoscopic surgery (VATS) is the treatment of choice for managing pleural cavity pathology, but its role in the postoperative phase of cardiac surgery remains unclear. Hence, we conducted a retrospective analysis to investigate the efficacy and safety of VATS evacuation of clotted blood from the pleural and pericardial cavity. The efficiency of this method was demonstrated.
Materials and Methods
Study sample
In this retrospective single-center analysis, all patients who underwent VATS to evacuate retained blood in the pleural and pericardial cavity after cardiac surgery procedures from April 2015 to September 2020 were included. All surgeons were experienced in both cardiac surgery and VATS procedures. Patient records were electronically obtained from the database of our institute. Informed consent was obtained from each patient, and the study was waived by the institutional ethics committee.
Diagnostics
If pleural effusion was suspected in the chest X-ray, pleural effusion volume, septate effusion, and the presence of clots were evaluated with sonography and computer tomography (CT) (Fig. 1). The CT scan provided information about hemothorax severity and the relationship of the effusion with great vessels and/or grafts. The decision for surgery was based on these radiological findings, clinical signs, and symptoms. These criteria included inability to wean from mechanical ventilation (MV), severe atelectasis of the lower lobe, increased inflammatory markers, dyspnea, and chest pain. VATS was not indicated in patients who (1) required urgent surgery due to acute bleeding and hemorrhagic shock; (2) were successfully managed with lavage and drainage tubes; (3) underwent re-sternotomy due to respiratory and/or circulatory instability; and (4) had bleedings on anastomotic sites of the cardiac surgery.
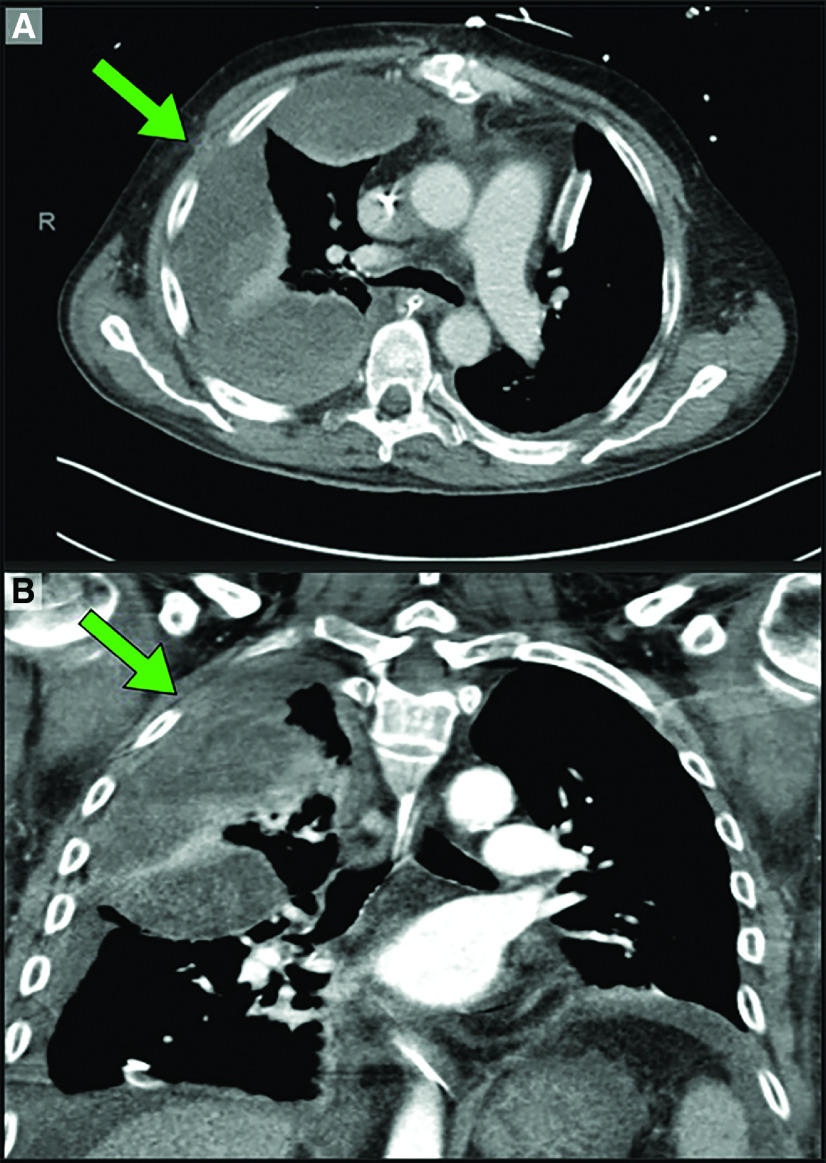
Fig. 1. CT scan showing hemothorax after minimally invasive mitral repair. (A) Axial view of the hemothorax. (B) Coronal view of the hemothorax. In both pictures, the hemothorax is marked with a green arrow.
Surgical procedure
All patients were placed in the lateral decubitus position. A 30° camera was used during the procedure. Uni portal, two-port, and three-port thoracoscopies were performed. If there was a thoracic drain, the incision site of the drain was used as a port insertion site. The pleural cavity was entered with considerable suction devices and a camera until enough free space was achieved in the cavity to insert additional ports. For the removal of clotted blood, we used graspers and large endoscopic suction. The entire pleural cavity, from the apex to the pleurodiaphragmatic angle, was inspected and evacuated. For pericardial exploration, the pericardium was incised 5 cm above the level of the phrenic nerve, and a pericardial window was made to prevent recurrent pericardial effusions. In the presence of soft adhesions, the anterior border of the pericardium was retracted from the sternum to explore the anterior pericardial space. Then, the camera was successfully inserted into the pericardium. With light pressure on the heart using a folded compress in a grasper, visualization could be achieved through the entire pericardial cavity. Caution was given to avoid injury of the intrapericardial coronary bypass grafts, the prosthesis anastomosed to the ascending or descending thoracic aorta, or cardiac structures including the right ventricle or displacement of pacer wires (Fig. 2). In patients with bilateral hemothorax, after completing the thoracoscopic evacuation of one side, we repositioned the patient to the contralateral decubitus position to perform the second thoracoscopic evacuation of the contralateral pleural cavity.
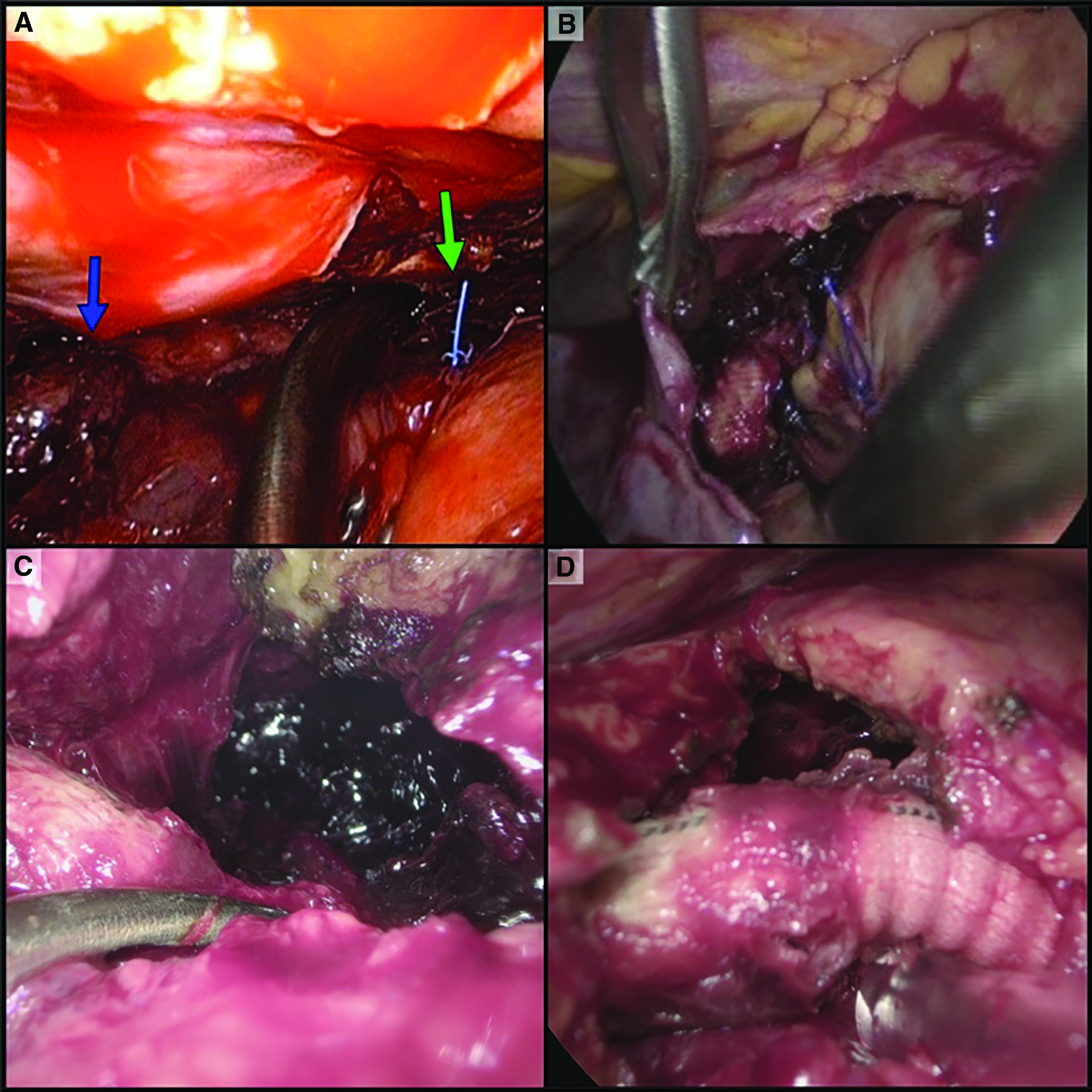
Fig. 2. Intraoperative view. (A) Diaphragm is on the left side, blue arrow indicating clotted blood, and green arrow indicating pacemaker wires. (B) Clots, fibrin, and empyema are visible in the pleural cavity surrounding the open pericardium. Hemopericardium evacuation was performed from the right side; threads of atrial cannulation are visible in the center. (C) Hemopericardium evacuation after LVAD implantation using VATS from the left side. Opened pericardium at the upper right corner, and clotted blood in the center. (D) Result of hemopericardium evacuation. Opened pericardium on the right side, and outflow graft (from left ventricle to aorta) in the center.
Statistical analysis
Categorical variables are presented as absolute numbers and percentages. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test, and presented as medians and interquartile ranges (IQRs). Statistical analysis was performed with SPSS Version 26 (IBM Corp., Armonk, NY, USA). Kaplan–Meier survival estimates, including visualization, were obtained using the open-source software, Jamovi version 1.2.22.0.
Results
Baseline characteristics
During the study period, 30 patients (30% female) underwent VATS for hemothorax and/or hemopericardium after cardiac surgery. Baseline characteristics are presented in Table 1. The median patient age was 70 (IQR 62–75) years, and the median BMI was 24.7 (IQR 22.8–29) kg/m2. Median EuroSCORE II was 2.3% (IQR 1.2–4.4). The median time between the initial cardiac surgery VATS was 17 (IQR 11–21) days, and 60% of the patients had a prolonged intensive care unit (ICU) stay after cardiac surgery and remained in the ICU prior to the VATS procedure. Between the initial cardiac procedure and VATS, 5 (IQR 2–8) units of packed red blood cells (PRBC) were transfused. MV and extracorporeal membrane oxygenation (ECMO) were needed in 7% of patients. Tracheostomy was performed in 20% of the patients. On the day of VATS, the median hemoglobin was 8.8 (IQR 8.1–9.4) g/dL, and the leukocyte count was 10.1 (IQR 7.8–13.5)/nL, procalcitonin (PCT) was 0.16 (0.08–0.54) ng/mL, and INR was 1.21 (IQR 1.03–1.48); further details are presented in Table 1.
Table 1. Baseline characteristics of 30 patients that underwent video-assisted thoracoscopic surgery.
| Age (years), median (IQR) | 70 (62–75) | ||
| Female gender | 9 (30) | ||
| BMI (kg/m2), median (IQR) | 24.7 (22.8–29) | ||
| Arterial hypertension | 19 (63) | ||
| Diabetes mellitus type 2 | 8 (27) | ||
| Insulin dependent diabetes mellitus | 0 (0) | ||
| Atrial fibrillation | 7 (23) | ||
| Nicotine user | 5 (17) | ||
| Chronic kidney disease† | 5 (17) | ||
| Dialysis | 1 (3) | ||
| COPD I | 1 (3) | ||
| COPD II | 3 (10) | ||
| COPD III | 2 (7) | ||
| Pulmonary hypertension | 3 (10) | ||
| Prior EF (percentage), median (IQR) | 55 (38–60) | ||
| Prior Euroscore II (percentage), median (IQR) | 2.3 (1.2–4.4) | ||
| PRBCs (units), median (IQR)†† | 5 (2–8) | ||
| FFPs (units), median (IQR)†† | 2 (0–4) | ||
| PCs (units), median (IQR)†† | 0 (0–3) | ||
| Hospitalization details | |||
| Time between initial cardiac surgery and VATS (d), median (IQR) | 17 (11–21) | ||
| Residing at ICU | 18 (60) | ||
| Mechanically ventilated | 2 (7) | ||
| Tracheostomy | 6 (20) | ||
| Intra-aortic balloon pump | 0 (0) | ||
| Extracorporeal membrane oxygenation | 2 (7) | ||
| Temporary ventricular support devices | 0 (0) | ||
| Laboratory parameters* | |||
| Hb (g/dL), median (IQR) | 8.8 (8.1–9.4) | ||
| Leucocytes (/nL), median (IQR) | 10.1 (7.8–13.5) | ||
| Platelet (G/L), median (IQR) | 336 (214–485) | ||
| PCT (ng/mL), median (IQR) | 0.16 (0.08–0.54) | ||
| INR (ratio), median (IQR) | 1.21 (1.03–1.48) | ||
| LDH (U/L), median (IQR) | 328 (252–447) | ||
| AST (U/L), median (IQR) | 44 (25–76.5) | ||
| ALT (U/L), median (IQR) | 47 (18–77.25) | ||
| Creatinine (mg/dL), median (IQR) | 0.99 (0.49–1.26) | ||
| BUN (mg/dL), median (IQR) | 56 (34–80) | ||
| Initial cardiac procedures | |||
| Coronary artery bypass graft | 15 (50) | ||
| Aortic valve repair | 6 (20) | ||
| Mitral valve repair | 9 (30) | ||
| Tricuspid valve repair | 2 (7) | ||
| Aortic aneurysm surgery | 2 (7) | ||
| LVAD implantation | 3 (10) | ||
| RVAD implantation | 1 (3) | ||
| Combined procedures | 12 (40) | ||
| Complications before VATS** | |||
| Pneumonia/bronchitis | 9 (30) | ||
| Septic shock | 5 (17) | ||
| Bleeding needing re-sternotomy | 5 (17) | ||
| Acute kidney failure needing dialysis | 10 (33) | ||
| Pulmonary artery embolism | 0 (0) | ||
| Ischemic stroke | 1 (3) | ||
| Hemorrhagic stroke | 1 (3) | ||
| Gastrointestinal bleeding | 0 (0) | ||
| Airway bleeding | 1 (3) | ||
| Pericardial tamponade | 3 (10) | ||
| Delirium | 4 (13) | ||
Percentages are shown in parentheses unless indicated as IQR.
†Including all patients with an MDRD-GFR<60 mL/min.
††Including all units since hospital admission until VATS surgery.
*Laboratory parameters were obtained before the VATS.
**Including complications between cardiac surgery and VATS.
ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BUN: blood urea nitrogen; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; FFP: fresh frozen plasma; ICU: intensive care unit; IQR: interquartile range; LDH: lactate dehydrogenase; LOS: length of stay; LVAD: left ventricular assist device; PC: platelet concentrate; PCT: procalcitonin; PRBC: packed right blood cells; RVAD: right ventricular assist device; VATS: video-assisted thoracic surgery
The initial cardiac procedures and related complications are shown in Table 1. The most frequent procedures were coronary artery bypass graft (CABG) (50%), mitral valve repair (30%), and aortic valve repair (20%). Combined procedures were performed in 40% of the patients. Patients who underwent CABG procedures received thrombotic prophylaxis low-molecular- weight-heparin once a day. However, in patients with valve and ventricular assist device operations, we administered intravenous heparin with a target activated partial thromboplastin time of 50–60 sec. Table 1 shows the complications occurred before VATS; 33% of the patients developed acute kidney failure with the need for dialysis after the initial cardiac surgery, and 17% suffered septic shock. More details can be found in Table 1.
A CT scan was conducted before the procedure in 90% of the patients. In the other 10% of patients, chest X-rays showed a considerable hemothorax, and there was no need for a CT scan. The hemothorax was located on the left side in 15 patients and the right side in 14 patients, and one patient had a bilateral hemothorax.
Outcomes
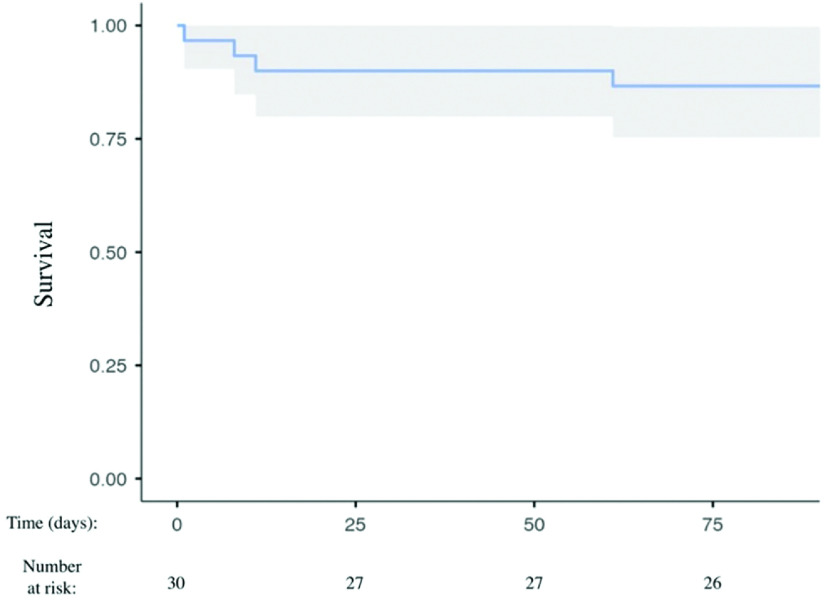
Outcomes are presented in Table 2. VATS for RBS was successful in all patients, and there was no mortality related to the VATS procedures. The overall mortality in the postoperative phase was 13%. Two patients suffered from multi-organ failure before VATS and died at 1 and 8 days after the VATS (Fig. 3). A third patient developed acute gastrointestinal bleeding and died 12 days after the VATS; the fourth patient underwent multiple re-operations due to coagulopathy disorders and LVAD implantation, and died after 61 days (Fig. 3). An additional VATS was needed in seven patients (three LVAD, one RVAD, and three mitral prostheses) who were anticoagulated with an INR target range of 2.5–3.5. The median length of hospital stay after VATS was 8 (IQR 5–14) days.
Table 2. Outcomes and clinical course.
| Mortality | 4 (13) | ||
| LOS in-hospital after VATS (days), median (IQR) | 8 (5–14) | ||
| Additional VATS needed | 7 (23) | ||
| Operative data during VATS | |||
| Operation time (min), median (IQR) | 120 (90–143) | ||
| PRBCs (units), median (IQR)† | 0 (0–1) | ||
| FFPs (units), median (IQR)† | 0 (0–0) | ||
| PCs (units), median (IQR)† | 0 (0–0) | ||
| Inotropes needed | 2 (7) | ||
| Complications after VATS | |||
| Sternal rewiring | 1 (3) | ||
| Complicated wound healing | 0 (0) | ||
| Pneumonia/bronchitis | 6 (20) | ||
| Septic shock | 4 (13) | ||
| Acute kidney failure needing dialysis | 6 (20) | ||
| Pulmonary artery embolism | 0 (0) | ||
| Ischemic stroke | 0 (0) | ||
| Hemorrhagic stroke | 0 (0) | ||
| Gastrointestinal bleeding | 1 (3) | ||
| Airway bleeding | 0 (0) | ||
| Pericardial tamponade | 0 (0) | ||
| Delirium | 5 (17) | ||
| Laboratory parameters before discharge/death | |||
| Hb (g/dL), median (IQR) | 9.3 (8.6–10) | ||
| Leucocytes (/nL), median (IQR) | 8.9 (6.2–10.3) | ||
| Platelet (G/L), median (IQR) | 272 (189–447) | ||
| PCT (ng/mL), median (IQR) | 0.15 (0.08–0.49) | ||
| INR (ratio), median (IQR) | 1.1 (0.37–1.24) | ||
| LDH (U/L), median (IQR) | 306 (256–340) | ||
| AST (U/L), median (IQR) | 44 (24–70) | ||
| ALT (U/L), median (IQR) | 39 (18–67) | ||
| Creatinine (mg/dL), median (IQR) | 0.90 (0.66–1.43) | ||
| BUN (mg/dL), median (IQR) | 42 (25–77) | ||
Percentages are shown in parentheses unless indicated as IQR.
†Including all units used on the day of VATS. ALT: alanine transaminase; AST: aspartate transaminase; BUN: blood urea nitrogen; FFP: fresh frozen plasma; ICU: intensive care unit; IQR: interquartile range; LDH: lactate dehydrogenase; LOS: length of stay; PC: platelet concentrate; PCT: procalcitonin; PRBC: packed right blood cells; VATS: video- assisted thoracic surgery
Fig. 3. Kaplan–Meier survival estimates for 90 days after VATS. Survivors/numbers at risk are illustrated as a blue line and confidence intervals are indicated by the gray area (VATS, video-assisted thoracic surgery).
Operative data and complications during or after VATS are also presented in Table 2. The median operation time was 120 (IQR 90–143) min, and the median amount of PRBC units used during VATS was 0 (IQR 0–1). A full pericardial exploration with hematoma evacuation was performed in 30% of the patients. Active bleeding sources could not be detected in any case. Therefore, conversion to a thoracotomy or sternotomy was not needed in any cases.
The most frequent complications were pneumonia or bronchitis (20%), acute kidney failure (needing dialysis) (20%), delirium (17%), and septic shock (13%). One patient suffered from sternal instability and underwent sternal rewiring 11 days after the VATS operation. No injuries to grafts or VAD connections from the previous cardiac surgery occurred.
Before discharge or death, the median Hb was 9.3 (IQR 8.6–10) g/dL, the leukocyte number was 89 (IQR 62–103)/nL, platelet count was 272 (IQR 189–447) G/L, PCT was 15% (IQR 8–42), and INR was 1.1 (IQR 0.37–1.24). More details are presented in Table 2.
Discussion
Blood loss after cardiac surgery6,7) in combination with inadequate blood evacuation due to clotting of drains8) leads to the presence of clots in the pleural and pericardial cavity, called RBS. Balzer et al. reported RBS incidences requiring intervention of up to 19%.4) Re-sternotomy is the standard approach to evacuate blood clots in the thorax and allows for thorough exploration of the pericardium. However, re-sternotomy is associated with several adverse events, including sternal instability and wound infection.6,7) In a meta-analysis, Biancari et al. demonstrated the association of re-sternotomy for bleeding after adult cardiac surgery with the increased risk of mortality, stroke, and sternal wound infection.9) In addition, RBS procedures are an independent risk factor for antibiotic requirement, wound infections, kidney disease, prolonged length of stay in the ICU, and composite major adverse events.10)
We investigated the outcomes of VATS in patients who developed RBS after cardiac surgery, because VATS may be a superior treatment option. Our results showed that VATS is a feasible, safe, and efficient approach to the evacuation of RBS in cardiac surgery patients, and no conversion to sternotomy or thoracotomy was needed. The safety of VATS was demonstrated; no procedure- related mortality was reported, and the risk of complications was low. Many patients resided in the ICU and received invasive therapies, including MV or ECMO. No sternal wound infections occurred after VATS, and only one patient required sternal rewiring due to mechanical instability.
Data on the feasibility and benefits of VATS after cardiac procedures remain scarce. Fiorelli et al.11) performed a systematic review of VATS pleurodesis to manage pleural effusion after coronary bypass surgery, and no-responded to repeated thoracentesis. Fiorelli and colleagues11) included seven retrospective, two observational studies, and four case series in their systematic review. They concluded that VATS is safe and effective, and its use could help prevent trapped lungs through the resection of adhesions and loculations. Georghiou et al.12) published their experience using VATS for pericardial fenestration for delayed effusions and tamponade after cardiac surgery. Similar to our experience and findings, Georghiou and colleagues12) demonstrated in their study that VATS for creating a pericardial window is a safe and effective treatment for loculated pericardial effusions secondary to cardiac surgery.
Interestingly, Monaco et al.13) published their results applying a modified right chest VATS to treat pericardial tamponade in 15 patients. In addition, Monaco et al.13) also demonstrated a modified VATS technique on the right chest using two trocars to be a feasible alternative approach for patients suffering from cardiac tamponade.
Bashir and colleagues described their experience by using VATS for hemothorax evacuation after cardiac surgery in eight patients.14) Complete removal of clotted blood and relief of the trapped lung were successful via VATS in seven patients. This study demonstrates that hemodynamically stable patients in the late postoperative period, with stable sternums and healed postoperative wounds, are good candidates for the VATS approach and benefit from the avoidance of re-sternotomy.14) Few other alternative approaches to re-sternotomy exist for the evacuation of coagulated retained blood. The subxiphoid approach is an alternative for thoracic evacuation, with the possibility of rapid conversion into an emergency sternotomy. Subxiphoid pericardiotomy or pericardial drainage is a rapid procedure with minimal morbidity.15) Therefore, surgical drainage of large pericardial effusions after cardiac surgery is already safe.16) However, the subxiphoid approach only allows for the evacuation of non-coagulated or non-complex septate pericardial effusions positioned in the inferior region, removing blood clots in the upper regions of the pericardium or the pleural cavity is not possible.
An essential benefit of VATS for retained blood evacuation is the minimization of the traumatized area. Postoperative pain is significantly lower after VATS compared with thoracotomy.17–19) Postoperative pain is a frequent cause of postoperative patient discomfort, limiting inspiration, leading to atelectasis.20) Decreased pain symptoms enable better inspiration and less coughing to avoid postoperative pneumonia and atelectasis. Kaseda et al. reported better postoperative lung function after VATS lobectomy than patients who received open thoracotomy.21,22) A small study by Salim and colleagues reported that VATS decreased the total length of hospital stay and the recurrence of pericardial effusion compared with the subxiphoid approach.23)
Several limitations to this study should be considered. The relatively small sample size and lack of a control group represent an important limitation. In contrast, as a single-center study, we could give detailed information on patient characteristics. Retrospective studies have a potential risk for selection bias, and results depend on accurate record-keeping. Cases with hemopericardium were few and always additional to the hemothorax. Therefore, the usefulness of VATS could not be sufficiently demonstrated for hemopericardium. Laboratory parameters were measured at only specific time points, which may not reflect more extended periods and could lead to misconceptions. Furthermore, patients with signs of cardiac tamponade and massive bleeding were not included in this analysis.
Conclusion
We demonstrated the feasibility and safety of VATS in patients with RBS after cardiac surgery. Our findings suggest that VATS may be an alternative approach to re-sternotomy for the evacuation of RBS in hemodynamically stable patients which could not be managed with drainage tubes.
Disclosure Statement
None declared.
References
- 1). Christensen MC, Krapf S, Kempel A, et al. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg 2009; 138: 687– 93. [DOI] [PubMed] [Google Scholar]
- 2). Kristensen KL, Rauer LJ, Mortensen PE, et al. Reoperation for bleeding in cardiac surgery. Interact Cardiovasc Thorac Surg 2012; 14: 709– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Boyle EM, Gillinov AM, Cohn WE, et al. Retained blood syndrome after cardiac surgery: a new look at an old problem. Innovations (Phila) 2015; 10: 296– 303. [DOI] [PubMed] [Google Scholar]
- 4). Balzer F, von Heymann C, Boyle EM, et al. Impact of retained blood requiring reintervention on outcomes after cardiac surgery. J Thorac Cardiovasc Surg 2016; 152: 595– 601.e4. [DOI] [PubMed] [Google Scholar]
- 5). Karmy-Jones R, Holevar M, Sullivan RJ, et al. Residual hemothorax after chest tube placement correlates with increased risk of empyema following traumatic injury. Can Respir J 2008; 15: 255– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Colson PH, Gaudard P, Fellahi JL, et al. Active bleeding after cardiac surgery: a prospective observational multicenter study. PLoS One 2016; 11: e0162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Al-Attar N, Johnston S, Jamous N, et al. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J Cardiothorac Surg 2019; 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Sirch J, Ledwon M, Püski T, et al. Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg 2016; 151: 832– 38.e2. [DOI] [PubMed] [Google Scholar]
- 9). Biancari F, Mikkola R, Heikkinen J, et al. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2012; 41: 50– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Tauriainen T, Kinnunen EM, Koski-Vähälä J, et al. Outcome after procedures for retained blood syndrome in coronary surgery. Eur J Cardio-Thoracic Surg 2017; 51: 1078– 85. [DOI] [PubMed] [Google Scholar]
- 11). Fiorelli A, Caronia F, Prencipe A, et al. The role of video-assisted thoracoscopic surgery for management of symptomatic pleural effusion after coronary artery bypass surgery: a best evidence topic report. J Thorac Dis 2017; 9: 2339– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Georghiou GP, Porat E, Fuks A, et al. Video-assisted pericardial fenestration for effusions after cardiac surgery. Asian Cardiovasc Thorac Ann 2009; 17: 480– 2. [DOI] [PubMed] [Google Scholar]
- 13). Monaco F, Barone M, David A, et al. Cardiac tamponade: a modified video-assisted thoracoscopic approach. Chir Ital 2009; 61: 321– 6. [PubMed] [Google Scholar]
- 14). Bashir A, Daraghma O, Brzezin´ski Z, et al. Video- assisted thoracic surgery in hemothorax evacuation after cardiac surgery or cardiac interventions. Kardiochir Torakochirurgia Pol 2017; 14: 154– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Mills SA, Julian S, Holliday RH, et al. Subxiphoid pericardial window for pericardial effusive disease. J Cardiovasc Surg (Torino) 1989; 30: 768– 73. [PubMed] [Google Scholar]
- 16). Aksöyek A, Tütün U, Ulus T, et al. Surgical drainage of late cardiac tamponade following open heart surgery. Thorac Cardiovasc Surg 2005; 53: 285– 90. [DOI] [PubMed] [Google Scholar]
- 17). Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001; 72: 362– 5. [DOI] [PubMed] [Google Scholar]
- 18). Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 1993; 56: 1285– 9. [DOI] [PubMed] [Google Scholar]
- 19). Langdon SE, Seery K, Kulik A. Contemporary outcomes after pericardial window surgery: impact of operative technique. J Cardiothorac Surg 2016; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008; 26: 355– 67, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000; 70: 1644– 6. [DOI] [PubMed] [Google Scholar]
- 22). Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012; 256: 487– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Salim EF, Rezk ME. Thoracoscopic versus subxiphoid pericardial window in patients with end-stage renal disease. J Egypt Soc Cardio-Thorac Surg 2018; 26: 212– 8. [Google Scholar]