Abstract
Purpose: The impact of chronic kidney disease (CKD) on the prognosis of transcatheter aortic valve replacement (TAVR) remains unclear. The purpose of this meta-analysis was to assess the impact of CKD and different stages of CKD on prognosis in patients undergoing TAVR.
Methods: As of June 2020, we performed a comprehensive literature search on relevant studies using PubMed, Embase, Cochrane Library, and Web of Science. Subsequently, we pooled the risk ratio (RR) of individual studies via random effects to analyze heterogeneity, quality assessment, and publication bias.
Results: A total of 20 studies, involving 133624 patients, were eligible for analysis. Patients with CKD had higher all-cause mortality at 30 days (RR: 1.39, 95% confidence interval [CI]: 1.31–1.47, P <0.001), 1 year (RR: 1.36, 95% CI: 1.24–1.49, P <0.001), and 2 years (RR: 1.2, 95% CI: 1.05–1.38, P = 0.009) of follow-up. Moreover, they also had higher acute kidney injury (AKI) (RR: 1.38, 95% CI: 1.16–1.63, P <0.001) and bleeding (RR: 1.33, 95% CI: 1.18–1.50, P <0.001) at 30 days. CKD3 alone also increased all-cause mortality at follow-ups. Risk of all-cause mortality increased with severity of CKD for stages 3, 4, and 5 at follow-up.
Conclusion: Patients with CKD are at an increased risk of all-cause mortality, AKI, and bleeding events after TAVR. Moreover, the mortality risk rises with increasing severity of CKD.
Keywords: chronic kidney disease, transcatheter aortic valve replacement, meta-analysis, prognosis
Introduction
With a rise in the global aging population, aortic stenosis (AS) has become one of the most common valvular diseases.1) Apart from affecting patient’s quality of life, severe AS can bring about death, in a relatively short period, if not treated with valve replacement.2) In the last decade, transcatheter aortic valve replacement (TAVR) has gained popularity as an alternative to surgical aortic valve replacement (SAVR) for patients, who are either inoperable or are at high risk to intermediate risk for surgery.3,4)
Chronic kidney disease (CKD) often coexists with AS, likely due to similar risk factors and pathophysiology.5) Recently, CKD was reported in approximately 75% of patients with severe AS.6) Mechanistically, it was demonstrated that CKD accelerates dystrophy calcification in the aortic valve, which contributes to severe AS 10 to 20 years earlier than in the general population.7) It is well known that CKD is detrimental to the course of valvular heart disease and prognosis of cardiovascular intervention.8,9) Moreover, the presence of CKD is also shown to increase both short- and long-term mortality in SAVR patients, with short-term mortality reaching as high as 21%.10)
The prognostic effects of CKD on TAVR, however, remain unclear. Moreover, little is known about the difference of prognosis among different CKD stages. High-quality meta-analysis is increasingly recognized as one of the key tools in the assessment of clinical effectiveness.11,12) At present, there is no meta-analysis on the outcome of preoperative CKD on long-term prognosis of TAVR. Therefore, our meta-analysis aimed to investigate the outcome of CKD, and different stages of CKD, on the short-, medium-, and long-term prognosis of TAVR patients.
Methods
The selected publications were systematically reviewed,13) according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines, and the complete research protocol was registered in the Prospero database (CRD42020200317).14,15) In addition, the methodology quality was assessed using the Assessment of Multiple Systematic Reviews tool.16,17)
Search strategy
We conducted an extensive and systematic computerized literature review, using the PubMed, Cochrane Library, EMBASE, and Web of Science databases, and searched from the inception of indicated databases till June 2020 using general terminology such as “TAVI,” “TAVR,” “transcatheter aortic valve implantation,” “transcatheter aortic valve replacement,” “chronic kidney disease,” “chronic kidney failure,” and “CKD.” To further ensure no relevant publications were overlooked, we also manually searched the list of references for publications that might meet our requirements.
Study selection
Two researchers independently conducted literature searches, qualification assessment, and data extraction. In case of disagreement, it was settled through mutual discussion or negotiation with a third party. The inclusion criteria for the meta-analysis were as follows: (1) report of clinical outcome of AS and CKD patients after TAVR and (2) participants without CKD served as controls. Alternately, the exclusion criteria included the following: (1) repeated publication or overlapping of patients; (2) unclear report or unable to calculate relevant results based on published data; (3) conference, reviews, case reports, and editorials; and (4) non-English language studies.
Study end points
The main outcome of our study was all-cause mortality after TAVR at the short-term (30 days), medium-term (1 year), and long-term (2 years) follow-ups. Secondary outcomes included stroke, bleeding, permanent pacemaker implantation (PPI), acute kidney injury (AKI), and major vascular complications at the short-term (30 days) follow-up. All outcomes after TAVR were defined according to the standard described by VARC.
Data extraction
We used standardized data sheets to extract data of patients and studies, including study type, first author, region, year, the number of patients, CKD definition, follow-up time, age, gender, left ventricular ejection fraction, past history, logistic European system for cardiac operative risk evaluation (EuroSCORE), Society of Thoracic Surgeons (STS) score, access site, and valve type.
Quality assessment
The risk of bias in the cohort study was assessed using the Newcastle Ottawa Scale.18) The quality score consisted of three main parts: the selection of study group, comparability of study group, and determination of the result of interest. A study with a score >7 (out of 9) was considered to have a low bias risk; moderate risk was 5–7 and high risk was <5.
Definition of CKD
CKD was defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, whereas CKD3 was eGFR ≥30–59 mL/min/1.72 m², CKD4 was eGFR ≥15–29 mL/min/1.72 m2, CKD5 was eGFR <15 mL/min/1.72 m2, and lastly, no CKD was normal kidney function to CKD2: eGFR ≥60 ml/min/1.73 m2.
Statistical analysis
The frequency of categorical variables and the standardized means with standard deviations of continuous variables were used for descriptive analysis. The risk ratio (RR) and 95% confidence interval (CI) of the results were performed using a meta-analysis for random effects models. The evaluation of the heterogeneity of different studies used the Cochrane Q-statistic to calculate the I² values, where <25%, 25%–50%, and >50%, respectively, indicated low, medium, and high heterogeneity. Sensitivity analysis further explored significant heterogeneity. Publication bias was assessed by funnel plot asymmetric analysis and Egger’s regression test. P values were bilateral, and P <0.05 was set as statistical significance threshold. Stata15.0 (StataCorp, College Station, TX, USA) statistical analysis software was used for data analysis.
Results
Study selection
As illustrated in Fig. 1, 1480 publications were identified in the preliminary search. In addition, 12 suitable publications were obtained from the list of references. After eliminating study duplicates, 1130 retrieved articles were screened by title and abstract. In all, 45 articles were read in full to determine their inclusion in the analysis. A total of 25 more publications were eliminated due to 11 lacking a control group, 13 not producing an outcome of interest, and 1 not being written in the English language. Finally, 20 articles, with a total of 133624 patients, fulfilled the inclusion criteria and were selected for analysis.19–38)
Fig. 1. Flow chart of the publication selection process, based on the PRISMA statement. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Study characteristics and quality assessment
A majority of the selected publications (10) were retrospective in nature and were published between the years 2012 and 2020. Table 1 summarizes the contents of these publications, including the study design, year of publication, country of research, and CKD definition. Based on the Newcastle Ottawa observational study bias risk scale (Table 2), the total score of 20 observational studies was >5, indicating that the bias risk was low.
Table 1. Study characteristics.
| Study | Year | Country | Study design | CKD group |
|---|---|---|---|---|
| D’Ascenzo et al.20) | 2013 | Italy | Prospective, multicenter | eGFR ≥60 mL/min/1.73 m2, 30–59 mL/min/1.73 m2, 15-29 mL/min/1.73 m2 |
| Wessely et al.19) | 2012 | Germany | Retrospective, single center | Pre-procedural eGFR ≤60 mL/min, equivalent to CKD stage ≥3 |
| Dumonteil et al.21) | 2013 | France | Retrospective, multicenter | Normal eGFR (≥90 mL/min), mild (60–89 mL/min), moderate (30–59 mL/min), and severe (<30 mL/min) CKD and those on chronic hemodialysis |
| Yamamoto et al.24) | 2013 | France | Prospective, multicenter | eGFR ≥60 ml/min/1.73 m² (CKD stage 1 + 2), 30–59 ml/min/1.73 m² (CKD stage 3), 15–29 ml/min/1.73 m² (CKD stage 4) |
| Goebel et al.22) | 2013 | Germany | Prospective, single center | Normal kidney function, CKD stage 3 and worse |
| Nguyen et al.23) | 2013 | USA | Retrospective, single center | Mild or normal (GFR >60), moderate (30 <GFR ≤60), severe or dialysis (GFR ≤30 or preoperative dialysis) |
| Allende et al.25) | 2014 | Canada | Retrospective, multicenter | CKD stages 1–2 (eGFR ≥60 mL/min/1.73 m2), stage 3 (30–59 mL/min/1.73 m2), stage 4 (15–29 mL/min/1.73 m2) and stage 5 (<15 mL/min/1.73 m2) |
| Rahman et al.27) | 2015 | UK | Retrospective, single center | CKD group (eGFR <60 mL/min/1.73 m2) and no-CKD group (eGFR >60 mL/min/1.73 m2) |
| Ferro et al.26) | 2015 | UK | Prospective, multicenter | eGFR: ≥60 (CKD stages 1–2), 45–59 (CKD stage 3a), 30–44 (CKD stage 3b), 15–29 (CKD stage 4), <15 mL/min/1.73 m2 or on dialysis (CKD stage 5) |
| Thourani et al.29) | 2016 | USA | Retrospective, multicenter | None/mild RD (GFR >60 mL/min), moderate RD (GFR 30–60 mL/min), severe RD (GFR ≤30 mL/min) |
| Codner et al.28) | 2016 | Europe | Prospective, multicenter | eGFR >60 mL/min/1.73 m2 (group I), 31–60 mL/min/1.73 m2 (group II), ≤30 mL/min/1.73 m2 (group III), dialysis (group IV) |
| Lüders et al.32) | 2017 | Germany | Retrospective, multicenter | Structural abnormalities or genetic traits that point to kidney disease (CKD stage 1), normal or mild reduced renal function (CKD stage 2), moderate renal insufficiency (CKD stage 3), severe renal insufficiency (CKD stage 4), end-stage renal failure (CKD stage 5). |
| Gupta et al.30) | 2017 | USA | Retrospective, multicenter | Patients with CKD were identified using ICD-9-CM codes 35.05 and 35.06 |
| Hansen et al.31) | 2017 | USA | Retrospective, multicenter | CKD stage 1: eGFR >90 mL/min/m2, stage 2: GFR 60–89 mL/min/m2, stage 3: eGFR of 30–59 mL/min/m2, stage 4: GFR of 15–29 mL/min/m2, stage 5: eGFR <15 mL/min/m2 |
| Franzone et al.33) | 2018 | Switzerland. | Prospective, multicenter | None or mild renal dysfunction: eGFR ≥60 mL/min/1.73 m2, moderate renal dysfunction: eGFR 30–59 mL/min/1.73 m2, severe renal dysfunction eGFR ≤30 mL/min/1.73 m2 |
| Pineda et al.34) | 2019 | USA | Prospective, multicenter | None/mild (eGFR >60 mL/min/1.73 m²), moderate/severe (eGFR ≤60 mL/min/1.73 m²) |
| Yap et al.38) | 2020 | Singapore | Prospective, single center | CKD 1 (eGFR ≥90 mL/min/1.73 m2), CKD 2 (eGFR 69–89 mL/ min/1.73 m2), CKD 3 (eGFR ≥30–59 mL/min/1.73 m2), CKD 4 (eGFR ≥15–29 mL/min/1.73 m2), CKD 5 or ESRF (eGFR <15 mL/min/1.73 m2) |
| Li et al.37) | 2021 | USA | Retrospective, single center | eGFR >60, eGFR = 30–60, eGFR <30 |
| Bandyopadhyay et al.35) | 2020 | International | Prospective, multicenter | No CKD: eGFR ≥60 ml/min/1.73 m2, mild CKD: eGFR 45–59 ml/min/1.73 m2, moderate/severe CKD: eGFR <45 ml/min/1.73 m2 |
| Gracia et al.36) | 2020 | USA | Prospective, single center | CrCl ≥60 mL/min, CrCl 30–60 mL/min, CrCl <30 mL/min |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; GFR: glomerular filtration rate; ICD-9-CM: international classification of diseases, ninth revision, clinical modification; ESRF: end stage renal failure; CrCl: creatinine clearance
Table 2. Baseline features of patients.
| Study | Total | Male | Age(Y) | DM | HTN | PVD | COPD | LVEF | Transfemoral | STS score | Logistic EuroSCORE | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D’Ascenzo et al.20) | 364 | 42.3 | 82.4 | 31.2 | 86.6 | 23.6 | NR | 52.4 | 84.2 | 6.6 | 23.2 | 7 |
| Wessely et al.19) | 183 | 44.8 | 81.1 | 30.0 | 84.2 | 12.2 | 23.9 | 58.7 | NR | NR | 23.5 | 8 |
| Dumonteil et al.21) | 942 | 53.8 | 81.0 | 28.5 | 69.5 | 25.3 | 34.5 | NR | 84.1 | NR | 20.9 | 7 |
| Yamamoto et al.24) | 642 | 48.1 | 83.6 | 22.6 | 70.6 | 28.5 | 29.1 | 51.0 | 67.1 | 6.8 | 19.9 | 7 |
| Goebel et al.22) | 270 | 44.4 | 81.6 | 27.6 | 96.3 | 41.6 | 22.1 | NR | NR | 14.0 | 33.5 | 6 |
| Nguyen et al.23) | 321 | 55.8 | 82.3 | 43.6 | 95.0 | 34.6 | 48.9 | 49.2 | NR | NR | NR | 8 |
| Allende et al.25) | 2075 | 49.9 | 80.1 | 30.1 | 78.8 | 20.1 | 29.8 | 54.7 | 73.7 | 6.5 | 29.9 | 8 |
| Rahman et al.27) | 118 | 57.6 | 81.3 | 22.0 | NR | 6.8 | 24.6 | NR | 77.1 | NR | 20.9 | 8 |
| Ferro et al.26) | 3696 | 53.5 | 82.3 | 22.4 | NR | NR | 27.0 | NR | 70.1 | NR | 18.9 | 8 |
| Thourani et al.29) | 2531 | 52.4 | 84.5 | 36.4 | 91.7 | 42.3 | 55.9 | 52.4 | 57.7 | 11.5 | NR | 9 |
| Codner et al.28) | 1204 | 44.5 | 81.6 | 31.7 | 91.8 | NR | 25.8 | 51.9 | 90.7 | 7.0 | NR | 7 |
| Lüders et al.32) | 28716 | 43.9 | 81.0 | 33.5 | 62.9 | 12.0 | 15.2 | NR | 69.7 | NR | 19.9 | 7 |
| Gupta et al.30) | 41025 | 52.3 | 81.1 | 34.6 | 79.5 | 29.7 | 33.2 | NR | NR | NR | NR | 7 |
| Hansen et al.31) | 44778 | 51.3 | 82.0 | 36.7 | 89.4 | 30.5 | NR | 57.0 | 71.9 | NR | NR | 7 |
| Franzone et al.33) | 927 | 46.9 | 82.6 | 26.0 | 84.0 | 11.3 | 13.3 | 53.9 | NR | 6.12 | 19.6 | 8 |
| Pineda et al.34) | 3733 | 54.1 | 83.2 | 37.3 | 92.8 | 45.4 | 54.3 | 53.9 | 80.5 | 8.9 | 22.1 | 7 |
| Yap et al.38) | 216 | 49.1 | 75.5 | 39.8 | 81.9 | 16.2 | 10.2 | NR | 77.8 | 6.5 | 16.1 | 7 |
| Li et al.37) | 733 | 49.9 | 82.0 | 30.5 | 85.9 | 36.4 | NR | NR | 67.8 | NR | NR | 8 |
| Bandyopadhyay et al.35) | 852 | 0 | 82.2 | 26.1 | 81.2 | 10.1 | 18.3 | 56.1 | 90.6 | 7.9 | NR | 7 |
| Gracia et al.36) | 298 | 51.7 | 79.9 | 38.9 | NR | 8.1 | 20.8 | NR | 99.7 | NR | NR | 7 |
Values are mean or % (n/N)
DM: diabetes mellitus; HTN: hypertension; LVEF: left ventricular ejection fraction; PVD: peripheral vascular disease; EuroSCORE: European system for cardiac operative risk evaluation; STS: society of thoracic surgeons; COPD: chronic obstructive pulmonary disease; NR: not reported; NOS: Newcastle-Ottawa scale
Patient characteristics
The baseline characteristics of patients, included in this study, are summarized in Table 2. A total of 133624 patients with AS received TAVR. The average age was 81.8 years (75.584.5 years), and among them, 49.9% (0–57.6%) were men. In 7 studies (among 12 reports), the average logistic EuroSCORE was >20%. In all 9 reported studies, the average STS score was >5%. Of the 133624 patients, 30.9% had diabetes mellitus (ranging from 22% to 43.6%), 84.2% had hypertension (ranging from 62.9% to 96.3%), 48.9% had peripheral vascular disease (ranging from 6.8% to 45.4%), and 25.8% had chronic obstructive pulmonary disease (ranging from 10.2% to 55.9%). Generally, the most common vascular access is the transfemoral approach. In the selected publications, only balloon-expandable valves were used in one study, only self-expandable valves were used in one study, and multiple valve types were used in the remaining studies.
All-cause mortality
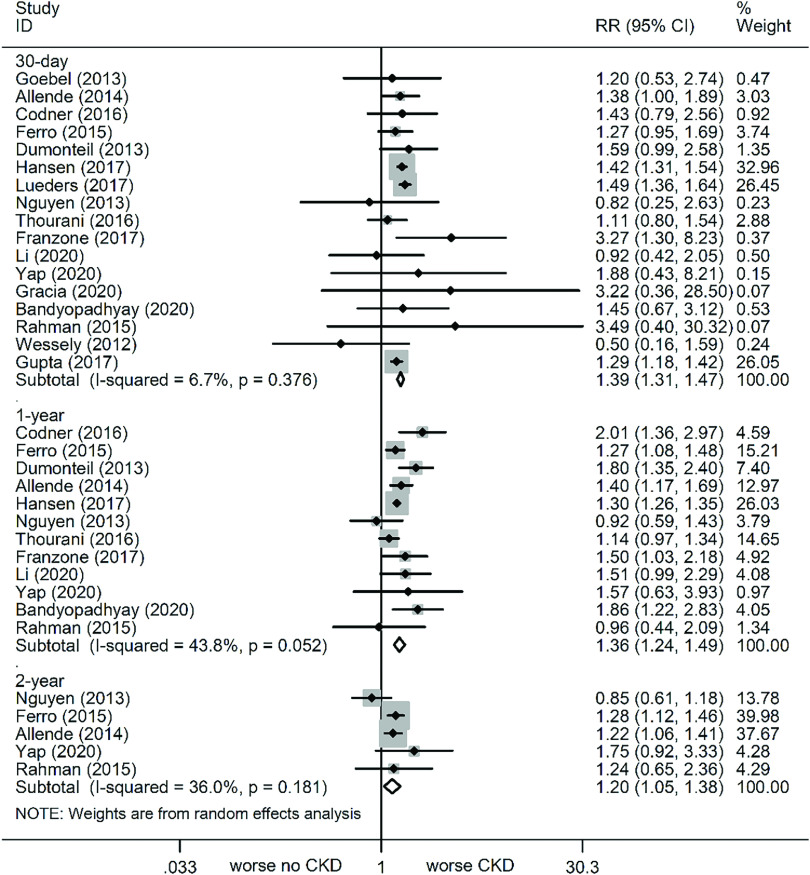
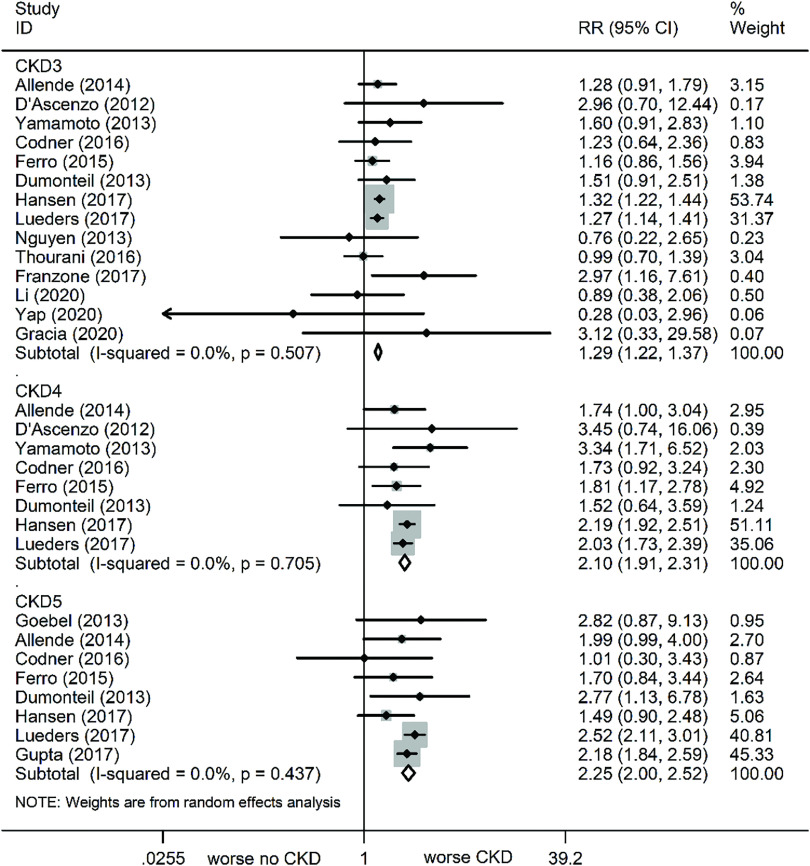
At the 30-day follow-up, all-cause mortality was markedly increased in all stage CKD patients, as compared to no CKD (Fig. 2). The relevant statistics are as follows: CKD (RR: 1.39, 95% CI: 1.31–1.47, P <0.001, I² = 6.7%), CKD3 (RR: 1.29, 95% CI: 1.22–1.37, P <0.001, I² = 0%), CKD4 (RR: 2.1, 95% CI: 1.91–2.31, P <0.001, I² = 0%), and CKD5 (RR: 2.25, 95% CI: 2.0–2.51, P <0.001, I² = 0%) (Fig. 3).
Fig. 2. Forest plots comparing all-cause mortality risk between CKD and non-CKD patients. CKD: chronic kidney disease; RR: risk ratio; CI: confidence interval.
Fig. 3. Forest plots comparing 30-day all-cause mortality risk in varying severity CKD patients. CKD: chronic kidney disease; RR: risk ratio; CI: confidence interval.
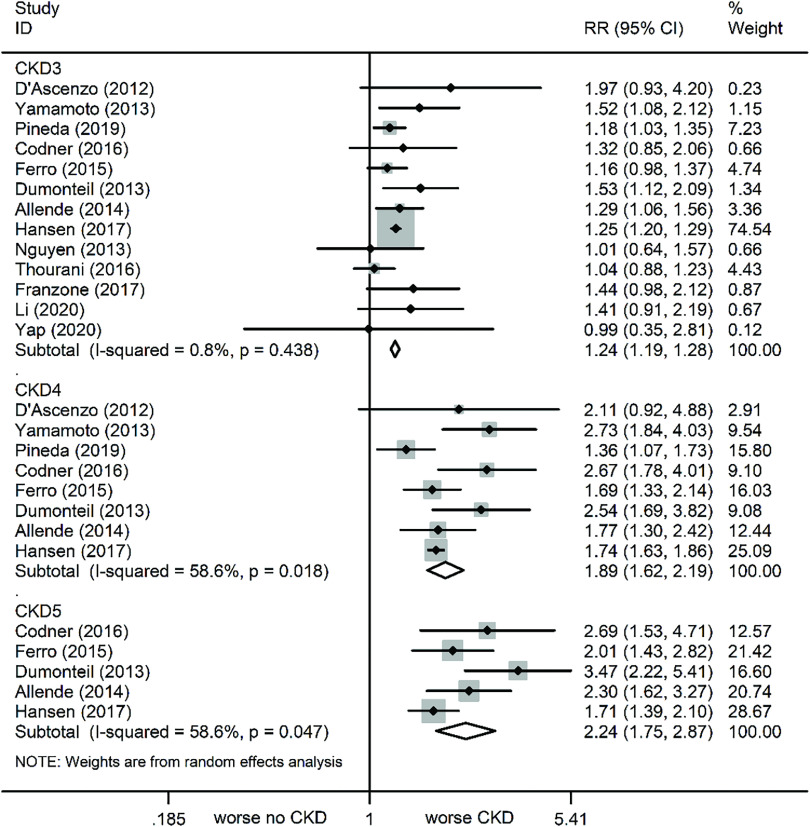
At the 1-year follow-up, all-cause mortality was again markedly increased in patients with varying stages of CKD versus no CKD (Fig. 2). The relevant statistics are as follows: CKD (RR: 1.36, 95% CI: 1.24–1.49, P <0.001, I² = 43.8%), CKD3 (RR: 1.24, 95% CI: 1.19–1.28, P <0.001, I² = 0.8%), CKD4 (RR: 1.89, 95% CI: 1.62–2.19, P <0.001, I² = 58.6%), and CKD5 (RR: 2.24, 95% CI: 1.75–2.87, P <0.001, I² = 58.6%) (Fig. 4). Of note, although sensitivity analyses were conducted one by one to exclude studies, the results remained unchanged.
Fig. 4. Forest plots comparing 1-year all-cause mortality risk in varying severity CKD patients. CKD: chronic kidney disease; RR: risk ratio; CI: confidence interval.
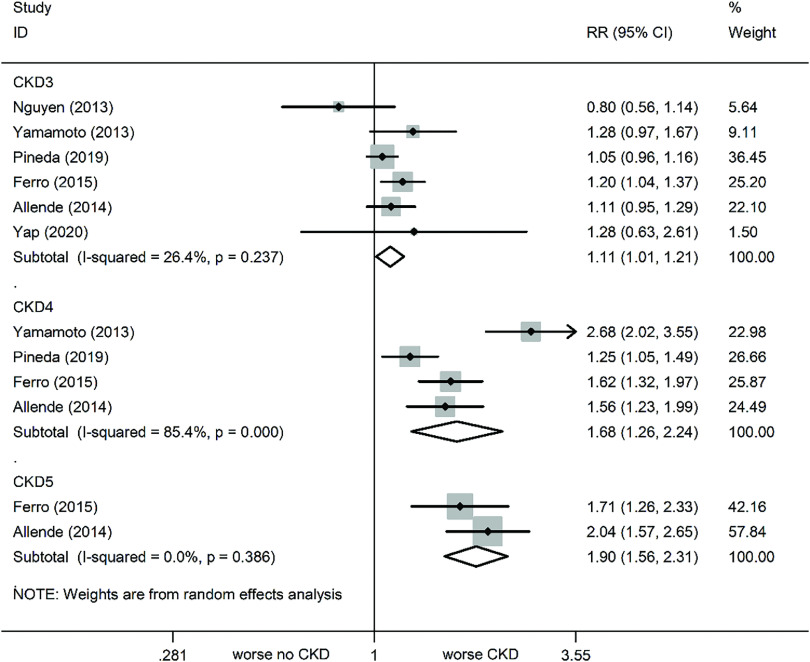
At the 2-year follow-up, all-cause mortality was still increased in patients with varying stages of CKD, as compared to no CKD (Fig. 2). The relevant statistics are as follows: CKD (RR: 1.2, 95% CI: 1.05–1.38, P = 0.009, I² = 36%), CKD3 (RR: 1.11, 95% CI: 1.01–1.21, P = 0.024, I² = 26.4%), CKD4 (RR: 1.68, 95% CI: 1.26–2.24, P <0.001, I² = 85.4%), and CKD5 (RR: 1.9, 95% CI: 1.56–2.31, P <0.001, I² = 0%) (Fig. 5). Subsequent sensitivity analyses failed to alter the results.
Fig. 5. Forest plots comparing 2-year all-cause mortality risk in varying severity CKD patients. CKD: chronic kidney disease; RR: risk ratio; CI: confidence interval.
Secondary outcomes
Bleeding
Based on our results, CKD patients of all stages are more prone to bleeding than patients without CKD (Table 3). The relevant statistics are as follows: CKD (RR: 1.33, 95% CI: 1.18–1.50, P <0.001, I² = 47.4%), CKD3 (RR: 1.26, 95% CI: 1.02–1.55, P = 0.034, I² = 55.3%), CKD4 (RR: 1.56, 95% CI: 1.07–2.26, P = 0.021, I² = 57.4%), and CKD5 (RR: 1.58, 95% CI: 1.05–2.38, P = 0.029, I² = 68.8%). Upon excluding each study one by one, the sensitivity analysis results still remained the same.
Table 3. Secondary outcomes.
| Outcomes | Studies | Patients | RE RR | 95% CI | P-value | I2 |
|---|---|---|---|---|---|---|
| AKI | ||||||
| CKD | 5 | 4267 | 1.38 | 1.16–1.63 | P <0.001 | 13.1 |
| CKD3 | 5 | 3738 | 1.28 | 1.11–1.48 | P = 0.001 | 0 |
| CKD4 | 4 | 2018 | 2.12 | 1.73–2.59 | P <0.001 | 0 |
| CKD5 | 2 | 1487 | 1.9 | 1.37–2.62 | P <0.001 | 0 |
| Bleeding | ||||||
| CKD | 10 | 53358 | 1.33 | 1.18–1.50 | P <0.001 | 47.4 |
| CKD3 | 9 | 10484 | 1.26 | 1.02–1.55 | P = 0.034 | 55.3 |
| CKD4 | 4 | 3444 | 1.56 | 1.07–2.26 | P = 0.021 | 57.4 |
| CKD5 | 4 | 30252 | 1.58 | 1.05–2.38 | P = 0.029 | 68.8 |
| Major vascular complications | ||||||
| CKD | 7 | 47759 | 1.05 | 0.97–1.13 | P = 0.203 | 0 |
| CKD3 | 6 | 5978 | 1.11 | 0.93–1.33 | P = 0.240 | 0 |
| CKD4 | 4 | 2018 | 1.26 | 0.88–1.81 | P = 0.211 | 0 |
| CKD5 | 3 | 28763 | 1.01 | 0.57–1.79 | P = 0.983 | 37.3 |
| Stroke | ||||||
| CKD | 9 | 49616 | 1.21 | 0.86–1.70 | P = 0.268 | 50.5 |
| CKD3 | 9 | 8267 | 1.26 | 0.95–1.67 | P = 0.112 | 0 |
| CKD4 | 5 | 3723 | 2.41 | 1.60–3.63 | P <0.001 | 0 |
| CKD5 | 5 | 30408 | 0.98 | 0.56–1.71 | P = 0.953 | 19.3 |
| PPI | ||||||
| CKD | 8 | 48027 | 1.09 | 0.96–1.25 | P = 0.192 | 38.8 |
| CKD3 | 5 | 5686 | 1.08 | 0.94–1.26 | P = 0.279 | 0 |
| CKD4 | 3 | 1872 | 0.77 | 0.54–1.11 | P = 0.161 | 0 |
| CKD5 | 4 | 28917 | 1.3 | 0.69–2.46 | P = 0.412 | 71.3 |
CI: confidence interval; RR: risk ratio; CKD: chronic kidney disease; AKI: acute kidney injury; PPI: permanent pacemaker implantation
AKI
CKD patients of all stages were at a higher risk for AKI, relative to patients without CKD (Table 3). The relevant statistics are as follows: CKD (RR: 1.38, 95% CI: 1.16–1.63, P <0.001, I² = 13.1%), CKD3 (RR: 1.28, 95% CI: 1.11–1.48, P = 0.001, I² = 0%), CKD4 (RR: 2.12, 95% CI: 1.73–2.59, P <0.001, I² = 0%), and CKD5 (RR: 1.9, 95% CI: 1.37–2.62, P <0.001, I² = 0%).
Stroke
According to the pooled results of short-term stroke, CKD4 patients who received TAVR were significantly more vulnerable to stroke than non-CDK patients who received TAVR (RR: 2.41, 95% CI: 1.6–3.63, P <0.001, I² = 0%) (Table 3). However, there was no discernible difference between other stages of CKD patients and non-CKD patients who received TAVR. The relevant statistics are as follows: CKD (RR: 1.21, 95% CI: 0.86–1.70, P = 0.268, I² = 50.5%), CKD3 (RR: 1.26, 95% CI: 0.95–1.67, P = 0.112, I² = 0%), and CKD5 (RR: 0.98, 95% CI: 0.56–1.71, P = 0.953, I² = 19.3%). Of note, one by one exclusion of studies did not alter the sensitivity analysis results.
Major vascular complications
There were no discernible differences in the major vascular complications between CKD of all stages and no CKD (Table 3). The relevant statistics are as follows: CKD (RR: 1.05, 95% CI: 0.97–1.13, P = 0.203, I² = 0%), CKD3 (RR: 1.11, 95% CI: 0.93–1.33, P = 0.240, I² = 0%), CKD4 (RR: 1.26, 95% CI: 0.88–1.81, P = 0.211, I² = 0%), and CKD5 (RR: 1.01, 95% CI: 0.57–1.79, P = 0.983, I² = 19.3%).
PPI
There were no discernible differences in the PPI risk between CKD of all stages and no CKD (Table 3). The relevant statistics are as follows: CKD (RR: 1.09, 95% CI: 0.96–1.25, P = 0.192, I² = 38.8%), CKD3 (RR: 1.08, 95% CI: 0.94–1.26, P = 0.279, I² = 0%), CKD4 (RR: 0.77, 95% CI: 0.54–1.11, P = 0.161, I² = 0%), and CKD5 (RR: 1.3, 95% CI: 0.69–2.46, P = 0.412, I² = 71.3%).
Publication bias
Funnel plots determined main outcome publication bias, and the results revealed good symmetry. The P-values obtained by the Egger’s regression test were 0.76 (30 days), 0.34 (1 year), and 0.83 (2 years), indicating the lack of publication bias.
Discussion
The current report, involving 20 studies on 133624 patients with 58315 events of CKD, is the first pooled analysis of the effect of CKD on long-term clinical outcome after TAVR. The main outcomes of this study were as follows. (1) Preoperative CKD increased all-cause mortality in patients with TAVR, according to the short-, medium- and long-term follow-ups. (2) Preoperative CKD elevated procedural complications, including AKI and bleeding, but no differences were observed in the major vascular complications, stroke, and PPI. (3) The all-cause mortality after TAVR was higher in patients with moderate CKD (CKD3) than in patients without CKD. (4) Lastly, the risk of all-cause mortality and bleeding increased with the severity of CKD.
With an increase in TAVR recommendations, it is essential to identify prognosis-related risk factors, among which is CKD. The relationship between CKD and prognosis after TAVR is controversial. Gupta et al.,30) for instance, conducted a national study of 41000 patients, who received TAVR between 2012 and 2014, in the United States. According to their report, CKD or end stage renal failure (ESRF) patients were more susceptible to in-hospital deaths than patients without CKD (3.8% vs. 4.5% vs. 8.3%, P <0.001). In another study, involving 42189 patients receiving TAVR from 2011 to 2014,39) 62.1% (n = 26229) did not have CKD or ESRD, 33.7% (n = 14252) were diagnosed with CKD, and 4% (n = 1708) had ESRD. Using the non-CKD/ESRD patients as reference, the in-hospital mortality of CKD patients (4.5% vs. 3.7%, odds ratio [OR] = 1.34, 95% CI: 1.20–1.31) and ESRD patients (8.2% vs. 3.7%, OR = 2.51, 95% CI: 2.02–3.12) were reported to be significantly elevated (both P <0.001). However, not all studies point to CKD as a critical independent predictor of mortality in TAVR recipients. Goebel et al.,22) for instance, demonstrated that CKD3 or higher patients did not exhibit an increase in the 30-day mortality rate after TAVR (7.0% vs. 7.1%, P = 0.97). Moreover, there have been conflicting results on the impact of CKD3 on clinical prognosis. While some studies reported that CKD3 is not a predictor of mortality after TAVR,28,40) remaining studies suggested otherwise.41,42). Bohbot et al.,43) for example, published the largest study on severe AS patients with CKD. Upon adjusting for the established outcome predictors, patients with moderate or severe CKD were shown to have higher all-cause mortality compared to those without CKD.
With our extensive review of all published data, we compiled substantial evidence that CKD, particularly CKD3, is associated with an enhanced all-cause mortality rate during follow-ups after TAVR. The most likely reasons for this may be as follows: First, the patients, receiving TAVR, have a higher incidence of coronary heart disease, systolic and diastolic heart failure, and conduction disturbance. Underlying CKD can exacerbate these cardiovascular abnormalities, thereby increasing the risk of death.25) Moreover, due to the advanced age of CKD patients, the logistic EuroSCORE and STS score were significantly higher, thus negatively impacting the survival of patients after TAVR.35,36) Conversely, Codner et al. published a report that failed to show an association between CKD3 and 1-year mortality rate (OR: 1.66; 95% CI: 0.95–2.9).28) However, Gargiulo et al.41) demonstrated an increased risk of 1-year all-cause mortality (OR: 1.34, 95% CI: 1.11–1.64) in CDK patients after TAVR. The discrepancy in results from different trials may be due to inadequate sample size and incomparable baseline demographics of patients.
With regard to TAVR surgical complications, Gupta et al.30) demonstrated an increased risk of major bleeding in CKD patients versus non-CVD patients (16.8% vs. 13.1%). Similarly, in our study, the incidence of post-TAVR bleeding in CKD patients was remarkably higher than in patients without CKD. The coagulation dysfunction in CKD has multifactorial mechanisms that include loss of normal platelet function, due to excess uremic toxins and metabolites; interaction of platelet–vascular wall affected by anemia; vascular structural changes caused by arteriosclerosis; impaired endothelial integrity; and insufficient anticoagulant excretion.44)
In the latest report by Bandyopadhyay et al.,35) AKI risk did not alter significantly between CKD patients and non-CKD patients after TAVR. Interestingly, in another study, CKD patients were reported to have a markedly elevated rate of AKI after TAVR, specifically, 34% AKI in CKD patients versus 10.6% in patients without CKD.30) Consistent with the later study, we also observed a significantly elevated AKI risk in CKD versus non-CKD patients. Among the contributing factors of AKI are the type and volume of contrast media used, hypotension caused by rapid ventricular pacing and balloon aortic valvuloplasty, microembolic events after catheter advancement, prosthesis implantation and valve expansion, and potential hemodynamic disorders associated with paravalvular regurgitation or arrhythmias.33) In multiple studies, AKI has been proposed to be an independent risk factor for increased mortality.25) Therefore, patients with severe CKD require appropriate procedural planning to reduce AKI and subsequent mortality risk.
Limitations
This meta-analysis had certain limitations. (1) There may have been some bias in the inclusion of research and registration data. (2) Some mortality data, extracted from the Kaplan–Meier curve, may have provided a less than accurate result. (3) We used a summary of events published in each study, rather than individual data. Therefore, confounding and selection bias cannot be ruled out. (4) Most of the studies reported only up to 1-year mortality, and few studies assessed long-term outcomes of CKD. (5) The research language was limited to English, which may lead to potential language bias.
Conclusion
In patients with AS, CKD increased the 30-day, 1-year, and 2-year all-cause mortality after TAVR. In addition, poor prognosis risk rises with increasing severity of CKD. Hence, our results contribute to the stratification of CKD-related risks in patients receiving TAVR. It is urgent and necessary to fully examine the specific prevention and management measures that would optimize the prognostic outcomes of AS and CKD patients undergoing TAVR.
Acknowledgment
The authors thank all participants who contribute to this study.
Funding
This study was supported by the Natural Science Foundation of Gansu Province (Grant No. 20JR10RA689).
Disclosure Statement
All authors have no conflicts of interest relevant to the topic in discussion.
References
- 1). Kanwar A, Thaden JJ, Nkomo VT. Management of patients with aortic valve stenosis. Mayo Clin Proc 2018; 93: 488– 508. [DOI] [PubMed] [Google Scholar]
- 2). Clark MA, Arnold SV, Duhay FG, et al. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis: results from a Medicare claims analysis. Circ Cardiovasc Qual Outcomes 2012; 5: 697– 704. [DOI] [PubMed] [Google Scholar]
- 3). Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385: 2485– 91. [DOI] [PubMed] [Google Scholar]
- 4). Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609– 20. [DOI] [PubMed] [Google Scholar]
- 5). Iung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart 2012; 98 Suppl 4: iv7– 13. [DOI] [PubMed] [Google Scholar]
- 6). Dellegrottaglie S, Saran R, Gillespie B, et al. Prevalence and predictors of cardiovascular calcium in chronic kidney disease (from the Prospective Longitudinal RRI-CKD Study). Am J Cardiol 2006; 98: 571– 6. [DOI] [PubMed] [Google Scholar]
- 7). London GM, Pannier B, Marchais SJ, et al. Calcification of the aortic valve in the dialyzed patient. J Am Soc Nephrol 2000; 11: 778– 83. [DOI] [PubMed] [Google Scholar]
- 8). Kahn MR, Robbins MJ, Kim MC, et al. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol 2013; 10: 261– 73. [DOI] [PubMed] [Google Scholar]
- 9). Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339– 52. [DOI] [PubMed] [Google Scholar]
- 10). Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation 2002; 105: 1336– 41. [DOI] [PubMed] [Google Scholar]
- 11). Yao L, Sun R, Chen YL, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol 2016; 74: 73– 9. [DOI] [PubMed] [Google Scholar]
- 12). Tian J, Zhang J, Ge L, et al. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol 2017; 85: 50– 8. [DOI] [PubMed] [Google Scholar]
- 13). Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Ge L, Tian JH, Li YN, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 2018; 93: 45– 55. [DOI] [PubMed] [Google Scholar]
- 15). Wang X, Chen Y, Yao L, et al. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol 2018; 98: 1– 8. [DOI] [PubMed] [Google Scholar]
- 16). Pieper D, Buechter RB, Li L, et al. Systematic review found AMSTAR, but not R(evised)-AMSTAR, to have good measurement properties. J Clin Epidemiol 2015; 68: 574– 83. [DOI] [PubMed] [Google Scholar]
- 17). Yan P, Yao L, Li H, et al. The methodological quality of robotic surgical meta-analyses needed to be improved: a cross-sectional study. J Clin Epidemiol 2019; 109: 20– 9. [DOI] [PubMed] [Google Scholar]
- 18). Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013; 7: e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Wessely M, Rau S, Lange P, et al. Chronic kidney disease is not associated with a higher risk for mortality or acute kidney injury in transcatheter aortic valve implantation. Nephrol Dial Transplant 2012; 27: 3502– 8. [DOI] [PubMed] [Google Scholar]
- 20). D’Ascenzo F, Moretti C, Salizzoni S, et al. 30 days and midterm outcomes of patients undergoing percutaneous replacement of aortic valve according to their renal function: a multicenter study. Int J Cardiol 2013; 167: 1514– 8. [DOI] [PubMed] [Google Scholar]
- 21). Dumonteil N, van der Boon RM, Tchetche D, et al. Impact of preoperative chronic kidney disease on short- and long-term outcomes after transcatheter aortic valve implantation: a Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC-Plus) initiative substudy. Am Heart J 2013; 165: 752– 60. [DOI] [PubMed] [Google Scholar]
- 22). Goebel N, Baumbach H, Ahad S, et al. Transcatheter aortic valve replacement: does kidney function affect outcome? Ann Thorac Surg 2013; 96: 507– 12. [DOI] [PubMed] [Google Scholar]
- 23). Nguyen TC, Babaliaros VC, Razavi SA, et al. Impact of varying degrees of renal dysfunction on transcatheter and surgical aortic valve replacement. J Thorac Cardiovasc Surg 2013; 146: 1399– 406; discussion 13406–7. [DOI] [PubMed] [Google Scholar]
- 24). Yamamoto M, Hayashida K, Mouillet G, et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol 2013; 62: 869– 77. [DOI] [PubMed] [Google Scholar]
- 25). Allende R, Webb JG, Munoz-Garcia AJ, et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur Heart J 2014; 35: 2685– 96. [DOI] [PubMed] [Google Scholar]
- 26). Ferro CJ, Chue CD, de Belder MA, et al. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): an analysis of the UK TAVI registry. Heart 2015; 101: 546– 52. [DOI] [PubMed] [Google Scholar]
- 27). Rahman MS, Sharma R, Brecker SJD. Transcatheter aortic valve implantation in patients with pre-existing chronic kidney disease. Int J Cardiol Heart Vasc 2015; 8: 9– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Codner P, Levi A, Gargiulo G, et al. Impact of renal dysfunction on results of transcatheter aortic valve replacement outcomes in a large multicenter cohort. Am J Cardiol 2016; 118: 1888– 96. [DOI] [PubMed] [Google Scholar]
- 29). Thourani VH, Forcillo J, Beohar N, et al. Impact of preoperative chronic kidney disease in 2,531 high-risk and inoperable patients undergoing transcatheter aortic valve replacement in the PARTNER trial. Ann Thorac Surg 2016; 102: 1172– 80. [DOI] [PubMed] [Google Scholar]
- 30). Gupta T, Goel K, Kolte D, et al. Association of chronic kidney disease with in-hospital outcomes of transcatheter aortic valve replacement. JACC Cardiovasc Interv 2017; 10: 2050– 60. [DOI] [PubMed] [Google Scholar]
- 31). Hansen JW, Foy A, Yadav P, et al. Death and dialysis after transcatheter aortic valve replacement: an analysis of the STS/ACC TVT registry. JACC Cardiovasc Interv 2017; 10: 2064– 75. [DOI] [PubMed] [Google Scholar]
- 32). Lüders F, Kaier K, Kaleschke G, et al. Association of CKD with outcomes among patients undergoing transcatheter aortic valve implantation. Clin J Am Soc Nephrol 2017; 12: 718– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Franzone A, Stortecky S, Pilgrim T, et al. Incidence and impact of renal dysfunction on clinical outcomes after transcatheter aortic valve implantation. Int J Cardiol 2018; 250: 73– 9. [DOI] [PubMed] [Google Scholar]
- 34). Pineda AM, Kevin Harrison J, Kleiman NS, et al. Clinical impact of baseline chronic kidney disease in patients undergoing transcatheter or surgical aortic valve replacement. Catheter Cardiovasc Interv 2019; 93: 740– 8. [DOI] [PubMed] [Google Scholar]
- 35). Bandyopadhyay D, Sartori S, Baber U, et al. The impact of chronic kidney disease in women undergoing transcatheter aortic valve replacement: analysis from the Women’s INternational Transcatheter Aortic Valve Implantation (WIN-TAVI) registry. Catheter Cardiovasc Interv 2020; 96: 198– 207. [DOI] [PubMed] [Google Scholar]
- 36). Gracia E, Wang TY, Callahan S, et al. Impact of severity of chronic kidney disease on management and outcomes following transcatheter aortic valve replacement with newer-generation transcatheter valves. J Invasive Cardiol 2020; 32: 25– 9. [DOI] [PubMed] [Google Scholar]
- 37). Li SX, Patel NK, Flannery LD, et al. Impact of bleeding after transcatheter aortic valve replacement in patients with chronic kidney disease. Catheter Cardiovasc Interv 2021; 97: E172– 8. [DOI] [PubMed] [Google Scholar]
- 38). Yap JJ, Tay JC, Ewe SH, et al. Impact of chronic kidney disease on outcomes in transcatheter aortic valve implantation. Ann Acad Med Singap 2020; 49: 273– 84. [PubMed] [Google Scholar]
- 39). Mohananey D, Griffin BP, Svensson LG, et al. Comparative outcomes of patients with advanced renal dysfunction undergoing transcatheter aortic valve replacement in the United States from 2011 to 2014. Circ Cardiovasc Interv 2017; 10: e005477. [DOI] [PubMed] [Google Scholar]
- 40). Allende R, Webb JG, Munoz-Garcia AJ, et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur Heart J 2014; 35: 2685– 96. [DOI] [PubMed] [Google Scholar]
- 41). Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189: 77– 8. [DOI] [PubMed] [Google Scholar]
- 42). Oguri A, Yamamoto M, Mouillet G, et al. Impact of chronic kidney disease on the outcomes of transcatheter aortic valve implantation: results from the FRANCE 2 registry. EuroIntervention 2015; 10: e1– 9. [DOI] [PubMed] [Google Scholar]
- 43). Bohbot Y, Candellier A, Diouf M, et al. Severe aortic stenosis and chronic kidney disease: outcomes and impact of aortic valve replacement. J Am Heart Assoc 2020; 9: e017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Lutz J, Menke J, Sollinger D, et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant 2014; 29: 29– 40. [DOI] [PubMed] [Google Scholar]