Abstract
Quinupristin-dalfopristin (Q-D), which is active against bacteria and Toxoplasma gondii, was examined for its activity against Pneumocystis carinii. After 72 h of incubation with rat P. carinii in an ATP cytotoxicity assay, the 50% inhibitory concentration of Q-D was 10.6 μg/ml, a level that can be achieved in serum with high-dose administration. Q-D administered intraperitoneally at doses of 50 to 200 mg per kg of body weight per day in the treatment and 100 mg/kg/day three times per week in the prophylaxis of pneumocystosis in immunosuppressed mice reduced the organism burden up to 15- and 302-fold, respectively. We conclude that Q-D has activity against P. carinii in vitro and in vivo.
Pneumocystis carinii is a leading cause of pneumonia in patients infected with human immunodeficiency virus and in other immunocompromised hosts. Anti-P. carinii drugs in clinical use (e.g., trimethoprim-sulfamethoxazole [TMP-SXT]), which have been available for many years, were originally developed to treat infections other than pneumocystosis (16, 27). Their development for use against P. carinii came mainly from rat and mouse models of pneumocystosis, which are reliable predictors of activity against the disease in humans (1, 9, 10, 22, 23, 28, 29, 30, 31). Recently, progress has also been made with in vitro techniques to screen drugs using rat P. carinii as the test organism (12, 13, 17, 32). Problems associated with anti-P. carinii agents include limited efficacy, increased toxicity, and developing resistance (16, 18, 26, 27). Given the lack of interest among pharmaceutical companies in developing new compounds, another strategy has been to investigate existing drugs used for other purposes for activity against the organism.
The present study is an example of the latter approach and involved quinupristin-dalfopristin (Q-D), a fixed combination of semisynthetic streptogramins which has been marketed primarily for the treatment of serious resistant enterococcal infections. Q-D has a spectrum of activity similar to that of azalides, ketolides, and macrolides (2, 5, 15, 21). A recent study has shown that similar to these compounds, Q-D is active against Toxoplasma gondii (19). Since drugs active against T. gondii are also active against P. carinii, we examined the effects of Q-D on P. carinii by in vitro and in vivo techniques.
P. carinii organisms used in vitro studies were purified from infected, immunosuppressed rats as described previously (8, 12, 13, 32). As determined by contour-clamped homogeneous electric field analysis, the organism preparations were predominantly P. carinii f. sp. carinii form 1; microscopically, about 95% of the developmental stages were trophs, and 5% were cysts. The organisms were cryopreserved, stored in liquid nitrogen, and cultured for contaminants before use. Q-D in powdered form for vitro testing was kindly provided by the manufacturer (Rhone Poulenc Rorer, Collegeville, Pa.). Candidate drugs were prepared in RPMI culture medium with dimethyl sulfoxide (final concentration, <0.2% [vol/vol]). They were then evaluated for their effects on P. carinii by the previously described ATP cytotoxicity assay, a bioluminescence reaction that measures the viability of the organism preparation maintained ex vivo for short periods of time. Organisms (108/ml, measured as total nuclei) were added with the drug or as controls (medium alone) in triplicate, and the ATP content was sampled after 24, 48, and 72 h of incubation at 35°C in 5% CO2. The ATP content was measured by the luciferin-luciferase assay with an AutoLumat LB 953 luminometer (Wallac, Inc., Gaithersburg, Md.) and expressed as relative light units. Activity of the drugs tested against P. carinii was expressed as the concentration of the agent needed to lower the ATP level by 50% compared to the level of the control (IC50). Pentamidine (2 μg/ml) and ampicillin (10 μg/ml) were routinely included as positive and negative control drugs, respectively, in the assay. The drugs were also analyzed for their potential human toxicity by studying their effects on A549 cells, a human lung carcinoma cell line. The IC50 of the drug for the A549 cells was compared with the IC50 for P. carinii. Based on their IC50s, drugs were classified using an activity scale ranging from very marked (<0.1 μg/ml) to inactive (≥50 μg/ml) (12, 32). Descriptive statistical analysis and the histograms were developed using GraphPad (San Francisco, Calif.) Software for Science.
Adult C3H/HeN mice (Charles River, Hollister, Calif.) were housed with infected mice under barrier conditions and administered corticosteroids to induce pneumocystosis as described (31, 32). When the infection reached moderate intensity (after 6 to 7 weeks), the mice were randomly divided into treatment and control groups. Q-D was administered intraperitoneally (i.p.) once daily and compared with TMP-SXT, the standard drug, which was given by oral gavage. The drugs were continued for 3 weeks, during which time the mice remained on the immunosuppressive regimen. Control animals receiving steroids (C-S) received no treatment or a placebo. Prophylaxis studies were similar to the treatment studies except that the drugs were administered throughout immunosuppression.
Drug effectiveness was based on lowering organism burden rather than survival, because some mice died from causes (e.g., drug toxicity) other than pneumocystosis (28, 29, 31, 32). The animals had to receive treatment for at least 7 days to be included in the data analysis, because it usually takes this long to observe an effect. The right lung was homogenized and stained with cresyl echt violet, which selectively stains P. carinii cysts, and the organisms were quantitated on a blinded basis. The lower limit of detection is 2.23 × 104 (log10 4.35) cysts/lung. The organism counts in the treatment and prophylaxis groups were compared with those in the C-S group. Statistical analysis for normally distributed data consisted of analysis of variance followed by Student's Neuman-Keuls test for multiple comparisons using GraphPad software. Nonparametric statistics were performed using the Kruskal-Wallis test followed by Dunn's multiple comparison test. The α value was set at 0.05. Drug activity was also classified using a scale ranging from ≥1,000-fold reduction (very marked) to <5-fold reduction (inactive) (28, 29, 32).
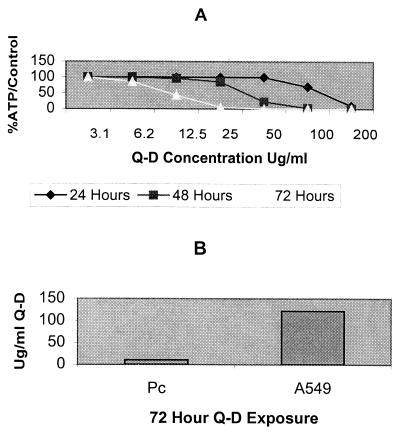
Q-D reduced the ATP pools in P. carinii in a dose- and time-dependent manner (Fig. 1A). Maximal effect was seen at 72 h with an IC50 of 10.6 μg/ml, which amounts to slight to moderate activity on our scoring scale. Toxicity evaluation revealed that the IC50 of Q-D for A549 cells was 122.0 μg/ml, compared with an IC50 of 10.6 μg/ml for P. carinii at 72 h, a >10-fold difference (Fig. 1B).
FIG. 1.
(A) Effects of different concentrations of Q-D on ATP levels in P. carinii at different time points as calculated by linear regression. The mean values of three separate experiments are presented. The mean IC50s were 116.6 μg/ml at 24 h, 22.1 μg/ml at 48 h, and 10.6 μg/ml at 72 h. Pentamidine, the positive control drug, reduced the ATP content by 90% at 24 h, 90% at 48 h, and 98% at 72 h in the three experiments. Ampicillin, the negative control drug, lowered the ATP content by 0% at 24 h, 2% at 48 h, and 2% at 72 h. (B) Effects of Q-D on ATP levels in P. carinii and the human lung carcinoma cell line A549 after 72 h of exposure. The IC50 of the drug for P. carinii was 10.6 μg/ml, compared with an IC50 of 122.0 μg/ml for A549 cells.
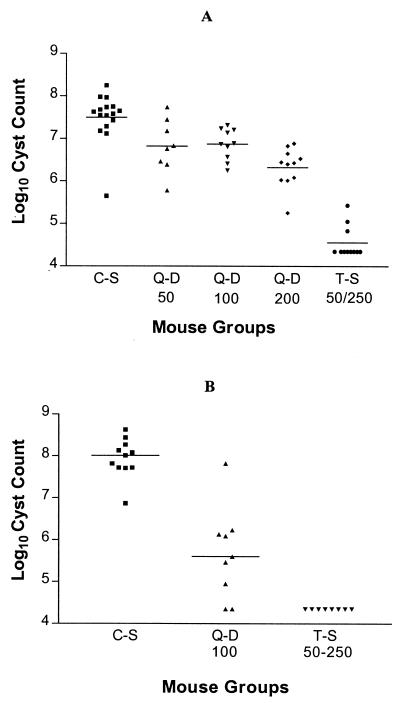
Q-D doses of 50 and 100 mg per kg of body weight per day in the treatment of pneumocystosis lowered the mean organism cyst count four- to fivefold, from log10 7.49 ± 0.57/lung in the C-S group to log10 6.82 ± 0.63 and log10 6.87 ± 0.37/lung, respectively (P < 0.01) (Fig. 2A). Q-D at 200 mg/kg/day reduced the organism count 15-fold (moderate activity), to log10 6.32 ± 0.46/lung (P < 0.001). This was greater than the reduction with the 50- and 100-mg/kg/day doses (P < 0.05). TMP-SXT at 50/250 mg/kg/day lowered the organism count 851-fold (marked activity), to log10 4.56 ± 0.38/lung (P < 0.001). This was greater than that achieved with the 200-mg/kg/day dose of Q-D (P < 0.001).
FIG. 2.
(A) Effects of drug treatment on P. carinii cyst counts in immunosuppressed mice with pneumocystosis. Q-D 50, Q-D 100, and Q-D 200, Q-D at 50, 100, and 200 mg/kg/day i.p.; T-S 50–250, TMP-SXT at 50/250 mg/kg/day orally. Horizontal lines show the means. (B) Effects of drug prophylaxis on P. carinii cyst counts in immunosuppressed mice. Q-D 100, Q-D 100 mg/kg/day i.p. three times per week; T-S 50–250, TMP-SXT at 50/250 μg/kg/day orally. Horizontal lines show the medians.
Nonparametric statistics were used to analyze the data for the prophylaxis experiment. Q-D administered i.p. at 100 mg/kg/day three times per week decreased the median P. carinii cyst count 302-fold (marked activity), from log10 8.01 cysts/lung in the C-S group to 5.53 organisms/lung (P < 0.01). For comparison purposes, TMP-SXT at 50/250 mg/kg/day as treatment during the last 3 weeks of the study lowered the median organism cyst count 4,570-fold (very marked activity) to undetectable levels (≤log10 4.35 cysts/lung) (P < 0.001). The differences between the results obtained with Q-D and TMP-SXT were not statistically significant.
Q-D caused transient, dose-related lethargy in the mice for the first few days but was otherwise well tolerated in both experiments. Q-D at 500 mg/kg/day i.p. resulted in the death of the mice within minutes after administration.
The in vitro data in the present study suggest that Q-D is less active against P. carinii than against T. gondii in tissue culture (19) and is also less active than standard anti-P. carinii drugs against the organism in the ATP assay (12, 13). However, the activity of Q-D is similar to that of some inhibitors of sterol biosynthesis, which are potential P. carinii drug targets (10, 11, 17, 25). Q-D administered to humans at higher-than-clinically-used doses (12.6 to 29.4 mg/kg) achieves levels in serum (10.7 to 24.2 μg/ml) that exceed the IC50 of 10.6 μg/ml (3).
The slight to moderate activity of Q-D in the treatment of established pneumocystosis in mice correlated well with the in vitro data. Q-D was much more effective in P. carinii prophylaxis, a finding that is consistent with our previous experience of studying drugs in this manner (29). Our results with Q-D were obtained in a model of chronic infection, whereas most studies of the drug have been performed in models of acute infection (6, 7). However, since Q-D given subcutaneously at 120 mg/kg results in a level in serum of 13.2 μg/ml (4), it seems likely that we achieved levels in serum that exceed the IC50 for P. carinii with our i.p. doses of 100 to 200 mg/kg.
Judging from its IC50 and need for parenteral administration, it appears unlikely that Q-D will be developed for its anti-P. carinii properties in humans. The present report is important because it extends studies of Q-D to a new organism and new type of animal model. More potent anti-P. carinii agents may be discovered among other streptogramins and, with the aid of the P. carinii genome project (24), may bring new insights into the mechanism of drug action. There may also be increased understanding of other interesting properties of Q-D, such as its short half-life, long postantibiotic effect, concentration in macrophages, and suppression of cytokine responses (2, 3, 4, 5, 6, 7, 14, 20, 21).
Acknowledgments
This study was supported by the Medical Research Service, Department of Veterans Affairs, and by Public Service contract AI 75319 and grant RO1 HL64570 from the National Institutes of Health.
We thank Randy Thomas and Diane Gillotte for excellent assistance.
REFERENCES
- 1.Bartlett M S, Queener S, Shaw M, Richardson J, Smith J. Pneumocystis carinii is resistant to imidazole antifungal agents. Antimicrob Agents Chemother. 1994;38:1859–1861. doi: 10.1128/aac.38.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebear C, Bouanchaud D H. A review of the in-vitro activity of quinupristin/dalfopristin against intracellular pathogens and mycoplasmas. J Antimicrob Chemother. 1997;39:59–62. doi: 10.1093/jac/39.suppl_1.59. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron M, Montay G. The pharmacokinetics of quinupristin/dalfopristin in laboratory animals and in humans. J Antimicrob Chemother. 1997;39:129–138. doi: 10.1093/jac/39.suppl_1.129. [DOI] [PubMed] [Google Scholar]
- 4.Berthaud N, Montay G, Conrad B J, Desnottes J F. Bacterial activity and kinetics of RP 59500 in a mouse model of Staphylococcus aureus septicaemia. J Antimicrob Chemother. 1995;36:365–373. doi: 10.1093/jac/36.2.365. [DOI] [PubMed] [Google Scholar]
- 5.Bryson H M, Spencer C M. Quinupristin-dalfopristin. Drugs. 1996;52:406–415. doi: 10.2165/00003495-199652030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Carbon C. Quinupristin/dalfopristin: a review of its activity in experimental animal models of infection. J Antimicrob Chemother. 1997;39:115–119. doi: 10.1093/jac/39.suppl_1.115. [DOI] [PubMed] [Google Scholar]
- 7.Carbon C. Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogens. Clin Infect Dis. 1998;27:28–32. doi: 10.1086/514619. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Cushion M T. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J Clin Microbiol. 1994;32:2791–2800. doi: 10.1128/jcm.32.11.2791-2800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comley J C W, Sterling A M. Effect of atovaquone and atovaquone drug combinations on prophylaxis of Pneumocystis carinii pneumonia in SCID mice. Antimicrob Agents Chemother. 1995;39:806–811. doi: 10.1128/aac.39.4.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contini C, Colombo D, Cultrera R, Prini E, Sechi T, Angelici E, Canipari R. Employment of terbinafine against Pneumocystis carinii infection in rat models. Br J Dermatol. 1996;26:30–32. doi: 10.1111/j.1365-2133.1996.tb15657.x. [DOI] [PubMed] [Google Scholar]
- 11.Contini C, Mangnaro M, Romani R, Tzantzoglou S, Poggesi I, Vulio V, Delia S, De Simone C. Activity of terbinafine against Pneumocystis carinii in vitro and its efficacy in the treatment of experimental pneumonia. J Antimicrob Chemother. 1994;34:727–735. doi: 10.1093/jac/34.5.727. [DOI] [PubMed] [Google Scholar]
- 12.Cushion M T, Chen F, Kloepfer N. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob Agents Chemother. 1997;41:379–384. doi: 10.1128/aac.41.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushion M T, Collins M, Hazra B, Kaneshiro E S. Effects of atovaquone and diospyrin-based drugs on the cellular ATP of Pneumocystis carinii f. sp. carinii. Antimicrob Agents Chemother. 2000;44:713–719. doi: 10.1128/aac.44.3.713-719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desnottes J F, Diallo N. Cellular uptake and intracellular bactericidal activity of RP 59500 in murine macrophages. J Antimicrob Chemother. 1992;30:107–115. doi: 10.1093/jac/30.suppl_a.107. [DOI] [PubMed] [Google Scholar]
- 15.Eldestin P H, Edelstein M A. In vitro activity of quinupristin/dalfopristin (Synercid, RP 59500) against Legionella spp. Diagn Microbiol Infect Dis. 2000;36:49–52. doi: 10.1016/s0732-8893(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 16.Fishman J A. Treatment of infection due to Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1309–1314. doi: 10.1128/aac.42.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshiro E S, Collins M S, Cushion M T. Inhibitors of sterol biosynthesis and amphotericin B reduce the viability of Pneumocystis carinii f. sp. carinii. Antimicrob Agents Chemother. 2000;44:1630–1638. doi: 10.1128/aac.44.6.1630-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazanjian P, Armstrong W, Hossler P A, Burman W, Richardson J, Lee C H, Crane L, Katz J, Meshnick S R. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J Infect Dis. 2000;182:551–557. doi: 10.1086/315719. [DOI] [PubMed] [Google Scholar]
- 19.Khan A A, Slifer T R, Araujo F G, Remington J S. Quinupristin-dalfopristin is active against Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:2043–2045. doi: 10.1128/aac.43.8.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan A A, Slifer T R, Araujo F G, Remington J S. Effect of quinupristin/dalfopristin on production of cytokines by human monocytes. J Infect Dis. 2000;182:356–358. doi: 10.1086/315655. [DOI] [PubMed] [Google Scholar]
- 21.Lamb H M, Figgitt D P, Faulds D. Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections. Drugs. 1999;58:1061–1097. doi: 10.2165/00003495-199958060-00008. [DOI] [PubMed] [Google Scholar]
- 22.Powles M A, Liberator P, Anderson J, Karkhanis Y, Dropinski J F, Bouffard F A, Balkovec J M, Fujioka H, Aikawa M, McFadden D, Schmatz D. Efficacy of Mk-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1985–1989. doi: 10.1128/aac.42.8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmatz D M, Powles M A, McFadden D, Nollstadt K, Bouffard F A, Dropinski J F, Liberator P, Andersen J. New semisynthetic pneumocandins with improved efficacies against Pneumocystis carinii in the rat. Antimicrob Agents Chemother. 1995;39:1320–1323. doi: 10.1128/aac.39.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smulian G A, Sesterhenn T, Tanaka R, Cushion M T. Genomic organization of signal transduction genes surrounding a putative 7-transmembrane pheromone receptor of Pneumocystis carinii f. sp. carinii. Genetics. 2001;157:991–1002. doi: 10.1093/genetics/157.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbina J A, Visbal G, Conteras L M, McLaughlin G, Docampo R. Inhibitors of Δ24(25) sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob Agents Chemother. 1997;41:1428–1432. doi: 10.1128/aac.41.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker D J, Wakefield A E, Dohn M N, Miller R F, Baughman R P, Hossler P A, Bartlett M S, Smith J W, Kazanjian P, Meshnick S R. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 1998;178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 27.Walzer P D. Pneumocystis carinii. In: Mandell G L, Bennett J E, Dolin P, editors. Principles and practice of infectious diseases. 5th ed. New York, N.Y: Churchill Livingstone, Inc.; 2000. pp. 2781–2795. [Google Scholar]
- 28.Walzer P D, Foy J, Runck J, Steele P, White M, Klein R S, Otter B A, Sundberg R J. Guanylhydrazones in treatment of Pneumocystis carinii pneumonia in immunosuppressed rats. Antimicrob Agents Chemother. 1994;38:2572–2576. doi: 10.1128/aac.38.11.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walzer P D, Foy J, Steele P, White M. Treatment of experimental pneumocystosis: review of seven years of experience and development of a new system for classifying antimicrobial drugs. Antimicrob Agents Chemother. 1992;36:1943–1950. doi: 10.1128/aac.36.9.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walzer P D, Runck J, Orr S, Foy J, Steele P, White M. Clinically used antimicrobial drugs against experimental pneumocystosis singly and in combination: analysis of drug interactions and efficacies. Antimicrob Agents Chemother. 1997;41:242–250. doi: 10.1128/aac.41.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walzer P D, Runck J, Steele P, White M, Linke M J, Sidman C L. Immunodeficient and immunosuppressed mice as models to test anti-Pneumocystis carinii drugs. Antimicrob Agents Chemother. 1997;41:251–258. doi: 10.1128/aac.41.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walzer, P. D., A. Ashbaugh, M. Collins, and M. T. Cushion. Anti-human immunodeficiency virus drugs are ineffective against Pneumocystis carinii in vitro and in vivo. J. Infect. Dis., in press. [DOI] [PubMed]