Abstract
Background:
Atherosclerosis is the most common cause of the cardiovascular disease. Saffron is a traditional food that affects many diseases and disorders. Therefore, the aim of this study was to identify the effects of Saffron (Crocus sativus L.) on quality of life (QOL) and appetite in patients with atherosclerosis.
Materials and Methods:
This was a randomized, double-blind, placebo-controlled clinical trial. A total of 63 participants with atherosclerosis were recruited from Emam Sajjad Hospital, Valiasr Hospital, and Zafaranieyh Clinic in Tehran, Iran. The participants were divided randomly into two groups. Participants received 100 mg/d saffron or placebo capsule for 6 weeks. QOL and appetite levels were measured by the McNew QOL questionnaire, and visual analog scale questionnaire, respectively. Furthermore, anthropometric indices of participants were measured before and after the intervention.
Results:
Statistical analysis showed that there was a statistically significant difference between atherosclerosis patients who received placebo and those who consumed saffron in terms of the physical domain (P = 0.008) and social domain (P = 0.012) of QOL. In the saffron group increased score in Total score Macnew (P < 0.001), physical domain (P = 0.025), and social domain (P < 0.001) was significant after the intervention. Moreover, the consumption of saffron did not significantly affect emotional domains of QOL, and appetite levels
Conclusion:
Saffron may be considered as a novel agent in patients with atherosclerosis to improve the QOL. A great deal of further research will be needed to critically validate the efficacy of saffron and its mechanisms in atherosclerosis.
Keywords: Appetite levels, atherosclerosis, cardiovascular disease, quality of life, saffron
INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of death in the world, accounting for almost 17 million deaths annually.[1] According to the studies conducted in Iran, the mortality rate from CVD was 46%.[2] CVD is mainly caused by atherosclerosis. Atherosclerosis is a chronic inflammatory disorder which can cause myocardial infarction (commonly known as a heart attack), stroke, ischemic heart disease, and sudden cardiac death.[1]
Saffron (Crocus sativus Linnaeus) is an herbal medicine belonging to the Iridaceae family. It is cultivated in various countries, such as Iran, Greece, Morocco, India, Spain, and Italy. Iran produces around 80% of the world's total for saffron.[3] Saffron is used as a food seasoning in Iranian traditional medicine as a relief for stomach pain, digestive aid, antidepressants, and colic pains.[3] The biological activity of saffron is related to carotenoid components, such as crocin, crocetin, picrocrocin, and safranal.[4]
Quality of life (QOL) is defined as an individual's perception based on expectations and different dimensions of standards, such as physical, psychological, and social factors.[5] Undesirable QOL is associated with the severity of the disease, less survival, increasing the hospital length of stay, and reduced functional activity of cardiac patients. Physical constraints and progressive symptoms can be affected by CVDs, so this condition can reduce QOL.[6] Appetite can be controlled by several organs, such as the brain, liver, and gastrointestinal system. The appetite and energy homeostasis is regulated in the hypothalamus. Inflammation is associated with appetite disorders.[7] Most previous studies showed that the saffron components in CVD patients decreased appetite, increased QOL, and improved depression.[7,8]
In the present study, we aimed to investigate the potential effects of saffron and its bioactive components on QOL and the appetite in patients with atherosclerosis. Given the useful effects of saffron on several diseases, such as CVDs, depression, etc; therefore, this clinical trial aimed to explore the relationship between saffron consumption and atherosclerosis that may improve the QOL and appetite levels.
MATERIALS AND METHODS
Study design and study population
Sixty-three out of 75 patients were willing to participate in the study. Male and female patients with atherosclerosis, aged 30–60 years were referred to Vali-e-Asr Hospital, Emam Sajjad Hospital, and Zafaranieh Clinic in Tehran, Iran. The atherosclerosis was confirmed by a cardiologist based on the results of angiography (coronary artery disease < 70% with the medical follow-up). At the beginning of the study, participants signed a consent form. Using the random allocation software, the patients were randomly divided into two groups: (1) Saffron group and (2) the placebo group. The participants in both groups were matched for age, sex, and body mass index.
Sample size
The required sample size with 95% confidence and 80% power based on the effect size 1.32 in the previous study for each group was 27 participants.[9] Due to the possibility of sample loss, 20% was added to the sample and 30 samples were estimated for each group. Finally, participants were randomly allocated to each group.
Saffron with high quality was purchased from Marand farms in East Azerbaijan and prepared at the Nutrition research center, Tabriz University of Medical Sciences. According to previous studies conducted on heart patients, the dose of this plant was selected 100 mg.[10] Supplementary capsules for the intervention group contained 50 mg of saffron and for the placebo group contained corn starch as placebo. Each patient received 2 capsules twice a day for 6 weeks.
Trial procedure
Patients should not change the treatment method as much as possible 2 months before the study. They did not consume any antioxidant and anti-inflammatory supplements at least 1 month before the start of the study. The study exclusion criteria were hepatic, renal or hematologic diseases, and infectious diseases, cancer, allergies, use of corticosteroids and immunosuppressive drugs, pregnancy, and lactation.
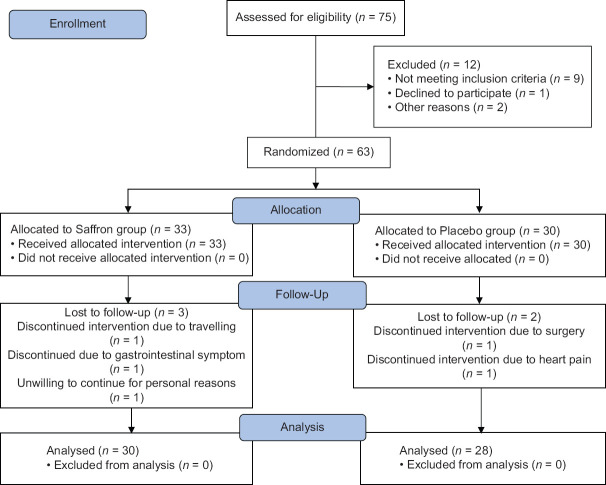
Before the intervention, demographic information (such as age, education, occupation, marital status, smoking, follow-up diet, duration of atherosclerosis, other diseases, types of drugs, and dietary supplements) was collected from the participants. The appetite questionnaire and McNew QOL questionnaire were completed by both groups and anthropometric measurements (including height, weight, waist circumference, hip circumference) were performed by the researcher. During the intervention, patients continued their diet, physical activity, and usual lifestyle. The patients were followed up by telephone once a week to ensure the complementary supplementation and emphasis on supplemental intake. Six months after the end of the intervention program, the questionnaires were completed again. Fifty-eight out of 63 patients participating in the study completed the study. Figure 1 shows the flowchart of the study.[11]
Figure 1.
Flowchart of design and protocol of the study[11]
The study protocol was approved by the Committee of Clinical Research Ethics of Tabriz University of Medical Sciences (TBZMED. REC.1394.841), and registered on the Iranian clinical trials website (IRCT201511192017N25).
Appetite status
The Visual analog scale questionnaire was used to assess the appetite levels. This questionnaire included 6 questions to express hunger, satiety, a particular desire for food, etc., by using a 100-millimeter line at the end of the line, depending on the question mark of the phrases between “no appetite” at one end and “uncontrollable appetite” at the other, with low, average, high, and very high points in between. The volunteers checked the questions before and at the end of the intervention.
The questionnaire was developed by Flint et al.[12] in the Fredrik Leaf School of Dentistry in Denmark, and its reliability was assessed in Iran's internal research and Cronbach's alpha value was 0.8.[13]
McNew Questionnaire
A specific questionnaire on QOL for cardiovascular patients (McNew Questionnaire) was used to assess the QOL of the study groups. This questionnaire was designed to evaluate the effects of cardiac diseases, especially coronary heart disease, on the physical, emotional, and social activities of the patients. The McNew questionnaire was applied to evaluate the patient's status for 2 weeks ago. The average response time for respondents was 10 min. 27 questions were completed by patients at the beginning and end of the study. A Seven-point Likert scale was used for questions and the response showed their position that included “always” to “not” at all. The highest score in each domain is 7 and the lowest score is 1, indicating high QOL and low QOL, respectively. Questions were categorized in three domains: Emotional functioning (n = 11 items), physical performance (n = 11 items), and social function (n = 13 items). Five questions in the physical domain were used to assess the symptoms of the disease, such as chest pain, dyspnea, fatigue, dizziness, and pain. The questions of the questionnaire were classified into one, two, or all three domains. The final score of QOL is calculated by obtaining the average of all questions. In Höfer et al.’ s study, a test-retest method was used to measure the reliability.[14] In Iran, Asadi-Lari et al. used this questionnaire to assess the QOL of heart patients.[15] Furthermore, the rate of coronary artery disease was examined as a factor determining QOL.
Statistical analysis
Statistical analysis was performed using SPSS (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). The outliers were identified by the Box plot method. The normal distribution of data was explored by using the Kolmogorov-Smirnov test. Paired t-test was used for finding the difference between before and after intervention and Independent t-test was used for evaluating placebo and Saffron groups. P < 0.05 was considered statistically significant. All variables were presented as Mean ± standard error of the mean.
RESULTS
Fifty-eight out of 63 patients participating in the study completed the study. In saffron Group 3 patients Lost to follow-up due to discontinued intervention due to traveling (n = 1), discontinued due to gastrointestinal symptom (n = 1), and unwilling to continue for personal reasons (n = 1). In placebo Group 2 patients lost to follow-up due to discontinued intervention due to surgery (n = 1) and discontinued intervention due to heart pain (n = 1). The general and clinical features of the participants are presented in Table 1. There was no statistically significant difference between the two groups in terms of age, sex, marital status, education level, weight variation, history of diagnosis, duration of coronary artery disease, and smoking.
Table 1.
The baseline demographic and clinical characteristics of two groups
| Group | Mean±SEM | P | |
|---|---|---|---|
|
| |||
| Variables | Intervention | Placebo | |
| Age | 51.6±1.18 | 53.71±1.33 | 0.092* |
| Height | 165.97±1.61 | 166.43±1.99 | 0.856* |
| Weight change | 1.33±0.15 | 1.46±0.13 | 0.523* |
| Weight change (%) | |||
| Increase | 7 (23) | 3 (10) | 0.339** |
| Decrease | 6 (20) | 9 (32) | |
| Stable | 17 (57) | 16 (58) | |
| Marital status (%) | |||
| Single | 0 (0) | 2 (7) | 0.36*** |
| Married | 27 (90) | 25 (89) | |
| Etc. | 3 (10) | 1 (4) | |
| Gender (%) | |||
| Male | 17 (57) | 12 (43) | 0.293** |
| Female | 13 (43) | 16 (57) | |
| Cigarette (%) | |||
| No | 28 (93) | 27 (96) | 0.526*** |
| Yes | 2 (7) | 1 (4) | |
| Past medical history (%) | |||
| No | 16 (54) | 19 (68) | 0.259** |
| Yes | 14 (46) | 9 (32) | |
| Duration heart disease (year) (%) | |||
| <1 | 14 (46) | 14 (50) | 0.504** |
| More than 1 | 16 (54) | 14 (50) | |
*Independent t-test; **Chi-square test; ***Fisher’s exact test. SEM=Standard error of the mean
As shown in Table 2, the mean total Macnew QOL score, emotional domain, physical domain, and social domain were increased in those who consumed saffron and decreased in the placebo group. Statistical analysis showed that there was a statistically significant difference between atherosclerosis patients who received placebo and those who consumed saffron in terms of the physical domain (P = 0.008) and social domain (P = 0.012) of QOL. Moreover, the consumption of saffron did not significantly affect emotional domains of QOL. In the saffron group increased score in Total score Macnew (P < 0.001), Physical domain (P = 0.025), and Social domain (P < 0.001) was significant after intervention at the end of the study.
Table 2.
Macnew questioner data in study groups
| Group | Mean±SE | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Time | Intervention group | Placebo group | Intervention* | Placebo* | Groups** | ||
|
|
|
||||||
| Variables | Before | After | Before | After | |||
| Total score macnew | 132.77±5.02 | 148.77±3.7 | 137.46±3.71 | 130.14±4.18 | 0.001 | 0.025 | 0.324 |
| Emotional domain | 64.67±2.92 | 73.9±2.36 | 66.89±2.15 | 62.86±2.46 | 0.059 | 0.088 | 0.065 |
| Physical domain | 70.5±2.65 | 79.9±1.79 | 73.07±2.01 | 67.5±2.71 | 0.025 | 0.083 | 0.008 |
| Social domain | 71.8±2.5 | 77.87±1.82 | 74.89±1.96 | 71.57±2.44 | 0.001 | 0.117 | 0.012 |
*Paired t-test; **Independent t-test. P<0.05 is significant. All data are expressed as mean±SE. SE=Standard error
Table 3 shows the appetite status for intervention and placebo groups. In the saffron group, the mean of hunger, the tendency to use the next promise, the tendency to consume salt, the tendency to consume sweet substances, and the tendency to use fatty substances were decreased and the mean of satiety was increased. The average hunger, tendency to consume saltines, and the tendency to use fatty substances were increased in the placebo group and the average sense of satiety was decreased, but no statistically significant difference was found in appetite status.
Table 3.
Appetite questioner parameters in study groups
| Group | Mean±SE | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Time | Intervention group | Placebo group | Intervention* | Placebo* | Groups** | ||
|
|
|
||||||
| Variables | Before | After | Before | After | |||
| hunger | 4.27±0.49 | 3.87±0.44 | 4.61±0.53 | 5.04±0.46 | 0.723 | 0.678 | 0.572 |
| Satiety | 5.47±0.51 | 6.03±0.49 | 6.07±0.35 | 5.36±0.45 | 0.521 | 0.345 | 0.385 |
| The desire to next meal | 6.1±0.44 | 5.23±0.47 | 5±0 | 4.75±0.08 | 0.545 | 0.725 | 0.649 |
| The tendency to consume salt | 2.57±0.47 | 2.53±0.52 | 2.46±0.43 | 2.79±0.43 | 0.785 | 0.632 | 0.652 |
| The tendency to consume sweet substances | 5.73±0.55 | 4.97±0.4 | 4.75±0.58 | 4.46±0.59 | 0.345 | 0.428 | 0.671 |
| The tendency to use fatty substances | 4.43±0.55 | 3.73±0.56 | 3.18±0.49 | 3.57±0.43 | 0.648 | 0.632 | 0.712 |
*Paired t-test; **Independent t-test. P<0.05 is significant. All data are expressed as mean±SE. SE=Standard error
DISCUSSION
In the present study, the intervention of saffron supplement improved Total score Macnew, physical domain, and social domain of QOL. Furthermore, saffron caused some changes in appetite status in atherosclerosis patients; however, these changes were not statistically significant. Increasing the consumption of saffron and carotenoids had positive effects on the QOL and appetite levels in patients. The consumption of saffron had no side effects. Saffron is dose-dependent supplement, and its higher dose is more efficient over a longer period. Different diets in various cultures can improve health or increase the risk of atherosclerosis.[16] Diet changes, physical activity, and stopping smoking can help you to prevent atherosclerosis. Medication will be needed to control the disease if lifestyle changes are not effective.[17] CVDs, especially coronary artery disease, have been identified as an important factor contributing to the QOL. Low QOL in patients led to pathogenicity and death.[18] Health-related QOL is associated with several factors, such as age, sex, level of education, physical activity, smoking, and abdominal obesity in CAD patients.[19] Several studies reported low QOL in women with CAD.[20] Patients with atherosclerosis suffer from disabilities in the physical, sexual, and social fields leading to mental problems, such as anxiety and depression.[21] Physical disability, sexual dysfunction, and psychological disorders led to lower levels of QOL. Therefore, an intervention leading to better physical, sexual, and psychological performance improved the level of QOL associated with individual health.
Abedimanesh et al. examined the effects of saffron aqueous extract and crocin on QOL in 58 patients with CAD. McNew-specific questionnaire was used to evaluate the QOL in cardiovascular patients. The results showed that the saffron aqueous extract and Crocin significantly improved the QOL in these patients.[8] Shahmansouri et al. conducted a clinical trial on patients with CAD and investigated the effects of saffron (30 mg daily) and fluoxetine on depression of patients for 6 weeks after percutaneous coronary intervention. Depression was evaluated using Hamilton's Depression Questioner. The results of Shahmansouri et al.'s study showed that saffron capsules like fluoxetine reduced depression in patients.[22] Previous studies compared the effectiveness of saffron with antidepressants, such as Selective Serotonin Reuptake Inhibitor (SSRI) and Tricyclic antidepressant. It seems that saffron with various mechanisms improved the mood and reduced the level of depression by modulating neurotransmitters in the brain such as serotonin, dopamine, glutamate, and norepinephrine.[23] SSRI, the best medication for depression, is commonly used to treat CAD patients. Obviously, these drugs have several side effects and heart problems,[24] while saffron can be effective like many antidepressants without side effects. Recently, another mechanism has been proposed for the role of saffron in reducing depression. The enzyme monoamine oxidase 1 is inhibited by some antidepressants. Talaei et al.'s study showed that saffron acted as an antidepressant by inhibiting monoamine oxidase enzymes. Therefore, saffron can effectively control many mental and emotional disorders.[25] CAD patients typically complained about sexual problems. Fluoxetine, as a type of SSRI drugs, causes sexual dysfunction in the long-term and increases sexual problems.[26] Regarding the effects of saffron on appetite, Crocin, and Crocetin, as inhibitors of pancreatic lipase, reduced the fat absorption from the diet.[27] Saffron affects appetite and food intake, and also anti-obesity effects of saffron contribute to antioxidant effects and increasing glucose and fat metabolism.[28] Crocin, as an important component of saffron, can influence appetite and food intake by affecting the intestine or brain mechanism. Crocin can be transformed into Crocetin during absorption from narrow intestine cells entering the bloodstream. Crocetin can also cross the blood-brain barrier and possibly affect the brain and starvation centers and alter appetite.[29]
The study of Mashmoul et al. aimed at investigating the effects of different doses of saffron hydroalcoholic extract and crocin, in comparison with orlistat weight loss drugs in rats. Obese rats were gavaged with saffron, crocin, and orlistat for 8 weeks. Compared to crocin, the saffron extract significantly decreased food intake and appetite in animals in the 2nd month of the study. This finding suggests that other factors, except for crocin, such as safranal, in the saffron extract can reduce appetite and food intake.[30]
In the study of Kianbakht and Dabaghian, all doses of saffron extract (25, 50, 100, 200 mg/kg) and crocin (5, 15, 30, 50 mg/kg) significantly reduced the effective intake of food,[29] while in the study of Mashmoul et al. a higher dose (80 mg/kg) was more effective.[30] These findings suggest that the effect of the saffron is dose-dependent. Comparison of our results with those of previous studies revealed that the consumption of saffron caused changes in appetite status and increased the satiety score. These changes were not statistically significant but were clinically significant.
The strength of our study was the high acceptance of saffron in atherosclerosis patients without side effects. This study included some limitations. Firstly, we did not examine the effects of various doses of saffron extracts, such as crosin, crocetin, and safranal. Second, the duration of intervention was short. Therefore, more studies are needed to assess the impact of saffron in the long-term.
CONCLUSIONS
The results of this study showed that the consumption of saffron could improve some aspects of QOL in patients with atherosclerosis. However, some results were not significant due to the probability of the effect of saffron in a dose-dependent manner and the short duration of 6 weeks. Furhermore, changes were observed in appetite status. Our findings suggested that saffron, as a nutrition adjuvant therapy, may be beneficial in the treatment of atherosclerosis. Further studies using a longer supplementation period and larger sample size are needed to detect precise mechanisms and other positive aspects of saffron in atherosclerosis patients.
Informed consent
Written informed consent was obtained from all participants included in the study.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Committee of Clinical Research Ethics of Tabriz University of Medical Sciences, Iran (Ethical code: TBZMED. REC.1395.1345), and registered at the Iranian registry of clinical trials (IRCT201511192017N25).
Financial support and sponsorship
The authors are grateful to the Nutrition Research Center of Tabriz University of Medical Sciences for their financial support (5/71/1207).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bekkering S, Joosten LA, van der Meer JW, Netea MG, Riksen NP. The epigenetic memory of monocytes and macrophages as a novel drug target in atherosclerosis. Clin Ther. 2015;37:914–23. doi: 10.1016/j.clinthera.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Sarrafzadegan N, Mohammmadifard N. Cardiovascular disease in Iran in the last 40 years: Prevalence, mortality, morbidity, challenges and strategies for cardiovascular prevention. Arch Iran Med. 2019;22:204–10. [PubMed] [Google Scholar]
- 3.Rahaiee S, Moini S, Hashemi M, Shojaosadati SA. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J Food Sci Technol. 2015;52:1881–8. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaglio R, Gentile C, Bonanno A, Vintaloro L, Perrone A, Mazza F, et al. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. International Journal of Dairy Technology. 2019;72:208–17. [Google Scholar]
- 5.Mostafavi S, Saeidi M, Heidari H. Effects of a comprehensive cardiac rehabilitation program on the quality of life of patients with cardio-vascular diseases. Journal of Research in Rehabilitation Sciences. 2012;7(4) [Google Scholar]
- 6.Yousefi P, Sabzevari S, Mohammadalizade S, Haghdoost A. Study of quality of life in heart failure hospitalized patients in Kerman medical university hospital in 2008. 2011;6:59–67. [Google Scholar]
- 7.Abedimanesh N, Bathaie SZ, Abedimanesh S, Motlagh B, Separham A, Ostadrahimi A. Saffron and crocin improved appetite, dietary intakes and body composition in patients with coronary artery disease. J Cardiovasc Thorac Res. 2017;9:200–8. doi: 10.15171/jcvtr.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abedimanesh N, Ostadrahimi A, Bathaie SZ, Abedimanesh S, Motlagh B, Jafarabadi MA, et al. Effects of saffron aqueous extract and its main constituent, crocin, on health-related quality of life, depression, and sexual desire in coronary artery disease patients: A double-blind, placebo-controlled, randomized clinical trial. Iranian Red Crescent Medical Journal. 2017;19(9) [Google Scholar]
- 9.Fadai F, Mousavi B, Ashtari Z, Ali beigi N, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry. 2014;47:156–61. doi: 10.1055/s-0034-1382001. [DOI] [PubMed] [Google Scholar]
- 10.Verma SK, Bordia A. Antioxidant property of Saffron in man. Indian J Med Sci. 1998;52:205–7. [PubMed] [Google Scholar]
- 11.Khatir SA, Bayatian A, Barzegari A, Roshanravan N, Safaiyan A, Pavon-Djavid G, et al. Saffron (Crocus sativus L.) Supplements Modulate Circulating MicroRNA (miR-21) in Atherosclerosis Patients; A Randomized, Double-Blind, Placebo-Controlled Trial. Iranian Red Crescent Medical Journal. 2018;20(10) [Google Scholar]
- 12.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 13.Yarahmadi H, Hamedinia M, Haghighi A, Jahandide A, Taher Z. The Effect of one session moderate and heavy resistance exercise on the appetite, food intake and energy expenditure in healthy men. Daneshvar Medicine. 2020;18:51–60. [Google Scholar]
- 14.Höfer S, Lim L, Guyatt G, Oldridge N. The MacNew heart disease health-related quality of life instrument: A summary. Health Qual Life Outcomes. 2004;2:3. doi: 10.1186/1477-7525-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asadi-Lari M, Javadi HR, Melville M, Oldridge NB, Gray D. Adaptation of the MacNew quality of life questionnaire after myocardial infarction in an Iranian population. Health Qual Life Outcomes. 2003;1:23. doi: 10.1186/1477-7525-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres N, Guevara-Cruz M, Velázquez-Villegas LA, Tovar AR. Nutrition and atherosclerosis. Arch Med Res. 2015;46:408–26. doi: 10.1016/j.arcmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Bergheanu S, Bodde M, Jukema J. Pathophysiology and treatment of atherosclerosis. Neth Heart J. 2017;25:231–42. doi: 10.1007/s12471-017-0959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohar A, Gijsberts CM, Haitjema S, Pasterkamp G, de Kleijn DP, Asselbergs FW, et al. Health-related quality of life and outcome in atherosclerosis – Does sex matter? Int J Cardiol. 2016;212:303–6. doi: 10.1016/j.ijcard.2016.03.149. [DOI] [PubMed] [Google Scholar]
- 19.De Smedt D, Clays E, Annemans L, Doyle F, Kotseva K, Pająk A, et al. Health related quality of life in coronary patients and its association with their cardiovascular risk profile: Results from the EUROASPIRE III survey. Int J Cardiol. 2013;168:898–903. doi: 10.1016/j.ijcard.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Dueñas M, Ramirez C, Arana R, Failde I. Gender differences and determinants of health related quality of life in coronary patients: A follow-up study. BMC Cardiovasc Disord. 2011;11:24. doi: 10.1186/1471-2261-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZJ, Guo M, Si TM, Jiang MM, Liu SM, Liu YY, et al. Association of depression with adverse cardiovascular events after percutaneous coronary intervention. Coron Artery Dis. 2013;24:589–95. doi: 10.1097/MCA.0b013e3283650234. [DOI] [PubMed] [Google Scholar]
- 22.Shahmansouri N, Farokhnia M, Abbasi SH, Kassaian SE, Noorbala Tafti AA, Gougol A, et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J Affect Disord. 2014;155:216–22. doi: 10.1016/j.jad.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50:761–86. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 24.Pizzi C, Rutjes AW, Costa GM, Fontana F, Mezzetti A, Manzoli L. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol. 2011;107:972–9. doi: 10.1016/j.amjcard.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Talaei A, Hassanpour Moghadam M, Sajadi Tabassi SA, Mohajeri SA. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord. 2015;174:51–6. doi: 10.1016/j.jad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Kashani L, Raisi F, Saroukhani S, Sohrabi H, Modabbernia A, Nasehi AA, et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebo-controlled study. Hum Psychopharmacol. 2013;28:54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 27.Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–22. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Mashmoul M, Azlan A, Khaza’ai H, Yusof BN, Noor SM. Saffron: A natural potent antioxidant as a promising anti-obesity drug. Antioxidants. 2013;2:293–308. doi: 10.3390/antiox2040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kianbakht S, Dabaghian FH. Anti-obesity and anorectic effects of saffron and its constituent crocin in obese Wistar rat. J Med Plants. 2015;1:25–33. [Google Scholar]
- 30.Mashmoul M, Azlan A, Yusof BNM, Khaza’ai H, Mohtarrudin N, Boroushaki MT. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. journal of functional foods. 2014;8:180–7. [Google Scholar]