Abstract
Background
Left atrial (LA) and left ventricular (LV) structural and functional parameters have independent prognostic values as predictors of atrial fibrillation (AF).
Purpose
To investigate the prognostic value of a left atrioventricular coupling index (LACI) and average annualized change in LACI (hereafter, ΔLACI) measured by cardiac MRI to predict incident AF in a population-based sample from the Multi-Ethnic Study of Atherosclerosis (MESA).
Materials and Methods
In a secondary analysis of the prospective MESA, 1911 study participants without clinically recognized AF and cardiovascular disease at baseline had LACI assessed with cardiac MRI at baseline (examination 1, 2000–2002) and 10 years later (examination 5, 2010–2012). LACI was defined as the ratio of LA to LV end-diastolic volumes. Univariable and multivariable Cox proportional hazard models were used to evaluate the associations of LACI and average ΔLACI with incident AF.
Results
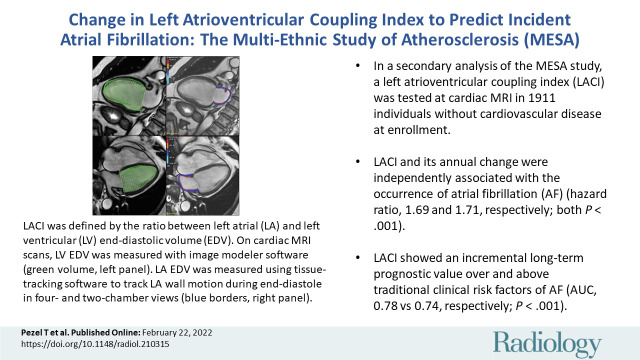
Among the 1911 participants (mean age, 59 years ± 9 [standard deviation]; 907 men), 87 incident AF events occurred over 3.9 years ± 0.9 after the second imaging (examination 5). After adjustment for traditional risk factors, greater LACI and ΔLACI were independently associated with AF (hazard ratio, 1.69 [95% CI: 1.46, 1.96] and 1.71 [95% CI: 1.50, 1.94], respectively; both P < .001). Adjusted models for LACI and ΔLACI showed improvement in model discrimination compared with currently used AF risk score (Cohort for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation, or CHARGE-AF, score) model (area under receiver operating characteristic curve [AUC], 0.78 vs 0.74; and AUC, 0.80 vs 0.74, respectively; both P < .001); and to the final model including individual LA or LV parameters for predicting AF incidence (AUC, 0.78 vs 0.76; and AUC, 0.80 vs 0.78, respectively; both P < .001).
Conclusion
Atrioventricular coupling (left atrioventricular coupling index [LACI]) and coupling change (annual change in LACI) were strong predictors for atrial fibrillation (AF) in a multiethnic population. Both had incremental prognostic value for predicting AF over traditional risk factors, and superior discrimination compared with the Cohort for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation, or CHARGE-AF, score and to individual left atrial or left ventricular parameters.
© RSNA, 2022
Online supplemental material is available for this article.
See also the editorial by Leiner in this issue.
Summary
In individuals without cardiovascular disease, the left atrioventricular coupling index and its annual change have incremental prognostic value to predict incident atrial fibrillation over traditional risk factors.
Key Results
■ In a secondary analysis of the prospective Multi-Ethnic Study of Atherosclerosis (MESA), a left atrioventricular coupling index (LACI) was tested at cardiac MRI in 1911 individuals without cardiovascular disease at enrollment.
■ LACI and its annual change were independently associated with the occurrence of atrial fibrillation (AF) (hazard ratio, 1.69 and 1.71, respectively; both P < .001).
■ LACI showed an incremental long-term prognostic value over and above traditional clinical risk factors of AF (area under receiver operating characteristic curve, 0.78 vs 0.74, respectively; P < .001).
Introduction
A trial fibrillation (AF) is the most common cardiac arrhythmia; 6–12 million people will be diagnosed with this condition in the United States by 2050 and 17.9 million in Europe by 2060 (1). AF is therefore a public health problem with important economic burden caused by morbidity and mortality associated with increased risks of stroke and heart failure (1–4).
To address the need for early detection of participants at risk for AF, several studies have assessed left atrial (LA) structure and function by cardiac MRI (5–7). Indeed, LA volumes, peak LA strain, and LA ejection fractions have shown prognostic value in predicting the occurrence of AF independent of traditional risk factors (5,6). These findings suggest that adverse LA remodeling facilitates initiation of AF by promoting ectopic triggers and altering the wavelength of re-entrant circuits (7). However, many studies emphasize that AF does not occur exclusively because of adverse LA remodeling (8–11). Indeed, left ventricular (LV) diastolic dysfunction has been established as a prognosticator of AF (8–11). Therefore, LV diastolic dysfunction may impair left heart performance by uncoupling functional performances of the two chambers leading to AF. Atrioventricular coupling is complex because LA and LV chamber filling, emptying, and active contraction are not synchronous.
Although LA and LV parameters have independent prognostic values for predicting AF, the inherently connected physiologic relationship between the LA and the LV (12,13) suggests that the assessment of left atrioventricular coupling alterations could better reflect left heart dysfunction and be a better predictor of incident AF.
Previous studies have also shown the superiority of longitudinal evaluations of change in LA parameters to predict AF (6,14–16). Therefore, longitudinal assessment of atrioventricular coupling could be more effective in risk stratification of incident AF among healthy individuals. On the basis of this rationale, we designed an analysis to examine the associations of the left atrioventricular coupling index (LACI) and change in LACI with incident AF in a prospective population study of individuals without history of heart disease at baseline. Specifically, we aimed to investigate the prognostic value of LACI and the average annualized change in LACI (hereafter, ΔLACI) measured at cardiac MRI, for predicting incident AF in the Multi-Ethnic Study of Atherosclerosis (MESA).
Materials and Methods
Study Sample
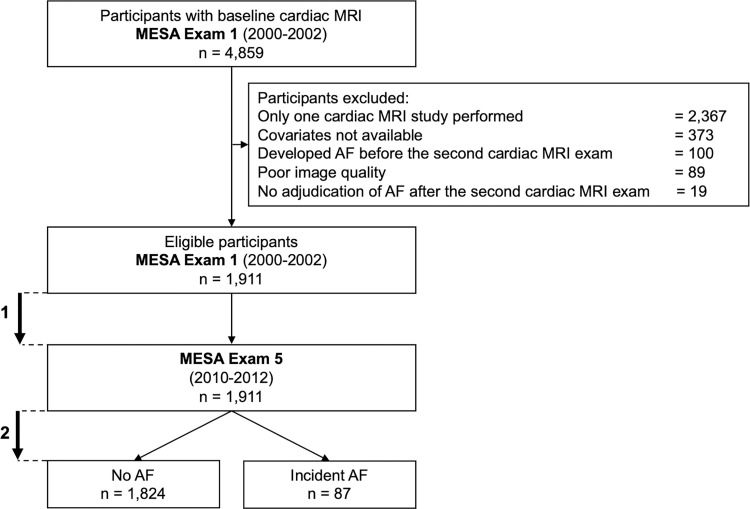
The MESA (ClinicalTrials.gov identifier: NCT00005487) is a prospective, population-based multiethnic (White, African American, Chinese American, and Hispanic) cohort study of subclinical cardiovascular disease, and the study design was previously described in detail (https://www.mesa-nhlbi.org) (17). In summary, between 2000 and 2002 (examination 1), 6814 men and women from age 45 to 84 years and free of clinical cardiovascular disease at enrollment were recruited from six U.S. field centers (Baltimore, Md; Chicago, Ill; Forsyth County, NC; Los Angeles County, Calif; Northern Manhattan, NY; and St Paul, Minn). Examination 1 was followed by examinations 2 (years 2002–2004), 3 (years 2004–2005), 4 (years 2005–2007), and 5 (years 2010–2012). The methods of baseline characteristics and outcome collection are detailed in Appendix E1 (online). In this secondary analysis of the prospective MESA, we included all consecutive participants with at least two cardiovascular MRI examinations (examinations 1 and 5 after 10 years). Participants provided written informed consent. Study protocols were approved by the institutional review boards of each participating field center, with Health Insurance Portability and Accountability Act guidelines compliance.
Participants were excluded if they did not undergo the second cardiac MRI examination, their images were missing or not of sufficient quality to allow measurement of LA and LV volumes, or they developed incident AF between examinations 1 and 5 (Fig 1).
Figure 1:
Flowchart of the study. Mean time between baseline and second cardiac MRI examinations, 9.6 years ± 0.4 (1). Mean time of AF follow-up: 3.9 years ± 0.9 after the second cardiac MRI examination (2). AF = atrial fibrillation, MESA = Multi-Ethnic Study of Atherosclerosis.
Cardiac MRI Protocol
The MESA cardiac MRI protocol has been described in detail previously (18). Briefly, cardiac MRI was performed at six MESA field centers by using 1.5‐T scanners (Appendix E2 [online]). Long-axis cine images were obtained at two- and four-chamber views by using electrocardiography-gated fast gradient-echo pulse sequences. A stack of short-axis cine images were acquired to encompass both ventricles, and LV end-diastolic volume was measured by using cardiac image modeler software (CIM version 6.0; University of Auckland, New Zealand). The cine images were acquired with a temporal resolution of approximately 50 msec. Cardiac MRI examinations were performed only in patients in sinus rhythm. MRI sequence parameters are detailed in Table E1 (online).
Image Analysis
Images were read at the central MESA cardiac MRI review center at Johns Hopkins University (Baltimore, Md) (Appendix E2 [online]). Multimodality tissue tracking software (MTT version 6.0; Toshiba Medical Systems) was used to quantify LA volume and strain at two- and four-chamber cine cardiac MRI (Appendix E3 [online]). This method has been validated previously with good to excellent intra- and interreader reproducibility (intraclass correlation, 0.88–0.98; P < .001) and good interstudy reproducibility (intraclass correlation, 0.44–0.82; (P value range, .02 to <.001) (19,20).
LACI Definition
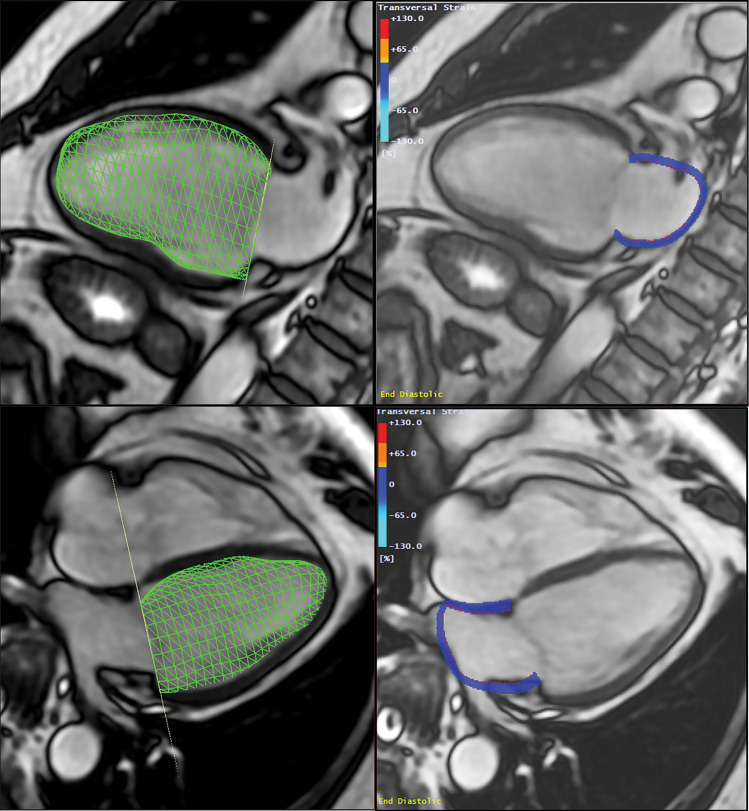
The LACI was defined for each participant by the ratio between the LA end-diastolic volume and the LV end-diastolic volume assessed at cardiac MRI (T.P., with 5 years of experience). The LV volume was measured from the stack of short-axis cine images, whereas the LA volume was measured from the two- and four-chamber views, as described previously (Fig 2). The LA and LV volumes were measured in the same end-diastolic phase defined by mitral valve closure.
Figure 2:
Method to assess the left atrioventricular coupling index (LACI) at cardiac MRI. LACI was defined by the ratio between the left atrial (LA) end-diastolic volume and the left ventricular (LV) end-diastolic volume. A stack of short-axis noncontrast cine MRI scans were acquired to encompass both ventricles, and LV end-diastolic volume was measured by using cardiac image modeler software (green volume, left panel). LA end-diastolic volume was measured by using multimodality tissue-tracking software to track LA wall motion during the end-diastole in the four-chamber and two-chamber views (pink borders, right panel).
The LACI value is expressed as a percentage, and a higher LACI indicates greater disproportion between the LA and LV volumes at ventricular end-diastole, which reflects greater impairment of left atrioventricular coupling. Moreover, the ΔLACI is defined by the annual difference in the LACI value measured at baseline, at examination 1 and the LACI value measured after 10 years at examination 5 (LACI10), and the ΔLACI value is expressed as a percentage per year.
Incident AF
Incident AF during the follow-up period was defined on the basis of study electrocardiograms and hospital discharge diagnosis International Classification of Diseases–9 codes, supplemented by Medicare claims (Appendix E4 [online]).
Statistical Analysis
Participant characteristics at baseline and after 10 years are presented as means for continuous variables and as counts and percentages for categorical variables (Table 1). Comparisons used the χ2 or Fisher exact test for categorical variables and the Student t test or Mann-Whitney Wilcoxon test, as appropriate, for continuous variables. We used Cox regression models to study the associations between the LACI, or ΔLACI, and the incident AF. The cumulative risk of incident AF over the follow-up years for the cohort, stratified by the LACI terciles, or ΔLACI terciles, was determined by using Kaplan-Meier curves.
Table 1:
Characteristics of Participants at Baseline and at Second Examination
The AF risk prediction model used was the Cohort for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation (CHARGE-AF) risk model (21). Two models were proposed. In model 1, we adjusted for the following CHARGE-AF risk factors at the second cardiac MRI examination after 10 years (ie, examination 5): age, sex, ethnicity, height, weight, systolic and diastolic blood pressure, use of antihypertensive medication, smoking status, diabetes, and the development of myocardial infarction and congestive heart failure (21). Model 2 included model 1 and the baseline value of the parameter assessed, measured for baseline differences when measuring change (22). The additional predictive value of the LACI and ΔLACI was calculated by the area under receiver operating characteristic curve increment compared with the CHARGE-AF score (21) and each LA or LV parameter. A two-tailed P value less than .05 was considered to indicate statistical significance. All data were analyzed (T.P. and C.O.W., with 25 years of experience) by using software (R version 3.6.1; R Project for Statistical Computing).
Results
Characteristics of Participants in MESA
Of the 4859 participants with baseline cardiac MRI records that included LA volume assessment (examination 1), 1911 participants (39.3%) returned to undergo a second cardiac MRI examination at examination 5 with LA, LV, and outcome data available after a mean time of 9.6 years ± 0.4 (standard deviation; mean age, 59 years ± 9; 907 men) (Fig 1). Among the 1911 patients who underwent a second cardiac examination at examination 5, 689 (36.1%) had hypertension with 568 (29.7%) administered antihypertensive therapy, 166 (8.7%) had diabetes mellitus, 230 (12.0%) were current smokers, and mean body mass index was 27.8 kg/m2 ± 4.9. The baseline characteristics of the study population at examination 5 after a mean time of 9.6 years ± 0.4, divided into those who developed AF or who did not, are presented in Table 1. After a mean follow-up time of 3.9 years ± 0.9 after the second cardiac MRI examination (examination 5), 87 participants developed incident AF. LA functional parameters were lower, and LV mass and LV volume was higher in participants with AF compared with those without AF.
LACI and Annualized Change in LACI
For the entire study sample, mean baseline LACI was 16.8% ± 7.9. At follow up, LACI10 was 25.5% ± 11.2 (mean ΔLACI, 1.2% ± 1.0 per year; Fig E1 [online]). ΔLACI and individual LA and LV parameters during 9.6 years ± 0.4 are shown in Table E2 (online). Whereas participants who developed AF had greater increase in LA volume (minimum ΔLAVI, 0.90 mL/m2 per year ± 1.07 vs 0.45 mL/m2 per year ± 0.76; P < .001) versus those who did not, LV end-diastolic volumes decreased similarly with aging in both groups (–0.59 mL/m2 per year ± 1.43 vs –0.65 mL/m2 per year ± 1.21; P = .70). Correlations between LA and LV end-diastolic volumes were low at both baseline and follow up (R2 = 0.15 and 0.10, respectively; both P < .001; Fig E2 [online]).
We found no evidence of a difference in mean LACI between women and men at baseline (LACI at baseline, 16.7% ± 8.3 vs 16.9% ± 7.5, respectively; P = .46). However, at follow up, mean LACI was higher in women than in men (LACI10, 26.2% ± 12.2 vs 24.8% ± 11.4, respectively; P = .01). Consistently, ΔLACI was higher in women than in men (1.01% per year ± 1.20 vs 0.84% per year ± 1.18, respectively; P = .002; Table E3 [online]).
LACI and Incident AF
The results of unadjusted and adjusted Cox proportional hazard models for LACI10 and LA and LV parameters measured after 10 years are in Table 2. LACI10 was positively associated with incident AF before and after adjustment for risk factors (adjusted hazard ratio [HR], 1.69; 95% CI: 1.46, 1.96; increment by 1 standard deviation; P < .001). LACI10 top tercile (LACI10 > 28.6%) was more strongly associated with AF incidence than the bottom tercile (<19.7%; log-rank P < .001; Fig 3A). By using an optimal cutoff point to predict incident AF (Fig E3 [online]), LACI10 greater than 30% was independently associated with incident AF before (HR, 2.62; 95% CI: 1.72, 4.00; P < .001) and after adjustment (adjusted HR, 1.91; 95% CI: 1.23, 2.95; P = .004; Fig 3B). LACI value at baseline measured at examination 1 was also independently associated with AF (HR, 1.81; 95% CI: 1.57, 2.01; P < .001; Table E4 [online]).
Table 2:
Univariable and Multivariable Analyses of Incident Atrial Fibrillation according to Left Atrioventricular Coupling Index and Other Left Atrial or Left Ventricular Parameters at Examination 5
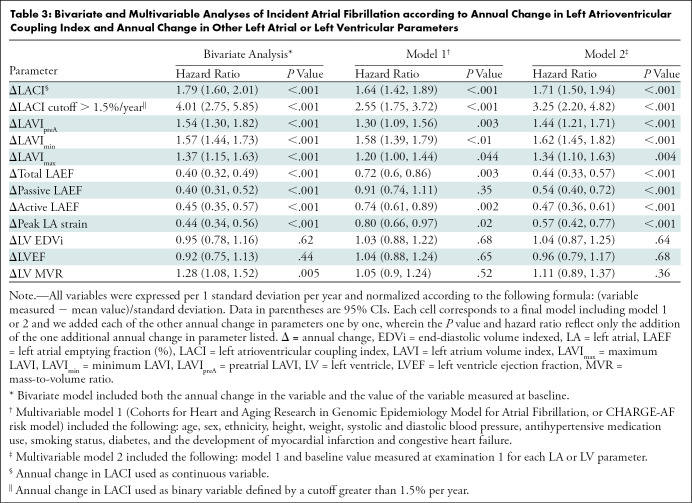
Figure 3:
Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified by (A) left atrioventricular coupling index (LACI) terciles and by (B) a LACI cutoff of 30%. (A) The cumulative hazard was greater in the third LACI value measured after 10 years (LACI10) tercile compared with the first tercile for incident AF (hazard ratio [HR], 2.48; 95% CI: 1.53, 3.87; P < .001). (B) The cumulative hazard was greater for participants with LACI10 greater than 30% compared with participants with LACI10 of 30% or less for incident AF (HR, 2.62; 95% CI: 1.72, 4.00; P < .001).
Annualized Change in LACI and Incident AF
Table 3 shows the results of bivariate and multivariable analyses for ΔLACI and main LA and LV parameters; unadjusted results are shown in Table E5 (online).
Table 3:
ivariate and Multivariable Analyses of Incident Atrial Fibrillation according to Annual Change in Left Atrioventricular Coupling Index and Annual Change in Other Left Atrial or Left Ventricular Parameters
Annual change in LACI was positively associated with AF before and after adjustment (LACI value at baseline HR, 1.76 [95% CI: 1.55, 2.00] and 1.79 [95% CI: 1.60, 2.01], respectively; both P < .001). After adjusting for CHARGE-AF risk factors (model 1) and LACI value at baseline (model 2), ΔLACI remained independently associated with incident AF (adjusted model 1 HR, 1.64 [95% CI: 1.42, 1.89] per 1 standard deviation increment; adjusted model 2 HR, 1.71 [95% CI: 1.50, 1.94] per 1 standard deviation increment; both P < .001). The top tercile (>1.3% per year) of ΔLACI was more strongly associated with incident AF than the bottom tercile (<0.4% per year; log-rank P < .001; Fig 4A).
Figure 4:
Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified by terciles of annual change (Δ) in left atrioventricular coupling index (LACI) (A) and by annual change in LACI with a cutoff of 1.5% per year (B). (A) The cumulative hazard was greater in the third tercile compared with the first tercile for incident AF (hazard ratio [HR], 2.52; 95% CI: 1.57, 3.96; P < .001). (B) The cumulative hazard was greater for participants with LACI greater than 1.5% per year compared with participants with annual change in LACI of 1.5% or less per year for incident AF (HR, 2.77; 95% CI: 1.82, 4.21; P < .001).
By using an optimal ΔLACI cutoff value greater than 1.5% per year to predict incident AF (Fig E4 [online]), an increase in ΔLACI greater than 1.5% per year remained independently associated with greater AF occurrence (adjusted model 1 HR, 2.55 [95% CI: 1.75, 3.72] per 1 standard deviation increment; adjusted model 2 HR, 3.25 [95% CI: 2.20, 4.82] per 1 standard deviation increment; both P < .001) (Fig 4B). In participants with LACI10 greater than 30%, the cumulative hazard was greater for participants with ΔLACI greater than 1.5% per year than for those with ΔLACI less than or equal to 1.5% per year (P < .001). However, among participants with LACI10 less than or equal to 30%, we found no evidence of differences between those with ΔLACI greater or less than 1.5% per year (P = .46) (Fig 5).
Figure 5:
Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified simultaneously by left atrioventricular coupling index (LACI) value measured after 10 years (LACI10) with a cutoff of 30% and an annual change (Δ) in LACI with a cutoff of 1.5% per year. In participants with a LACI10 greater than 30%, the cumulative hazard was greater for participants with annual change in LACI greater than 1.5% per year than for those with annual change LACI of 1.5% or less per year (hazard ratio [HR], 2.20; 95% CI: 1.08, 4.15; P < .001). However, among participants with LACI10 of 30% or less, we found no evidence of differences between those with annual change in LACI greater than or less than 1.5% per year (HR, 1.19; 95% CI: 0.87, 1.89; P = .46).
Atrioventricular Coupling Improvement of AF Risk Prediction
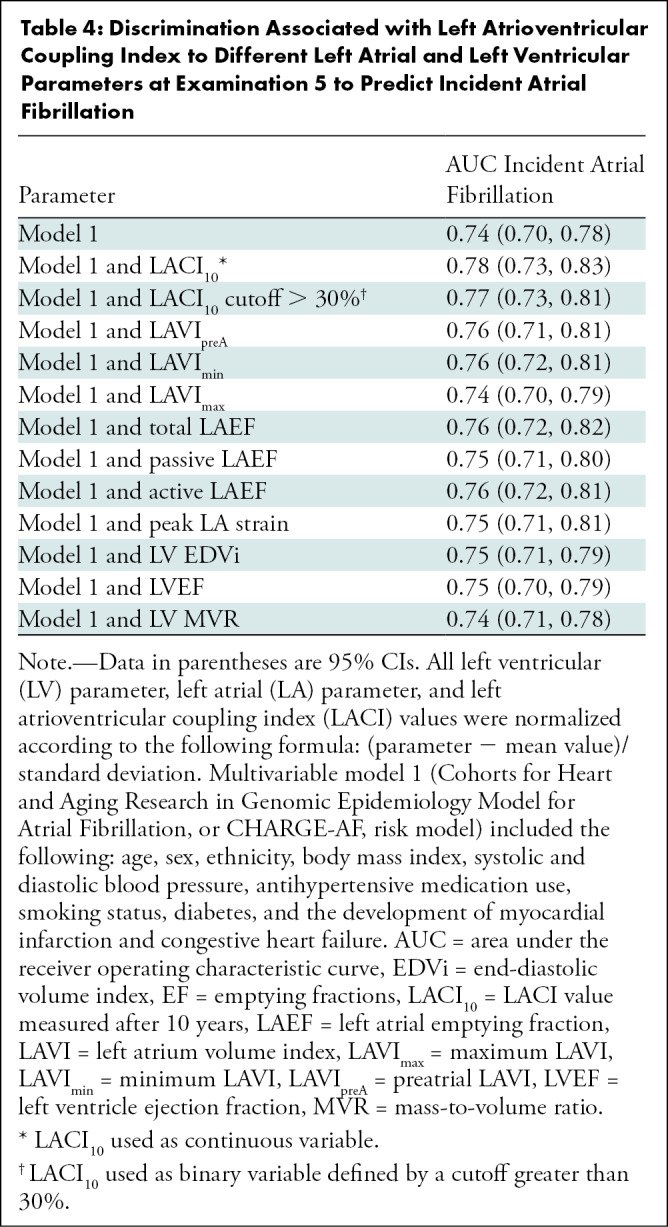
The multivariable model with the LACI10 showed improvement in model discrimination compared with the multivariable model with CHARGE-AF risk factors for predicting incident AF (area under receiver operating characteristic curve, 0.78 vs 0.74, respectively; P < .001). Follow-up examination LACI10 also showed better discrimination for incident AF than the multivariable model with individual LA or LV parameter and the CHARGE-AF score risk factors (Table 4).
Table 4:
Discrimination Associated with Left Atrioventricular Coupling Index to Different Left Atrial and Left Ventricular Parameters at Examination 5 to Predict Incident Atrial Fibrillation
Improvement in Risk Prediction with Addition of Average Annualized Change in LACI
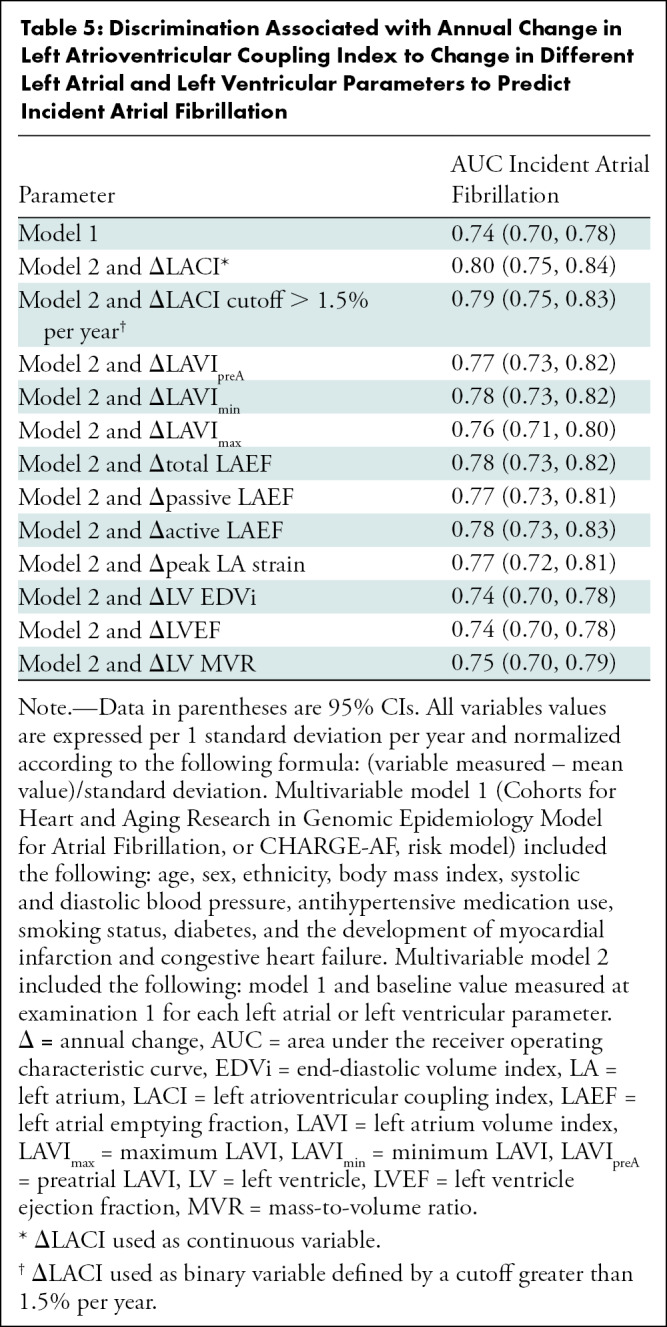
After adjustment, ΔLACI showed improvement in model discrimination compared with the multivariable model with CHARGE-AF risk factors for predicting incident AF (area under receiver operating characteristic curve, 0.80 vs 0.74, respectively; P < .001). ΔLACI also showed better discrimination for incident AF than did the multivariable model with average annualized changes in LA or LV parameters (Table 5).
Table 5:
Discrimination Associated with Annual Change in Left Atrioventricular Coupling Index to Change in Different Left Atrial and Left Ventricular Parameters to Predict Incident Atrial Fibrillation
Discussion
In our study, we demonstrated the predictive value of both a left atrioventricular coupling index (LACI) and the average annualized change in LACI (ΔLACI) for predicting incident atrial fibrillation (AF) in a multiethnic population free of clinical cardiovascular disease at enrollment. LACI and ΔLACI showed strong associations with incident AF (hazard ratio [HR] for both, 1.7; P < .001). Moreover, LACI and ΔLACI were stronger independent predictors of incident AF than the Cohort for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation (CHARGE-AF) score and individual left atrial (LA) or left ventricular (LV) parameters, resulting in improved discrimination for incident AF (area under receiver operating characteristic curve, 0.78 vs 0.74, respectively; and area under receiver operating characteristic curve, 0.80 vs 0.74, respectively; both P < .001). The increase in LA volume relative to that of the LV at end-diastole reflects impaired LV compliance, leading to a reduction of LA reservoir function, which were independent predictors of incident AF (23). We also investigated the best LACI and ΔLACI cutoff points to predict incident AF and found that LACI greater than 30% and ΔLACI greater than 1.5% per year were independently associated with incident AF (HR 1.91 and HR 3.25, both P < .001). Interestingly, the occurrence of AF was higher for participants with a ΔLACI greater than 1.5% per year than for participants with a ΔLACI less than or equal to 1.5% per year among participants with a LACI greater than 30% (8.8% vs 3.8%, respectively; P < .001). However, among participants with LACI less than or equal to 30%, we found no evidence of no differences between those with ΔLACI greater than 1.5% per year versus those with ΔLACI less than or equal to 1.5%% per year (2.5% vs 1.3%; P = .46). Whereas the Multi-Ethnic Study of Atherosclerosis showed the excellent prognostic value of CHARGE-AF score (24), our results suggest that the assessment of LACI and ΔLACI provides additional and relevant information to accurately stratify the risk of incident AF above CHARGE-AF risk factors in a population without known cardiovascular disease.
A previous report (7) suggested that adverse LA remodeling facilitates both initiation and maintenance of AF by promoting ectopic triggers and altering the wavelength of the re-entrant arrhythmic circuit. Knowing the crucial role of cardiac MRI data in predicting AF (25), LACI appears to reflect an earlier stage of LA remodeling than individual LA parameters, with stronger prognostic value for predicting incident AF before and after adjustment for traditional risk factors. In line with previous reports (8,10,11), these results suggest that AF may not occur exclusively because of impaired LA or LV structure or function but may also be susceptible to uncoupling of LA and LV structure and function as markers of early LV diastolic dysfunction or early LA myopathy.
Our findings are also consistent with those of previous echocardiography studies that assessed left atrioventricular coupling (26,27), and those of a previous cardiac MRI study performed in 40 healthy individuals that investigated the effects of aging on left atrioventricular coupling and LV filling (28). In that study, older individuals had larger LA and smaller LV volumes with larger LA-to-LV end-diastolic volume ratios (27% ± 6 vs 19% ± 3, respectively; P < .001) and preserved LV ejection fraction. Moreover, in a canine model of early-stage hypertensive heart failure with preserved LV ejection fraction, left heart atrioventricular coupling assessed at cardiac MRI was impaired, and the curvilinear LA end-reservoir pressure-volume relationship was shifted upward and leftward, indicating reduced LA compliance (29). A recent study (30) described a LACI measured at echocardiography as a prognosticator of death in patients with heart failure with reduced LV ejection fraction or degenerative mitral disease and regurgitation.
Regarding the optimal time of the cardiac cycle at which the LACI should be assessed, some reports have emphasized the important interaction between LA and LV performance, particularly during the LV end diastole (12,13). Furthermore, a recent investigation has suggested that LA end-diastolic volume (31,32) or change in LA end-diastolic volume (14,33) is more closely correlated with diastolic function (13), and therefore more robust than maximal LA volume (at LV end systole) for predicting AF. However, a rise in LA end-diastolic volume (LA minimum volume) has been reported as a strong predictor of AF (14,31), reflecting the hemodynamic interactions between LA and LV during LV diastole (23). Indeed, at the beginning of LV diastole, passive filling begins as a rotating blood flow pattern within the LA, gradually decreasing to a halt when pressures between the two chambers equalize. This passive filling pattern generates an early diastolic blood flow vortex inside the LV that is stronger than the original flow rotational pattern from the LA. The resultant buildup of kinetic energy expands the LV to a greater diastolic volume than it would in the absence of such vortex phenomenon (34). Such mechanisms underlie the important hemodynamic interactions between LA and LV during LV diastole, possibly in part explaining the particular prognostic value of LACI.
Our study had limitations. First, LACI was investigated as a diagnostic tool for early detection of AF risk in asymptomatic participants without known cardiovascular disease. Hence, LACI may not be as an ideal assessment tool for participants with pronounced LA and LV enlargement secondary to advanced structural heart disease. For these reasons, the extension of our findings to populations with established cardiovascular disease requires further investigation, including probably the assessment of another LACI cutoff in these patients. Second, incident AF was on the basis of diagnosis discharge codes, which may underestimate AF incidence because many AF cases can be asymptomatic. Third, ΔLACI was averaged across 10 years, thus assuming linearity over time. This method may not have fully captured the variation in year-to-year measurements, therefore providing additional precedence for further investigation. Fourth, we used two-dimensional methods instead of three-dimensional methods to measure LA volumes from two-chamber and four-chamber cine MRI, which may have underestimated true volumes by 11.5%–20% (35). Moreover, these LA volumes were performed with only one software program, which limited the generalizability of the findings. Fifth, knowing that cardiovascular MRI is not a widely accessible test in routine, the use of LACI should be investigated in echocardiography, particularly with the advent of three-dimensional echocardiography. Finally, although the mechanisms by which incident AF is associated with left atrioventricular coupling impairment are not entirely elucidated by our observational data, our results may provide valuable clues to AF pathophysiologic structure in human populations.
In conclusion, in a large multiethnic population (ie, the Multi-Ethnic Study of Artherosclerosis) free of clinical cardiovascular disease at baseline, impaired left atrioventricular coupling reflected as greater left atrioventricular coupling index (LACI) and annualized change in LACI, or ΔLACI, measured at cardiac MRI were associated with higher risk of incident atrial fibrillation (AF) during a 4-year median follow-up. The addition of LACI and ΔLACI to risk prediction models for incident AF improved model discrimination for incident AF risk. Future studies should validate these findings to better understand the role of left atrioventricular coupling in AF pathophysiologic structure and risk prediction.
S.R.H. supported by the National Institutes of Health, American Heart Association grants. P.H. supported by the National Institutes of Health. J.A.C.L. supported by the National Institutes of Health.
Disclosures of Conflicts of Interest: T.P. No relevant relationships. B.A.V. Institutional grant from Cannon Medical Systems, Myocardial Solutions. T.Q. No relevant relationships. S.R.H. Data safety monitoring board for University at California, San Francisco. Y.K. No relevant relationships. H.D.d.V. Data safety monitoring board at Johns Hopkins. C.O.W. No relevant relationships. W.S.P. NIH grant to Johns Hopkins. P.H. No relevant relationships. D.A.B. Editor-in-chief of Radiology. J.A.C.L. Member of the Radiology editorial board.
Abbreviations:
- AF
- atrial fibrillation
- CHARGE-AF
- Cohort for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation
- HR
- hazard ratio
- LA
- left atrium
- LACI
- left atrioventricular coupling index
- LACI10
- LACI value measured after 10 years
- LV
- left ventricle
- MESA
- Multi-Ethnic Study of Atherosclerosis
References
- 1. Lippi G , Sanchis-Gomar F , Cervellin G . Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge . Int J Stroke 2021. ; 16 ( 2 ): 217 – 221 [Published correction appears in Int J Stroke 2020 Jan 28:1747493020905964]. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS , Alonso A , Benjamin EJ , et al . Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association . Circulation 2020. ; 141 ( 9 ): e139 – e596 . [DOI] [PubMed] [Google Scholar]
- 3. Staerk L , Sherer JA , Ko D , Benjamin EJ , Helm RH . Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes . Circ Res 2017. ; 120 ( 9 ): 1501 – 1517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung MK , Eckhardt LL , Chen LY , et al . Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association . Circulation 2020. ; 141 ( 16 ): e750 – e772 . [DOI] [PubMed] [Google Scholar]
- 5. Habibi M , Lima JAC , Khurram IM , et al . Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study . Circ Cardiovasc Imaging 2015. ; 8 ( 2 ): e002769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sardana M , Lessard D , Tsao CW , et al . Association of Left Atrial Function Index with Atrial Fibrillation and Cardiovascular Disease: The Framingham Offspring Study . J Am Heart Assoc 2018. ; 7 ( 7 ): e008435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nattel S , Burstein B , Dobrev D . Atrial remodeling and atrial fibrillation: mechanisms and implications . Circ Arrhythm Electrophysiol 2008. ; 1 ( 1 ): 62 – 73 . [DOI] [PubMed] [Google Scholar]
- 8. Tsang TSM , Gersh BJ , Appleton CP , et al . Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women . J Am Coll Cardiol 2002. ; 40 ( 9 ): 1636 – 1644 . [DOI] [PubMed] [Google Scholar]
- 9. Fatema K , Barnes ME , Bailey KR , et al . Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study . Eur J Echocardiogr 2009. ; 10 ( 2 ): 282 – 286 . [DOI] [PubMed] [Google Scholar]
- 10. Nagarakanti R , Ezekowitz M . Diastolic dysfunction and atrial fibrillation . J Interv Card Electrophysiol 2008. ; 22 ( 2 ): 111 – 118 . [DOI] [PubMed] [Google Scholar]
- 11. Tsang TSM , Barnes ME , Gersh BJ , Bailey KR , Seward JB . Risks for atrial fibrillation and congestive heart failure in patients >/ = 65 years of age with abnormal left ventricular diastolic relaxation . Am J Cardiol 2004. ; 93 ( 1 ): 54 – 58 . [DOI] [PubMed] [Google Scholar]
- 12. Bowman AW , Kovács SJ . Left atrial conduit volume is generated by deviation from the constant-volume state of the left heart: a combined MRI-echocardiographic study . Am J Physiol Heart Circ Physiol 2004. ; 286 ( 6 ): H2416 – H2424 . [DOI] [PubMed] [Google Scholar]
- 13. Barbier P , Solomon SB , Schiller NB , Glantz SA . Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function . Circulation 1999. ; 100 ( 4 ): 427 – 436 . [DOI] [PubMed] [Google Scholar]
- 14. Lim DJ , Ambale-Ventakesh B , Ostovaneh MR , et al . Change in left atrial function predicts incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis . Eur Heart J Cardiovasc Imaging 2019. ; 20 ( 9 ): 979 – 987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaziri SM , Larson MG , Benjamin EJ , Levy D . Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study . Circulation 1994. ; 89 ( 2 ): 724 – 730 . [DOI] [PubMed] [Google Scholar]
- 16. Thomas L , Abhayaratna WP . Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance . JACC Cardiovasc Imaging 2017. ; 10 ( 1 ): 65 – 77 . [DOI] [PubMed] [Google Scholar]
- 17. Bild DE , Bluemke DA , Burke GL , et al . Multi-Ethnic Study of Atherosclerosis: objectives and design . Am J Epidemiol 2002. ; 156 ( 9 ): 871 – 881 . [DOI] [PubMed] [Google Scholar]
- 18. Natori S , Lai S , Finn JP , et al . Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity . AJR Am J Roentgenol 2006. ; 186 ( 6 Suppl 2 ): S357 – S365 . [DOI] [PubMed] [Google Scholar]
- 19. Zareian M , Ciuffo L , Habibi M , et al . Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. To ACY , Flamm SD , Marwick TH , Klein AL . Clinical utility of multimodality LA imaging: assessment of size, function, and structure . JACC Cardiovasc Imaging 2011. ; 4 ( 7 ): 788 – 798 . [DOI] [PubMed] [Google Scholar]
- 21. Alonso A , Krijthe BP , Aspelund T , et al . Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium . J Am Heart Assoc 2013. ; 2 ( 2 ): e000102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yanez ND 3rd , Kronmal RA , Shemanski LR , Psaty BM ; Cardiovascular Health Study . A regression model for longitudinal change in the presence of measurement error . Ann Epidemiol 2002. ; 12 ( 1 ): 34 – 38 . [DOI] [PubMed] [Google Scholar]
- 23. Abhayaratna WP , Fatema K , Barnes ME , et al . Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age . Am J Cardiol 2008. ; 101 ( 11 ): 1626 – 1629 . [DOI] [PubMed] [Google Scholar]
- 24. Bundy JD , Heckbert SR , Chen LY , Lloyd-Jones DM , Greenland P . Evaluation of Risk Prediction Models of Atrial Fibrillation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) . Am J Cardiol 2020. ; 125 ( 1 ): 55 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arai AE . New Insights from Major Prospective Cohort Studies with Cardiovascular Magnetic Resonance (CMR) . Curr Cardiol Rep 2015. ; 17 ( 6 ): 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takeuchi M , Kitano T , Nabeshima Y , Otsuji Y , Otani K . Left ventricular and left atrial volume ratio assessed by three-dimensional echocardiography: Novel indices for evaluating age-related change in left heart chamber size . Physiol Rep 2019. ; 7 ( 23 ): e14300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spevack DM , Blum L , Malhotra D , et al . Ratio of left atrial to left ventricular size: an anatomical marker of the diastolic left ventricular pressure-volume relationship . Echocardiography 2008. ; 25 ( 4 ): 366 – 373 . [DOI] [PubMed] [Google Scholar]
- 28. Germans T , Götte MJW , Nijveldt R , et al . Effects of aging on left atrioventricular coupling and left ventricular filling assessed using cardiac magnetic resonance imaging in healthy subjects . Am J Cardiol 2007. ; 100 ( 1 ): 122 – 127 . [DOI] [PubMed] [Google Scholar]
- 29. Zakeri R , Moulay G , Chai Q , et al . Left Atrial Remodeling and Atrioventricular Coupling in a Canine Model of Early Heart Failure With Preserved Ejection Fraction . Circ Heart Fail 2016. ; 9 ( 10 ): e003238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benfari G , Essayagh B , Nistri S , et al . Left Atrial Volumetric/Mechanical Coupling Index: A Novel Predictor of Outcome in Heart Failure With Reduced Ejection Fraction . Circ Cardiovasc Imaging 2021. ; 14 ( 1 ): e011608 . [DOI] [PubMed] [Google Scholar]
- 31. Prasad SB , Guppy-Coles K , Stanton T , et al . Relation of Left Atrial Volumes in Patients With Myocardial Infarction to Left Ventricular Filling Pressures and Outcomes . Am J Cardiol 2019. ; 124 ( 3 ): 325 – 333 . [DOI] [PubMed] [Google Scholar]
- 32. Habibi M , Chahal H , Opdahl A , et al . Association of CMR-measured LA function with heart failure development: results from the MESA study . JACC Cardiovasc Imaging 2014. ; 7 ( 6 ): 570 – 579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habibi M , Samiei S , Ambale Venkatesh B , et al . Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis) . Circ Cardiovasc Imaging 2016. ; 9 ( 8 ): e004299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sengupta PP , Narula J . À LA mode atrioventricular mechanical coupling . JACC Cardiovasc Imaging 2014. ; 7 ( 1 ): 109 – 111 . [DOI] [PubMed] [Google Scholar]
- 35. Vardoulis O , Monney P , Bermano A , et al . Single breath-hold 3D measurement of left atrial volume using compressed sensing cardiovascular magnetic resonance and a non-model-based reconstruction approach . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]



![Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified by (A) left atrioventricular coupling index (LACI) terciles and by (B) a LACI cutoff of 30%. (A) The cumulative hazard was greater in the third LACI value measured after 10 years (LACI10) tercile compared with the first tercile for incident AF (hazard ratio [HR], 2.48; 95% CI: 1.53, 3.87; P < .001). (B) The cumulative hazard was greater for participants with LACI10 greater than 30% compared with participants with LACI10 of 30% or less for incident AF (HR, 2.62; 95% CI: 1.72, 4.00; P < .001).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7fcd/9081516/9e06eb67bab6/radiol.210315.fig3.jpg)
![Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified by terciles of annual change (Δ) in left atrioventricular coupling index (LACI) (A) and by annual change in LACI with a cutoff of 1.5% per year (B). (A) The cumulative hazard was greater in the third tercile compared with the first tercile for incident AF (hazard ratio [HR], 2.52; 95% CI: 1.57, 3.96; P < .001). (B) The cumulative hazard was greater for participants with LACI greater than 1.5% per year compared with participants with annual change in LACI of 1.5% or less per year for incident AF (HR, 2.77; 95% CI: 1.82, 4.21; P < .001).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7fcd/9081516/28dd8fc15490/radiol.210315.fig4.jpg)
![Kaplan-Meier survival curves for incident atrial fibrillation (AF) stratified simultaneously by left atrioventricular coupling index (LACI) value measured after 10 years (LACI 10) with a cutoff of 30% and an annual change (Δ) in LACI with a cutoff of 1.5% per year. In participants with a LACI10 greater than 30%, the cumulative hazard was greater for participants with annual change in LACI greater than 1.5% per year than for those with annual change LACI of 1.5% or less per year (hazard ratio [HR], 2.20; 95% CI: 1.08, 4.15; P < .001). However, among participants with LACI10 of 30% or less, we found no evidence of differences between those with annual change in LACI greater than or less than 1.5% per year (HR, 1.19; 95% CI: 0.87, 1.89; P = .46).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7fcd/9081516/82983071ebe1/radiol.210315.fig5.jpg)